Development of a Green Polymeric Membrane for Sodium Diclofenac Removal from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Poly(Vinyl Alcohol)-Based Membrane Preparation
2.2. Optimization of Crosslinking Conditions Using DOE and Statistical Analyses
2.3. Evaluation and Characterization Techniques
2.3.1. Spectroscopic Characterization
2.3.2. Thermal Characterization
2.3.3. Physical and Morphological Characterization
2.3.4. Filtration Experiments
3. Results and Discussion
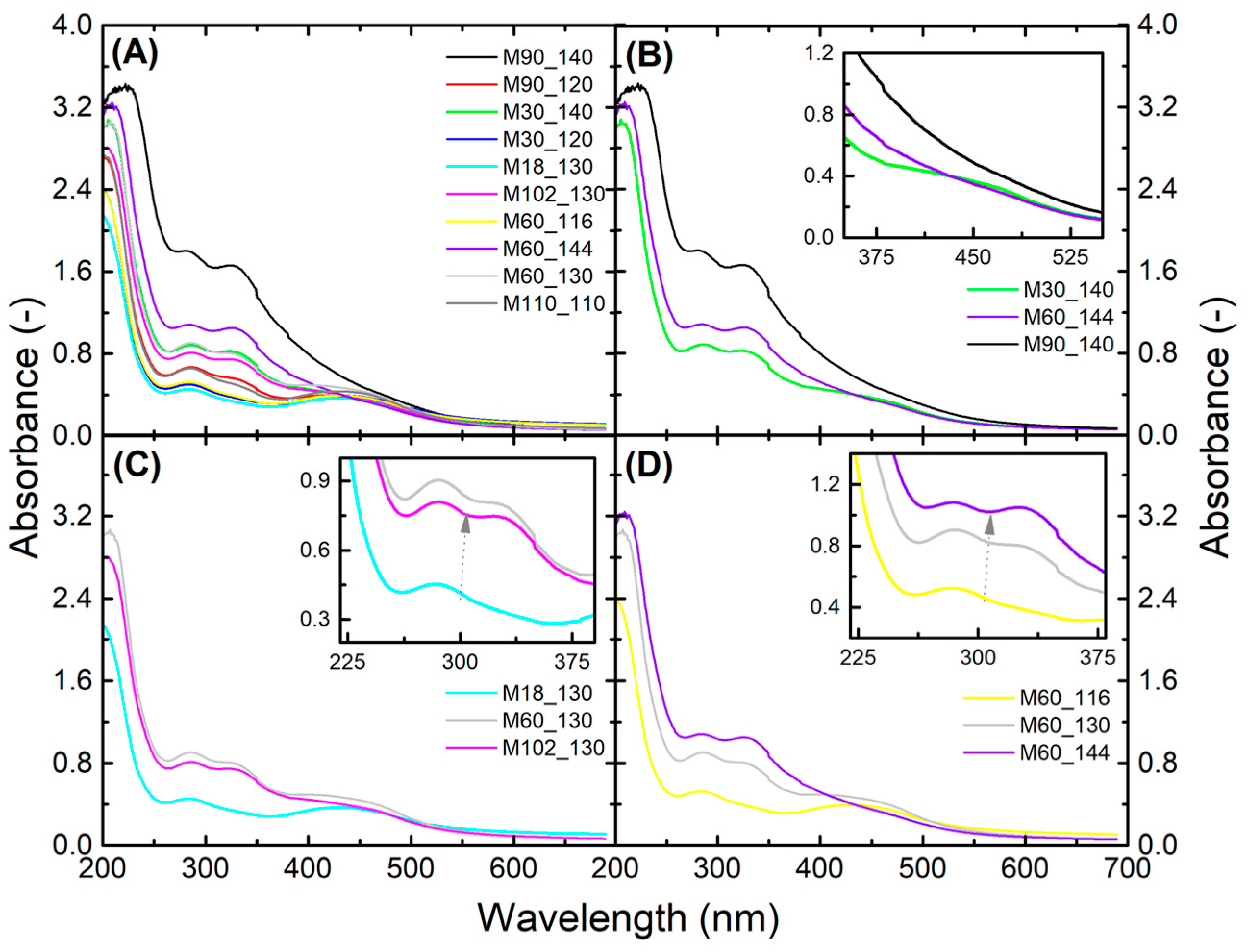
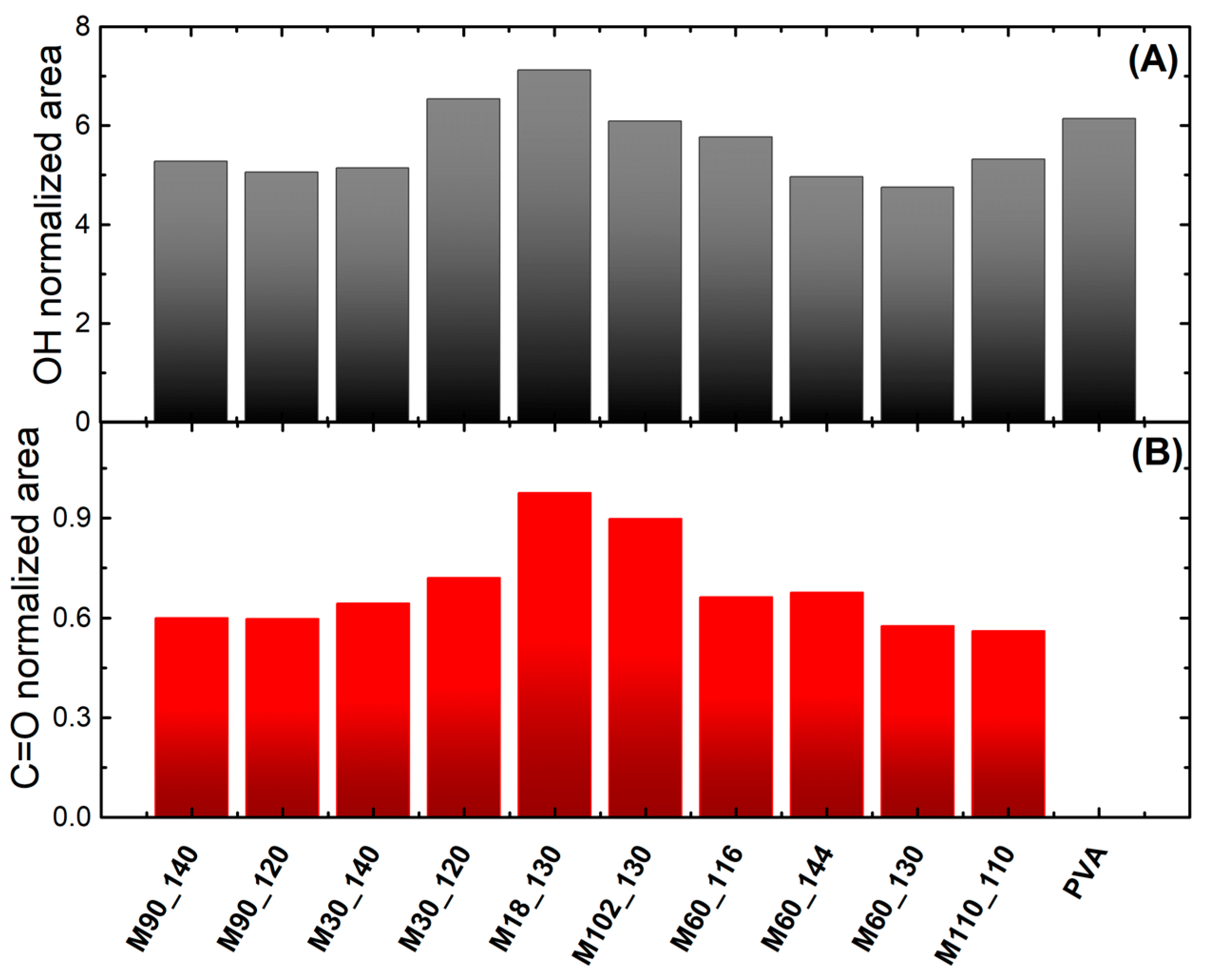
3.1. Spectroscopic Characterization Using Ultraviolet-Visible and Fourier-Transform Infrared Spectroscopies
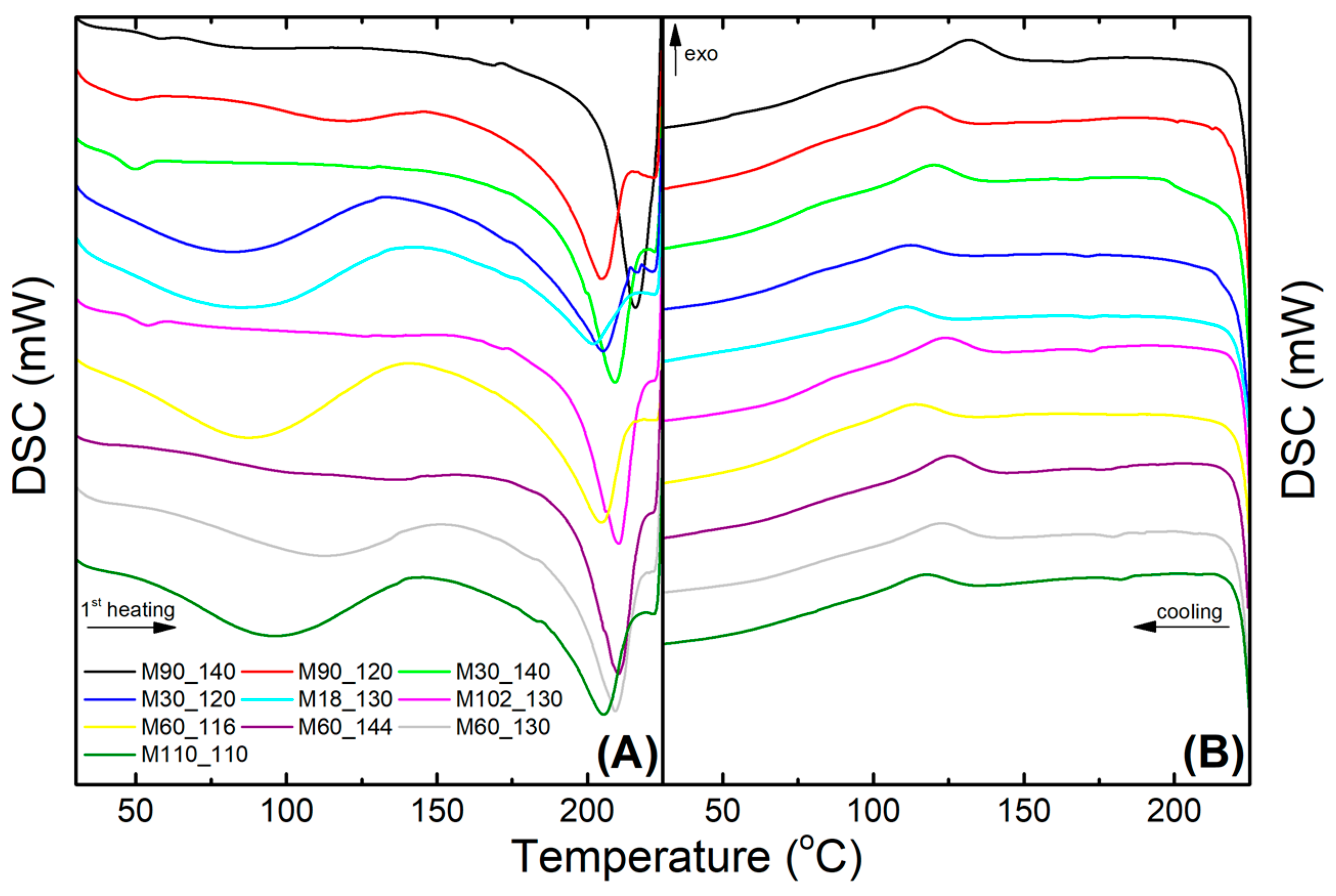
3.2. Thermal Characterization Using Differential Scanning Calorimetry
3.3. Physical Characterization via Swelling and Water Contact Angle Analyses
3.4. Membrane Performance in Filtration Experiments and Statistical Analysis
3.5. Desirability Function
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Harby, N.F.; El-Batouti, M.; Elewa, M.M. Prospects of polymeric nanocomposite membranes for water purification and scalability and their health and environmental impacts: A review. Nanomaterials 2022, 12, 3637. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Cuprys, A.K.; Maletskyi, Z.; Ratnaweera, H. Treatment trends and combined methods in removing pharmaceuticals and personal care products from wastewater—A review. Membranes 2023, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Directive (eu) 2020/2184 of 16 December 2020 on the Quality of Water Intended for Human Consumption; European Union: Brussels, Belgium, 2020. [Google Scholar]
- United States Environmental Protection Agency. Epa 815-r-16-006: Summary of Nominations for the Fourth Contaminant Candidate List (ccl 4); Environmental Protection Agency: Washington, DC, USA, 2016.
- Vebber, M.C.; da Silva Crespo, J.; Giovanela, M. Self-assembled thin films of paa/pah/tio2 for the photooxidation of ibuprofen. Part I: Optimization of photoactivity using design of experiments and surface response methodology. Chem. Eng. J. 2019, 360, 1447–1458. [Google Scholar] [CrossRef]
- Vebber, M.C.; Aguzzoli, C.; Beltrami, L.V.R.; Fetter, G.; da Silva Crespo, J.; Giovanela, M. Self-assembled thin films of paa/pah/tio2 for the photooxidation of ibuprofen. Part II: Characterization, sensitization, kinetics and reutilization. Chem. Eng. J. 2019, 361, 1487–1496. [Google Scholar] [CrossRef]
- Kerwald, J.; Vebber, M.C.; Aguzzoli, C.; da Silva Crespo, J.; Giovanela, M. Influence of silver nanoparticle deposition on self-assembled thin films of weak polyelectrolytes/tio2 for bezafibrate photodegradation through central composite experimental design. J. Environ. Chem. Eng. 2020, 8, 103619. [Google Scholar] [CrossRef]
- Antunes, M.; Esteves, V.I.; Guégan, R.; Crespo, J.S.; Fernandes, A.N.; Giovanela, M. Removal of diclofenac sodium from aqueous solution by isabel grape bagasse. Chem. Eng. J. 2012, 192, 114–121. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B.S.M. Removal of contaminants from water by membrane filtration: A review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Sahu, A.; Dosi, R.; Kwiatkowski, C.; Schmal, S.; Poler, J.C. Advanced polymeric nanocomposite membranes for water and wastewater treatment: A comprehensive review. Polymers 2023, 15, 540. [Google Scholar] [CrossRef]
- Divya, S.; Oh, T.H. Polymer nanocomposite membrane for wastewater treatment: A critical review. Polymers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Nain, A.; Sangili, A.; Hu, S.-R.; Chen, C.-H.; Chen, Y.-L.; Chang, H.-T. Recent progress in nanomaterial-functionalized membranes for removal of pollutants. iScience 2022, 25, 104616. [Google Scholar] [CrossRef] [PubMed]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of diclofenac from wastewater: A comprehensive review of detection, characteristics and tertiary treatment techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, M.; Lee, H.-J.; A Ganzoury, M.; Zhang, N.; de Lannoy, C.-F. Nanocomposite polymeric membranes for organic micropollutant removal: A critical review. ACS EST Eng. 2022, 2, 1574–1598. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef] [PubMed]
- Reyes, N.J.D.G.; Geronimo, F.K.F.; Yano, K.A.V.; Guerra, H.B.; Kim, L.-H. Pharmaceutical and personal care products in different matrices: Occurrence, pathways, and treatment processes. Water 2021, 13, 1159. [Google Scholar] [CrossRef]
- Licona, K.P.M.; Geaquinto, L.R.d.O.; Nicolini, J.V.; Figueiredo, N.G.; Chiapetta, S.C.; Habert, A.C.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Huang, Z.; Gong, B.; Huang, C.-P.; Pan, S.-Y.; Wu, P.; Dang, Z.; Chiang, P.-C. Performance evaluation of integrated adsorption-nanofiltration system for emerging compounds removal: Exemplified by caffeine, diclofenac and octylphenol. J. Environ. Manag. 2019, 231, 121–128. [Google Scholar] [CrossRef]
- Żyłła, R.; Boruta, T.; Gmurek, M.; Milala, R.; Ledakowicz, S. Integration of advanced oxidation and membrane filtration for removal of micropollutants of emerging concern. Process Saf. Environ. Prot. 2019, 130, 67–76. [Google Scholar] [CrossRef]
- Cuhorka, J.; Wallace, E.; Mikulášek, P. Removal of micropollutants from water by commercially available nanofiltration membranes. Sci. Total Environ. 2020, 720, 137474. [Google Scholar] [CrossRef]
- Maryam, B.; Buscio, V.; Odabasi, S.U.; Buyukgungor, H. A study on behavior, interaction and rejection of paracetamol, diclofenac and ibuprofen (phacs) from wastewater by nanofiltration membranes. Environ. Technol. Innov. 2020, 18, 100641. [Google Scholar] [CrossRef]
- Grand View Research. Membrane Separation Technology Market Size, Share & Trends Analysis Report by Technology (Microfiltration, Ultrafiltration, Nanofiltration, Reverse Osmosis), by Application, by Region, and Segment Forecasts, 2022—2030. Report id: Gvr-4-68038-503-8; Grand View Research: San Francisco, CA, USA, 2022. [Google Scholar]
- The Insight Partners. South America Membrane Separation Systems Market—Forecast to 2028, id: Tip17679790; The Insight Partners: New York, NY, USA, 2023. [Google Scholar]
- Matin, A.; Jillani, S.M.S.; Baig, U.; Ihsanullah, I.; Alhooshani, K. Removal of pharmaceutically active compounds from water sources using nanofiltration and reverse osmosis membranes: Comparison of removal efficiencies and in-depth analysis of rejection mechanisms. J. Environ. Manag. 2023, 338, 117682. [Google Scholar] [CrossRef]
- Beuscher, U.; Kappert, E.J.; Wijmans, J.G. Membrane research beyond materials science. J. Membr. Sci. 2022, 643, 119902. [Google Scholar] [CrossRef]
- Jiang, S.; Ladewig, B.P. Green synthesis of polymeric membranes: Recent advances and future prospects. Curr. Opin. Green Sustain. Chem. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Gautam, L.; Warkar, S.G.; Ahmad, S.I.; Kant, R.; Jain, M. A review on carboxylic acid cross-linked polyvinyl alcohol: Properties and applications. Polym. Eng. Sci. 2022, 62, 225–246. [Google Scholar] [CrossRef]
- The World Commission on Environment and Development. Our Common Future; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- American Chemical Society (ACS). What Is Green Chemistry? Available online: https://www.acs.org/greenchemistry/what-is-green-chemistry.Html (accessed on 26 April 2023).
- Figoli, A.; Marino, T.; Simone, S.; Di Nicolò, E.; Li, X.M.; He, T.; Tornaghi, S.; Drioli, E. Towards non-toxic solvents for membrane preparation: A review. Green Chem. 2014, 16, 4034–4059. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, C.; Huang, Q.; Liu, H.; Zhao, J. Progress on polymeric hollow fiber membrane preparation technique from the perspective of green and sustainable development. Chem. Eng. J. 2021, 403, 126295. [Google Scholar] [CrossRef]
- Naziri Mehrabani, S.A.; Vatanpour, V.; Koyuncu, I. Green solvents in polymeric membrane fabrication: A review. Sep. Purif. Technol. 2022, 298, 121691. [Google Scholar] [CrossRef]
- Abu Bakar, N.H.H.; Tan, W.L. Natural composite membranes for water remediation: Toward a sustainable tomorrow. In Renewable Energy and Sustainable Technologies for Building and Environmental Applications: Options for a Greener Future; Ahmad, M.I., Ismail, M., Riffat, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–49. [Google Scholar]
- Xie, W.; Li, T.; Tiraferri, A.; Drioli, E.; Figoli, A.; Crittenden, J.C.; Liu, B. Toward the next generation of sustainable membranes from green chemistry principles. ACS Sustain. Chem. Eng. 2021, 9, 50–75. [Google Scholar] [CrossRef]
- Kim, D.; Nunes, S.P. Green solvents for membrane manufacture: Recent trends and perspectives. Curr. Opin. Green Sustain. Chem. 2021, 28, 100427. [Google Scholar] [CrossRef]
- Marino, T.; Blasi, E.; Tornaghi, S.; Di Nicolò, E.; Figoli, A. Polyethersulfone membranes prepared with rhodiasolv® polarclean as water soluble green solvent. J. Membr. Sci. 2018, 549, 192–204. [Google Scholar] [CrossRef]
- Marino, T.; Galiano, F.; Molino, A.; Figoli, A. New frontiers in sustainable membrane preparation: Cyrene™ as green bioderived solvent. J. Membr. Sci. 2019, 580, 224–234. [Google Scholar] [CrossRef]
- Wang, H.H.; Jung, J.T.; Kim, J.F.; Kim, S.; Drioli, E.; Lee, Y.M. A novel green solvent alternative for polymeric membrane preparation via nonsolvent-induced phase separation (nips). J. Membr. Sci. 2019, 574, 44–54. [Google Scholar] [CrossRef]
- Zou, D.; Hu, C.; Drioli, E.; Zhong, Z. Engineering green and high-flux poly(vinylidene fluoride) membranes for membrane distillation via a facile co-casting process. J. Membr. Sci. 2022, 655, 120577. [Google Scholar] [CrossRef]
- Xie, W.; Tiraferri, A.; Liu, B.; Tang, P.; Wang, F.; Chen, S.; Figoli, A.; Chu, L.-Y. First exploration on a poly(vinyl chloride) ultrafiltration membrane prepared by using the sustainable green solvent polarclean. ACS Sustain. Chem. Eng. 2020, 8, 91–101. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Gan, Z.-Q.; Bao, R.-Y.; Ke, K.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Green and robust superhydrophilic electrospun stereocomplex polylactide membranes: Multifunctional oil/water separation and self-cleaning. J. Membr. Sci. 2020, 593, 117420. [Google Scholar] [CrossRef]
- Oldal, D.G.; Topuz, F.; Holtzl, T.; Szekely, G. Green electrospinning of biodegradable cellulose acetate nanofibrous membranes with tunable porosity. ACS Sustain. Chem. Eng. 2023, 11, 994–1005. [Google Scholar] [CrossRef]
- Reino Olegário da Silva, D.A.; Bosmuler Zuge, L.C.; de Paula Scheer, A. Preparation and characterization of a novel green silica/pva membrane for water desalination by pervaporation. Sep. Purif. Technol. 2020, 247, 116852. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Xia, M.; Cai, M.; Nie, Z.; Gao, J. Green multifunctional pva composite hydrogel-membrane for the efficient purification of emulsified oil wastewater containing pb2+ ions. Sci. Total Environ. 2023, 856, 159271. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, F.; Zhang, J.; Wang, Y.; Yang, S.; Xing, D. Gradient crosslinking optimization for the selective layer to prepare polyvinyl alcohol (pva) nanofiltration (nf) membrane: The enhanced filtration performance and potential rejection for edcs. J. Membr. Sci. 2023, 675, 121548. [Google Scholar] [CrossRef]
- Razmgar, K.; Nasiraee, M. Polyvinyl alcohol-based membranes for filtration of aqueous solutions: A comprehensive review. Polym. Eng. Sci. 2022, 62, 25–43. [Google Scholar] [CrossRef]
- Sapalidis, A.A. Porous polyvinyl alcohol membranes: Preparation methods and applications. Symmetry 2020, 12, 960. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research progress of polyvinyl alcohol water-resistant film materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Ko, C.-Y.; Chang, K.-C.; Chen, C.-H. Influences of processing and sterilizing strategies on reduced silver nanoparticles in poly(vinyl alcohol) electrospun membranes: Optimization and preservation of antibacterial activity. Mater. Chem. Phys. 2020, 254, 123300. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Michele, A.; Paschkowski, P.; Hänel, C.; Tovar, G.E.M.; Schiestel, T.; Southan, A. Acid catalyzed crosslinking of polyvinyl alcohol for humidifier membranes. J. Appl. Polym. Sci. 2022, 139, 51606. [Google Scholar] [CrossRef]
- Sonker, A.K.; Rathore, K.; Nagarale, R.K.; Verma, V. Crosslinking of polyvinyl alcohol (pva) and effect of crosslinker shape (aliphatic and aromatic) thereof. J. Polym. Environ. 2018, 26, 1782–1794. [Google Scholar] [CrossRef]
- do Nascimento, F.C.; de Aguiar, L.C.V.; Costa, L.A.T.; Fernandes, M.T.; Marassi, R.J.; Gomes, A.d.S.; de Castro, J.A. Formulation and characterization of crosslinked polyvinyl alcohol (pva) membranes: Effects of the crosslinking agents. Polym. Bull. 2021, 78, 917–929. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and thermally crosslinked pva-based membranes: Effect on swelling and transport behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Gohil, J.M.; Dutta, K. Poly(vinyl alcohol)-based membranes for fuel cell and water treatment applications: A review on recent advancements. Polym. Adv. Technol. 2021, 32, 4175–4203. [Google Scholar] [CrossRef]
- Katz, M.G.; Wydeven, T., Jr. Selective permeability of pva membranes—I: Radiation-crosslinked membranes. J. Appl. Polym. Sci. 1981, 26, 2935–2946. [Google Scholar] [CrossRef]
- Katz, M.G.; Wydeven, T., Jr. Selective permeability of pva membranes—II: Heat-treated membranes. J. Appl. Polym. Sci. 1982, 27, 79–87. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Angumeenal, A.R.; Venkappayya, D. An overview of citric acid production. LWT Food Sci. Technol. 2013, 50, 367–370. [Google Scholar] [CrossRef]
- Yu, D.; Feng, Y.-Y.; Xu, J.-X.; Kong, B.-H.; Liu, Q.; Wang, H. Fabrication, characterization, and antibacterial properties of citric acid crosslinked pva electrospun microfibre mats for active food packaging. Packag. Technol. Sci. 2021, 34, 361–370. [Google Scholar] [CrossRef]
- Raota, C.S.; Cerbaro, A.F.; Salvador, M.; Delamare, A.P.L.; Echeverrigaray, S.; da Silva Crespo, J.; da Silva, T.B.; Giovanela, M. Green synthesis of silver nanoparticles using an extract of ives cultivar (vitis labrusca) pomace: Characterization and application in wastewater disinfection. J. Environ. Chem. Eng. 2019, 7, 103383. [Google Scholar] [CrossRef]
- Mohsin, M.; Hossin, A.; Haik, Y. Thermal and mechanical properties of poly(vinyl alcohol) plasticized with glycerol. J. Appl. Polym. Sci. 2011, 122, 3102–3109. [Google Scholar] [CrossRef]
- Sau, S.; Pandit, S.; Kundu, S. Crosslinked poly (vinyl alcohol): Structural, optical and mechanical properties. Surf. Interfaces 2021, 25, 101198. [Google Scholar] [CrossRef]
- Merck, J.Z.; Raota, C.S.; Duarte, J.; Baldasso, C.; Crespo, J.d.S.; Giovanela, M. Development of poly(vinyl alcohol)-based membranes by the response surface methodology for environmental applications. Rev. Eletrôn. Gestão Educ. Tecnol. Ambient. 2020, 24, e5. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2013. [Google Scholar]
- Canevarolo Júnior, S.V. Ciência dos Polímeros: Um Texto Básico Para Tecnólogos e Engenheiros; Artiiber: São Paulo, Brasil, 2006. [Google Scholar]
- Birck, C.; Degoutin, S.; Tabary, N.; Miri, V.; Bacquet, M. New crosslinked cast films based on poly(vinyl alcohol): Preparation and physico-chemical properties. Express Polym. Lett. 2014, 8, 941–952. [Google Scholar] [CrossRef]
- Gugliuzza, A. Membrane swelling. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2. [Google Scholar]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A Comprehensive Review on Polymeric Nano-Composite Membranes for Water Treatment. J. Membr. Sci. Technol. 2018, 8, 179. [Google Scholar] [CrossRef]
- Raicopol, M.D.; Andronescu, C.; Voicu, S.I.; Vasile, E.; Pandele, A.M. Cellulose acetate/layered double hydroxide adsorptive membranes for efficient removal of pharmaceutical environmental contaminants. Carbohydr. Polym. 2019, 214, 204–212. [Google Scholar] [CrossRef]
- Nadour, M.; Boukraa, F.; Benaboura, A. Removal of diclofenac, paracetamol and metronidazole using a carbon-polymeric membrane. J. Environ. Chem. Eng. 2019, 7, 103080. [Google Scholar] [CrossRef]
- ECHA, European Chemicals Agency. Substance Infocard: Sodium [2-[(2,6-dichlorophenyl)amino]phenyl]acetate. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.035.754 (accessed on 4 January 2023).
- Selim, A.; Toth, A.J.; Fozer, D.; Haaz, E.; Valentínyi, N.; Nagy, T.; Keri, O.; Bakos, L.P.; Szilágyi, I.M.; Mizsey, P. Effect of silver-nanoparticles generated in poly (vinyl alcohol) membranes on ethanol dehydration via pervaporation. Chin. J. Chem. Eng. 2019, 27, 1595–1607. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. Ph-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (pva)/nano silver hydrogels. Colloids Surf. B Biointerfaces 2020, 188, 110757. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Qi, G.; Li, C.; Xu, P.; Zheng, T.; Zhu, X.; Kenny, J.M.; Puglia, D.; Ma, P. Highly transparent pva/nanolignin composite films with excellent uv shielding, antibacterial and antioxidant performance. React. Funct. Polym. 2021, 162, 104873. [Google Scholar] [CrossRef]
- Abd El-Kader, F.H.; Gafer, S.A.; Basha, A.F.; Bannan, S.I.; Basha, M.A.F. Thermal and optical properties of gelatin/poly(vinyl alcohol) blends. J. Appl. Polym. Sci. 2010, 118, 413–420. [Google Scholar] [CrossRef]
- Krukowski, S.; Karasiewicz, M.; Kolodziejski, W. Convenient uv-spectrophotometric determination of citrates in aqueous solutions with applications in the pharmaceutical analysis of oral electrolyte formulations. J. Food Drug Anal. 2017, 25, 717–722. [Google Scholar] [CrossRef]
- Zahlan, H.; Saeed, W.S.; Alrasheed, R.; Alandes, N.M.; Aouak, T. Synthesis of poly (citric acid-co-glycerol) and its application as an inhibitor of caco3 deposition. Materials 2019, 12, 3800. [Google Scholar] [CrossRef] [PubMed]
- Tisserat, B.; O’kuru, R.H.; Hwang, H.; Mohamed, A.A.; Holser, R. Glycerol citrate polyesters produced through heating without catalysis. J. Appl. Polym. Sci. 2012, 125, 3429–3437. [Google Scholar] [CrossRef]
- Nataraj, D.; Reddy, R.; Reddy, N. Crosslinking electrospun poly (vinyl) alcohol fibers with citric acid to impart aqueous stability for medical applications. Eur. Polym. J. 2020, 124, 109484. [Google Scholar] [CrossRef]
- Yang, W.; He, X.; Luzi, F.; Dong, W.; Zheng, T.; Kenny, J.M.; Puglia, D.; Ma, P. Thermomechanical, antioxidant and moisture behaviour of pva films in presence of citric acid esterified cellulose nanocrystals. Int. J. Biol. Macromol. 2020, 161, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Fattoum, A.; Arous, M.; Pedicini, R.; Carbone, A.; Charnay, C. Conductivity and dielectric relaxation in crosslinked pva by oxalic and citric acids. Polym. Sci. Ser. A 2015, 57, 321–329. [Google Scholar] [CrossRef]
- Mandal, P.; Stokes, K.; Hernández, G.; Brandell, D.; Mindemark, J. Influence of binder crystallinity on the performance of si electrodes with poly(vinyl alcohol) binders. ACS Appl. Energy Mater. 2021, 4, 3008–3016. [Google Scholar] [CrossRef]
- Shi, J.J.; Yang, E.L. Green electrospinning and crosslinking of polyvinyl alcohol/citric acid. J. Nano Res. 2015, 32, 32–42. [Google Scholar] [CrossRef]
- Wen, L.; Liang, Y.; Lin, Z.; Xie, D.; Zheng, Z.; Xu, C.; Lin, B. Design of multifunctional food packaging films based on carboxymethyl chitosan/polyvinyl alcohol crosslinked network by using citric acid as crosslinker. Polymer 2021, 230, 124048. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Han, Q. Modulation of swelling of pva hydrogel by polymer and crosslinking agent concentration. Polym. Bull. 2023, 80, 1303–1320. [Google Scholar] [CrossRef]
- Harland, R.S.; Peppas, N.A. Solute diffusion in swollen membranes vii. Diffusion in semicrystalline networks. Colloid Polym. Sci. 1989, 267, 218–225. [Google Scholar] [CrossRef]
- Thomas, L.V.; Arun, U.; Remya, S.; Nair, P.D. A biodegradable and biocompatible pva-citric acid polyester with potential applications as matrix for vascular tissue engineering. J. Mater. Sci. Mater. Med. 2008, 20, 259. [Google Scholar] [CrossRef]
- Medhat Bojnourd, F.; Pakizeh, M. The effect of preparation parameters on performance of polyvinyl alcohol thin-film composite membrane: Experimental study, modeling, and optimization. Polym. Adv. Technol. 2018, 29, 1150–1160. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. Membrane distillation: Principles, applications, configurations, design, and implementation. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 55–106. [Google Scholar]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, P.; Zhang, X.; Li, J.; Luo, Y.; Zhang, W.; Ma, J.; Zhang, T. Constructing anti-scaling and anti-wetting polyvinyl alcohol layers through spray-coating with improved water permeability in membrane distillation. Desalination 2023, 545, 116161. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, V.J.H.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yusuf, N.M.; Ooi, B.S. Preparation and modification of poly (vinyl) alcohol membrane: Effect of crosslinking time towards its morphology. Desalination 2012, 287, 35–40. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Evaluation of polyvinyl alcohol (pva) loading in the pva/titanium dioxide (tio2) thin film coating on polyvinylidene fluoride (pvdf) membrane for the removal of textile dyes. Chemosphere 2020, 257, 127144. [Google Scholar] [CrossRef] [PubMed]
- Żyłła, R.; Foszpańczyk, M.; Kamińska, I.; Kudzin, M.; Balcerzak, J.; Ledakowicz, S. Impact of polymer membrane properties on the removal of pharmaceuticals. Membranes 2022, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Cardoso, M.; Martins, R.C.; Quinta-Ferreira, R.M.; Gando-Ferreira, L.M. Removal of a mixture of pharmaceuticals sulfamethoxazole and diclofenac from water streams by a polyamide nanofiltration membrane. Water Sci. Technol. 2020, 81, 732–743. [Google Scholar] [CrossRef] [PubMed]
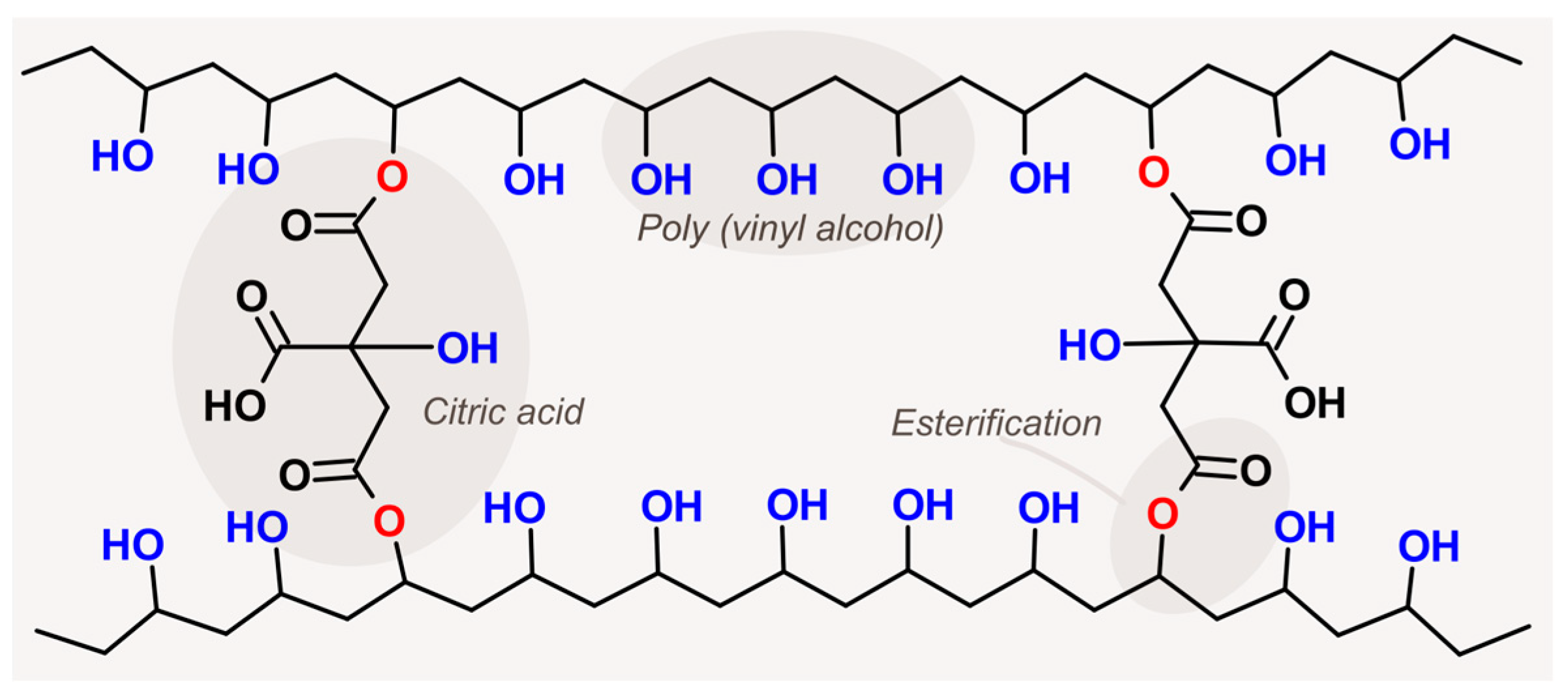

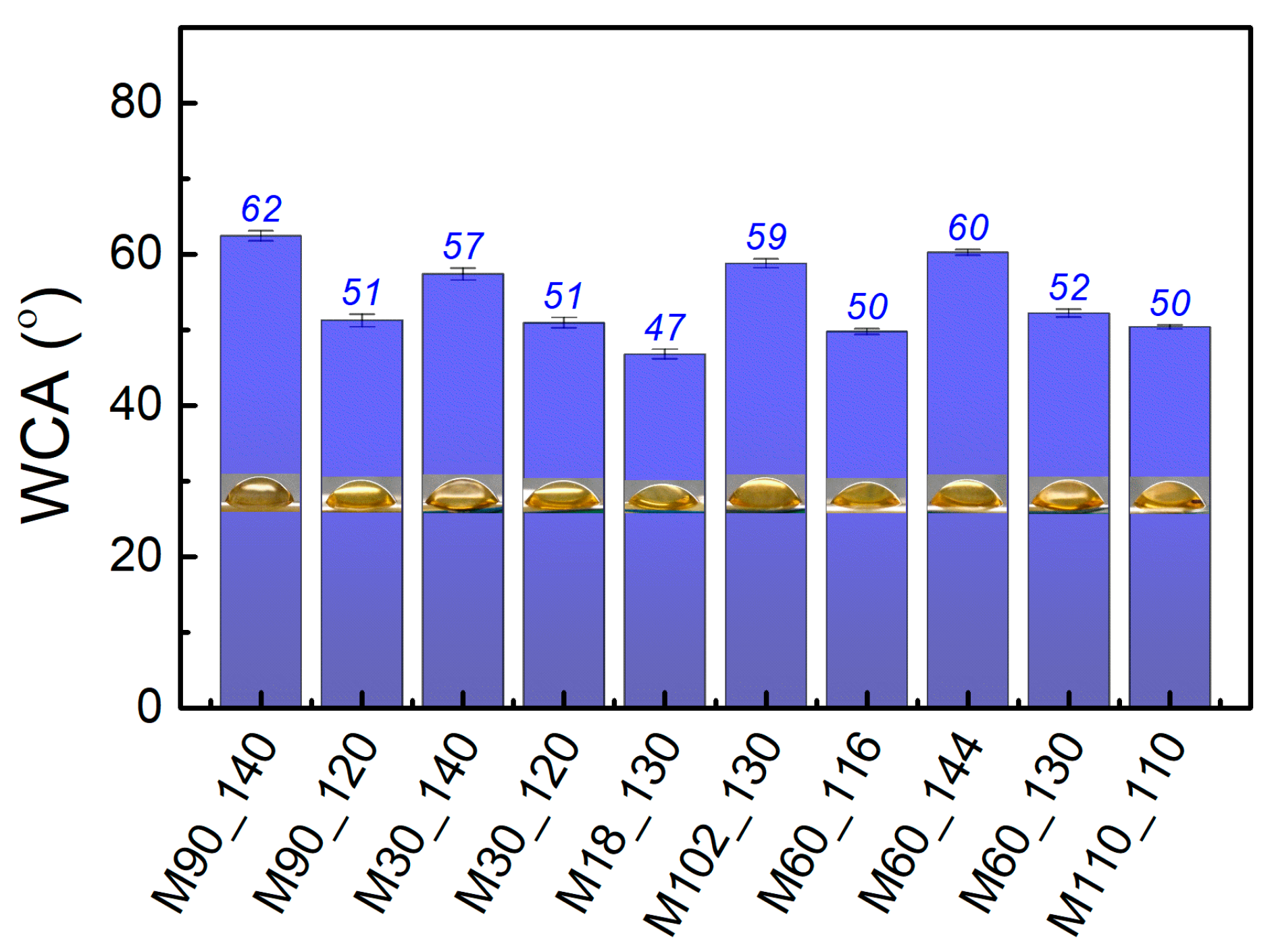
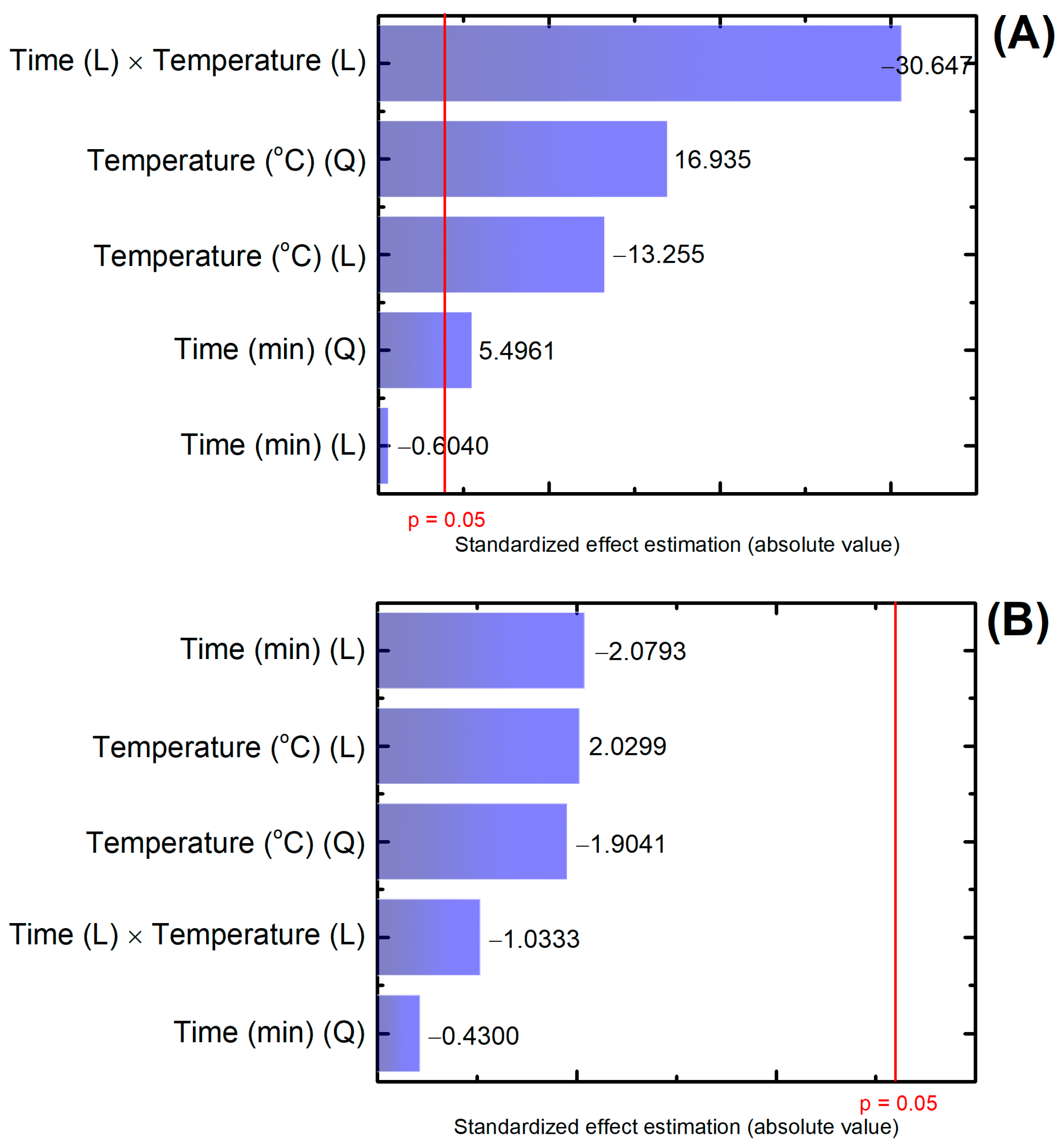


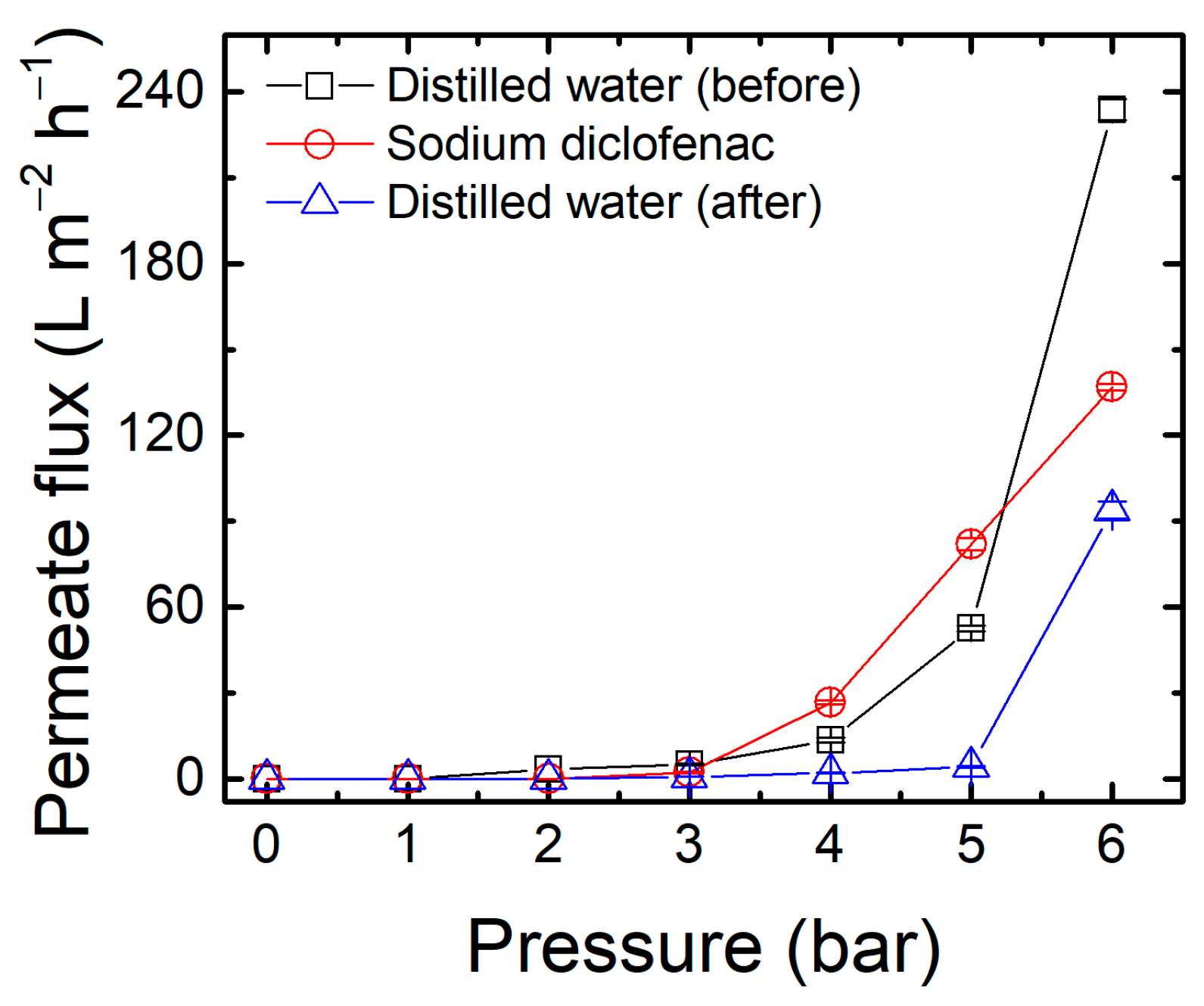
| Membranes 1 | Factor Codes 2 | Time (min) | Temperature (°C) | |
|---|---|---|---|---|
| x1 | x2 | |||
| M90_140 | +1 | +1 | 90 | 140 |
| M90_120 | +1 | −1 | 90 | 120 |
| M30_140 | −1 | +1 | 30 | 140 |
| M30_120 | −1 | −1 | 30 | 120 |
| M18_130 | −√2 | 0 | 18 | 130 |
| M102_130 | +√2 | 0 | 102 | 130 |
| M60_116 | 0 | −√2 | 60 | 116 |
| M60_144 | 0 | +√2 | 60 | 144 |
| M60_130 #1 | 0 | 0 | 60 | 130 |
| M60_130 #2 | 0 | 0 | 60 | 130 |
| M60_130 #3 | 0 | 0 | 60 | 130 |
| M110_110 3 | - | - | 110 | 110 |
| Membranes | Tg (°C) | Tf (°C) | ΔHf (J g−1) | Xc (%) | Tc (°C) |
|---|---|---|---|---|---|
| M90_140 | 42.1 | 204.4 | 75.5 | 46.4 | 116.5 |
| M90_120 | 53.7 | 215.9 | 83.9 | 51.6 | 131.5 |
| M30_140 | 44.6 | 208.9 | 83.9 | 51.6 | 119.5 |
| M30_120 | 45.8 | 205.2 | 80.7 | 49.7 | 111.7 |
| M18_130 | 46.2 | 201.7 | 56.4 | 34.7 | 110.6 |
| M102_130 | 49.6 | 210.4 | 81.5 | 50.1 | 123.3 |
| M60_116 | 45.1 | 204.4 | 75.4 | 46.3 | 113.3 |
| M60_144 | 62.6 | 210.3 | 75.3 | 46.3 | 125.0 |
| M60_130 | 60.9 | 209.2 | 75.2 | 46.2 | 121.8 |
| M110_110 | 53.9 | 205.5 | 55.6 | 34.2 | 116.7 |
| Membranes | SM (%) | SD (%) |
|---|---|---|
| M90_140 | 29.9 ± 5.5 | 36.3 ± 3.2 |
| M90_120 | 29.6 ± 4.2 | 37.4 ± 1.3 |
| M30_140 | 30.0 ± 1.9 | 38.3 ± 1.7 |
| M30_120 | 23.9 ± 3.4 | 25.5 ± 2.6 |
| M18_130 | 28.9 ± 1.8 | 23.8 ± 1.1 |
| M102_130 | 41.8 ± 6.0 | 39.6 ± 1.5 |
| M60_116 | 31.9 ± 3.8 | 36.7 ± 1.5 |
| M60_144 | 33.4 ± 3.1 | 38.8 ± 2.3 |
| M60_130 | 33.0 ± 3.1 | 38.7 ± 2.6 |
| M110_110 | 35.2 ± 3.6 | 46.5 ± 4.5 |
| Membranes | Permeate Flux (L m−2 h−1) | Rejection (%) |
|---|---|---|
| M90_140 | 0.95 | - |
| M90_120 | 363 | 0.8 |
| M30_140 | 1.19 | - |
| M30_120 | 24.0 | 3.7 |
| M18_130 | 9.1 | 49.3 |
| M102_130 | 3.6 | 21.3 |
| M60_116 | 68.0 | 11.0 |
| M60_144 | 73.6 | 20.7 |
| M60_130 #1 | 14.2 | 33.3 |
| M60_130 #2 | 22.8 | 15.7 |
| M60_130 #3 | 10.2 | 30.8 |
| Membranes | MWCO 1 (Da) | DCF (g L−1) | R 2 (%) | Pressure (bar) | Permeate Flux/Hydraulic Permeability | Ref. |
|---|---|---|---|---|---|---|
| AFC 30 (PCI Membranes) | 100–150 | 0.02 | 99.2 | 25–30 | 6.04 L m−2 h−1 bar−1 | [21] |
| AFC 40 (PCI Membranes) | 200–400 | 0.02 | 99.4 | 15–20 | 7.11 L m−2 h−1 bar−1 | [21] |
| BW30 (Dow FilmTech) | ≈100 | 0.01 | 98 | 20 | - | [18] |
| DL (GE Osmonics) | ~150–300 | 0.16 | 94 | 10 | 3.2 L m−2 h−1 bar−1 | [100] |
| HL (GE Osmonics) | 150–300 | 1.0 | 90 | 10 | - | [20] |
| 0.16 | 99 | 10 | 9.5 L m−2 h−1 bar−1 | [100] | ||
| NF10 (Hydranautics) | 3000 | 0.1 | 9.7 | 8 | 0.003 L m−2 h−1 | [22] |
| NF50 (Hydranautics) | 1000 | 0.1 | 43.3 | 8 | 0.0007 L m−2 h−1 | [22] |
| NF90 (Dow FilmTech) | 200–400 | 0.01 | 98 | 20 | - | [18] |
| 0.16 | 98 | 10 | 8.7–11.3 L m−2 h−1 bar−1 | [100] | ||
| NF270 (Dow FilmTech) | 200–400 | 0.001 | 91 | 6.9 | - | [19] |
| 1.0 | 100 | 10 | - | [20] | ||
| 0.16 | 92 | 10 | 13.5–18.5 L m−2 h−1 bar−1 | [100] | ||
| NFX (Synder Filtration) | ~150–300 | 0.16 | ~100 | 10 | 4.2–5.5 L m−2 h−1 bar−1 | [100] |
| TS40 (Trisep Corp) | ~200 | 0.16 | 99 | 10 | 4.2 L m−2 h−1 bar−1 | [100] |
| TS80 (Trisep Corp) | 100–200 | 0.06 | ~100 | 5 | 36 L m−2 h−1 | [101] |
| 0.16 | 99 | 10 | 4.2 L m−2 h−1 bar−1 | [100] | ||
| PVA + citric acid, AgNPs, and glycerol | - | 0.01 | 44 | 3 | 2.2 L m−2 h−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raota, C.S.; Crespo, J.d.S.; Baldasso, C.; Giovanela, M. Development of a Green Polymeric Membrane for Sodium Diclofenac Removal from Aqueous Solutions. Membranes 2023, 13, 662. https://doi.org/10.3390/membranes13070662
Raota CS, Crespo JdS, Baldasso C, Giovanela M. Development of a Green Polymeric Membrane for Sodium Diclofenac Removal from Aqueous Solutions. Membranes. 2023; 13(7):662. https://doi.org/10.3390/membranes13070662
Chicago/Turabian StyleRaota, Camila Suliani, Janaina da Silva Crespo, Camila Baldasso, and Marcelo Giovanela. 2023. "Development of a Green Polymeric Membrane for Sodium Diclofenac Removal from Aqueous Solutions" Membranes 13, no. 7: 662. https://doi.org/10.3390/membranes13070662
APA StyleRaota, C. S., Crespo, J. d. S., Baldasso, C., & Giovanela, M. (2023). Development of a Green Polymeric Membrane for Sodium Diclofenac Removal from Aqueous Solutions. Membranes, 13(7), 662. https://doi.org/10.3390/membranes13070662








