Evaluation between Biodegradable Magnesium Metal GBR Membrane and Bovine Graft with or without Hyaluronate
Abstract
1. Introduction
2. Materials and Methods
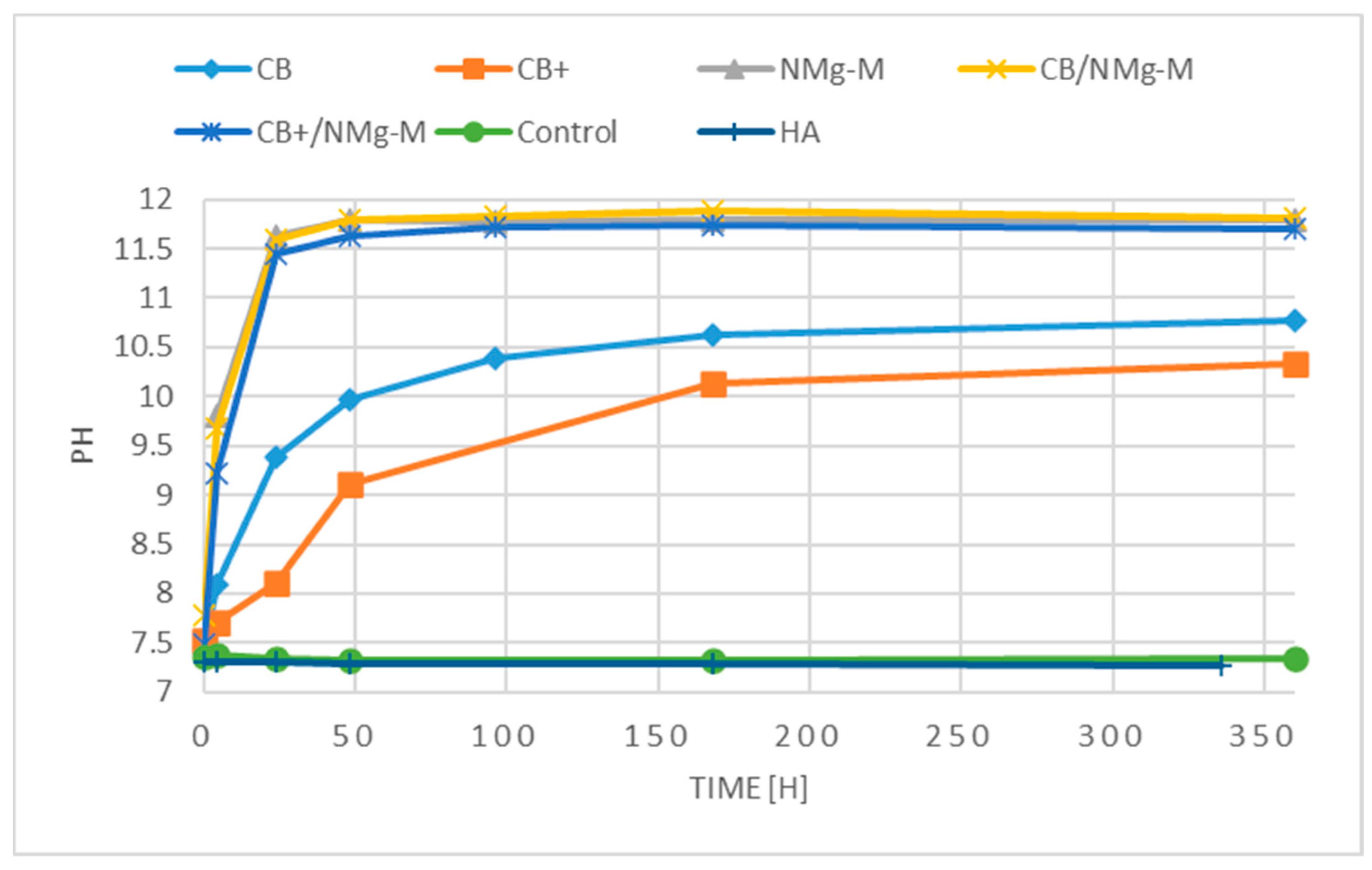
2.1. In Vitro Interactions
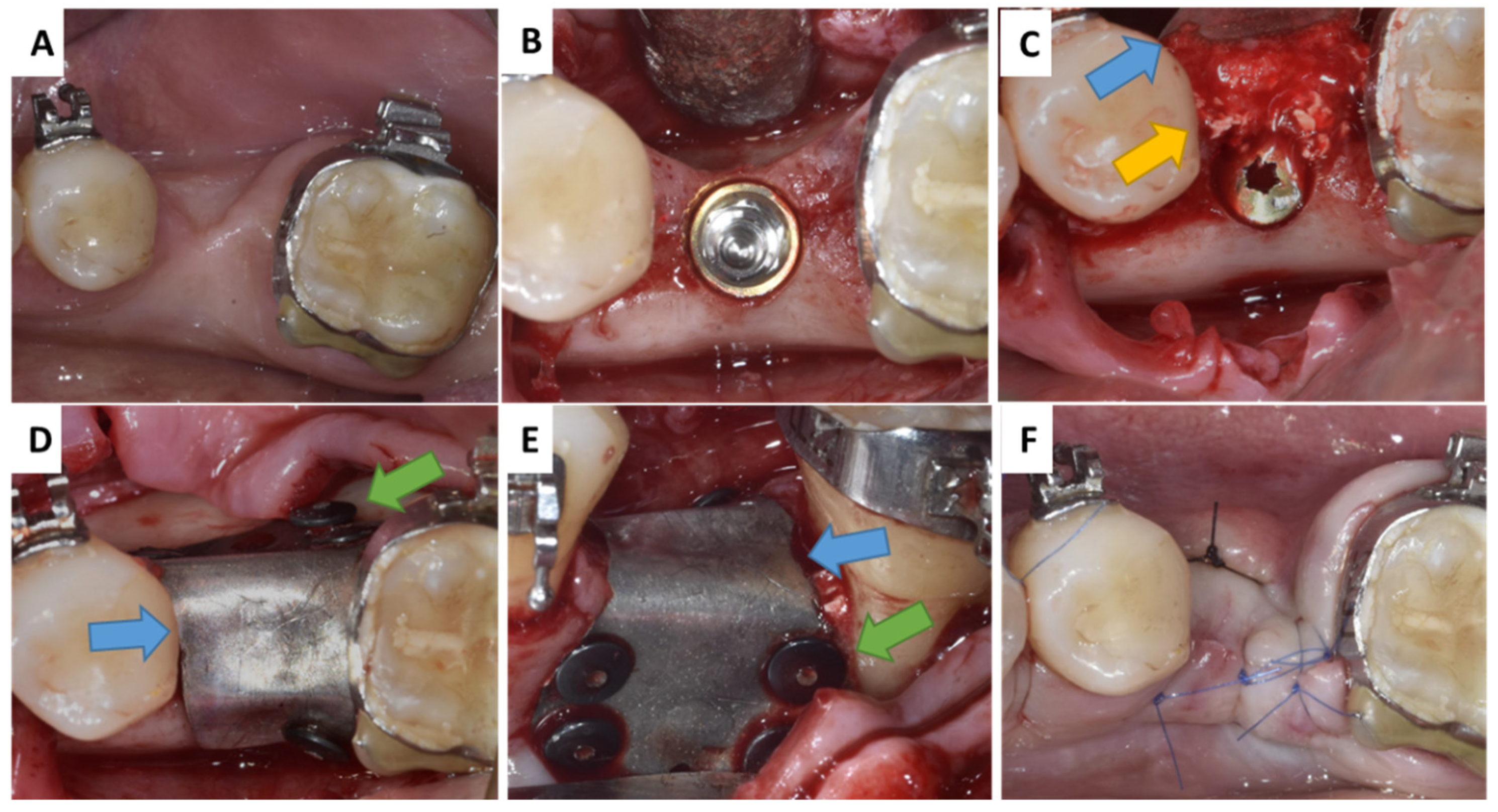
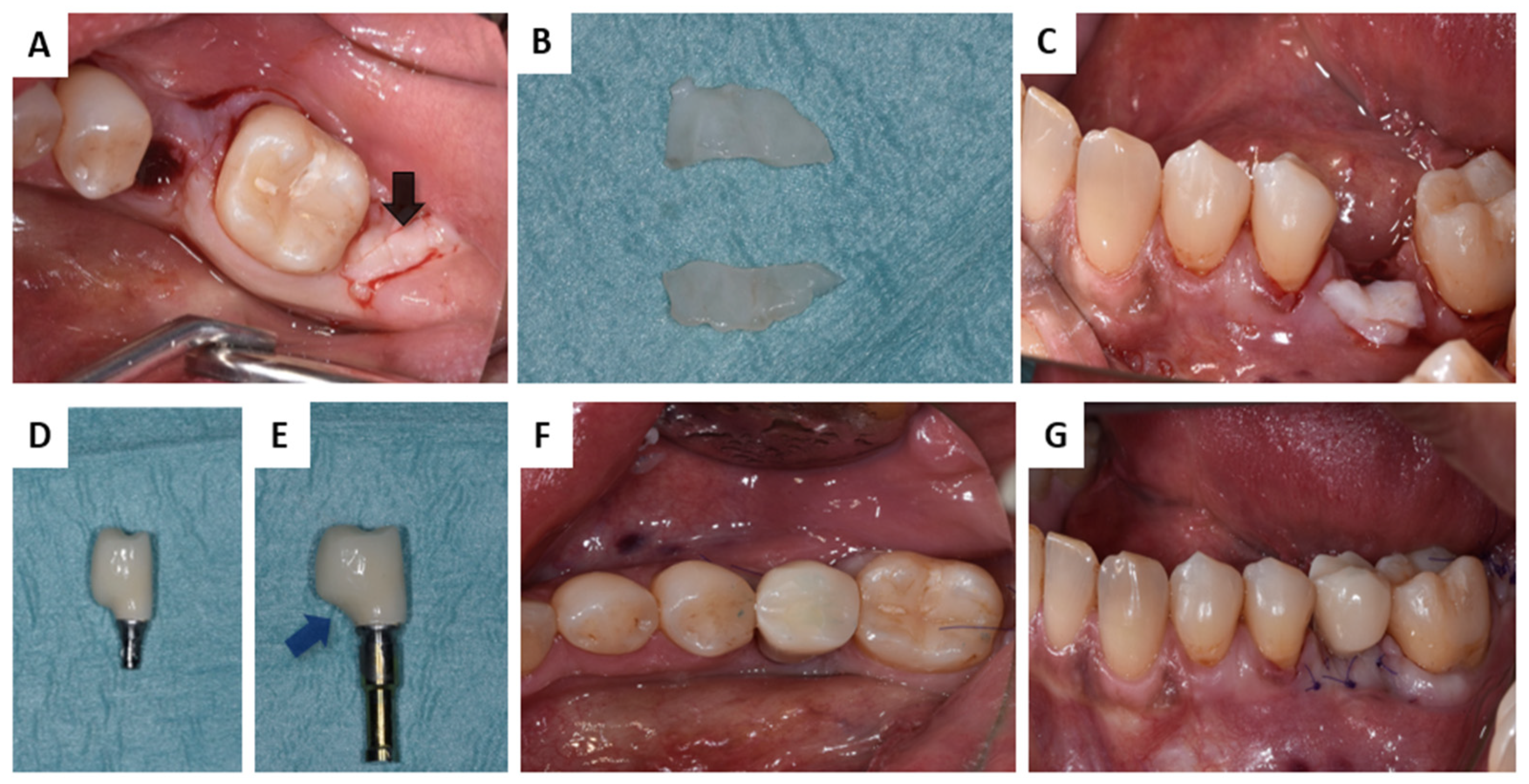
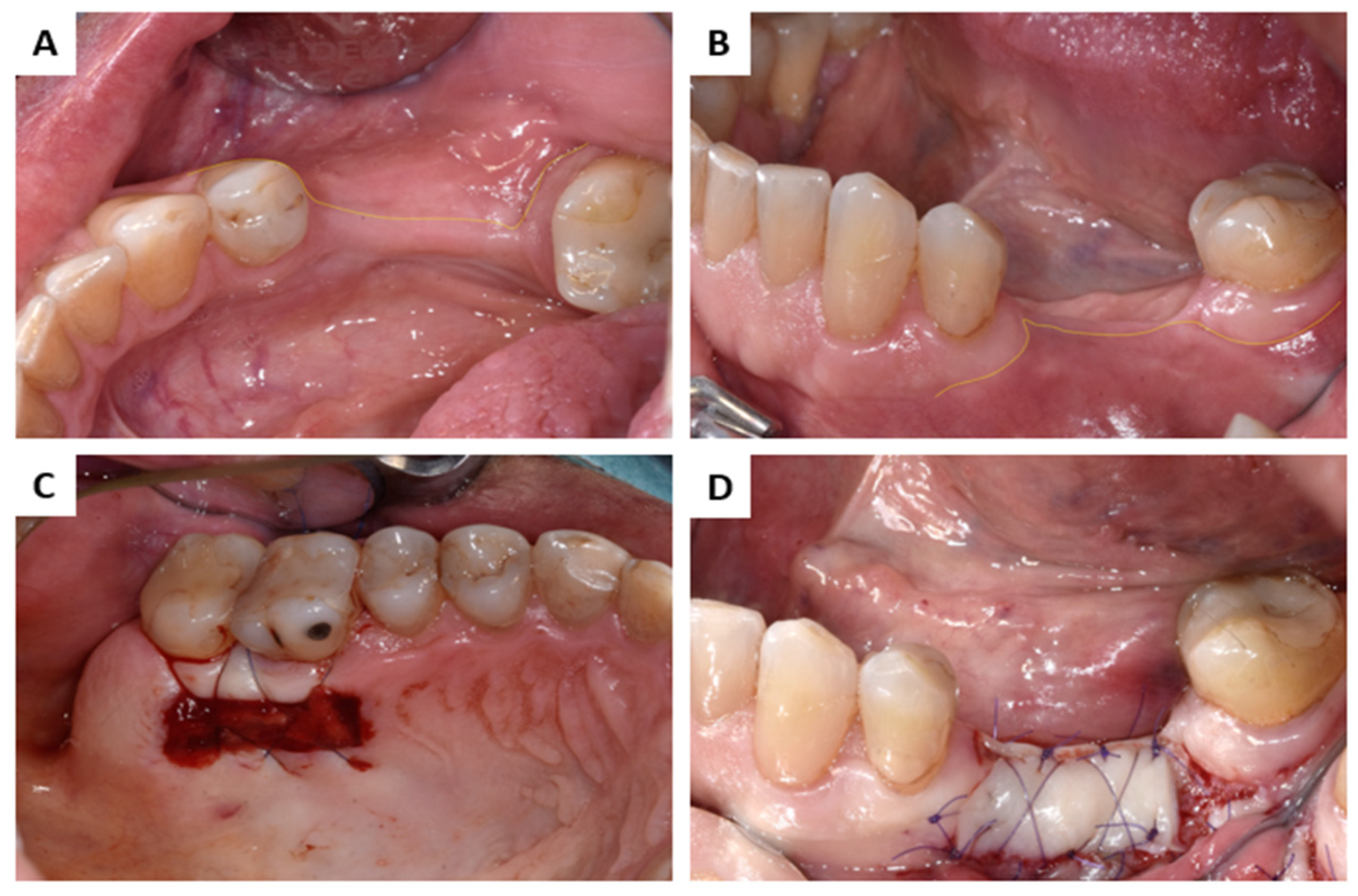
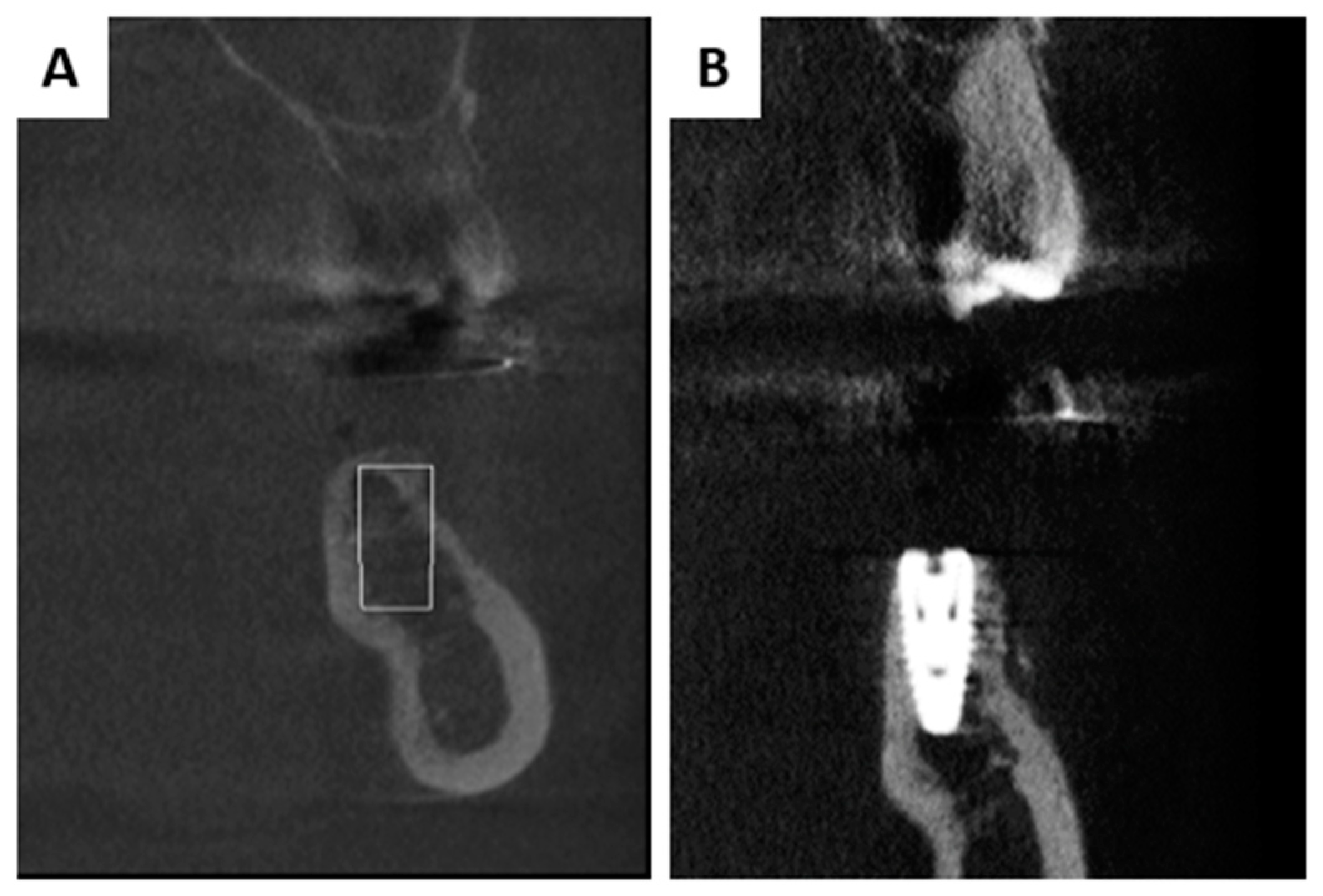
2.2. Interactions in Patients
3. Results
3.1. In Vitro Interactions
3.2. Interactions in Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.-K.; Ku, J.-K. Ridge Augmentation in Implant Dentistry. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, B.; Jaunich, M.; Müller, W.D.; Beuer, F.; Zafiropoulos, G.G.; Houshmand, A. Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Fienitz, T.; Moses, O.; Klemm, C.; Happe, A.; Ferrari, D.; Kreppel, M.; Ormianer, Z.; Gal, M.; Rothamel, D. Histological and Radiological Evaluation of Sintered and Non-Sintered Deproteinized Bovine Bone Substitute Materials in Sinus Augmentation Procedures. A Prospective, Randomized-Controlled, Clinical Multicenter Study. Clin. Oral Investig. 2017, 21, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Tawil, G.; Tawil, P.; Khairallah, A. Sinus Floor Elevation Using the Lateral Approach and Bone Window Repositioning I: Clinical and Radiographic Results in 102 Consecutively Treated Patients Followed from 1 to 5 Years. Int. J. Oral Maxillofac. Implant. 2016, 31, 827–834. [Google Scholar] [CrossRef]
- Lorean, A.; Mazor, Z.; Barbu, H.; Mijiritsky, E.; Levin, L. Nasal Floor Elevation Combined with Dental Implant Placement: A Long-Term Report of up to 86 Months. Int. J. Oral Maxillofac. Implant. 2014, 29, 705–708. [Google Scholar] [CrossRef]
- Murugan, R.; Rao, K.P.; Kumar, T.S.S. Heat-Deproteinated Xenogeneic Bone from Slaughterhouse Waste: Physico-Chemical Properties. Bull. Mater. Sci. 2003, 26, 523–528. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Smeets, R.; Happe, A.; Fienitz, T.; Mazor, Z.; Zöller, J. Sinusbodenelevation Mit Einem Gesinterten, Natürlichen Knochenmineral. Z. Fur Zahnarztl. Implantol. 2011, 1, 60–67. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; de Buitrago, J.G.; Padial-Molina, M.; Fernández-Barbero, J.E.; Ata-Ali, J.; O’Valle, F. Histopathological Comparison of Healing after Maxillary Sinus Augmentation Using Xenograft Mixed with Autogenous Bone versus Allograft Mixed with Autogenous Bone. Clin. Oral Implant. Res. 2018, 29, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Marchetti, C.; Iezzi, G.; Piattelli, A.; Worthington, H.; Pellegrino, G.; Esposito, M. Vertical Ridge Augmentation of the Atrophic Posterior Mandible with Interpositional Bloc Grafts: Bone from the Iliac Crest vs. Bovine Anorganic Bone. Clinical and Histological Results up to One Year after Loading from a Randomized-Controlled Clinical Trial. Clin. Oral Implant. Res. 2009, 20, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, C.; Beretta, M.; Salina, S.; Santoro, F. Reduction of Autogenous Bone Graft Resorption by Means of Bio-Oss Coverage: A Prospective Study—PubMed. Int. J. Periodontics Restor. Dent 2005, 25, 19–25. [Google Scholar]
- Canullo, L.; Del Fabbro, M.; Khijmatgar, S.; Panda, S.; Ravidà, A.; Tommasato, G.; Sculean, A.; Pesce, P. Dimensional and Histomorphometric Evaluation of Biomaterials Used for Alveolar Ridge Preservation: A Systematic Review and Network Meta-Analysis. Clin. Oral Investig. 2022, 26, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Kyyak, S.; Pabst, A.; Heimes, D.; Kämmerer, P.W. The Influence of Hyaluronic Acid Biofunctionalization of a Bovine Bone Substitute on Osteoblast Activity In Vitro. Materials 2021, 14, 2885. [Google Scholar] [CrossRef]
- Pröhl, A.; Batinic, M.; Alkildani, S.; Hahn, M.; Radenkovic, M.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Bone Healing Capacity of a Novel Bone Grafting Material Combined with Hyaluronic Acid. Int. J. Mol. Sci. 2021, 22, 4818. [Google Scholar] [CrossRef]
- Rakašević, D.; Šćepanović, M.; Mijailović, I.; Mišić, T.; Janjić, B.; Soldatović, I.; Marković, A. Reconstructive Peri-Implantitis Therapy by Using Bovine Bone Substitute with or without Hyaluronic Acid: A Randomized Clinical Controlled Pilot Study. J. Funct. Biomater. 2023, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Chapanov, K.; Deliverska, E.; Zafiropoulos, G.; Trajkovski, B. Lateral Sinus Augmentation by Using Natural Bovine Bone Substitute with Hyaluronate. Int. J. Dent. Biomater. Res. 2022, 1, 8–12. [Google Scholar] [CrossRef]
- Frenkel, J.S. The Role of Hyaluronan in Wound Healing. Int. Wound J. 2014, 11, 159. [Google Scholar] [CrossRef]
- Kyyak, S.; Blatt, S.; Wiesmann, N.; Smeets, R.; Kaemmerer, P.W. Hyaluronic Acid with Bone Substitutes Enhance Angiogenesis In Vivo. Materials 2022, 15, 3839. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Tommasato, G.; Pesce, P.; Ravidà, A.; Khijmatgar, S.; Sculean, A.; Galli, M.; Antonacci, D.; Canullo, L. Sealing Materials for Post-Extraction Site: A Systematic Review and Network Meta-Analysis. Clin. Oral Investig. 2022, 26, 1137–1154. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, C.; Shi, H.; Luo, X.; Sun, H.; Wang, Q.; Zhang, D. Advances in Barrier Membranes for Guided Bone Regeneration Techniques. Front. Bioeng. Biotechnol. 2022, 10, 921576. [Google Scholar] [CrossRef]
- Annunziata, M.; Nastri, L.; Cecoro, G.; Guida, L. The Use of Poly-d,l-Lactic Acid (PDLLA) Devices for Bone Augmentation Techniques: A Systematic Review. Molecules 2017, 22, 2214. [Google Scholar] [CrossRef] [PubMed]
- Rasia dal Polo, M.; Poli, P.P.; Rancitelli, D.; Beretta, M.; Maiorana, C. Alveolar Ridge Reconstruction with Titanium Meshes: A Systematic Review of the Literature. Med. Oral Patol. Oral Y Cir. Bucal 2014, 19, e639–e646. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and Options Using Resorbable versus Nonresorbable Membranes for Successful Guided Bone Regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef]
- Kölliker, R.; Hicklin, S.P.; Hirsiger, C.; Liu, C.C.; Janett, F.; Schmidlin, P.R. In Vitro Evaluation of the Permeability of Different Resorbable Xenogeneic Membranes after Collagenolytic Degradation. Membranes 2022, 12, 787. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zöller, J. Biocompatibility and Biodegradation of a Native Porcine Pericardium Membrane: Results of in Vitro and in Vivo Examinations—PubMed. Int. J. Oral Maxillofac. Implant. 2012, 27, 146–154. [Google Scholar]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of Differently Cross-Linked Collagen Membranes: An Experimental Study in the Rat. Clin. Oral Implant. Res. 2005, 16, 369–378. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable Magnesium Barrier Membrane Used for Guided Bone Regeneration in Dental Surgery. Bioact. Mater. 2022, 14, 152–168. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Analysis of a Pure Magnesium Membrane Degradation Process and Its Functionality When Used in a Guided Bone Regeneration Model in Beagle Dogs. Materials 2022, 15, 3106. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable Magnesium Fixation Screw for Barrier Membranes Used in Guided Bone Regeneration. Bioact. Mater. 2022, 14, 15–30. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Biodegradation of a Magnesium Alloy Fixation Screw Used in a Guided Bone Regeneration Model in Beagle Dogs. Materials 2022, 15, 4111. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Hesse, B.; Stojanovic, S.; Seim, C.; Weitkamp, T.; Batinic, M.; Goerke, O.; Kačarević, Ž.P.; Rider, P.; Najman, S.; et al. Biocompatibility Analyses of HF-Passivated Magnesium Screws for Guided Bone Regeneration (GBR). Int. J. Mol. Sci. 2021, 22, 12567. [Google Scholar] [CrossRef] [PubMed]
- Elad, A.; Rider, P.; Rogge, S.; Witte, F.; Tadić, D.; Kačarević, Ž.P.; Steigmann, L. Application of Biodegradable Magnesium Membrane Shield Technique for Immediate Dentoalveolar Bone Regeneration. Biomedicines 2023, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Blašković, M.; Butorac Prpić, I.; Blašković, D.; Rider, P.; Tomas, M.; Čandrlić, S.; Botond Hangyasi, D.; Čandrlić, M.; Perić Kačarević, Ž. Guided Bone Regeneration Using a Novel Magnesium Membrane: A Literature Review and a Report of Two Cases in Humans. J. Funct. Biomater. 2023, 14, 307. [Google Scholar] [CrossRef]
- Carboneras, M.; García-Alonso, M.C.; Escudero, M.L. Biodegradation Kinetics of Modified Magnesium-Based Materials in Cell Culture Medium. Corros. Sci. 2011, 53, 1433–1439. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In Vivo Study of Magnesium Plate and Screw Degradation and Bone Fracture Healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef]
- Razavi, M.; Huang, Y. Assessment of Magnesium-Based Biomaterials: From Bench to Clinic. Biomater. Sci. 2019, 7, 2241–2263. [Google Scholar] [CrossRef]
- Shan, X.; Xu, Y.; Kolawole, S.K.; Wen, L.; Qi, Z.; Xu, W.; Chen, J. Degradable Pure Magnesium Used as a Barrier Film for Oral Bone Regeneration. J. Funct. Biomater. 2022, 13, 298. [Google Scholar] [CrossRef]
- Seitz, J.M.; Eifler, R.; Bach, F.W.; Maier, H.J. Magnesium Degradation Products: Effects on Tissue and Human Metabolism. J. Biomed. Mater. Res. A 2014, 102, 3744–3753. [Google Scholar] [CrossRef]
- Xin, Y.; Huo, K.; Tao, H.; Tang, G.; Chu, P.K. Influence of Aggressive Ions on the Degradation Behavior of Biomedical Magnesium Alloy in Physiological Environment. Acta Biomater. 2008, 4, 2008–2015. [Google Scholar] [CrossRef]
- Hassan, H.W.; Grasso, V.; Korostynska, O.; Khan, H.; Jose, J.; Mirtaheri, P. An Overview of Assessment Tools for Determination of Biological Magnesium Implant Degradation. Med. Eng. Phys. 2021, 93, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Alpar, B.; Leyhausen, G.; Günay, H.; Geurtsen, W. Compatibility of Resorbable and Nonresorbable Guided Tissue Regeneration Membranes in Cultures of Primary Human Periodontal Ligament Fibroblasts and Human Osteoblast-like Cells. Clin. Oral Investig. 2000, 4, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of Recent Developments in the Field of Magnesium Corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Riachi, F.; Naaman, N.; Tabarani, C.; Aboelsaad, N.; Aboushelib, M.N.; Berberi, A.; Salameh, Z. Influence of Material Properties on Rate of Resorption of Two Bone Graft Materials after Sinus Lift Using Radiographic Assessment. Int. J. Dent. 2012, 2012, 737262. [Google Scholar] [CrossRef]
- Huynh, A.; Priefer, R. Hyaluronic Acid Applications in Ophthalmology, Rheumatology, and Dermatology. Carbohydr. Res. 2020, 489, 107950. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic Acid: Perspectives in Dentistry. A Systematic Review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef]
- Zafiropoulos, G.; Trajkovski, B. Socket Preservation with High-Density Polytetrafluoroethylene Barrier Membrane during Open Healing. Int. J. Dent. Biomater. Res. 2022, 1, 13–19. [Google Scholar] [CrossRef]
- Cvetanovska Stojcheva, D.; Aleksandrovska, A.; Petrevska, P.; Micevski, B. Alveolar Ridge Augmentation by Open Healing with High-Density Polytetrafluoroethylene Membrane. Int. J. Dent. Biomater. Res. 2022, 1, 1–7. [Google Scholar] [CrossRef]
- Zafiropoulos, G.; Kačarević, Z.P.; Qasim, S.S.B.; Trajkovski, B. Open-Healing Socket Preservation with a Novel Dense Polytetrafluoroethylene (DPTFE) Membrane: A Retrospective Clinical Study. Medicina 2020, 56, 216. [Google Scholar] [CrossRef]
- Papi, P.; Di Murro, B.; Tromba, M.; Passarelli, P.C.; D’addona, A.; Pompa, G. The Use of a Non-Absorbable Membrane as an Occlusive Barrier for Alveolar Ridge Preservation: A One Year Follow-Up Prospective Cohort Study. Antibiotics 2020, 9, 110. [Google Scholar] [CrossRef]
- Saad, S.; Qasim, B.; Al-Asfour, A.A.; Abuzayeda, M.; Mohamed, A.M.; Trajkovski, B.; Murray, C.A.; Zafiropoulos, G.-G. Differences in Mechanical and Physicochemical Properties of Several PTFE Membranes Used in Guided Bone Regeneration. Materials 2023, 16, 904. [Google Scholar] [CrossRef]
- Nkenke, E.; Vairaktaris, E.; Knipfer, C.; Stelzle, F.; Schwarz, S.; Eyüpoglu, I.; Ganslandt, O.; Leis, T. Prospective Assessment of Complications Associated with Ultrasound Activated Resorbable Pin Osteosynthesis in Pediatric Craniofacial Surgery: Preliminary Results. Neurocirugia 2011, 22, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Riffault, M.; Hoey, D.; Duffy, B.; Curtin, J.; Jaiswal, S. Biomimetic Hyaluronic Acid-Lysozyme Composite Coating on AZ31 Mg Alloy with Combined Antibacterial and Osteoinductive Activities. ACS Biomater. Sci. Eng. 2017, 3, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Labour, M.N.; Hoey, D.; Duffy, B.; Curtin, J.; Jaiswal, S. Enhanced Corrosion Resistance and Cytocompatibility of Biomimetic Hyaluronic Acid Functionalised Silane Coating on AZ31 Mg Alloy for Orthopaedic Applications. J. Mater. Sci. Mater. Med. 2018, 29, 144. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.Y.; Lee, S.H.; Lee, M.H.; Lee, K.B. Stabilized Loading of Hyaluronic Acid-Containing Hydrogels into Magnesium-Based Cannulated Screws. ACS Biomater. Sci. Eng. 2020, 6, 715–726. [Google Scholar] [CrossRef]
- Rohr, N.; Brunner, C.; Bellon, B.; Fischer, J.; de Wild, M. Characterization of a Cotton-Wool like Composite Bone Graft Material. J. Mater. Sci. Mater. Med. 2022, 33, 61. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The Role and Significance of Magnesium in Modern Day Research-A Review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.M.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blašković, M.; Blašković, D.; Hangyasi, D.B.; Peloza, O.C.; Tomas, M.; Čandrlić, M.; Rider, P.; Mang, B.; Kačarević, Ž.P.; Trajkovski, B. Evaluation between Biodegradable Magnesium Metal GBR Membrane and Bovine Graft with or without Hyaluronate. Membranes 2023, 13, 691. https://doi.org/10.3390/membranes13080691
Blašković M, Blašković D, Hangyasi DB, Peloza OC, Tomas M, Čandrlić M, Rider P, Mang B, Kačarević ŽP, Trajkovski B. Evaluation between Biodegradable Magnesium Metal GBR Membrane and Bovine Graft with or without Hyaluronate. Membranes. 2023; 13(8):691. https://doi.org/10.3390/membranes13080691
Chicago/Turabian StyleBlašković, Marko, Dorotea Blašković, David Botond Hangyasi, Olga Cvijanović Peloza, Matej Tomas, Marija Čandrlić, Patrick Rider, Berit Mang, Željka Perić Kačarević, and Branko Trajkovski. 2023. "Evaluation between Biodegradable Magnesium Metal GBR Membrane and Bovine Graft with or without Hyaluronate" Membranes 13, no. 8: 691. https://doi.org/10.3390/membranes13080691
APA StyleBlašković, M., Blašković, D., Hangyasi, D. B., Peloza, O. C., Tomas, M., Čandrlić, M., Rider, P., Mang, B., Kačarević, Ž. P., & Trajkovski, B. (2023). Evaluation between Biodegradable Magnesium Metal GBR Membrane and Bovine Graft with or without Hyaluronate. Membranes, 13(8), 691. https://doi.org/10.3390/membranes13080691











