Progress on Improved Fouling Resistance-Nanofibrous Membrane for Membrane Distillation: A Mini-Review
Abstract
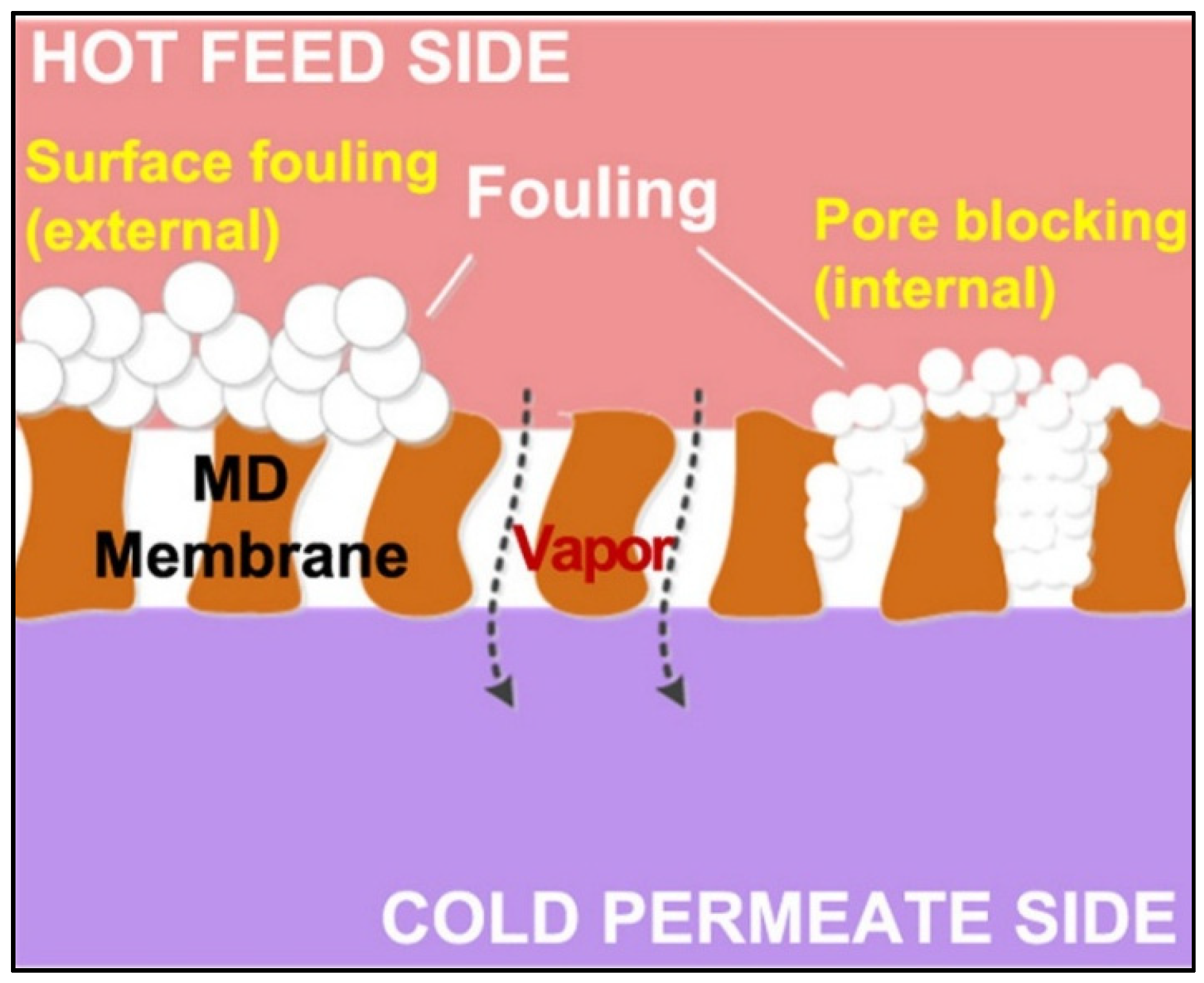
1. Overview of Fouling in Membrane Distillation
1.1. Organic Fouling
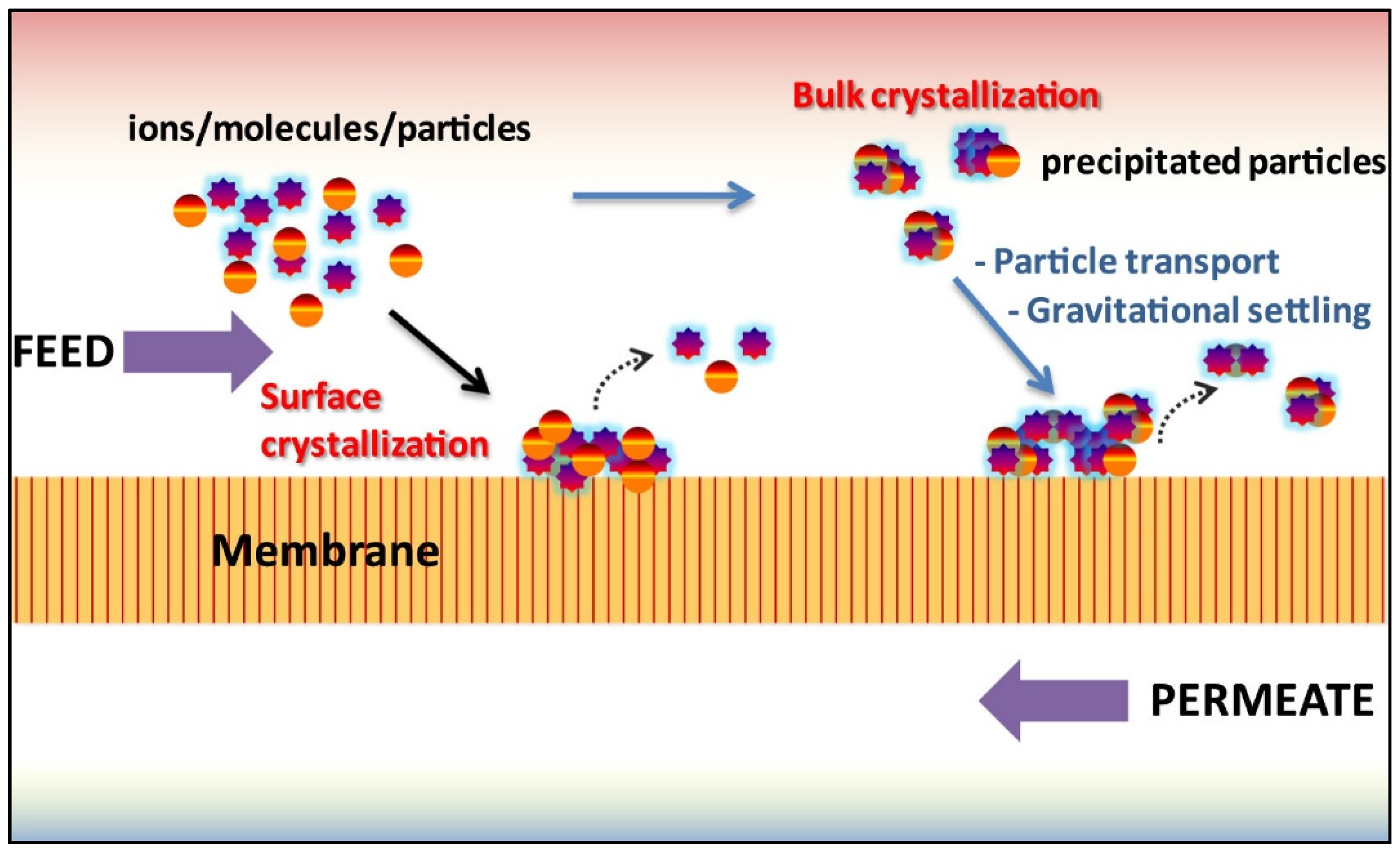
1.2. Inorganic Fouling
1.3. Biofouling
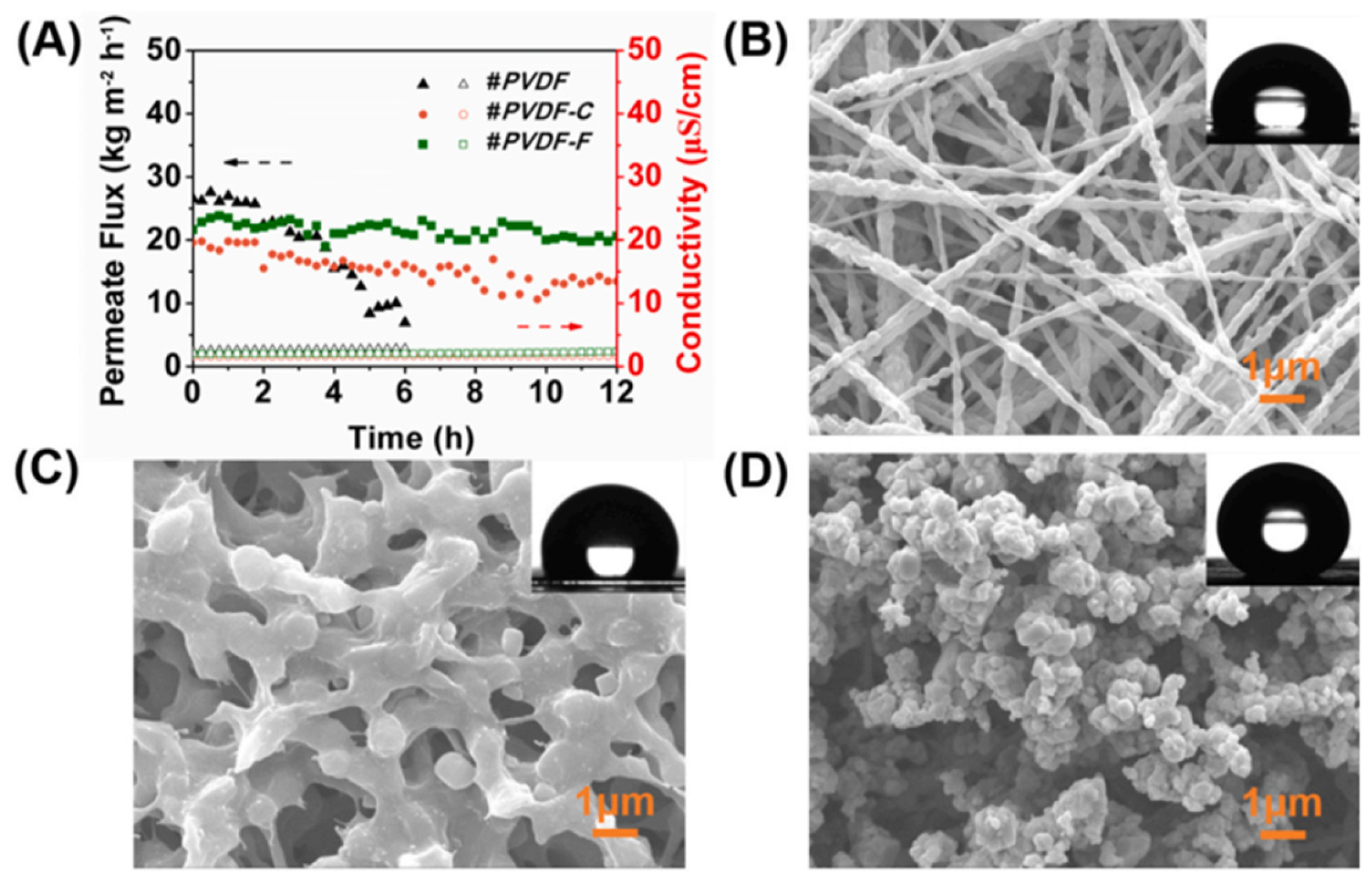
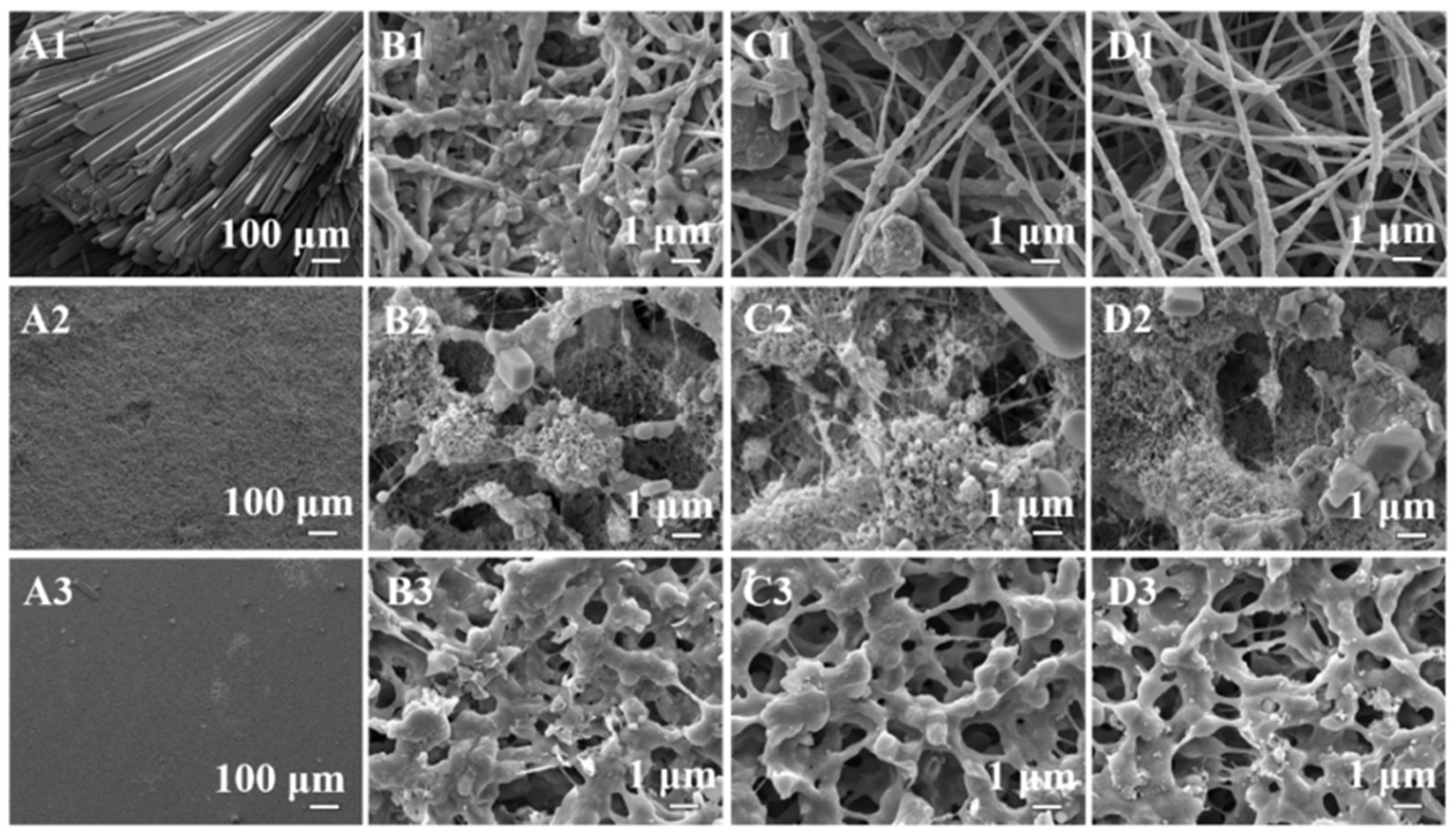
2. Development of Antifouling Nanofibrous Membranes for Membrane Distillation
2.1. Superhydrophobic Nanofibrous Membrane
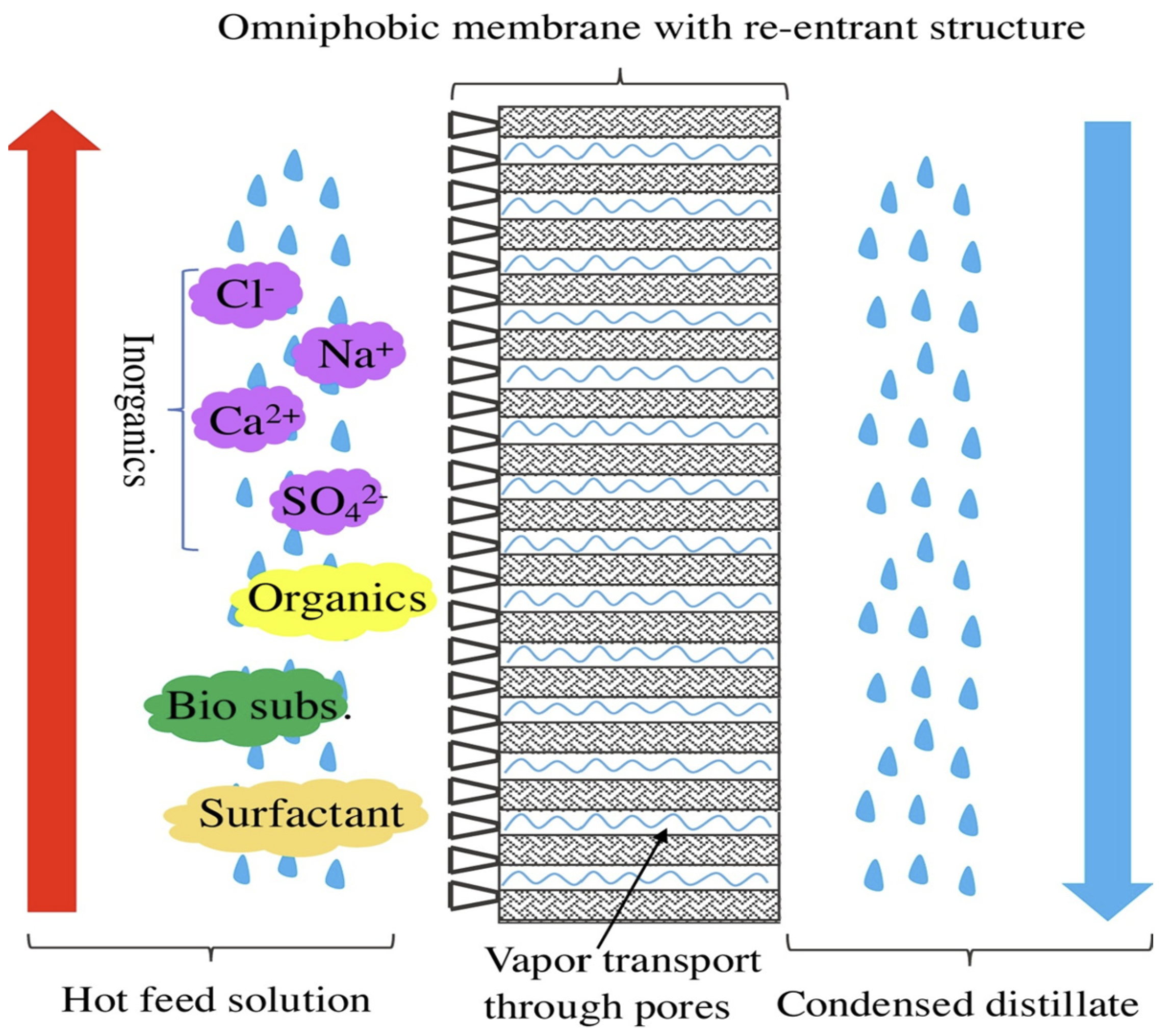
2.2. Omniphobic Nanofibrous Membrane
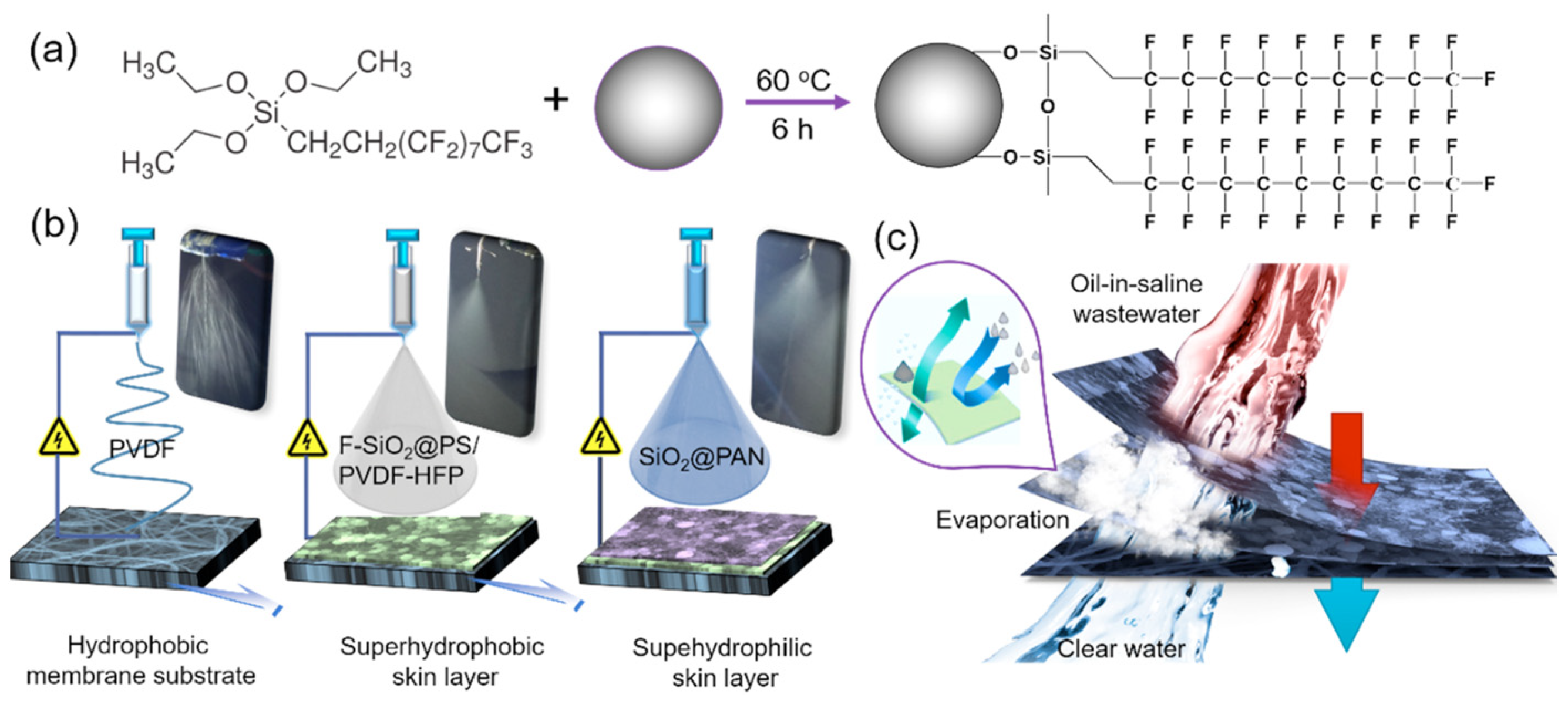
2.3. Janus Nanofibrous Membrane
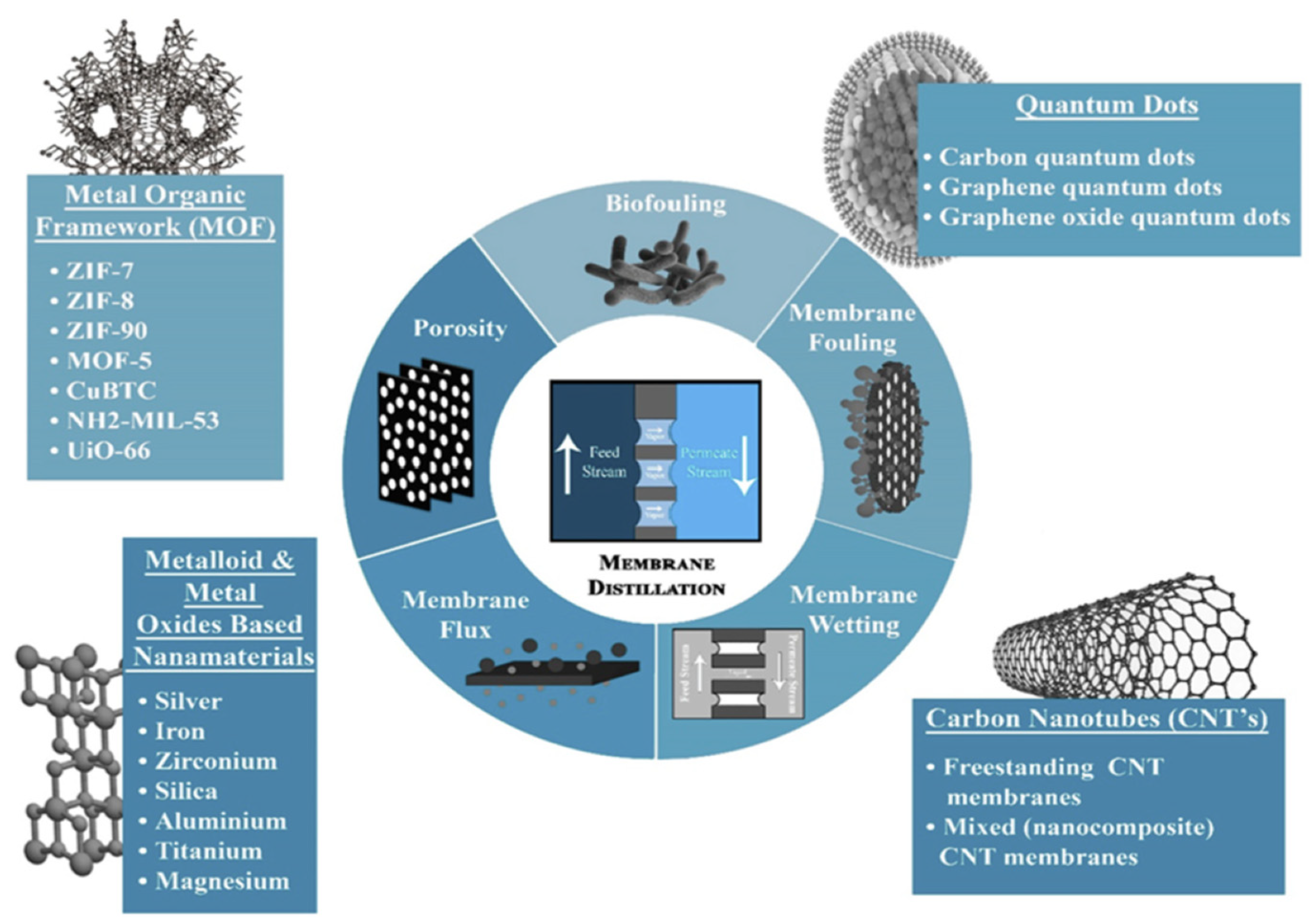
3. Modifications on Nanofiber Membrane for Membrane Distillation
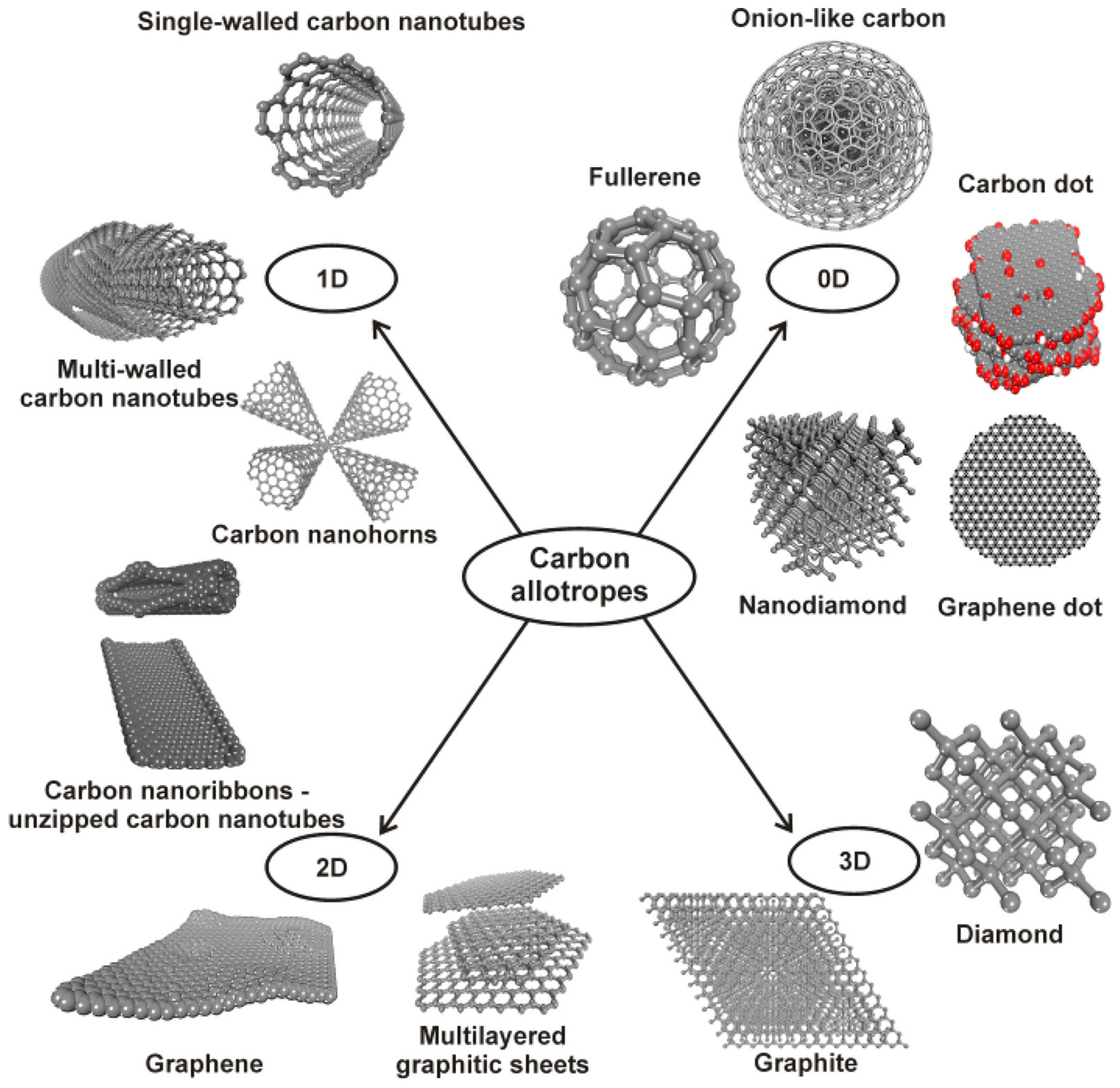
3.1. Carbon-Based Materials
3.2. Metal–Organic Frameworks
3.3. Metalloid and Metal Oxides-Based Nanoparticles
4. Future Way Forward and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuzil, N.S.; Othman, N.H.; Alias, N.H.; Marpani, F.; Mat Shayuti, M.S.; Shahruddin, M.Z.; Mohd Razlan, M.R.; Abd Rahman, N.; Lau, W.J.; Othman, M.H.D.; et al. MoS2-TiO2 coated PVDF-based hollow fiber membranes for permeate flux enhancement in membrane distillation. J. Environ. Chem. Eng. 2023, 11, 109866. [Google Scholar] [CrossRef]
- Shi, D.; Gong, T.; Qing, W.; Li, X.; Shao, S. Unique Behaviors and Mechanism of Highly Soluble Salt-Induced Wetting in Membrane Distillation. Environ. Sci. Technol. 2022, 56, 14788–14796. [Google Scholar] [CrossRef]
- Hou, D.; Yuan, Z.; Tang, M.; Wang, K.; Wang, J. Effect and mechanism of an anionic surfactant on membrane performance during direct contact membrane distillation. J. Membr. Sci. 2020, 595, 117495. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, L.; Zhu, L. In-situ electric-enhanced membrane distillation for simultaneous flux-increasing and anti-wetting. J. Membr. Sci. 2021, 630, 119305. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, Y.; Quan, X.; Zhao, H.; Chen, S.; Yi, G.; Du, L. High desalination permeability, wetting and fouling resistance on superhydrophobic carbon nanotube hollow fiber membrane under self-powered electrochemical assistance. J. Membr. Sci. 2016, 514, 501–509. [Google Scholar] [CrossRef]
- Mohammad Ameen, N.A.; Ibrahim, S.S.; Alsalhy, Q.F.; Figoli, A. Highly Saline Water Desalination Using Direct Contact Membrane Distillation (DCMD): Experimental and Simulation Study. Water 2020, 12, 1575. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Han, M.; Dong, T.; Hou, D.; Yao, J.; Han, L. Carbon nanotube based Janus composite membrane of oil fouling resistance for direct contact membrane distillation. J. Membr. Sci. 2020, 607, 118078. [Google Scholar] [CrossRef]
- Kharraz, J.A.; An, A.K. Patterned superhydrophobic polyvinylidene fluoride (PVDF) membranes for membrane distillation: Enhanced flux with improved fouling and wetting resistance. J. Membr. Sci. 2020, 595, 117596. [Google Scholar] [CrossRef]
- Khoshnevisan, S.; Bazgir, S. Treatment of dye wastewater by direct contact membrane distillation using superhydrophobic nanofibrous high-impact polystyrene membranes. Int. J. Environ. Sci. Technol. 2021, 18, 1513–1528. [Google Scholar] [CrossRef]
- Santos, P.G.; Scherer, C.M.; Fisch, A.G.; Rodrigues, M.A.S. Petrochemical wastewater treatment: Water recovery using membrane distillation. J. Clean. Prod. 2020, 267, 121985. [Google Scholar] [CrossRef]
- Goh, S.; Zhang, J.; Liu, Y.; Fane, A.G. Fouling and wetting in membrane distillation (MD) and MD-bioreactor (MDBR) for wastewater reclamation. Desalination 2013, 323, 39–47. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, K.J.; Chung, T.-S. An omniphobic slippery membrane with simultaneous anti-wetting and anti-scaling properties for robust membrane distillation. J. Membr. Sci. 2020, 595, 117572. [Google Scholar] [CrossRef]
- Han, M.; Wang, Y.; Yao, J.; Liu, C.; Chew, J.W.; Wang, Y.; Dong, Y.; Han, L. Electrically conductive hydrophobic membrane cathode for membrane distillation with super anti-oil-fouling capability: Performance and mechanism. Desalination 2021, 516, 115199. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, Z.; Chang, H.; Fan, G.; Wang, Q.; Fu, X.; Qu, F.; Liang, H. Integrated membrane electrochemical reactor-membrane distillation process for enhanced landfill leachate treatment. Water Res. 2023, 230, 119559. [Google Scholar] [CrossRef]
- An, X.; Liu, Z.; Hu, Y. Amphiphobic surface modification of electrospun nanofibrous membranes for anti-wetting performance in membrane distillation. Desalination 2018, 432, 23–31. [Google Scholar] [CrossRef]
- Lu, C.; Su, C.; Cao, H.; Horseman, T.; Duan, F.; Li, Y. Nanoparticle-free and self-healing amphiphobic membrane for anti-surfactant-wetting membrane distillation. J. Environ. Sci. 2021, 100, 298–305. [Google Scholar] [CrossRef]
- Liao, X.; Wang, Y.; Liao, Y.; You, X.; Yao, L.; Razaqpur, A.G. Effects of different surfactant properties on anti-wetting behaviours of an omniphobic membrane in membrane distillation. J. Membr. Sci. 2021, 634, 119433. [Google Scholar] [CrossRef]
- Hou, D.; Ding, C.; Fu, C.; Wang, D.; Zhao, C.; Wang, J. Electrospun nanofibrous omniphobic membrane for anti-surfactant-wetting membrane distillation desalination. Desalination 2019, 468, 114068. [Google Scholar] [CrossRef]
- Singh, V.; Tyagi, R. Unique Micellization and CMC Aspects of Gemini Surfactant: An Overview. J. Dispers. Sci. Technol. 2014, 35, 1774–1792. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Matsuura, T.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Othman, N.H.; Abdullah, N.; Paiman, S.H.; et al. Photocatalytic nanofiber-coated alumina hollow fiber membranes for highly efficient oilfield produced water treatment. Chem. Eng. J. 2019, 360, 1437–1446. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Hussein, S.N.C.M.; Dollah, A. Supported graphene oxide hollow fibre membrane for oily wastewater treatment. AIP Conf. Proc. 2017, 1901, 020008. [Google Scholar] [CrossRef]
- Junaidi, N.F.D.; Othman, N.H.; Shahruddin, M.Z.; Alias, N.H.; Marpani, F.; Lau, W.J.; Ismail, A.F. Fabrication and characterization of graphene oxide–polyethersulfone (GO–PES) composite flat sheet and hollow fiber membranes for oil–water separation. J. Chem. Technol. Biotechnol. 2020, 95, 1308–1320. [Google Scholar] [CrossRef]
- Makanjuola, O.; Ahmed, F.; Janajreh, I.; Hashaikeh, R. Development of a dual-layered PVDF-HFP/cellulose membrane with dual wettability for desalination of oily wastewater. J. Membr. Sci. 2019, 570–571, 418–426. [Google Scholar] [CrossRef]
- Hou, D.; Wang, Z.; Wang, K.; Wang, J.; Lin, S. Composite membrane with electrospun multiscale-textured surface for robust oil-fouling resistance in membrane distillation. J. Membr. Sci. 2018, 546, 179–187. [Google Scholar] [CrossRef]
- Tang, M.; Hou, D.; Ding, C.; Wang, K.; Wang, D.; Wang, J. Anti-oil-fouling hydrophobic-superoleophobic composite membranes for robust membrane distillation performance. Sci. Total Environ. 2019, 696, 133883. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Ding, C.; Li, K.; Lin, D.; Wang, D.; Wang, J. A novel dual-layer composite membrane with underwater-superoleophobic/hydrophobic asymmetric wettability for robust oil-fouling resistance in membrane distillation desalination. Desalination 2018, 428, 240–249. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Zhong, L.; Song, C.; Shi, W.; Cui, F.; Wang, W. Breathable and asymmetrically superwettable Janus membrane with robust oil-fouling resistance for durable membrane distillation. J. Membr. Sci. 2018, 563, 602–609. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; García-Payo, M.C.; Khayet, M.; El Kharraz, J.; Chaouch, M. Application of direct contact membrane distillation for textile wastewater treatment and fouling study. Sep. Purif. Technol. 2019, 209, 815–825. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, P.; Panda, A.B.; Shahi, V.K. Photocatalytic TiO2 incorporated PVDF-co-HFP UV-cleaning mixed matrix membranes for effective removal of dyes from synthetic wastewater system via membrane distillation. J. Environ. Chem. Eng. 2021, 9, 105904. [Google Scholar] [CrossRef]
- An, A.K.; Guo, J.; Lee, E.-J.; Jeong, S.; Zhao, Y.; Wang, Z.; Leiknes, T. PDMS/PVDF hybrid electrospun membrane with superhydrophobic property and drop impact dynamics for dyeing wastewater treatment using membrane distillation. J. Membr. Sci. 2017, 525, 57–67. [Google Scholar] [CrossRef]
- Buffle, J.; Wilkinson, K.J.; Stoll, S.; Filella, M.; Zhang, J. A generalized description of aquatic colloidal interactions: the three-colloidal component approach. Environ. Sci. Technol. 1998, 32, 2887–2899. [Google Scholar] [CrossRef]
- Zen, Y.; Wei, J.; Krantz, W.B. Effect of humic-acid fouling on membrane distillation. J. Membr. Sci. 2016, 504, 263–273. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, L.; Wang, Z.; Fan, H.; Wang, J.; Huang, H. Humic acid fouling mitigation by ultrasonic irradiation in membrane distillation process. Sep. Purif. Technol. 2015, 154, 328–337. [Google Scholar] [CrossRef]
- Csiszár, E.; Galambos, I.; Békássy-Molnár, E.; Vatai, G. Ultrafiltration of humic acid containing well-water in pilot scale: New mass transfer model for transient flow regime. Desalination 2006, 199, 512–514. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Wang, W.; Feng, P.; Yang, Q.; Wang, W.; Wang, X. Effects of sodium, magnesium, and calcium salts on the coagulation performance of cucurbit uril for humic acid removal from synthetic seawater. Desalination 2016, 386, 77–83. [Google Scholar] [CrossRef]
- Klavins, M.; Purmalis, O. Surface activity of humic acids depending on their origin and humification degree. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2013, 687, 493–499. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Vigneswaran, S. Interaction of humic substances on fouling in membrane distillation for seawater desalination. Chem. Eng. J. 2015, 262, 946–957. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A.G. Humic acid fouling in the membrane distillation process. Desalination 2005, 174, 63–72. [Google Scholar] [CrossRef]
- Deng, L.; Liu, K.; Li, P.; Sun, D.; Ding, S.; Wang, X.; Hsiao, B.S. Engineering construction of robust superhydrophobic two-tier composite membrane with interlocked structure for membrane distillation. J. Membr. Sci. 2020, 598, 117813. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, G.; Jeanne, J.; Tian, M.; Wang, R. Development of robust and superhydrophobic membranes to mitigate membrane scaling and fouling in membrane distillation Development of robust and superhydrophobic membranes to mitigate membrane scaling and fouling in membrane distillation. J. Membr. Sci. 2020, 601, 117962. [Google Scholar] [CrossRef]
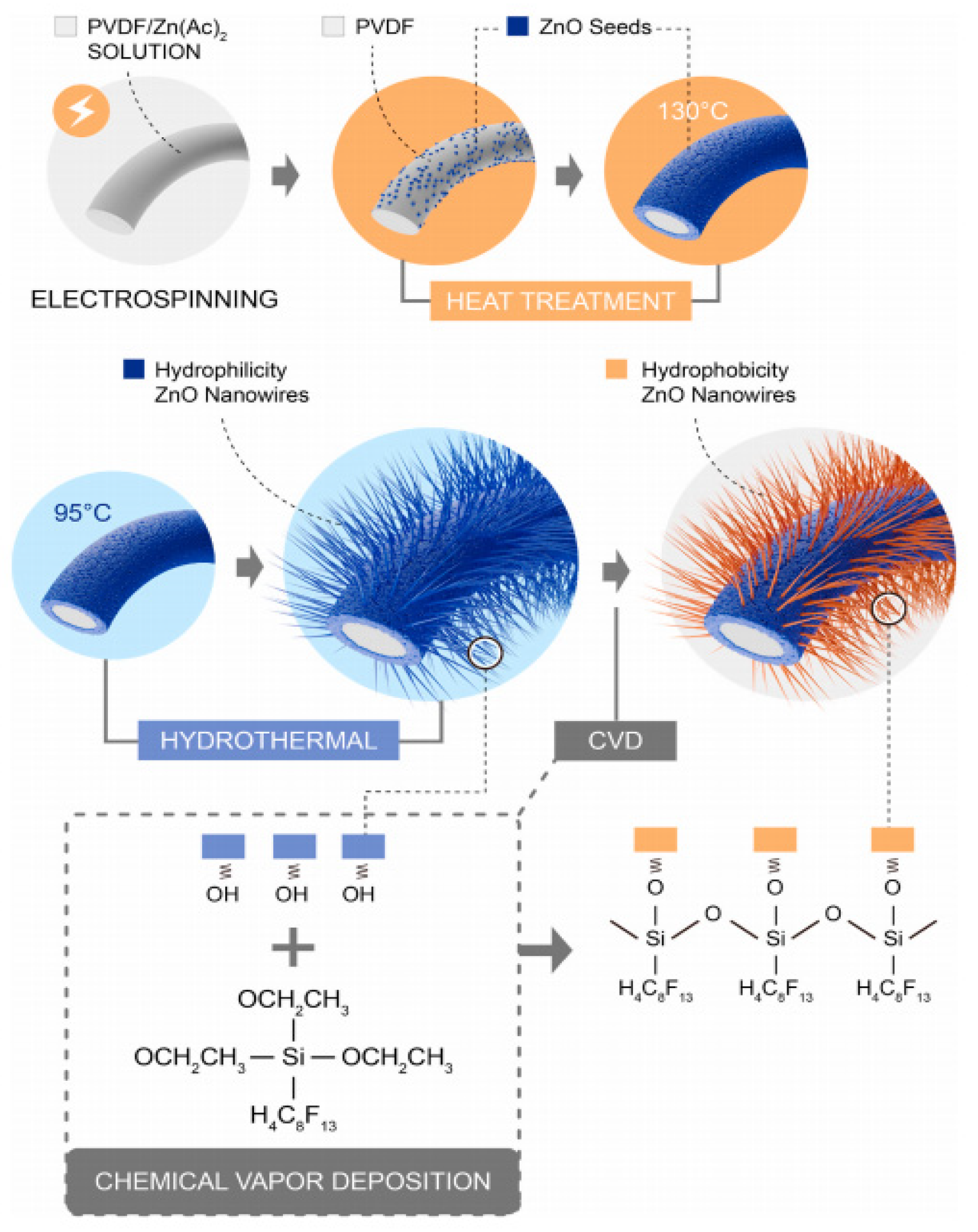
- Pan, T.; Liu, J.; Deng, N.; Li, Z.; Wang, L.; Xia, Z.; Fan, J.; Liu, Y. ZnO Nanowires@PVDF nanofiber membrane with superhydrophobicity for enhanced anti-wetting and anti-scaling properties in membrane distillation. J. Membr. Sci. 2021, 621, 118877. [Google Scholar] [CrossRef]
- Hyun Park, S.; Hoon Kim, J.; Ju Moon, S.; Drioli, E.; Moo Lee, Y. Enhanced, hydrophobic, fluorine-containing, thermally rearranged (TR) nanofiber membranes for desalination via membrane distillation. J. Membr. Sci. 2018, 550, 545–553. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Guo, H.; Liu, Y.; Wang, Y.; Yin, H.; Li, X.; Song, J.; Nghiem, L.D.; He, T. Scaling mitigation in membrane distillation: From superhydrophobic to slippery. Desalination 2019, 466, 36–43. [Google Scholar] [CrossRef]
- Li, J.; Ren, L.F.; Zhou, H.S.; Yang, J.; Shao, J.; He, Y. Fabrication of superhydrophobic PDTS-ZnO-PVDF membrane and its anti-wetting analysis in direct contact membrane distillation (DCMD) applications. J. Membr. Sci. 2021, 620, 118924. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-h.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Rao, U.; Posmanik, R.; Hatch, L.E.; Tester, W.; Walker, S.L.; Barsanti, K.C.; Jassby, D. Coupling hydrothermal liquefaction and membrane distillation to treat anaerobic digestate from food and dairy farm waste. Bioresour. Technol. 2018, 267, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Krivorot, M.; Kushmaro, A.; Oren, Y.; Gilron, J. Factors affecting biofilm formation and biofouling in membrane distillation of seawater. J. Membr. Sci. 2011, 376, 15–24. [Google Scholar] [CrossRef]
- Zeng, Q. Size matching effect on Wenzel wetting on fractal surfaces. Results Phys. 2018, 10, 588–593. [Google Scholar] [CrossRef]
- Yan, K.K.; Jiao, L.; Lin, S.; Ji, X.; Lu, Y.; Zhang, L. Superhydrophobic electrospun nanofiber membrane coated by carbon nanotubes network for membrane distillation. Desalination 2018, 437, 26–33. [Google Scholar] [CrossRef]
- Feng, D.; Li, X.; Wang, Z. Comparison of omniphobic membranes and Janus membranes with a dense hydrophilic surface layer for robust membrane distillation. J. Membr. Sci. 2022, 660, 120858. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, L.; Horseman, T.; Liu, Z.; Zeng, G.; Li, Z.; Lin, S.; Wang, W. Superhydrophobic-omniphobic membrane with anti-deformable pores for membrane distillation with excellent wetting resistance. J. Membr. Sci. 2021, 620, 118768. [Google Scholar] [CrossRef]
- Ren, L.; Chen, J.; Lu, Q.; Han, J.; Wu, H. Anti-biofouling nanofiltration membrane constructed by in-situ photo-grafting bactericidal and hydrophilic polymers. J. Membr. Sci. 2021, 617, 118658. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G.A. A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation. Membranes 2022, 12, 1271. [Google Scholar] [CrossRef]
- Gou, X.; Guo, Z. Surface topographies of biomimetic superamphiphobic materials: Design criteria, fabrication and performance. Adv. Colloid Interface Sci. 2019, 269, 87–121. [Google Scholar] [CrossRef]
- Ferrari, M.; Ravera, F. Surfactants and wetting at superhydrophobic surfaces: Water solutions and non aqueous liquids. Adv. Colloid Interface Sci. 2010, 161, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhao, J.; Fan, Y.; Wan, Z.; Lu, L.; Zhang, Z.; Ming, W.; Ren, L. Shape memory superhydrophobic surface with switchable transition between “Lotus Effect” to “Rose Petal Effect”. Chem. Eng. J. 2020, 382, 12298. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Z. Biomimetic superhydrophobic surfaces with transition metals and their oxides: A review. J. Bionic Eng. 2017, 14, 401–439. [Google Scholar] [CrossRef]
- Ren, L.F.; Xia, F.; Chen, V.; Shao, J.; Chen, R.; He, Y. TiO2-FTCS modified superhydrophobic PVDF electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Desalination 2017, 423, 1–11. [Google Scholar] [CrossRef]
- Xue, X.; Tan, G.; Zhu, Z. All-Polymer and Self-Roughened Superhydrophobic PVDF Fibrous Membranes for Stably Concentrating Seawater by Membrane Distillation. Appl. Mater. Interfaces 2021, 13, 45977–45986. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiao, Z.; Liu, Y.; Li, X.; Yin, H.; Volkov, A.; He, T. Understanding the fouling/scaling resistance of superhydrophobic/omniphobic membranes in membrane distillation. Desalination 2021, 499, 114864. [Google Scholar] [CrossRef]
- Utech, S.; Bley, K.; Aizenberg, J.; Vogel, N. Tailoring re-entrant geometry in inverse colloidal monolayers to control surface wettability. J. Mater. Chem. A Mater. Energy Sustain. 2015, 4, 6853–6859. [Google Scholar] [CrossRef]
- Ni, T.; Lin, J.; Kong, L.; Zhao, S. Omniphobic membranes for distillation: Opportunities and challenges. Chin. Chem. Lett. 2021, 32, 3298–3306. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Robust omniphobic surfaces. Proc. Natl. Acad. Sci. USA 2008, 105, 18200–18205. [Google Scholar] [CrossRef] [PubMed]
- Whyman, G.; Bormashenko, E. How to make the Cassie wetting state stable? Langmuir 2011, 27, 8171–8176. [Google Scholar] [CrossRef]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.B.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Liu, J.; Li, X.; Li, Y.; Tezel, F.H.; Li, B.; Wang, S. Nanocoated amphiphobic membrane for flux enhancement and comprehensive anti-fouling performance in direct contact membrane distillation. J. Membr. Sci. 2018, 567, 166–180. [Google Scholar] [CrossRef]
- Lin, S.; Nejati, S.; Boo, C.; Hu, Y.; Osuji, C.O.; Elimelech, M. Omniphobic Membrane for Robust Membrane Distillation. Environ. Sci. Technol. Lett. 2014, 1, 443–447. [Google Scholar] [CrossRef]
- Afsari, M.; Shon, H.K.; Tijing, L.D. Janus membranes for membrane distillation: Recent advances and challenges. Adv. Colloid Interface Sci. 2021, 289, 102362. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Du, X.; Tong, T.; Cath, T.Y.; Lee, J. Antiwetting and antifouling Janus membrane for desalination of saline oily wastewater by membrane distillation. ACS Appl. Mater. Interfaces 2019, 11, 18456–18465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, F.; Cao, W.; Wan, Y. Hydrophilic/hydrophobic Janus membranes with a dual-function surface coating for rapid and robust membrane distillation desalination. Desalination 2020, 491, 114561. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, L.; Chen, X.; Zheng, W.; Zuo, J.; Zeng, G.; Wang, W. Monolithic and self-roughened Janus fibrous membrane with superhydrophilic/omniphobic surface for robust antifouling and antiwetting membrane distillation. J. Membr. Sci. 2020, 615, 18–21. [Google Scholar] [CrossRef]
- Wu, X.Q.; Wu, X.; Wang, T.Y.; Zhao, L.; Truong, Y.B.; Ng, D.; Zheng, Y.M.; Xie, Z. Omniphobic surface modification of electrospun nanofiber membrane via vapor deposition for enhanced anti-wetting property in membrane distillation. J. Membr. Sci. 2020, 606, 118075. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.C.; Matsuura, T.; Gopal, R.; Kaur, S.; Ramakrishna, S.; Khayet, M. Production of drinking water from saline water by air-gap membrane distillation using polyvinylidene fluoride nanofiber membrane. J. Membr. Sci. 2008, 311, 1–6. [Google Scholar] [CrossRef]
- Peng, S.; Jin, G.; Li, L.; Li, K.; Srinivasan, M.; Ramakrishna, S.; Chen, J. Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment. Chem. Soc. Rev. 2016, 45, 1225–1241. [Google Scholar] [CrossRef]
- Madalosso, H.B.; Machado, R.; Hotza, D.; Marangoni, C. Membrane surface modification by electrospinning, coating, and plasma for membrane distillation applications: A state-of-the-art review. Adv. Eng. Mater. 2021, 23, 1438–1656. [Google Scholar] [CrossRef]
- Ray, S.S.; Bakshi, H.S.; Dangayach, R.; Singh, R.; Deb, C.K.; Ganesapillai, M.; Chen, S.S.; Purkait, M.K. Recent developments in nanomaterials-modified membranes for improved membrane distillation performance. Membranes 2020, 10, 140. [Google Scholar] [CrossRef]
- Chang, H.; Liu, B.; Pawar, Z.Z.; Yan, Z.; Crittenden, J.C.; Vidic, R.D. A critical review of membrane wettability in membrane distillation from the perspective on interfacial interactions.pdf. Environ. Sci. Technol. 2021, 55, 1395–1418. [Google Scholar] [CrossRef]
- Chun-You, P.; Xua, G.-R.; Xua, K.; Zhaoa, H.-L.; Wua, Y.-Q.; Sua, H.-C.; Xua, J.-M.; Dasb, R. Electrospun nanofibrous membranes in membrane distillation: Recent developmemt and guture perspectives. Sep. Purif. Technol. 2019, 221, 44–63. [Google Scholar] [CrossRef]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-based nanomaterials: Synthesis and prospective applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Essalhi, M.; Khayet, M.; Tesfalidet, S.; Alsultan, M.; Tavajohi, N. Desalination by direct contact membrane distillation using mixed matrix electrospun nanofibrous membranes with carbon-based nanofillers: A strategic improvement. Chem. Eng. J. 2021, 426, 131316. [Google Scholar] [CrossRef]
- Leaper, S.; Avendaño Cáceres, E.O.; Luque-Alled, J.M.; Cartmell, S.H.; Gorgojo, P. POSS-Functionalized Graphene Oxide/PVDF Electrospun Membranes for Complete Arsenic Removal Using Membrane Distillation. ACS Appl. Polym. Mater. 2021, 3, 1854–1865. [Google Scholar] [CrossRef]
- Fouladivanda, M.; Karimi-Sabet, J.; Abbasi, F.; Moosavian, M.A. Step-by-step improvement of mixed-matrix nanofiber membrane with functionalized graphene oxide for desalination via air-gap membrane distillation. Sep. Purif. Technol. 2021, 256, 117809. [Google Scholar] [CrossRef]
- Li, W.; Deng, L.; Huang, H.; Zhou, J.; Liao, Y.; Qiu, L.; Yang, H.; Yao, L. Janus photothermal membrane as an energy generator and a mass-transfer accelerator for high-efficiency solar-driven membrane distillation. ACS Appl. Mater. Interfaces 2021, 13, 26861–26869. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shi, J.; Ning, X.; Long, Y.Z.; Zheng, J. The High Flux of Superhydrophilic-Superhydrophobic Janus Membrane of cPVA-PVDF/PMMA/GO by Layer-by-Layer Electrospinning for High Efficiency Oil-Water Separation. Polymers 2022, 14, 621. [Google Scholar] [CrossRef]
- Yang, G.; Ng, D.; Huang, Z.; Zhang, J.; Gray, S.; Xie, Z. Janus hollow fibre membranes with intrusion anchored structure for robust desalination and leachate treatment in direct contact membrane distillation. Desalination 2023, 551, 116423. [Google Scholar] [CrossRef]
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal-organic frameworks supported on nanofiber for desalination by direct contact membrane distillation. ACS Appl. Mater. Interfaces 2018, 10, 11251–11260. [Google Scholar] [CrossRef] [PubMed]
- Qadir, N.U.; Said, S.A.M.; Bahaidarah, H.M. Structural stability of metal organic frameworks in aqueous media—Controlling factors and methods to improve hydrostability and hydrothermal cyclic stability. Microporous Mesoporous Mater. 2015, 201, 61–90. [Google Scholar] [CrossRef]
- Xie, L.H.; Xu, M.M.; Liu, X.M.; Zhao, M.J.; Li, J.R. Hydrophobic metal–organic frameworks: Assessment, construction, and diverse applications. Adv. Sci. 2020, 7, 1901758. [Google Scholar] [CrossRef]
- Le, T.; Chen, X.; Dong, H.; Tarpeh, W.; Perea-Cachero, A.; Coronas, J.; Martin, S.M.; Mohammad, M.; Razmjou, A.; Esfahani, A.R.; et al. An Evolving insight into metal organic framework-functionalized membranes for water and wastewater treatment and resource recovery. Ind. Eng. Chem. Res. 2021, 60, 6869–6907. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Yang, F.; Cong, Y.; Lan, C.Q. Triple-layered nanofibrous metal-organic framework-based membranes for desalination by direct contact membrane distillation. ACS Sustain. Chem. Eng. 2020, 8, 6601–6610. [Google Scholar] [CrossRef]
- Chen, L.; Li, F.; Jiang, L.; He, F.; Wei, Y. UiO-66-NH2/PVA composite Janus membrane with a dense hydrophilic surface layer for strong resistance to fouling and wettability in membrane distillation. J. Water Process Eng. 2022, 48, 102887. [Google Scholar] [CrossRef]
- Abd Aziz, M.H.; Dzarfan Othman, M.H.; Alias, N.H.; Nakayama, T.; Shingaya, Y.; Hashim, N.A.; Kurniawan, T.A.; Matsuura, T.; Rahman, M.A.; Jaafar, J. Enhanced omniphobicity of mullite hollow fiber membrane with organosilane-functionalized TiO2 micro-flowers and nanorods layer deposition for desalination using direct contact membrane distillation. J. Membr. Sci. 2020, 607, 118137. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Fan, X.; Liu, Z.; Song, Y.; Wang, Y.; Tao, P.; Song, C.; Shao, M. In-situ silica nanoparticle assembly technique to develop an omniphobic membrane for durable membrane distillation. Desalination 2021, 499, 114832. [Google Scholar] [CrossRef]
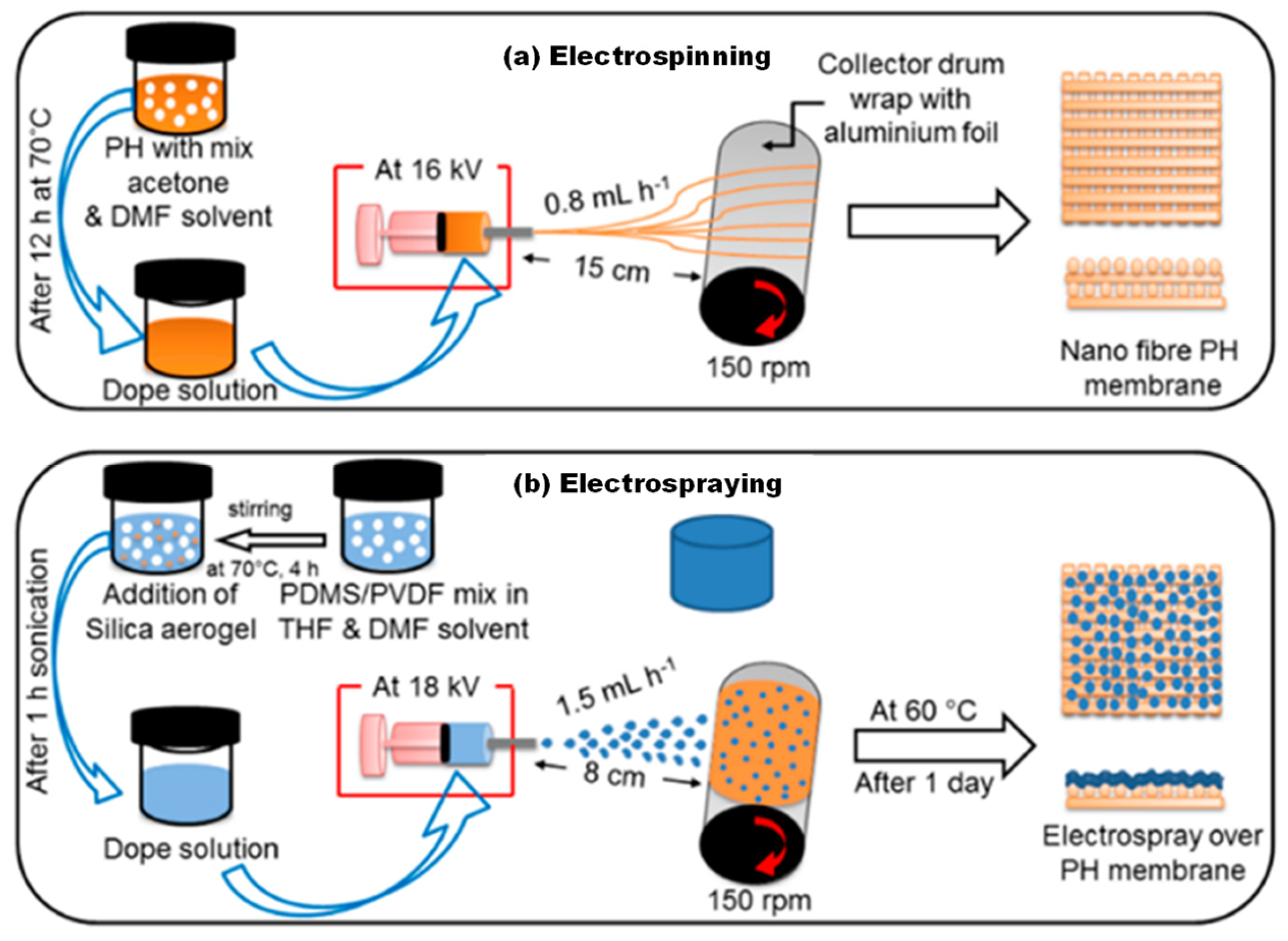
- Deka, B.J.; Lee, E.J.; Guo, J.; Kharraz, J.; An, A.K. Electrospun nanofiber membranes incorporating pdms-aerogel superhydrophobic coating with enhanced flux and improved antiwettability in membrane distillation. Environ. Sci. Technol. 2019, 53, 4948–4958. [Google Scholar] [CrossRef]
- Deka, B.J.; Guo, J.; An, A.K. Robust dual-layered omniphobic electrospun membrane with anti-wetting and anti-scaling functionalised for membrane distillation application. J. Membr. Sci. 2021, 624, 119089. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Anithaa, S.; Brabub, B.; Thiruvadigal, D.J.; John, D.; Gopalakrishnanb, C.; Natarajanc, T.S. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym. 2013, 97, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Gao, S.; Huang, Y.; Zhang, M.; Xiao, C. Study on photothermal PVDF/ATO nanofiber membrane and its membrane distillation performance. J. Membr. Sci. 2019, 582, 203–210. [Google Scholar] [CrossRef]

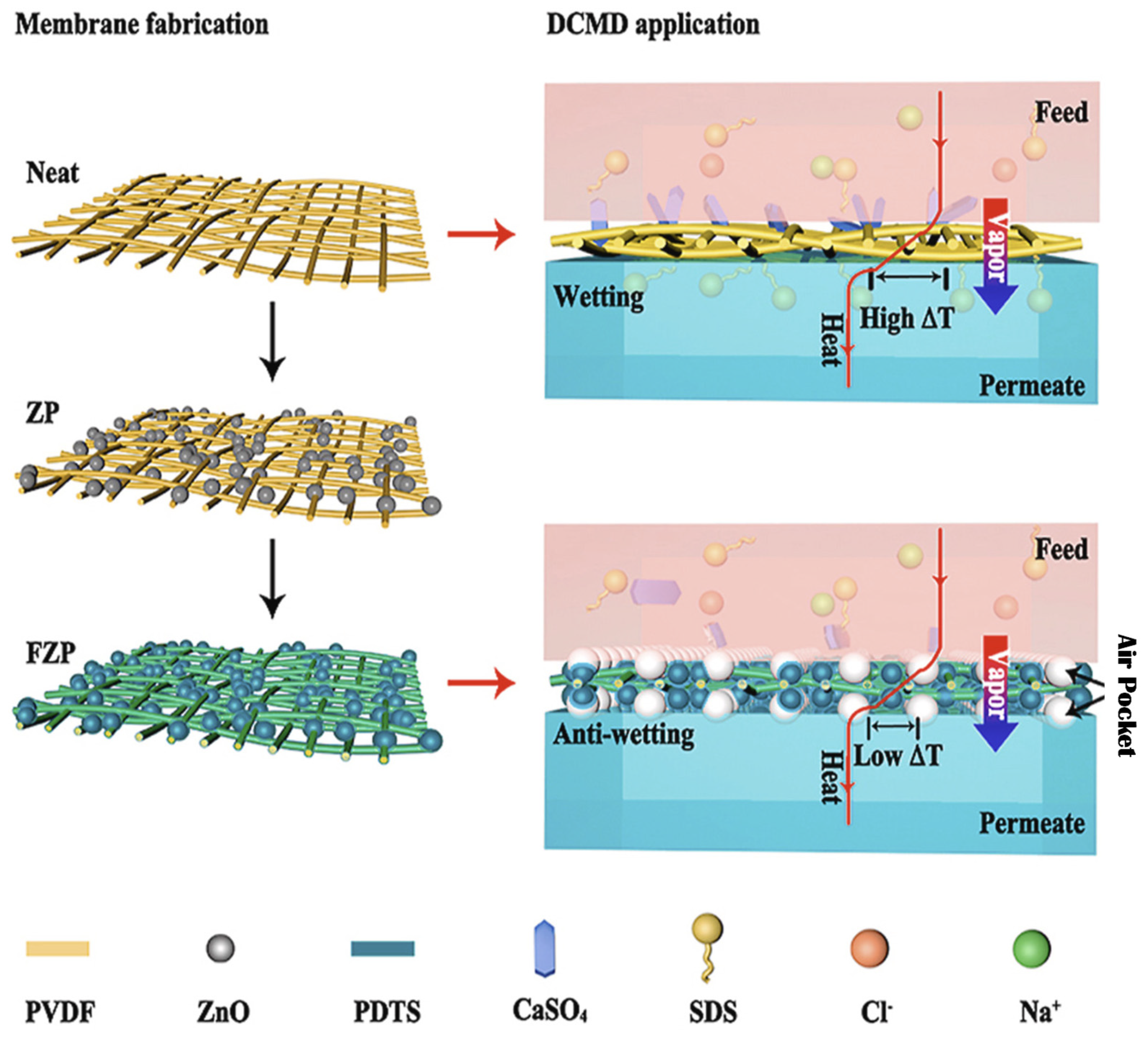




| Nanofiber-Based Membrane | Nano- Materials | MD Type | Category | Contact Angle | Flux (kg/m2 h) | Salt Rejection | Ref. |
|---|---|---|---|---|---|---|---|
| PVDF | CNT | VMD | Carbon | 159.3° | 28.4 | >99.99% | [53] |
| PVDF | MWCNT | DCMD | Carbon | 142.0° | 74.7 | 99.995% | [85] |
| PVDF | GO | DCMD | Carbon | 119.0° | 28 L | >99.90% | [86] |
| PVDF-HFP | GO | AGMD | Carbon | 162.0° | 21.1 | 99.99% | [87] |
| PP | GO | DCMD | Carbon | 120.0° | 3.4 | 99.60% | [90] |
| PVDF-HFP | Carbon spheres | SDMD | Carbon | 152.0° | 1.29 | 99.99% | [88] |
| PVDF | F300 | DCMD | MOF | 138.06° | 2.87 | 99.99% | [91] |
| PAN/ PVDF | MOF-808 | DCMD | MOF | 140.0° | 4.4 | 99.97% | [95] |
| PTFE | UiO-66-NH2 | DCMD | MOF | 54.0° | 21.3 | 99.99% | [96] |
| PVDF-HFP/APTES | Si | DCMD | Metalloid | 154.9° | 16.5 | ~100.00% | [98] |
| PVDF-HFP | Si | DCMD | Metalloid | 170.0° | 34.6 | 99.90% | [99] |
| PVDF-HFP-FAS | ZnO | DCMD | Metal oxide | >161.0° | 22.7 | >99.90% | [100] |
| PVDF | ZnO | DCMD | Metal oxide | 150.0° | 15.7 | 99.90% | [44] |
| PVDF | ATO | VMD | Metal oxide | 125.5° | 27.0 | 99.00% | [103] |
| PVDF | TiO2 | DCMD | Metal oxide | 157.1° | 73.4 | 99.99% | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.Z.; Alias, N.H.; Aziz, M.H.A.; Jaafar, J.; Othman, F.E.C.; Chew, J.W. Progress on Improved Fouling Resistance-Nanofibrous Membrane for Membrane Distillation: A Mini-Review. Membranes 2023, 13, 727. https://doi.org/10.3390/membranes13080727
Tan YZ, Alias NH, Aziz MHA, Jaafar J, Othman FEC, Chew JW. Progress on Improved Fouling Resistance-Nanofibrous Membrane for Membrane Distillation: A Mini-Review. Membranes. 2023; 13(8):727. https://doi.org/10.3390/membranes13080727
Chicago/Turabian StyleTan, Yong Zen, Nur Hashimah Alias, Mohd Haiqal Abd Aziz, Juhana Jaafar, Faten Ermala Che Othman, and Jia Wei Chew. 2023. "Progress on Improved Fouling Resistance-Nanofibrous Membrane for Membrane Distillation: A Mini-Review" Membranes 13, no. 8: 727. https://doi.org/10.3390/membranes13080727
APA StyleTan, Y. Z., Alias, N. H., Aziz, M. H. A., Jaafar, J., Othman, F. E. C., & Chew, J. W. (2023). Progress on Improved Fouling Resistance-Nanofibrous Membrane for Membrane Distillation: A Mini-Review. Membranes, 13(8), 727. https://doi.org/10.3390/membranes13080727











