Fabrication of PES Modified by TiO2/Na2Ti3O7 Nanocomposite Mixed-Matrix Woven Membrane for Enhanced Performance of Forward Osmosis: Influence of Membrane Orientation and Feed Solutions
Abstract
1. Introduction
2. Experimental Information
2.1. Raw Materials
2.2. Woven Support Permeability
2.3. Manufacture and Characterization of TiO2/Na2Ti3O7 (TNT) Nanocomposite
2.4. Preparation of PES/TNT Nanocomposite MMW FO Membranes
2.5. Characterization of PES/TNT Nanocomposite MMW FO Membrane
2.5.1. Morphology and Functional Groups
2.5.2. Water Uptake Rate, Contact Angle, Tortuosity, Porosity, and Structural Parameter
2.6. The Performance of PES/TNT Nanocomposite MMW FO Membrane
2.6.1. Effect of TNT wt.% Ratio on the PES MMWFO Membrane Performance
2.6.2. Effect of Loaded TNT wt.% Ratios and PES/TNT MMWFO Membrane Orientations on FO Performance
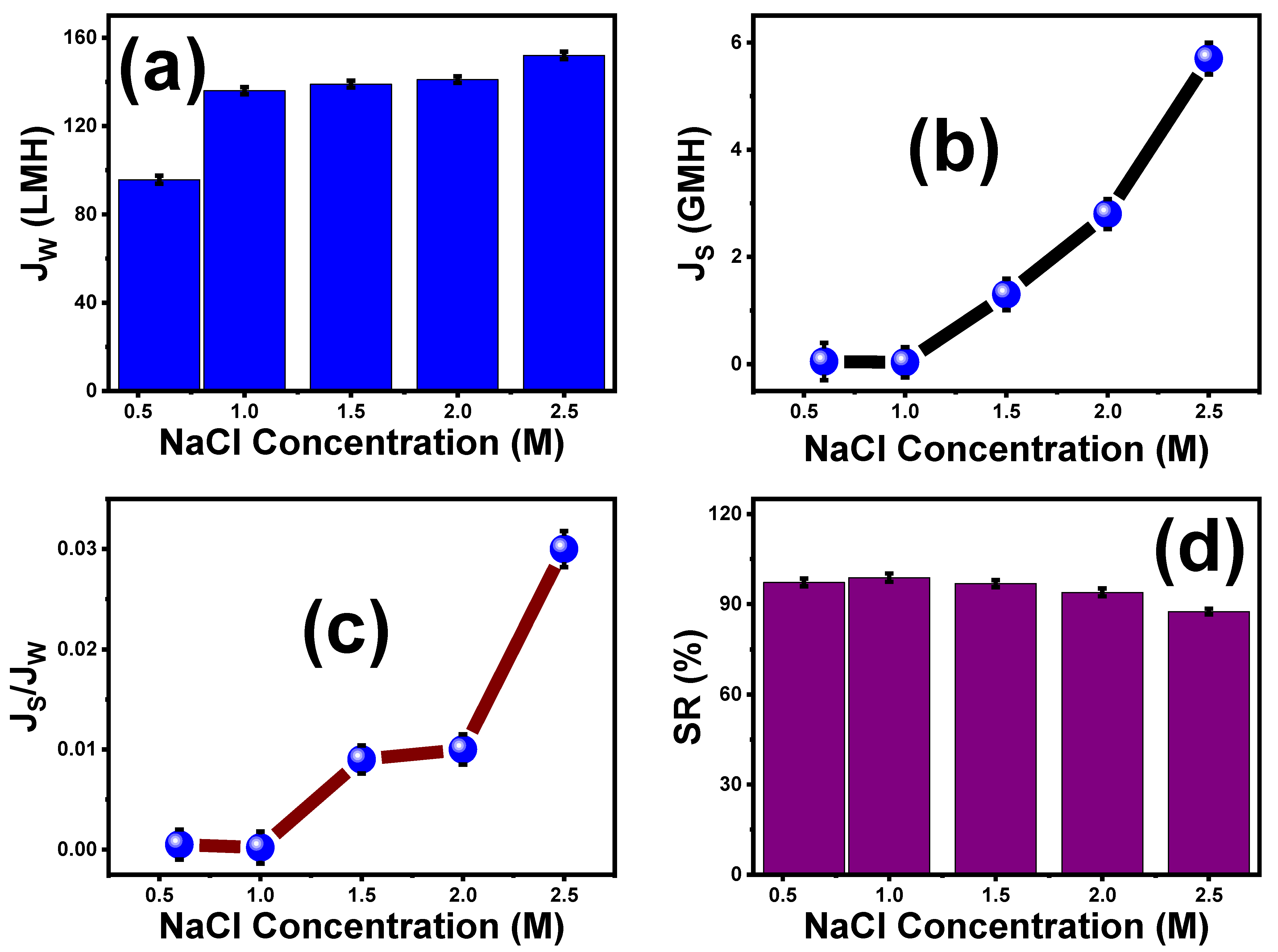
2.6.3. Impact of Different NaCl Concentrations as DS on the Performance of Optimal ST3 Membrane
2.7. MMWFO Membrane (ST3) Applications
2.7.1. Effect of Various Feed Types on the Performance of MMWFO Membranes
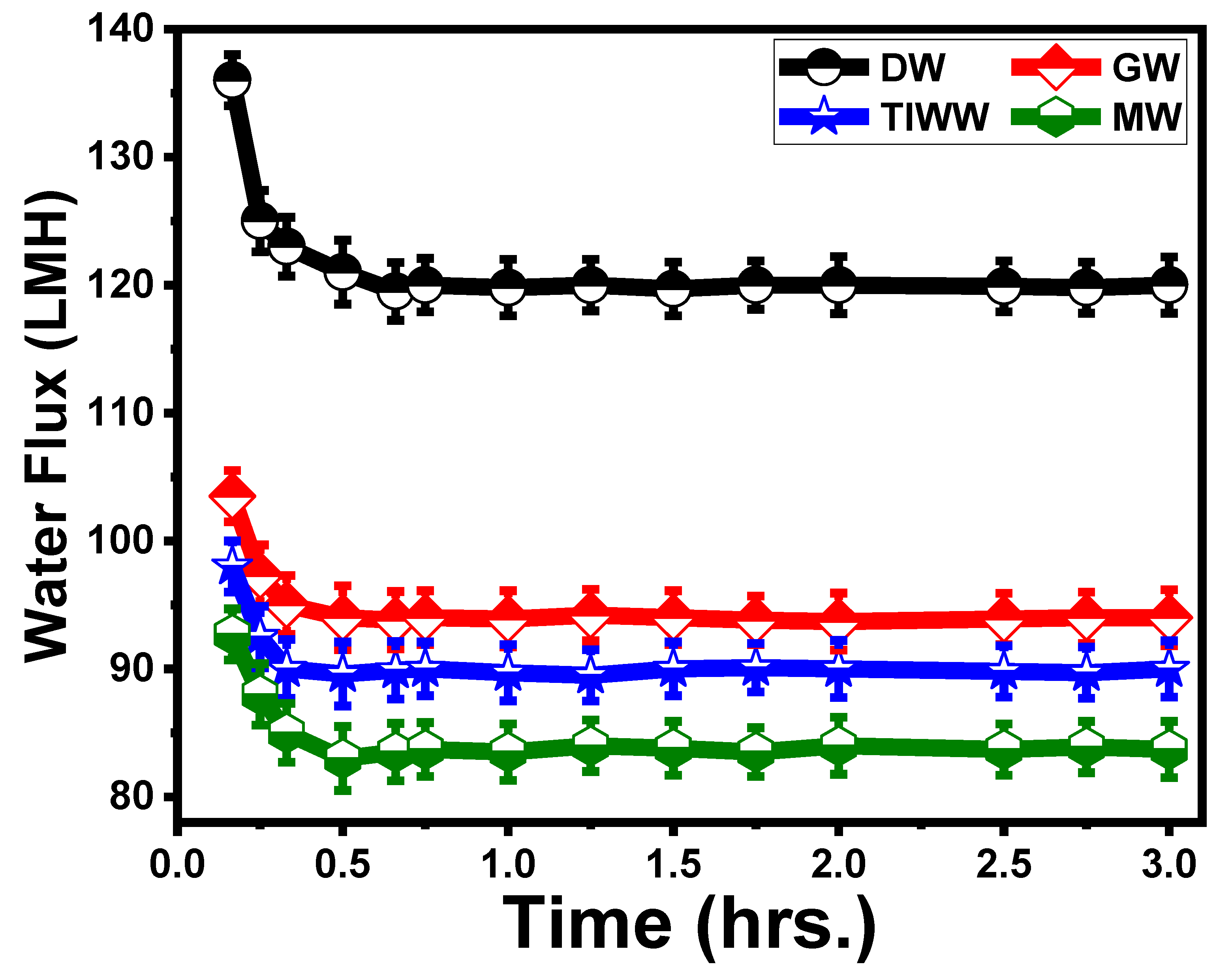
2.7.2. Effect of Time on the Flux of ST3 Membrane
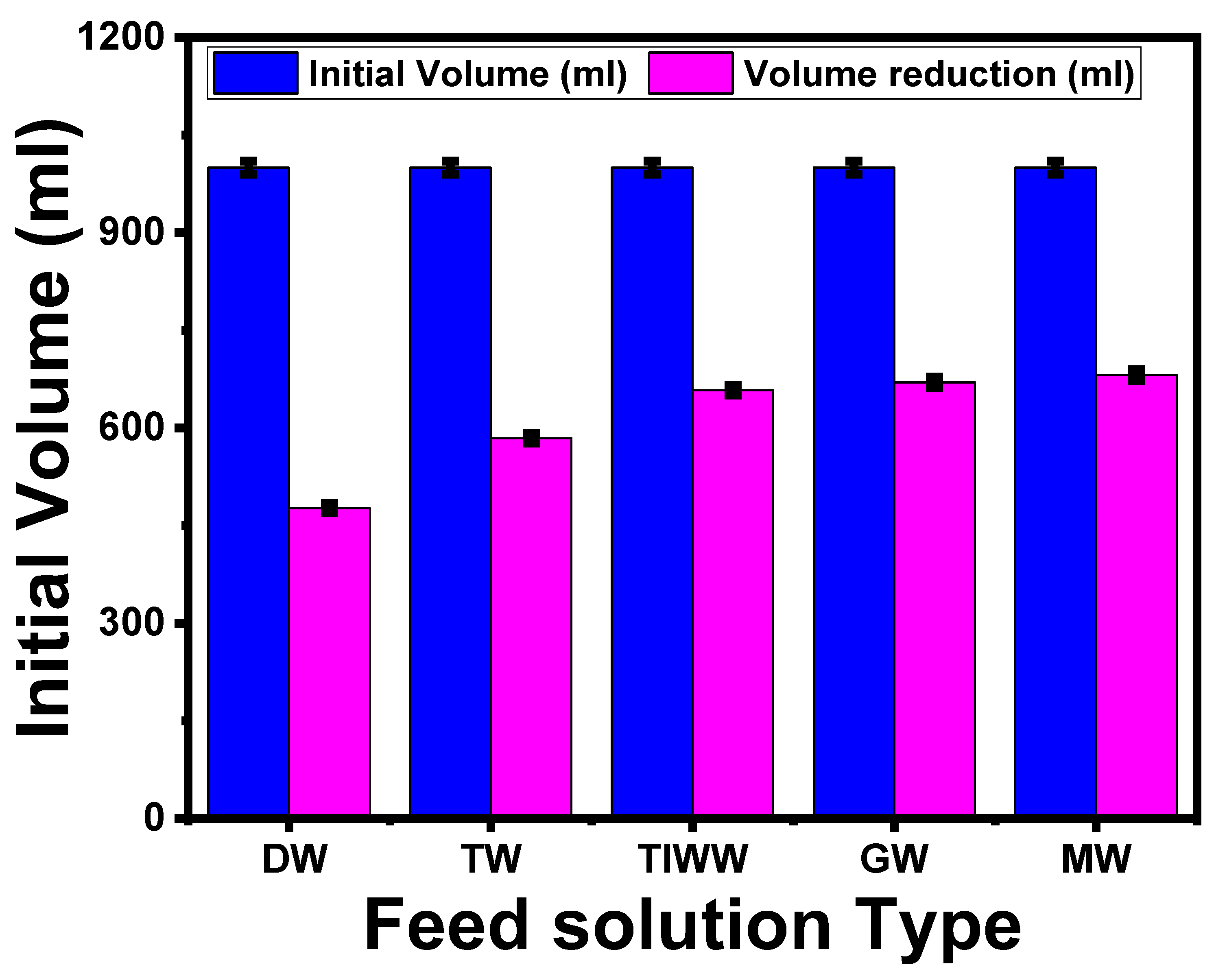
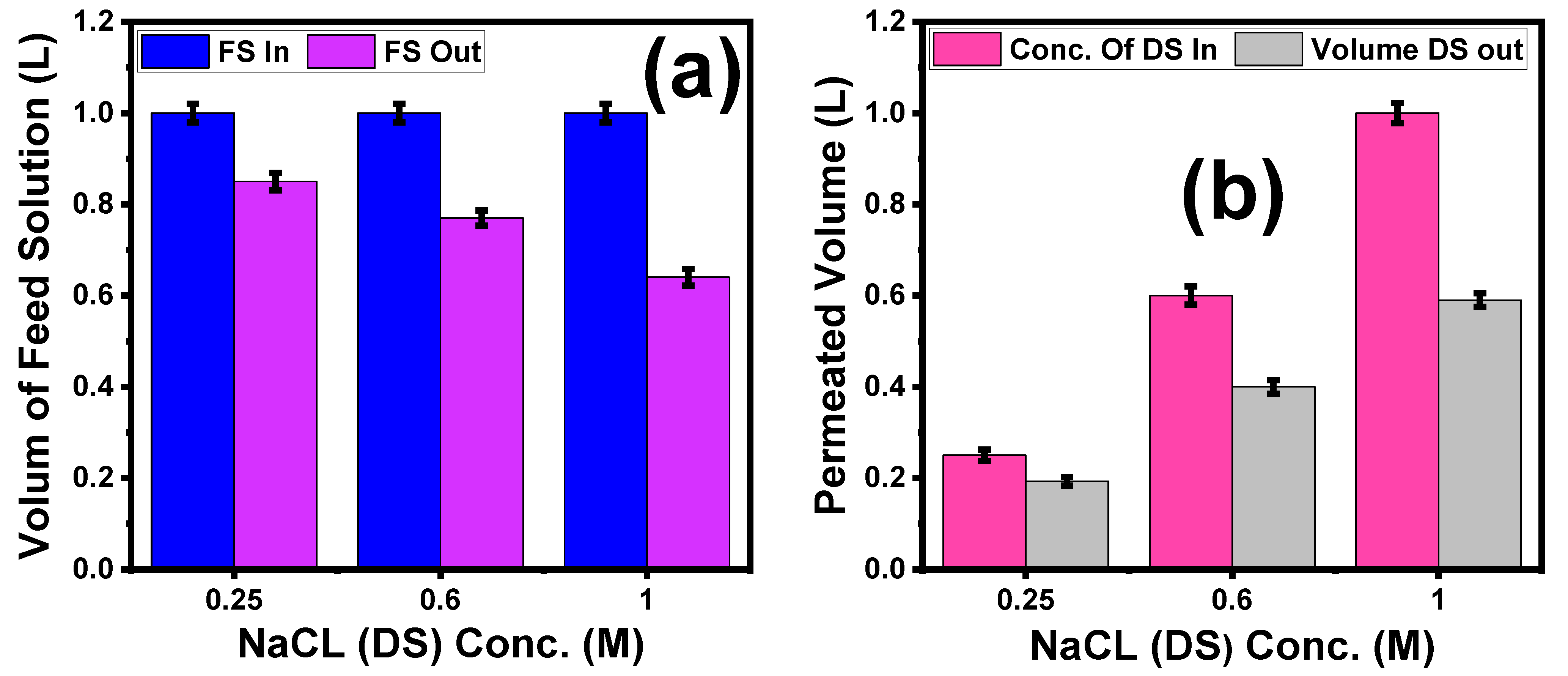
2.7.3. Effect of Different NaCl Concentrations on the Volume Reduction of Textile Wastewater
3. Results and Discussion
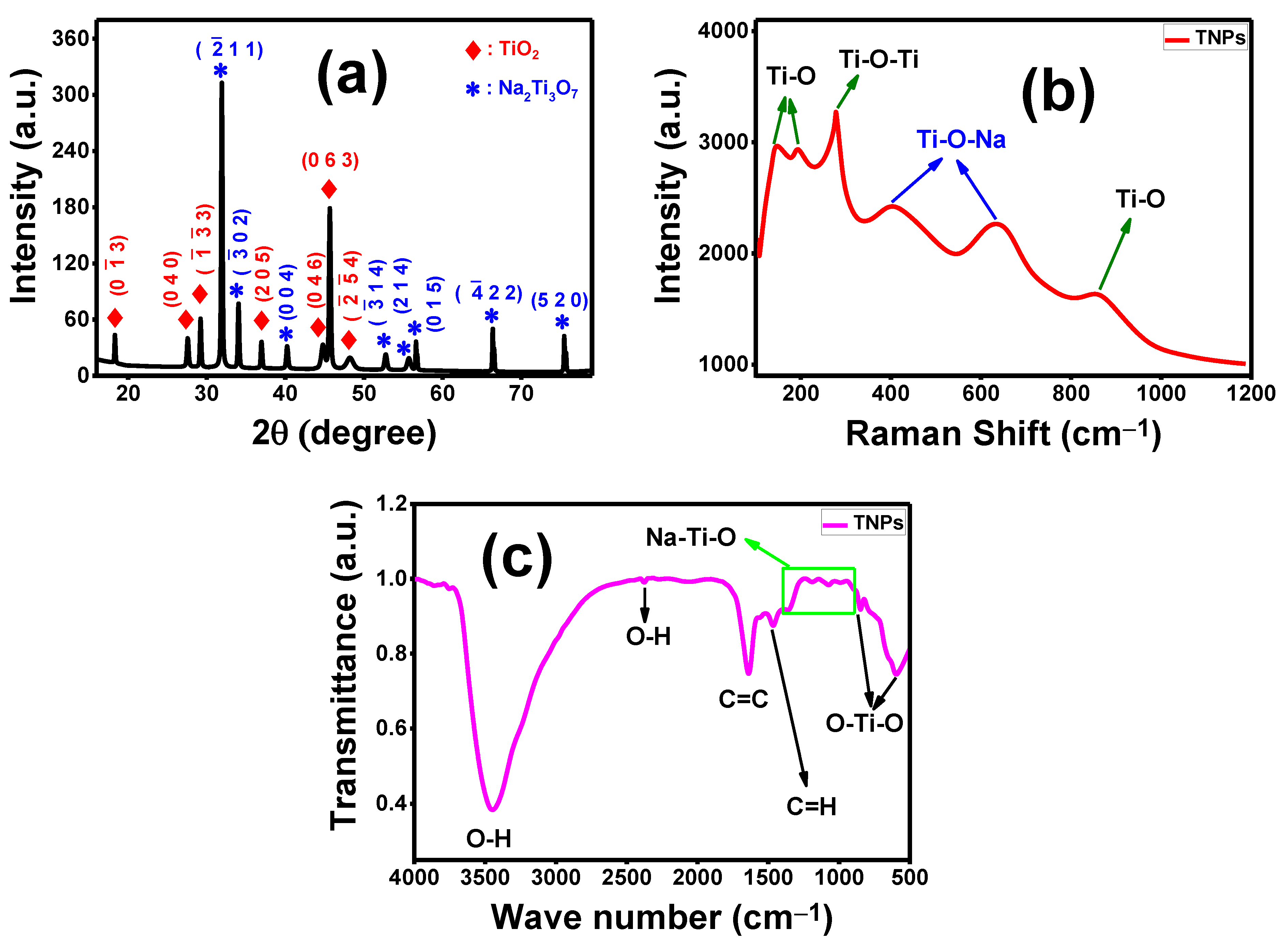
3.1. Characterization of Fabricated TNT Nanocomposite
3.1.1. Structural Analysis of TNT Nanocomposite
3.1.2. Raman Spectroscopy
3.1.3. Infrared Spectroscopy (FTIR) Analysis of TNT Nanocomposite
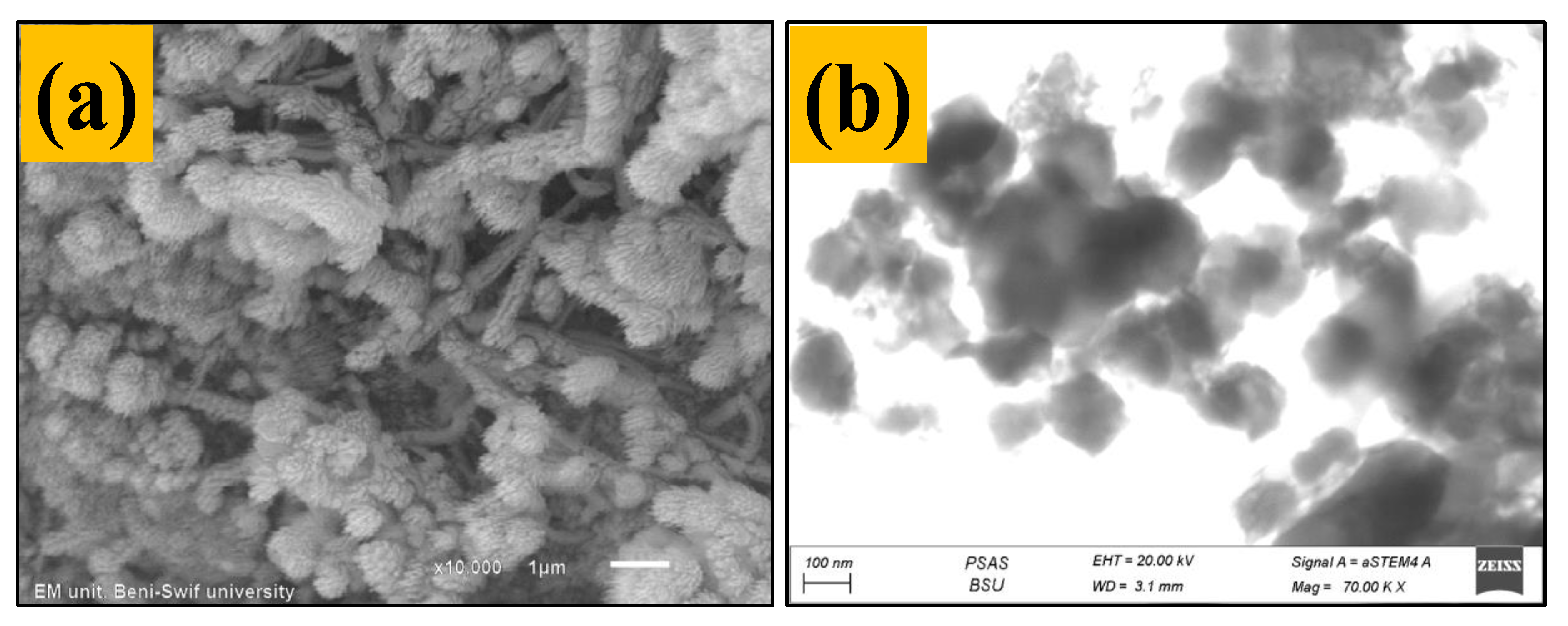
3.1.4. SEM and TEM Analysis for Fabricated TNT Nanocomposite
3.2. PES/TNT Nanocomposite MMWFO Membrane Characterization
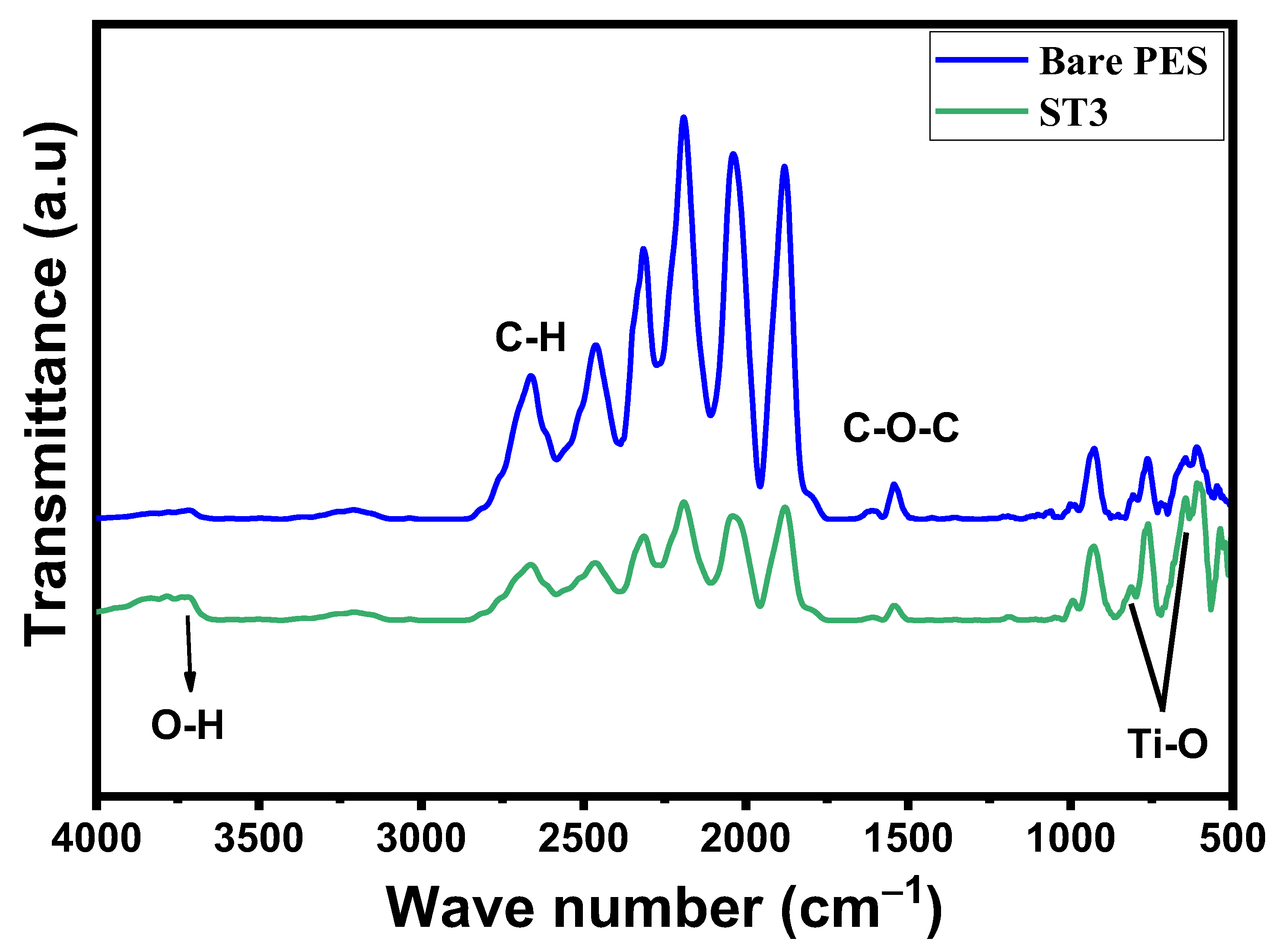
3.2.1. FTIR and Functional Group Analysis of PES/TNT Nanocomposite MMWFO Membranes
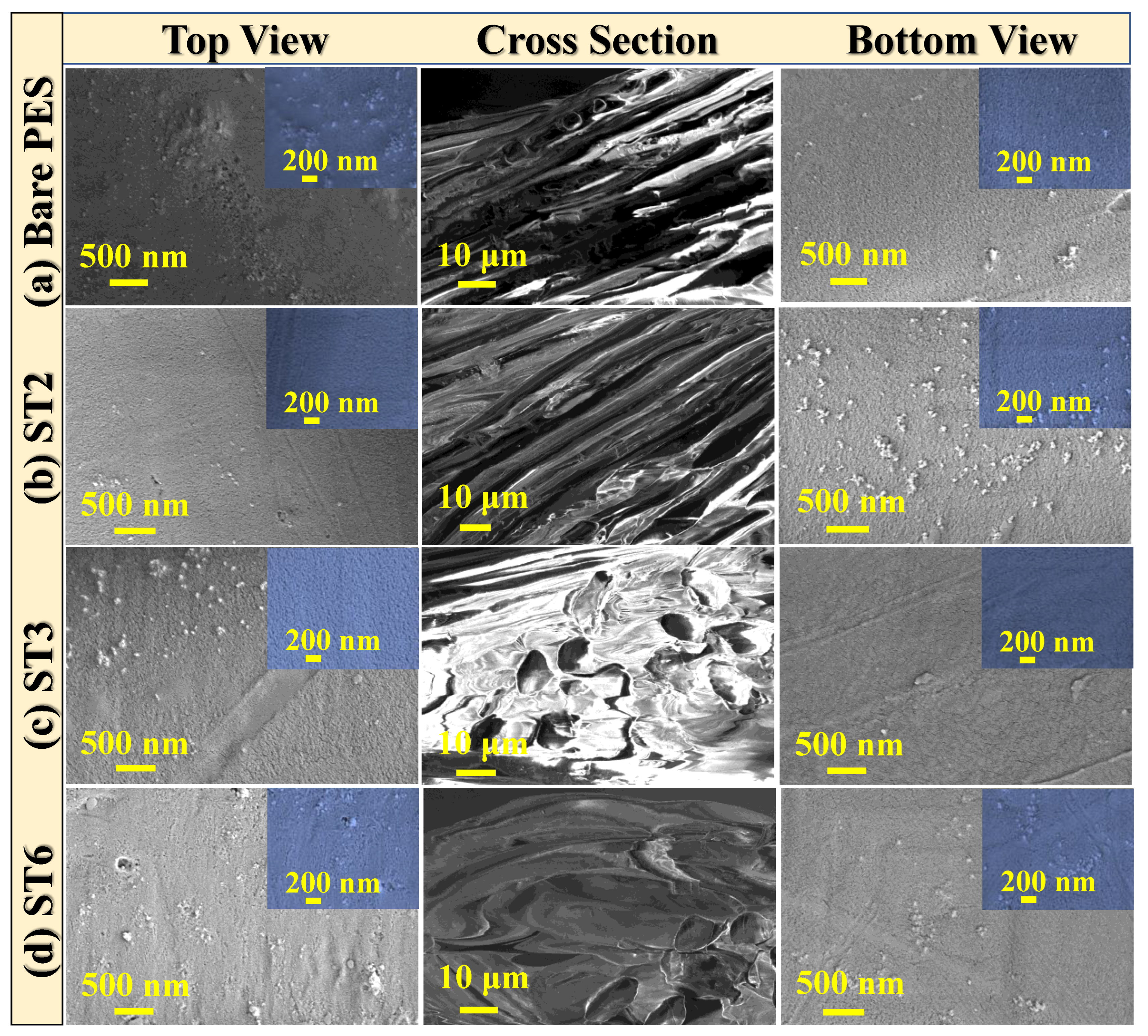
3.2.2. Surface Morphology of PES/TNT MMWFO Membranes
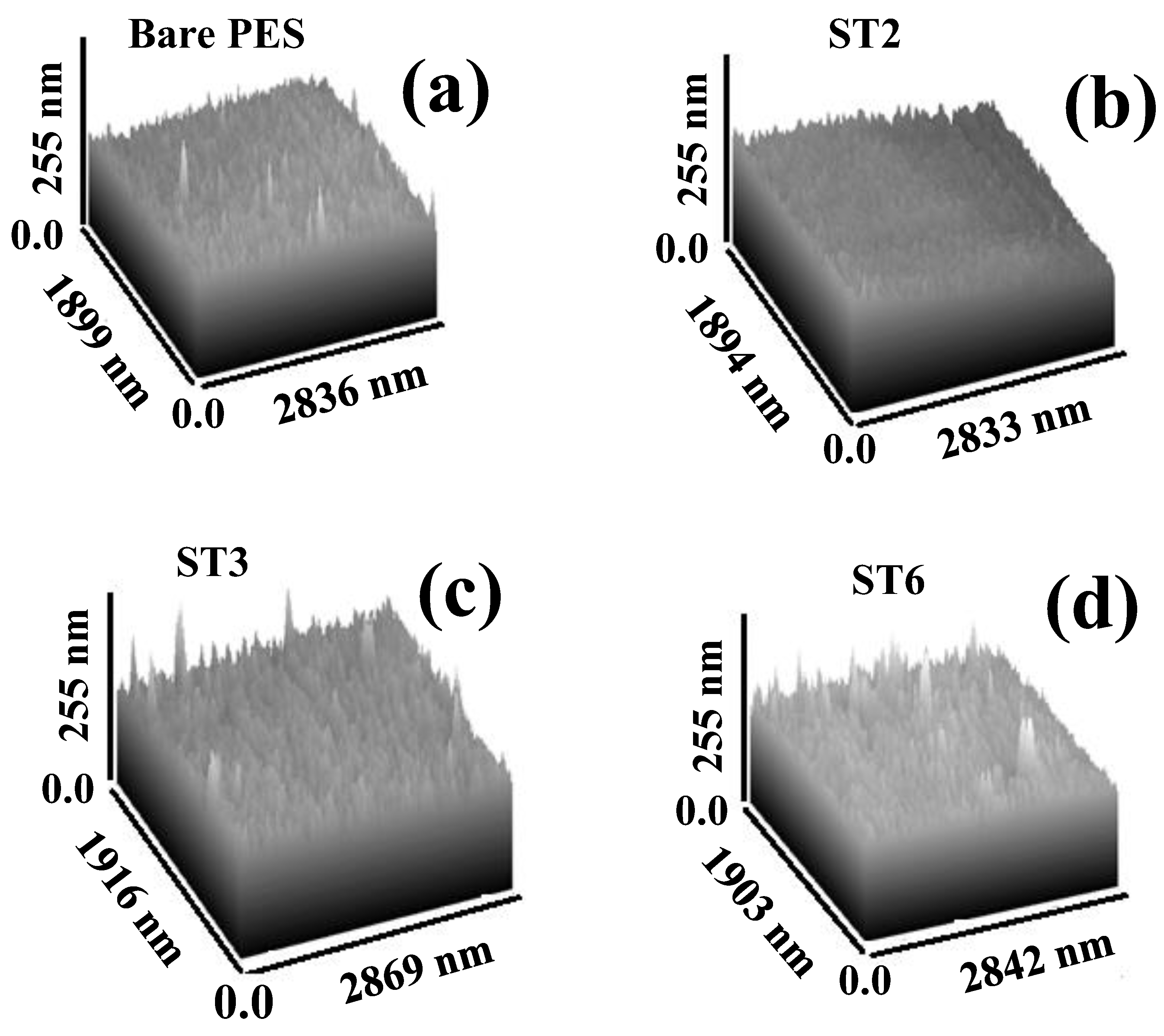
3.2.3. Atomic Force Microscopy (AFM) Analysis and Roughness Parameters
4. Evaluating the Role of TNPs in Improving Membrane Contact Angles, Water Uptake, and Porosity Measurements of Membrane
4.1. Contact Angles
4.2. Water Uptake
4.3. Porosity
5. Membrane Performance
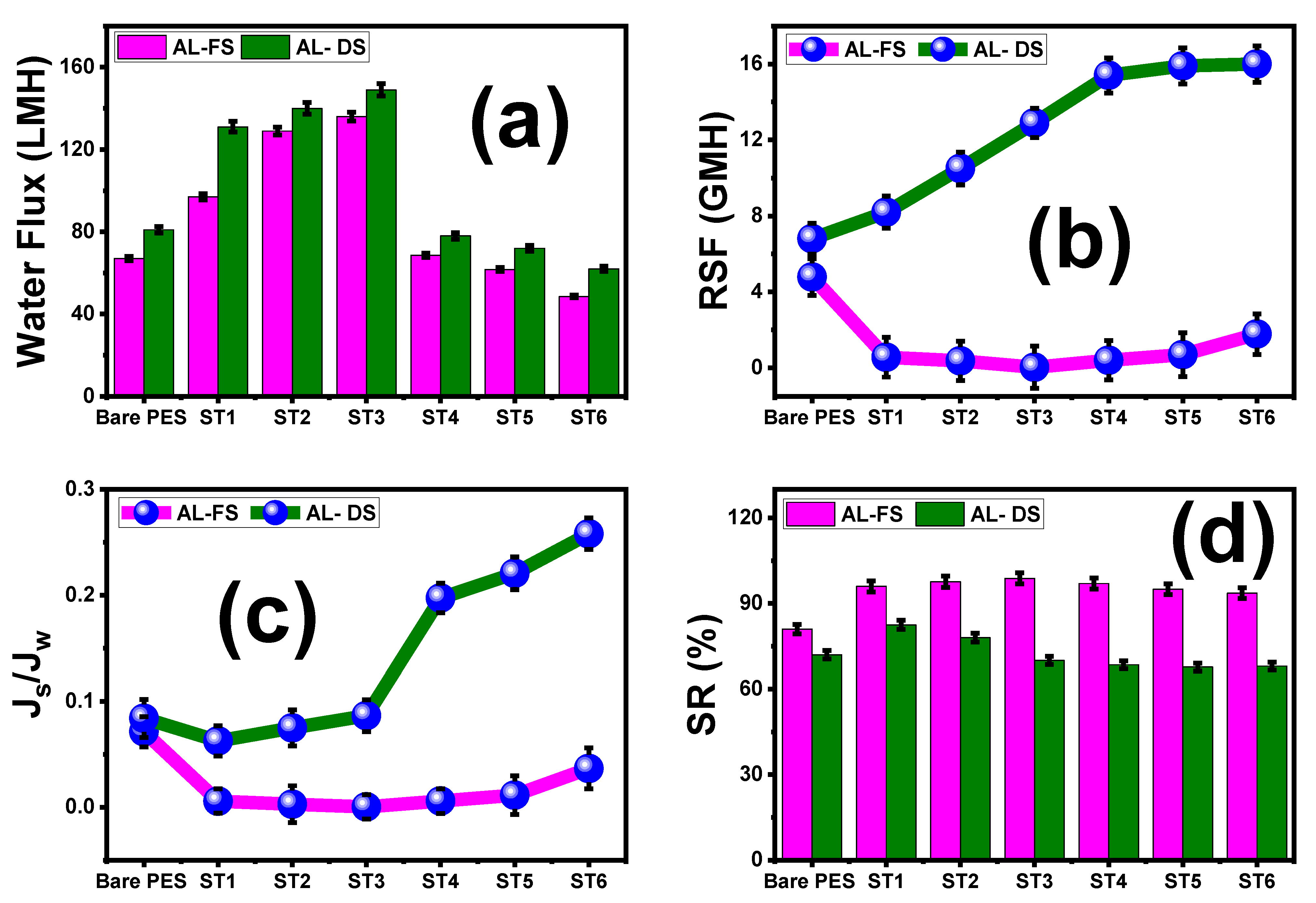
5.1. Effect of Loaded TNT wt.% Ratios and PES/TNT MMWFO Membrane Orientations on FO Performance
5.2. Effect of Various NaCl Concentrations on the Performance of ST3 Membrane
5.3. MMWFO Membrane (ST3) Application
5.3.1. Effect of Different Feed Types on MMWFO Membrane Performance
5.3.2. Effect of Time on ST3 Membrane Flux
5.3.3. Effect of Different NaCl Concentrations on the Volume Reduction of Textile Wastewater
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farhat, S.; Bali, M.; Kamel, F. Membrane autopsy to provide solutions to operational problems of Jerba brackish water desalination plant. Desalination 2018, 445, 225–235. [Google Scholar] [CrossRef]
- Mohamad Shirajuddin, T.; Muhammad, N.S.; Abdullah, J. Optimization problems in water distribution systems using Non-dominated Sorting Genetic Algorithm II: An overview. Ain Shams Eng. J. 2023, 14, 101932. [Google Scholar] [CrossRef]
- Blankert, B.; Huisman, K.T.; Martinez, F.D.; Vrouwenvelder, J.S.; Picioreanu, C. Are commercial polyamide seawater and brackish water membranes effectively charged? J. Membr. Sci. Lett. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Lin, W.; Xiao, K.; Chen, D.; Luo, S.; Huang, X. Significant insights of Cu and Fe as key metals to cause RO membrane fouling under coal-mining wastewater treatment. Desalination 2023, 555, 116517. [Google Scholar] [CrossRef]
- Lakra, R.; Bilad, M.R.; Balakrishnan, M.; Basu, S. Development of high-performance CuBTC MOF-based forward osmosis (FO) membranes and their cleaning strategies. Chem. Eng. Res. Des. 2023, 190, 566–579. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Braytee, A.; Altaee, A.; HosseinZadeh, A.; Samal, A.K.; Zhou, J.L.; Khan, J.A.; Bartocci, P.; Fantozzi, F. Evaluation of machine learning algorithms to predict internal concentration polarization in forward osmosis. J. Membr. Sci. 2022, 646, 120257. [Google Scholar] [CrossRef]
- Huang, M.; You, W.; Zhang, L.; Li, Y.; Niu, M.; Lv, Y. Structure and circuit modeling of frequency domain polarization characteristics for porous composite material. Compos. Sci. Technol. 2022, 224, 109457. [Google Scholar] [CrossRef]
- Sabharwal, M.; Secanell, M. Understanding the effect of porosity and pore size distribution on low loading catalyst layers. Electrochim. Acta 2022, 419, 140410. [Google Scholar] [CrossRef]
- Kahrizi, M.; Lin, J.; Ji, G.; Kong, L.; Song, C.; Dumée, L.F.; Sahebi, S.; Zhao, S. Relating forward water and reverse salt fluxes to membrane porosity and tortuosity in forward osmosis: CFD modelling. Sep. Purif. Technol. 2020, 241, 116727. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, Y.; Wang, Q.; Mi, B. Carbon nanotube-supported polyamide membrane with minimized internal concentration polarization for both aqueous and organic solvent forward osmosis process. J. Membr. Sci. 2020, 611, 118273. [Google Scholar] [CrossRef]
- Qasim, M.; Mohammed, F.; Aidan, A.; Darwish, N.A. Forward osmosis desalination using ferric sulfate draw solute. Desalination 2017, 423, 12–20. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward Osmosis Application in Manufacturing Industries: A Short Review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Abdikheibari, S.; Lei, W.; Dumée, L.F.; Barlow, A.J.; Baskaran, K. Novel thin film nanocomposite membranes decorated with few-layered boron nitride nanosheets for simultaneously enhanced water flux and organic fouling resistance. Appl. Surf. Sci. 2019, 488, 565–577. [Google Scholar] [CrossRef]
- Akther, N.; Phuntsho, S.; Chen, Y.; Ghaffour, N.; Shon, H.K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 2019, 584, 20–45. [Google Scholar] [CrossRef]
- Liu, X.; Ng, H.Y. Fabrication of layered silica–polysulfone mixed matrix substrate membrane for enhancing performance of thin-film composite forward osmosis membrane. J. Membr. Sci. 2015, 481, 148–163. [Google Scholar] [CrossRef]
- Liu, X.; Ng, H.Y. Double-blade casting technique for optimizing substrate membrane in thin-film composite forward osmosis membrane fabrication. J. Membr. Sci. 2014, 469, 112–126. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Rezakazemi, M.; Mohammadi, T. Fundamentals and Measurement Techniques for Gas Transport in Polymers. In Transport Properties of Polymeric Membranes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 391–423. [Google Scholar] [CrossRef]
- Ismail, A.F. Preparation of Carbon Membranes for Gas Separation. Compr. Membr. Sci. Eng. 2010, 1, 275–290. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Salim, W.; Ho, W.W. Recent developments on nanostructured polymer-based membranes. Curr. Opin. Chem. Eng. 2015, 8, 76–82. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Wang, H.; Xu, T. Graphene oxide modified graphitic carbon nitride as a modifier for thin film composite forward osmosis membrane. J. Membr. Sci. 2015, 475, 281–289. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.M.; Chen, G.; Chung, W.J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Qi, S.; Zhao, Y.; Gao, Y.; Tang, C.Y. Nanocomposite substrates for controlling internal concentration polarization in forward osmosis membranes. J. Membr. Sci. 2013, 441, 54–62. [Google Scholar] [CrossRef]
- Liu, W.; Ni, J.; Yin, X. Synergy of photocatalysis and adsorption for simultaneous removal of Cr(VI) and Cr(III) with TiO2 and titanate nanotubes. Water Res. 2014, 53, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, N.; Kumar, P.; Goswami, A.; Perdicakis, B.; Shankar, K.; Sadrzadeh, M. Robust Polymer Nanocomposite Membranes Incorporating Discrete TiO2 Nanotubes for Water Treatment. Nanomaterials 2019, 9, 1186. [Google Scholar] [CrossRef]
- Shaban, M.; AbdAllah, H.; Said, L.; Hamdy, H.S.; Abdel Khalek, A. Titanium dioxide nanotubes embedded mixed matrix PES membranes characterization and membrane performance. Chem. Eng. Res. Des. 2015, 95, 307–316. [Google Scholar] [CrossRef]
- Shaban, M.; Ashraf, A.M.; AbdAllah, H.; Abd El-Salam, H.M. Titanium dioxide nanoribbons/multi-walled carbon nanotube nanocomposite blended polyethersulfone membrane for brackish water desalination. Desalination 2018, 444, 129–141. [Google Scholar] [CrossRef]
- Celik Madenli, E.; Yanar, N.; Choi, H. Enhanced antibacterial properties and suppressed biofilm growth on multi-walled carbon nanotube (MWCNT) blended polyethersulfone (PES) membranes. J. Environ. Chem. Eng. 2021, 9, 104755. [Google Scholar] [CrossRef]
- Tang, C.Y.; She, Q.; Lay, W.C.L.; Wang, R.; Fane, A.G. Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J. Membr. Sci. 2010, 354, 123–133. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Park, M.J.; Bae, T.H.; Yang, Y.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Melamine-based covalent organic framework-incorporated thin film nanocomposite membrane for enhanced osmotic power generation. Desalination 2019, 459, 10–19. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Liu, B.; Huang, Y.; Song, A.; Lin, Y.; Wang, M.; Li, X.; Cao, H. Gel polymer electrolyte based on polymethyl methacrylate matrix composited with methacrylisobutyl-polyhedral oligomeric silsesquioxane by phase inversion method. Electrochim. Acta 2018, 278, 1–12. [Google Scholar] [CrossRef]
- Piluharto, B.; Suendo, V.; Ciptati, T.; Radiman, C.L. Strong correlation between membrane effective fixed charge and proton conductivity in the sulfonated polysulfone cation-exchange membranes. Ionics 2011, 17, 229–238. [Google Scholar] [CrossRef]
- Gholami, N.; Mahdavi, H. Nanofiltration composite membranes of polyethersulfone and graphene oxide and sulfonated graphene oxide. Adv. Polym. Technol. 2018, 37, 3529–3541. [Google Scholar] [CrossRef]
- Alves, V.D.; Coelhoso, I.M. Effect of membrane characteristics on mass and heat transfer in the osmotic evaporation process. J. Membr. Sci. 2004, 228, 159–167. [Google Scholar] [CrossRef]
- Wang, R.; Shi, L.; Tang, C.Y.; Chou, S.; Qiu, C.; Fane, A.G. Characterization of novel forward osmosis hollow fiber membranes. J. Membr. Sci. 2010, 355, 158–167. [Google Scholar] [CrossRef]
- Sinulingga, K.; Sirait, M.; Marpaung, T.M. Effect of Temperature Variation Characteristics Of Crystal Structure and Morphology of Nano TiO2 Coating On Metal Anti-Corrosion Coating by Sol-Gel Method Spin Coating. J. Phys. Conf. Ser. 2020, 1485, 012063. [Google Scholar] [CrossRef]
- Ashraf, T.; Alfryyan, N.; Nasr, M.; Ahmed, S.A.; Shaban, M. Removal of Scale-Forming Ions and Oil Traces from Oil Field Produced Water Using Graphene Oxide/Polyethersulfone and TiO2 Nanoribbons/Polyethersulfone Nanofiltration Membranes. Polymers 2022, 14, 2572. [Google Scholar] [CrossRef]
- Zayed, M.; Nasser, N.; Shaban, M.; Alshaikh, H.; Hamdy, H.; Ahmed, A.M. Effect of Morphology and Plasmonic on Au/ZnO Films for Efficient Photoelectrochemical Water Splitting. Nanomaterials 2021, 11, 2338. [Google Scholar] [CrossRef]
- Zayed, M.; Ahmed, A.M.; Shaban, M. Synthesis and characterization of nanoporous ZnO and Pt/ZnO thin films for dye degradation and water splitting applications. Int. J. Hydrogen Energy 2019, 44, 17630–17648. [Google Scholar] [CrossRef]
- Mohamed, W.S.; Hasaneen, M.F.; Shokr, E.K. Influence of (Ar + O2) atmosphere and CdCl2 coating heat treatment on physical properties of CdS thin film for solar cell applications. Mater. Res. Express 2017, 4, 046406. [Google Scholar] [CrossRef]
- El-Gharbawy, S.A.; Al-Dossari, M.; Zayed, M.; Saudi, H.A.; Hassaan, M.Y.; Alfryyan, N.; Shaban, M. Fabrication and Characterization of Nanostructured Rock Wool as a Novel Material for Efficient Water-Splitting Application. Nanomaterials 2022, 12, 2169. [Google Scholar] [CrossRef]
- Huang, J.; Meng, R.; Zu, L.; Wang, Z.; Feng, N.; Yang, Z.; Yu, Y.; Yang, J. Sandwich-like Na0.23TiO2 nanobelt/Ti3C2 MXene composites from a scalable in situ transformation reaction for long-life high-rate lithium/sodium-ion batteries. Nano Energy 2018, 46, 20–28. [Google Scholar] [CrossRef]
- Ming, K.; Zhang, Z.; Li, H. In situ growth of NaTiO2 nanotubes on Ti3C2Fx for enhanced sodium ion batteries. Mater. Lett. 2022, 309, 131457. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, V.; Vilotijević, M.; Jokanović, B.; Jenko, M.; Anžel, I.; Stamenković, D.; Lazic, V.; Rudolf, R. Investigations of corrosion on the surface of titanium substrate caused by combined alkaline and heat treatment. Corros. Sci. 2014, 82, 180–190. [Google Scholar] [CrossRef]
- Yahya, G.A.; El, G.; Abd, D.; Yahya, E.-R. Studies on Some Lithium-Borate Glasses Containing Iron and Copper. Turkish J. Phys. 2003, 27, 255–262. [Google Scholar]
- Silim, H.A. Composition Effect on Some Physical Properties and FTIR Spectra of Alumino—Borate Glasses Containing Lithium, Sodium, Potassium and Barium Oxides. Egypt. J. Solids 2006, 29, 293–302. [Google Scholar] [CrossRef]
- Pal Singh, G.; Kaur, P.; Kaur, S.; Singh, D.P. Role of WO3 in structural and optical properties of WO3–Al2O3–PbO–B2O3 glasses. Phys. B Condens. Matter 2011, 406, 4652–4656. [Google Scholar] [CrossRef]
- Khalil, K.M.S. Cerium modified MCM-41 nanocomposite materials via a nonhydrothermal direct method at room temperature. J. Colloid Interface Sci. 2007, 315, 562–568. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, S.P.; Mudahar, G.S.; Thind, K.S. Synthesis and Optical Characterization of Silver Doped Sodium Borate Glasses. N. J. Glas. Ceram. 2012, 2012, 111–115. [Google Scholar] [CrossRef]
- Chu, C.L.; Chung, C.Y.; Zhou, J.; Pu, Y.P.; Lin, P.H. Fabrication and characteristics of bioactive sodium titanate/titania graded film on NiTi shape memory alloy. J. Biomed. Mater. Res. Part A 2005, 75A, 595–602. [Google Scholar] [CrossRef]
- Dukhin, A.S.; Goetz, P.J. Fundamentals of Interface and Colloid Science. Stud. Interface Sci. 2010, 24, 21–89. [Google Scholar] [CrossRef]
- Chai, M.N.; Isa, M.I.N. The Oleic Acid Composition Effect on the Carboxymethyl Cellulose Based Biopolymer Electrolyte. J. Cryst. Process Technol. 2013, 3, 1–4. [Google Scholar] [CrossRef]
- Forfang, K.; Zimmermann, B.; Kosa, G.; Kohler, A.; Shapaval, V. FTIR Spectroscopy for Evaluation and Monitoring of Lipid Extraction Efficiency for Oleaginous Fungi. PLoS ONE 2017, 12, e0170611. [Google Scholar] [CrossRef] [PubMed]
- Kavalenka, M.N.; Vüllers, F.; Kumberg, J.; Zeiger, C.; Trouillet, V.; Stein, S.; Ava, T.T.; Li, C.; Worgull, M.; Hölscher, H. Adaptable bioinspired special wetting surface for multifunctional oil/water separation. Sci. Rep. 2017, 7, 39970. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Nanocomposite-Based Aminated Polyethersulfone and Carboxylate Activated Carbon for Environmental Application. A Real Sample Analysis. C 2018, 4, 30. [Google Scholar] [CrossRef]
- Sirinupong, T.; Youravong, W.; Tirawat, D.; Lau, W.J.; Lai, G.S.; Ismail, A.F. Synthesis and characterization of thin film composite membranes made of PSF-TiO2/GO nanocomposite substrate for forward osmosis applications. Arab. J. Chem. 2018, 11, 1144–1153. [Google Scholar] [CrossRef]
- Bolis, V.; Busco, C.; Ciarletta, M.; Distasi, C.; Erriquez, J.; Fenoglio, I.; Livraghi, S.; Morel, S. Hydrophilic/hydrophobic features of TiO2 nanoparticles as a function of crystal phase, surface area and coating, in relation to their potential toxicity in peripheral nervous system. J. Colloid Interface Sci. 2012, 369, 28–39. [Google Scholar] [CrossRef]
- Choi, J.H.; Jegal, J.; Kim, W.N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Ali, S.I.A.; Ismail, A.; Yusoff, N.I.M.; Karim, M.R.; Al-Mansob, R.A.; Alhamali, D.I. Physical and rheological properties of acrylate–styrene–acrylonitrile modified asphalt cement. Constr. Build. Mater. 2015, 93, 326–334. [Google Scholar] [CrossRef]
- Adibah Raja Ahmad, R.; Harun, Z.; Hafeez Azhar, F.; Hussin, R.; Faiz Mohd Zin, M.; Sazali, N.; Syamsul Bahri, S.; Riduan Jamaluddin, M.; Misdan, N.; Kamdi, Z.; et al. Polymer mixed membrane with microflower TiO2 as additive for photocatalyst in organic compound. Mater. Today Proc. 2021, 46, 2122–2130. [Google Scholar] [CrossRef]
- Iesako, R.; Yoshioka, T.; Nakagawa, K.; Shintani, T.; Matsuoka, A.; Kamio, E.; Matsuyama, H. Organic solvent permeation characteristics of TiO2-ZrO2 composite nanofiltration membranes prepared using organic chelating ligand to control pore size and surface property. Sep. Purif. Technol. 2022, 297, 121458. [Google Scholar] [CrossRef]
- Ma, D.; Zou, X.; Zhao, Z.; Zhou, J.; Li, S.; Yin, H.; Wang, J. Hydrophilic PAA-g-MWCNT/TiO2@PES nano-matrix composite membranes: Anti-fouling, antibacterial and photocatalytic. Eur. Polym. J. 2022, 168, 111006. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H. Synergetic effects of dodecylamine-functionalized graphene oxide nanoparticles on antifouling and antibacterial properties of polysulfone ultrafiltration membranes. J. Water Process Eng. 2021, 42, 102120. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Lemos, H.G.; Ragio, R.A.; Conceição, A.C.S.; Venancio, E.C.; Mierzwa, J.C.; Subtil, E.L. Assessment of mixed matrix membranes (MMMs) incorporated with graphene oxide (GO) for co-treatment of wastewater and landfill leachate (LFL) in a membrane bioreactor (MBR). Chem. Eng. J. 2021, 425, 131772. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Han, M.; Wang, D.; Han, S.; Liu, Z.; Zhou, N.; Shang, R.; Xie, C. Graphene Oxide Incorporated Forward Osmosis Membranes With Enhanced Desalination Performance and Chlorine Resistance. Front. Chem. 2020, 7, 877. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Alfryyan, N.; Ali, S.S.; Abd El-Salam, H.M.; Shaban, M. Preparation, characterization, and performance of PES/GO woven mixed matrix nanocomposite forward osmosis membrane for water desalination. RSC Adv. 2022, 12, 25654–25668. [Google Scholar] [CrossRef]
- Phuntsho, S.; Sahebi, S.; Majeed, T.; Lotfi, F.; Kim, J.E.; Shon, H.K. Assessing the major factors affecting the performances of forward osmosis and its implications on the desalination process. Chem. Eng. J. 2013, 231, 484–496. [Google Scholar] [CrossRef]
- Khoo, Y.S.; Lau, W.J.; Chamani, H.; Matsuura, T.; Ismail, A.F. Water flux increase by inverting the membrane from its normal position—Is it occurring in FO and PRO? J. Water Process Eng. 2020, 37, 101366. [Google Scholar] [CrossRef]
- Akther, N.; Yuan, Z.; Chen, Y.; Lim, S.; Phuntsho, S.; Ghaffour, N.; Matsuyama, H.; Shon, H. Influence of graphene oxide lateral size on the properties and performances of forward osmosis membrane. Desalination 2020, 484, 114421. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Tan, Y.H.; Chong, C.Y.; Krause-Rehberg, R.; Awad, S. Tailor-made thin film nanocomposite membrane incorporated with graphene oxide using novel interfacial polymerization technique for enhanced water separation. Chem. Eng. J. 2018, 344, 524–534. [Google Scholar] [CrossRef]
- Padaki, M.; Emadzadeh, D.; Masturra, T.; Ismail, A.F. Antifouling properties of novel PSf and TNT composite membrane and study of effect of the flow direction on membrane washing. Desalination 2015, 362, 141–150. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.S. High performance thin-film composite forward osmosis hollow fiber membranes with macrovoid-free and highly porous structure for sustainable water production. Environ. Sci. Technol. 2012, 46, 7358–7365. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, T.S.; Weber, M.; Maletzko, C.; Warzelhan, V. A sulfonated polyphenylenesulfone (sPPSU) as the supporting substrate in thin film composite (TFC) membranes with enhanced performance for forward osmosis (FO). Chem. Eng. J. 2013, 220, 15–23. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhou, Z.; Xie, J.; Lee, J.Y. Hydrophilic Mineral Coating of Membrane Substrate for Reducing Internal Concentration Polarization (ICP) in Forward Osmosis. Sci. Rep. 2016, 6, 19593. [Google Scholar] [CrossRef]
- Lambrechts, R.; Sheldon, M.S. Performance and energy consumption evaluation of a fertiliser drawn forward osmosis (FDFO) system for water recovery from brackish water. Desalination 2019, 456, 64–73. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. Characterization of humic acid fouled reverse osmosis and nanofiltration membranes by transmission electron microscopy and streaming potential measurements. Environ. Sci. Technol. 2007, 41, 942–949. [Google Scholar] [CrossRef]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S.; Kandasamy, J. Fertiliser drawn forward osmosis desalination: The concept, performance and limitations for fertigation. Rev. Environ. Sci. Bio/Technol. 2011, 11, 147–168. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Fane, A.G.; Waite, T.D. Nanofiltration of natural organic matter: Removal, fouling and the influence of multivalent ions. Desalination 1998, 118, 109–122. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. The role of foulant–foulant electrostatic interaction on limiting flux for RO and NF membranes during humic acid fouling—Theoretical basis, experimental evidence, and AFM interaction force measurement. J. Membr. Sci. 2009, 326, 526–532. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Guan, C.Y.; Liu, C.X.; Lang, W.Z.; Wang, Y. Construction of SiO2@MWNTs incorporated PVDF substrate for reducing internal concentration polarization in forward osmosis. J. Membr. Sci. 2018, 564, 328–341. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, S.; Liu, C.X.; Shen, L.; Ding, C.; Guan, C.Y.; Wang, Y. Confining migration of amine monomer during interfacial polymerization for constructing thin-film composite forward osmosis membrane with low fouling propensity. Chem. Eng. Sci. 2019, 207, 54–68. [Google Scholar] [CrossRef]
- Kuang, W.; Liu, Z.; Yu, H.; Kang, G.; Jie, X.; Jin, Y.; Cao, Y. Investigation of internal concentration polarization reduction in forward osmosis membrane using nano-CaCO3 particles as sacrificial component. J. Membr. Sci. 2016, 497, 485–493. [Google Scholar] [CrossRef]
- Cho, Y.H.; Han, J.; Han, S.; Guiver, M.D.; Park, H.B. Polyamide thin-film composite membranes based on carboxylated polysulfone microporous support membranes for forward osmosis. J. Membr. Sci. 2013, 445, 220–227. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, K.Y.; Chung, T.S. Dual-layer hollow fibers with enhanced flux as novel forward osmosis membranes for water production. Environ. Sci. Technol. 2009, 43, 2800–2805. [Google Scholar] [CrossRef]
- Setiawan, L.; Wang, R.; Li, K.; Fane, A.G. Fabrication of novel poly(amide–imide) forward osmosis hollow fiber membranes with a positively charged nanofiltration-like selective layer. J. Membr. Sci. 2011, 369, 196–205. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Tajik, S. Fabrication of thin film composite forward osmosis membrane using electrospun polysulfone/polyacrylonitrile blend nanofibers as porous substrate. Desalination 2018, 425, 68–76. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Ismail, A.F.; Rahbari-Sisakht, M. Synthesis and characterization of thin film nanocomposite forward osmosis membrane with hydrophilic nanocomposite support to reduce internal concentration polarization. J. Membr. Sci. 2014, 449, 74–85. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
| Membrane ID | PES (wt.%) | NMP (wt.%) | TNT (wt.%) | PEG (wt.%) |
|---|---|---|---|---|
| Bare PES | 22 | 77.5 | 0 | 0.5 |
| ST1 | 22 | 77.496 | 0.004 | 0.5 |
| ST2 | 22 | 77.492 | 0.008 | 0.5 |
| ST 3 | 22 | 77.49 | 0.01 | 0.5 |
| ST 4 | 22 | 77.46 | 0.04 | 0.5 |
| ST 5 | 22 | 77.42 | 0.08 | 0.5 |
| ST 6 | 22 | 77.4 | 0.1 | 0.5 |
| Parameter | Textile Industrial Wastewater (TIWW) | Low-Strength Gray Water (GW) | Municipal Water (MW) |
|---|---|---|---|
| pH | 7.5 | 7.7 | 7.3 |
| COD g/L | 1.194 | 0.28 | 0.39 |
| TSS g/L | 0.860 | 1 | 1.8 |
| TDS g/L | 0.56 | 0.585 | 0.6 |
| Conductivity | 1.084 | 1.018 | 1.052 |
| PO4 g/L | 0.09 | 0.009 | 0.14 |
| Total Nitrogen g/L | - | - | 0.04 |
| Sulfide g/L | - | - | 4.4 |
| Compound Name | Anorthic TiO2 | Monoclinic Na2Ti3O7 | ||||||
|---|---|---|---|---|---|---|---|---|
| ( ) | (040) | ( ) | (063) | ( ) | ( ) | ( ) | (520) | |
| 2θ (o) | 18.33 | 27.57 | 29.20 | 45.63 | 31.91 | 34.03 | 66.37 | 75.47 |
| d-spacing (Å) | 4.84 | 3.23 | 3.05 | 1.99 | 2.80 | 2.63 | 1.41 | 1.26 |
| I/Io (%) | 9.34 | 8.95 | 16.55 | 57.45 | 100 | 22.98 | 18.55 | 15.96 |
| FWHM (β) | 0.118 | 0.236 | 0.157 | 0.197 | 0.157 | 0.157 | 0.118 | 0.118 |
| Microstrain × 10−3 | 3.19 | 4.20 | 2.64 | 2.04 | 2.40 | 2.24 | 0.79 | 0.67 |
| D (nm) | 71.14 | 36.16 | 54.47 | 45.72 | 54.81 | 55.11 | 83.91 | 88.80 |
| TC | 0.58 | 0.56 | 1.03 | 3.58 | 4.31 | 0.99 | 0.80 | 0.69 |
| δ × 10−3 (dis/nm2) | 0.20 | 0.76 | 0.34 | 0.45 | 0.33 | 0.33 | 0.14 | 0.13 |
| Sample | Ra (nm) | Rq (nm) | ||
|---|---|---|---|---|
| Top | Bottom | Top | Bottom | |
| Bare PES | 16.5 | 14.6 | 18.3 | 16.7 |
| ST2 | 16.7 | 15.8 | 18.4 | 18.5 |
| ST3 | 17.9 | 19.4 | 19.8 | 20.8 |
| ST6 | 17.5 | 14.9 | 17.9 | 15.4 |
| Samples | TNP Wt.% | Water Uptake (%) | Porosity (%) | Contact Angle (°) | Thickness (µm) | Tortuosity (τ) | S Value |
|---|---|---|---|---|---|---|---|
| Bare PES | 0 | 49 | 45 | 78 | 167 | 5.3 | 19.6 |
| ST1 | 0.004 | 64 | 54 | 73 | 166 | 3.9 | 11.98 |
| ST2 | 0.008 | 70 | 60 | 68 | 153 | 3.2 | 8.16 |
| ST3 | 0.01 | 77 | 74 | 63 | 152 | 2.1 | 4.3 |
| ST4 | 0.04 | 52 | 52 | 58 | 153 | 4.2 | 12.1 |
| ST 5 | 0.08 | 46 | 53 | 55 | 154 | 4.1 | 10.6 |
| ST 6 | 0.1 | 30 | 31 | 51 | 154 | 9.2 | 45.7 |
| Parameter | FO (Al-FS) Mode | PRO (Al-DS) Mode | ||||||
|---|---|---|---|---|---|---|---|---|
| Jw (LMH) | Js (GMH) | Js/Jw × 10−3 | SR% | Jw (LMH) | Js (GMH) | Js/Jw × 10−3 | SR% | |
| Bare PES | 67 | 4.78 | 71.3 | 81 | 81 | 6.8 | 83.9 | 72 |
| ST1 | 97 | 0.56 | 5.8 | 96 | 131 | 8.2 | 62.6 | 82.5 |
| ST2 | 129 | 0.37 | 2.9 | 97.6 | 140 | 10.5 | 75 | 78 |
| ST3 | 136 | 0.035 | 0.3 | 98.8 | 149 | 12.9 | 86.6 | 70 |
| ST4 | 68.6 | 0.4 | 5.8 | 97 | 78 | 15.4 | 197 | 68.5 |
| ST5 | 61.7 | 0.7 | 11.4 | 95 | 72 | 15.9 | 220 | 67.7 |
| ST6 | 48.6 | 1.78 | 36.6 | 93.6 | 62 | 16 | 258 | 68 |
| Parameter | Textile Industrial Wastewater (TIWW) | Low-Strength Gray Water (GW) | Municipal Water (MW) |
|---|---|---|---|
| pH | 7.7 | 7.7 | 7.5 |
| COD g/L | 1.815 | 1.8 | 0.57 |
| TSS g/L | 1.307 | 1.3 | 0.26 |
| TDS g/L | 0.854 | 0.836 | 0.883 |
| Conductivity | 1.648 | 1.848 | 1.70 |
| Membrane | Jw (LMH) | Js (GMH) | Js/Jw (g/L) | Test Conditions (FS/DS, Membrane Orientation) | Ref. |
|---|---|---|---|---|---|
| TiO2/Na2Ti3O7/PES | 136 | 0.035 | 0.3 × 10−3 | DI water/1 M NaCl, FO mode | This work |
| 149 | 12.9 | 86.6 | DI water/1 M NaCl, PRO mode | ||
| SiO2@MWNTs incorporated into polyvinylidene difluoride (PVDF) | 22.10 | 4.10 | 0.19 | DI water/1.0 M NaCl, FO mode | [84] |
| Polysulfone (PSF)/TiO2 nanocomposite | 31.20 | 6.66 | 0.21 | 10 mM NaCl/ 0.5 M NaCl, PRO mode | [19] |
| NMP added to coagulation bath PES | 62.70 | 10.30 | 0.16 | DI water/2.0 M NaCl, PRO mode | [75] |
| p-TiO2 incorporated into polyvinylidene difluoride (PVDF) | 18.70 | 4.50 | 0.24 | DI water/1 M NaCl, FO mode | [85] |
| CaCO3 incorporated into PSF | 25.40 | 57.00 | 2.24 | DI water/2 M NaCl, FO mode | [86] |
| Zeolite incorporated into PSF | 85 | 55.00 | 0.65 | DI water/2 M NaCl, PRO mode | [23] |
| Polyamide thin-film composite membranes based on carboxylated polysulfone (TFC-cPSf flat sheet) | 18 | 2.2 | 0.12 | DI water/1 M MgCl2, FO mode | [87] |
| 27 | 5.5 | 0.09 | DI water/1 M MgCl2, PRO mode | ||
| Dual-layer polybenzimidazole-polyether sulfone (PBI-PES) nanofiltration (NF) hollow-fiber membranes | 24.2 | - | - | DI water/5 M MgCl2, FO mode | [88] |
| 33.8 | - | - | DI water/5 M MgCl2, PRO mode | ||
| Polyamide-imide (PAI) material as the porous substrate, followed by polyelectrolyte post-treatment using polyethyleneimine (PEI) | 11.7 | 3.9 | 0.33 | DI water/1.5 M MgCl2, FO mode | [89] |
| 17.3 | 16.6 | 0.96 | DI water/1.5 M MgCl2, PRO mode | ||
| (TFC) FO membrane with hydrophilic mineral (CaCO3)-coated polyether sulfone (PES) | 52 | 16.8 | 0.32 | DI water/2 M NaCl, FO mode | [77] |
| Polysulfone/polyacrylonitrile blend nanofibers as a porous substrate | 38.3 | 10.1 | 0.27 | DI water/1 M NaCl, FO mode | [90] |
| Thin-film nanocomposite (TFN) polyamide (commercial CTA-W) | 14 | 5.6 | 0.4 | DI water/1 M NaCl, FO mode | [91] |
| CTA-NW (commercial) | 4.4 | 0.6 | 0.14 | 10 mM NaCl /0.5 M NaCl, FO mode | [92] |
| 8.19 | 2.8 | 0.34 | 10 mM NaCl /0.5 M NaCl, PRO mode | ||
| CTA-HW (commercial) | 9.03 | 5.3 | 0.59 | 10 mM NaCl /0.5 M NaCl, FO mode | |
| 15.4 | 9.4 | 0.61 | 10 mM NaCl /0.5 M NaCl, PRO mode | ||
| CTA-W (commercial) | 5 | 2.9 | 0.58 | 10 mM NaCl /0.5 M NaCl, FO mode | |
| 6.55 | 4.8 | 0.73 | 10 mM NaCl /0.5 M NaCl, PRO mode |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Senani, G.M.; Nasr, M.; Zayed, M.; Ali, S.S.; Alshaikh, H.; Abd El-Salam, H.M.; Shaban, M. Fabrication of PES Modified by TiO2/Na2Ti3O7 Nanocomposite Mixed-Matrix Woven Membrane for Enhanced Performance of Forward Osmosis: Influence of Membrane Orientation and Feed Solutions. Membranes 2023, 13, 654. https://doi.org/10.3390/membranes13070654
Al-Senani GM, Nasr M, Zayed M, Ali SS, Alshaikh H, Abd El-Salam HM, Shaban M. Fabrication of PES Modified by TiO2/Na2Ti3O7 Nanocomposite Mixed-Matrix Woven Membrane for Enhanced Performance of Forward Osmosis: Influence of Membrane Orientation and Feed Solutions. Membranes. 2023; 13(7):654. https://doi.org/10.3390/membranes13070654
Chicago/Turabian StyleAl-Senani, Ghadah M., Mervat Nasr, Mohamed Zayed, Sahar S. Ali, Hind Alshaikh, Hanafy M. Abd El-Salam, and Mohamed Shaban. 2023. "Fabrication of PES Modified by TiO2/Na2Ti3O7 Nanocomposite Mixed-Matrix Woven Membrane for Enhanced Performance of Forward Osmosis: Influence of Membrane Orientation and Feed Solutions" Membranes 13, no. 7: 654. https://doi.org/10.3390/membranes13070654
APA StyleAl-Senani, G. M., Nasr, M., Zayed, M., Ali, S. S., Alshaikh, H., Abd El-Salam, H. M., & Shaban, M. (2023). Fabrication of PES Modified by TiO2/Na2Ti3O7 Nanocomposite Mixed-Matrix Woven Membrane for Enhanced Performance of Forward Osmosis: Influence of Membrane Orientation and Feed Solutions. Membranes, 13(7), 654. https://doi.org/10.3390/membranes13070654










