Influence of Nitrosyl Iron Complex with Thiosulfate Ligands on Therapeutically Important Targets Related to Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fluorescent Probes Assay
2.3. Tissue Preparation
2.4. Luminol-Amplified Chemiluminescence Assay
2.5. Aldose Reductase (ALR2) Inhibitory Activity
2.6. Antiglycation Assay
2.7. Acute Toxicity Test Procedure
2.8. Statistical Analysis
3. Results and Discussion
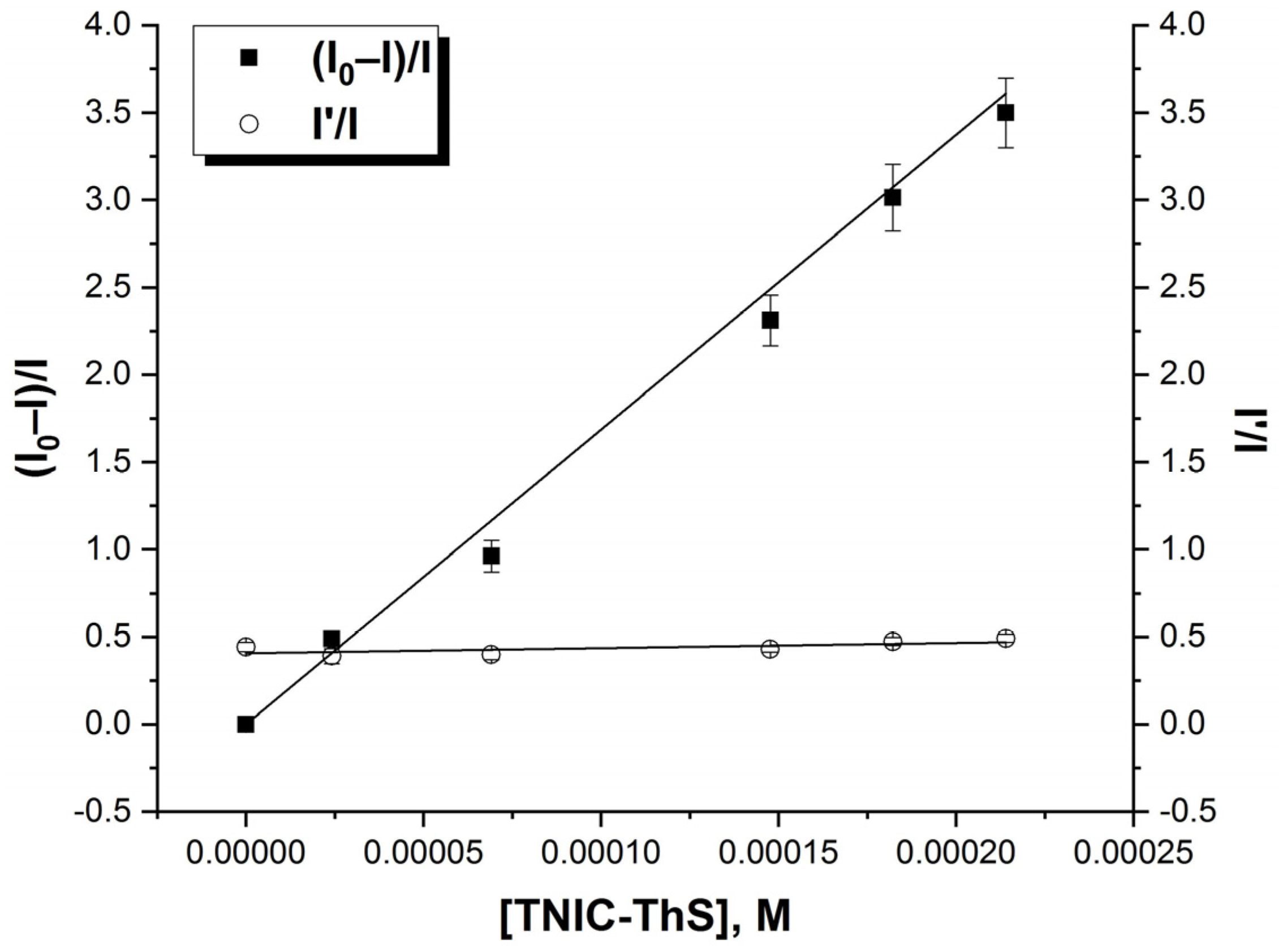
3.1. Interaction of TNIC-ThS with PC Liposomes
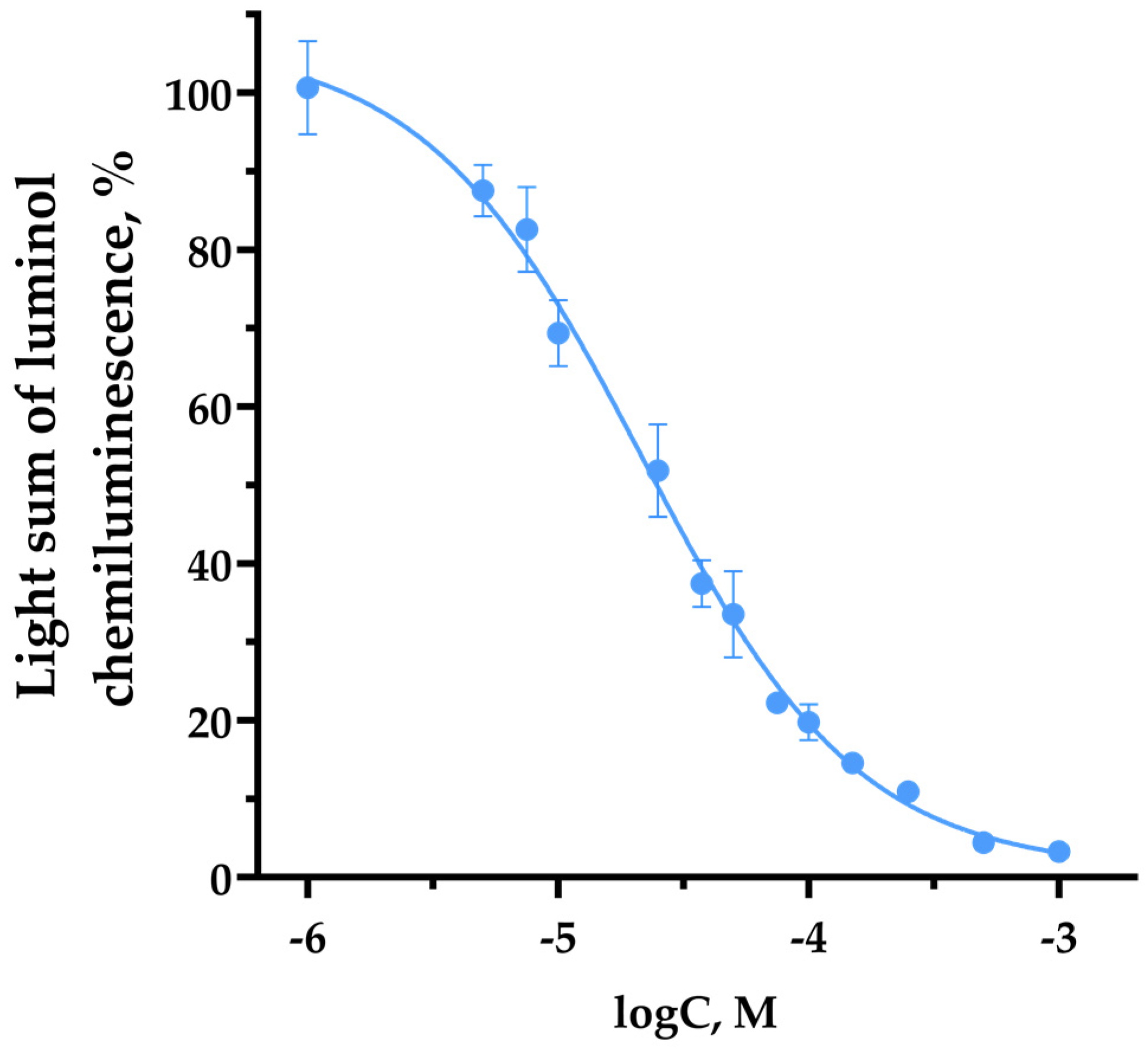
3.2. TNIC-ThS Radical Scavenging Capacity in Luminol Chemiluminescence Assay
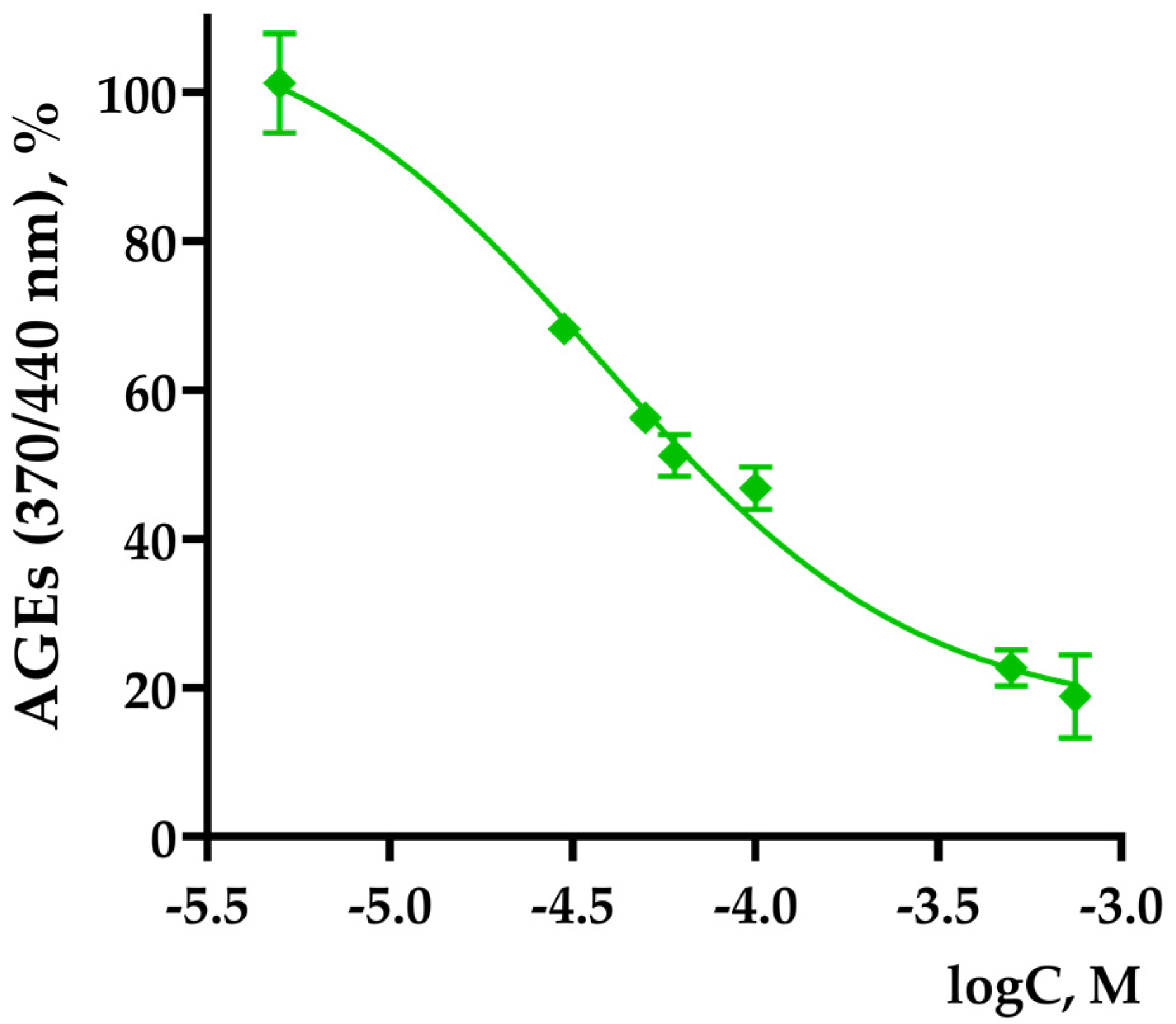
3.3. TNIC Antiglycating Activity
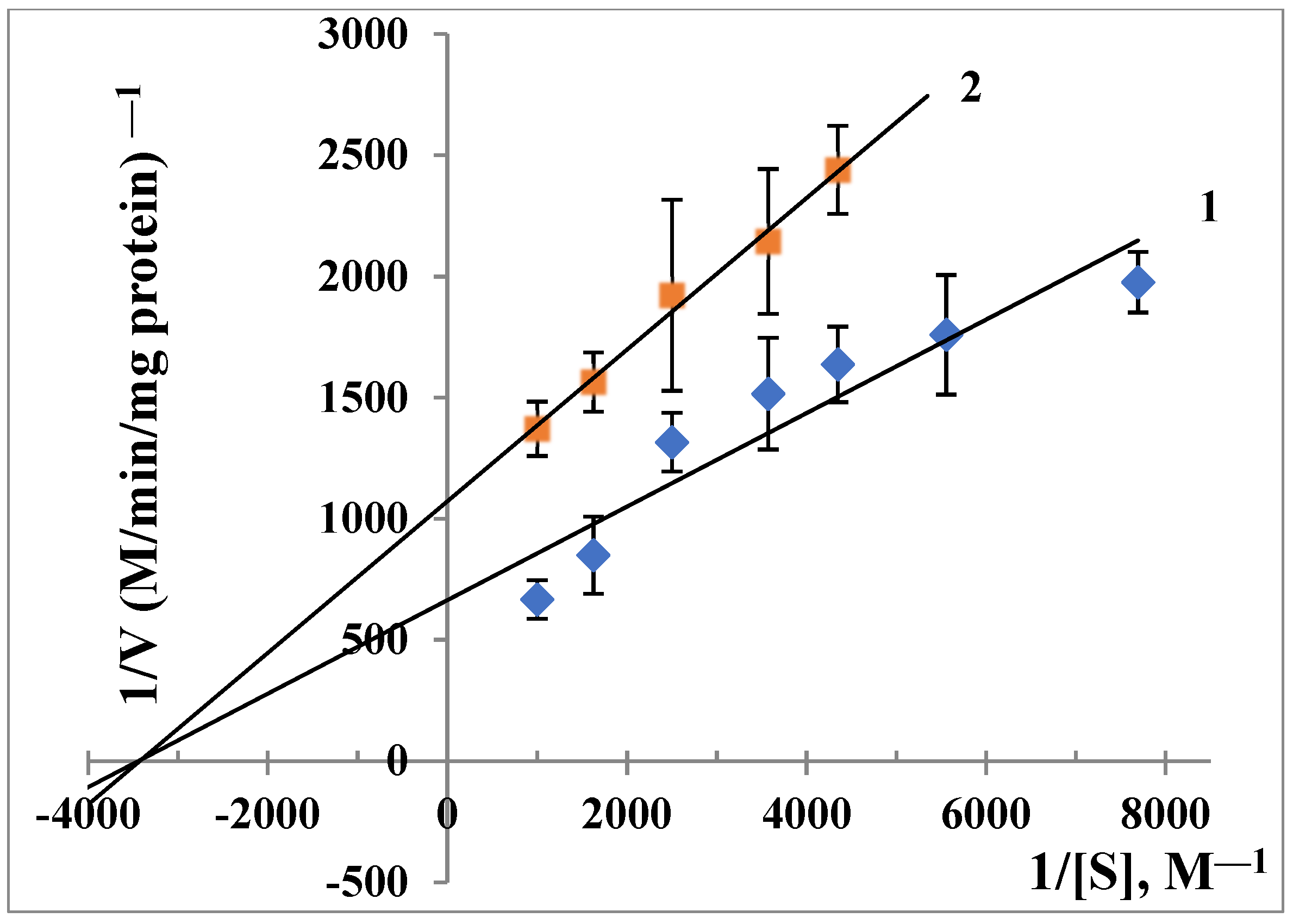
3.4. ALR2 Inhibitory Activity
3.5. Acute Toxicity of the TNIC-ThS in Mice
- no change in the mice’s condition and no behavioral changes;
- good intensity of motor actions;
- no tonic convulsions;
- normal response to touch, pain, sound, and light stimuli;
- no changes in frequency, depth of respiratory movements, and heart rate;
- skin and hair condition was good;
- no changes in color of mucous membranes and pupil size.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation IDF. Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/ (accessed on 18 June 2023).
- World Health Organization. Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2019. Available online: https://apps.who.int/iris/rest/bitstreams/1233344/retrieve (accessed on 18 June 2023).
- Chaudhury, A.; Duvoor, C.; Dendi, V.S.R.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, S.N.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007, 3, 853–876. [Google Scholar] [PubMed]
- Tessari, P.; Cecchet, D.; Cosma, A.; Vettore, M.; Coracina, A.; Millioni, R.; Iori, E.; Puricelli, L.; Avogaro, A.; Vedovato, M. Nitric oxide synthesis in subjects with type 2 diabetes and nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Michell, B.J.; Griffiths, J.E.; Mitchelhill, K.I.; Rodriguez-Crespo, I.; Tiganis, T.; Bozinovski, S.; de Montellano, P.R.; Kemp, B.E.; Pearson, R.B. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999, 9, 845–848. [Google Scholar] [CrossRef]
- Honing, M.L.; Morrison, P.J.; Banga, J.D.; Stroes, E.S.; Rabelink, T.J. Nitric oxide availability in diabetes mellitus. Diabetes Metab. Rev. 1998, 14, 241–249. [Google Scholar] [CrossRef]
- Sciacca, M.; Chillemi, R.; Sciuto, S.; Greco, V.; Messineo, C.; Kotler, S.A.; Lee, D.K.; Brender, J.R.; Ramamoorthy, A.; La Rosa, C.; et al. A blend of two resveratrol derivatives abolishes hIAPP amyloid growth and membrane damage. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1793–1802. [Google Scholar] [CrossRef]
- Bredt, D.S.; Snyder, S.H. Nitric oxide: A physiologic messenger molecule. Annu. Rev. Biochem. 1994, 63, 175–195. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Förstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflug. Arch. 2010, 459, 923–939. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Verbeuren, T.J.; Van de Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Lee, P.H.; Lei, C.C.; Ho, C.; Shih, Y.H.; Lin, C.L. Nitric oxide donors rescue diabetic nephropathy through oxidative-stress-and nitrosative-stress-mediated Wnt signaling pathways. J. Diabetes Investig. 2015, 6, 24–34. [Google Scholar] [CrossRef]
- Henstridge, D.C.; Drew, B.G.; Formosa, M.F.; Natoli, A.K.; Cameron-Smith, D.; Duffy, S.J.; Kingwell, B.A. The effect of the nitric oxide donor sodium nitroprusside on glucose uptake in human primary skeletal muscle cells. Nitric Oxide 2009, 21, 126–131. [Google Scholar] [CrossRef]
- Paolisso, G.; Tagliamonte, M.R.; Marfella, R.; Verrazzo, G.; D’Onofrio, F.; Giugliano, D. L-arginine but not d-arginine stimulates insulin-mediated glucose uptake. Metabolism 1997, 46, 1068–1073. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Mitchelhill, K.I.; Michell, B.J.; Stapleton, D.; Rodriguez-Crespo, I.; Witters, L.A.; Power, D.A.; de Montellano, P.R.O.; Kemp, B.E. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999, 29, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Serezhenkov, V.A.; Kuznetsov, I.S.; Romantsova, T.I.; Kuznetsova, M.I.; Vanin, A.F. Antidiabetes drug metformin is a donor of nitric oxide: ESR measurement of efficiency. Biofizika 2011, 56, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.; Jacobs, T.F. Metformin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK518983 (accessed on 18 June 2023).
- Sanina, N.A.; Aldoshin, S.M. Structure and properties of iron nitrosyl complexes with functionalized sulfur-containing ligands. Russ. Chem. Bull. Int. Ed. 2011, 60, 1223–1251. [Google Scholar] [CrossRef]
- Sanina, N.A.; Aldoshin, S.M.; Rudneva, T.N.; Golovina, N.I.; Shilov, G.V.; Shul’ga, Y.M.; Martynenko, V.M.; Ovanesyan, N.S. Synthesis, Structure and Solid-Phase Transformations of Fe Nitrosyl Complex Na2[Fe2(S2O3)2(NO)4] 4H2O. Russ. J. Coord. Chem. 2005, 31, 301–306. [Google Scholar] [CrossRef]
- Timoshin, A.A.; Vanin, A.F.; Orlova, T.R.; Sanina, N.A.; Ruuge, E.K.; Aldoshin, S.M.; Chazov, E.I. Protein-bound dinitrosyl–iron complexes appearing in blood of rabbit added with a low-molecular di-nitrosyl–iron complex: EPR studies. Nitric Oxide 2007, 16, 286–293. [Google Scholar] [CrossRef]
- Tat’yanenko, L.V.; Kotelnikov, A.I.; Dobrokhotova, O.V.; Saratovskikh, E.A.; Sanina, N.A.; Rudneva, T.N.; Aldoshin, S.M. Effect of nitrosyl iron–sulfur complexes on the activity of hydrolytic enzymes. Pharm. Chem. J. 2009, 43, 525–529. [Google Scholar] [CrossRef]
- Aldoshin, S.M.; Bezrukov, V.V.; Gorban, E.N.; Koltover, V.K.; Sanina, N.A. Iron-sulfur [2Fe–2S] nitrosyl complexes as new trend in synthesis of donors of nitric oxide for anti-aging therapy. Free Radic. Biol. Med. 2017, 112, 54. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Dobretsov, G.E. Fluorescent Probes in the Investigation of Biological Membranes; Nauka: Moscow, Russia, 1980. [Google Scholar]
- Poletaeva, D.A.; Soldatova, Y.V.; Smolina, A.V.; Savushkin, M.A.; Klimanova, E.N.; Sanina, N.A.; Faingold, I.I. The Influence of Cationic Nitrosyl Iron Complex with Penicillamine Ligands on Model Membranes, Membrane-Bound Enzymes and Lipid Peroxidation. Membranes 2022, 12, 1088. [Google Scholar] [CrossRef]
- Poletaeva, D.A.; Kotelnikova, R.A.; Mischenko, D.V.; Rybkin, A.Y.; Smolina, A.V.; Faingol’d, I.I.; Troshin, P.A.; Kornev, A.B.; Khakina, E.A.; Kotel’nikov, A.I. Estimation of Membrane Activity of WaterSoluble Polysubstituted Fullerene Derivatives by Luminescence Methods. Nanotechnol. Russ. 2012, 7, 302–307. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P.; Piro, M.C.; De Leo, T. Enhanced Luminescence Study of Liver Homogenate Response to Oxidative Stress. Arch. Physiol. Biochem. 1995, 103, 187–195. [Google Scholar] [CrossRef]
- Motaal, A.A.; Askary, H.E.; Crockett, S.; Kunert, O.; Sakr, B.; Shaker, S.; Grigore, A.; Albulescu, R.; Bauer, R. Aldose reductase inhibition of a saponin-rich fraction and new furostanol saponin derivatives from Balanites aegyptiaca. Phytomedicine 2015, 22, 829–836. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Chen, Y.F.; Roan, H.Y.; Lii, C.K.; Huang, Y.C.; Wang, T.-S. Relationship between Antioxidant and Antiglycation Ability of Saponins, Polyphenols, and Polysaccharides in Chinese Herbal Medicines Used to Treat Diabetes. J. Med. Plants Res. 2011, 5, 2322–2331. [Google Scholar]
- Séro, L.; Sanguinet, L.; Blanchard, P.; Dang, B.; Morel, S.; Richomme, P.; Séraphin, D.; Derbré, S. Tuning a 96-Well Microtiter Plate Fluorescence-Based Assay to Identify AGE Inhibitors in Crude Plant Extracts. Molecules 2013, 18, 14320–14339. [Google Scholar] [CrossRef]
- Matsuura, N.; Aradate, T.; Sasaki, C.; Kojima, H.; Ohara, M.; Hasegawa, J.; Ubukata, M. Screening System for the Maillard Reaction Inhibitor from Natural Product Extracts. J. Health Sci. 2002, 48, 520–526. [Google Scholar] [CrossRef]
- Mironov, A.N.; Bunyatyan, N.D.; Vasiliev, A.N.; Verstakova, O.L.; Zhuravleva, M.V.; Lepakhin, V.K.; Korobov, N.V.; Merkulov, V.A.; Orekhov, S.N.; Sakaeva, I.V.; et al. Rukovodstvo Po Provedeniyu Doklinicheskikh Issledovaniy Lekarstvennykh Sredstv. (Guidelines for Conducting Preclinical Studies of Drugs, Part 1); Grif & K.: Moscow, Russia, 2012. (In Russian) [Google Scholar]
- Peetla, C.; Stine, A.; Labhasetw, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Barceló, F.; Prades, J.; Funari, S.S.; Frau, J.; Alemany, R.; Escribá, P.V. The hypotensive drug 2-hydroxyoleic acid modifies the structural properties of model membranes. Mol. Membr. Biol. 2004, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Preetha, A.; Huilgol, N.; Banerjee, R. Comparison of paclitaxel penetration in normal and cancerous cervical model monolayer membranes. Colloids Surf. B Biointerfaces 2006, 53, 179–186. [Google Scholar] [CrossRef]
- Luchini, A.; Vitiello, G. Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies. Biomimetics 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Galla, H.J.; Sackmann, E. Lateral diffusion in the hydrophobic region of membranes: Use of pyrene excimers as optical probes. Biochim. Biophys. Acta 1974, 339, 103–115. [Google Scholar] [CrossRef]
- Brocklehurst, J.R.; Freedman, R.B.; Hancock, D.J.; Radda, G.K. Membrane studies with polarity-dependent and excimer-forming fluorescent probes. Biochem. J. 1970, 116, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Kachel, K.; Asuncion-Punzalan, E.; London, E. The location of fluorescence probes with charged groups in model membranes. Biochim. Biophys. Acta 1998, 1374, 63–76. [Google Scholar] [CrossRef]
- Kotel’nikov, A.I.; Kuznetsov, S.N.; Fogel, V.R.; Likhtenshteĭn, G.I. Investigation of the microstructure of biological systems by triplet label. Mol. Biol. 1979, 1, 152–159. [Google Scholar]
- Faingold, I.I.; Poletaeva, D.A.; Soldatova, Y.V.; Smolina, A.V.; Pokidova, O.V.; Kulikov, A.V.; Sanina, N.A.; Kotelnikova, R.A. Effects of albumin-bound nitrosyl iron complex with thiosulfate ligands on lipid peroxidation and activities of mitochondrial enzymes in vitro. Nitric Oxide 2021, 117, 46–52. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Nishikawa, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 2007, 9, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; de Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Pannangpetch, P.; Kukongviriyapan, V.; Kongyingyoes, B.; Kukongviriyapan, U. Antihyperglycemic, Antioxidant and Antiglycation Activities of Mulberry Leaf Extract in Streptozotocin-Induced Chronic Diabetic Rats. Plant Foods Hum. Nutr. 2009, 64, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Radbakhsh, S.; Ganjali, S.; Moallem, S.A.; Guest, P.C.; Sahebkar, A. Antioxidant Effects of Trehalose in an Experimental Model of Type 2 Diabetes. Adv. Exp. Med. Biol. 2021, 1328, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef]
- Pang, G.M.; Li, F.X.; Yan, Y.; Zhang, Y.; Kong, L.L.; Zhu, P.; Wang, K.F.; Zhang, F.; Liu, B.; Lu, C. Herbal medicine in the treatment of patients with type 2 diabetes mellitus. Chin. Med. J. 2019, 132, 78–85. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Spinas, G.A. The Dual Role of Nitric Oxide in Islet β-Cells. Physiology 1999, 14, 49–54. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Demircan, N.; Gurel, A.; Armutcu, F.; Unalacak, M.; Aktunc, E.; Atmaca, H. The evaluation of serum cystatin C, malondialdehyde, and total antioxidant status in patients with metabolic syndrome. Med. Sci. Monit. 2008, 14, 97–101. [Google Scholar]
- Calabrese, V.; Cornelius, C.; Leso, V.; Trovato-Salinaro, A.; Ventimiglia, B.; Cavallaro, M.; Scuto, M.; Rizza, S.; Zanoli, L.; Neri, S.; et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim. Biophys. Acta 2012, 1822, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Faingold, I.I.; Kotelnikova, R.A.; Smolina, A.V.; Poletaeva, D.A.; Soldatova, Y.V.; Pokidova, O.V.; Sadkov, A.P.; Sanina, N.A.; Aldoshin, S.M. Antioxidant Activity of Tetranitrosyl Iron Complex with Thiosulfate Ligands and Its Effect on Catalytic Activityof Mitochondrial Enzymes In vitro. Dokl. Biochem. Biophys. 2019, 488, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Hwang, S.H.; Wang, Z.; Quispe, Y.N.G.; Lim, S.S.; Yu, J.M. Evaluation of Aldose Reductase, Protein Glycation, and Antioxidant Inhibitory Activities of Bioactive Flavonoids in Matricaria recutita L. and Their Structure-Activity Relationship. J. Diabetes Res. 2018, 2018, 3276162. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowski, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef]
- Jagdale, A.D.; Bavkar, L.N.; More, T.A.; Joglekar, M.M.; Arvindekar, A.U. Strong inhibition of the polyol pathway diverts glucose flux to protein glycation leading to rapid establishment of secondary complications in diabetes mellitus. J. Diabetes Complicat. 2016, 30, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Subratty, A.H.; Ahmed, N. Inhibition of glucose- and fructose-mediated protein glycation by infusions and ethanolic extracts of ten culinary herbs and spices. Asian Pac. J. Trop. Biomed. 2016, 6, 492–500. [Google Scholar] [CrossRef]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.A.; Rasheed, Z. Non-enzymatic glycation of proteins: From diabetes to cancer. Biomed. Khim. 2010, 56, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J. Diabetes Res. 2014, 2014, 137919. [Google Scholar] [CrossRef]
- Meenatchi, P.; Purushothaman, A.; Maneemegalai, S. Antioxidant, antiglycation and insulinotrophic properties of Coccinia grandis (L.) in vitro: Possible role in prevention of diabetic complications. J. Tradit. Complement. Med. 2016, 2, 54–64. [Google Scholar] [CrossRef]
- Huang, J.S.; Chuang, L.Y.; Guh, J.Y.; Huang, Y.J. Effects of nitric oxide and antioxidants on advanced glycation end products-induced hypertrophic growth in human renal tubular cells. Toxicol. Sci. 2009, 111, 109–119. [Google Scholar] [CrossRef]
- Asahi, K.; Ichimori, K.; Nakazawa, H.; Izuhara, Y.; Inagi, R.; Watanabe, T.; Miyata, T.; Kurokawa, K. Nitric oxide inhibits the formation of advanced glycation end products. Kidney Int. 2000, 58, 1780–1787. [Google Scholar] [CrossRef]
- Thakur, S.; Gupta, S.K.; Ali, V.; Singh, P.; Verma, M. Aldose Reductase: A cause and a potential target for the treatment of diabetic complications. Arch. Pharm. Res. 2021, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Schemmel, K.E.; Padiyara, R.S.; D’Souza, J.J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: A review. J. Diabetes Complicat. 2010, 24, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Chung, S.S. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999, 13, 23–30. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Minchenko, A.G.; Vasupuram, R.; White, L.; Abatan, O.I.; Kumagai, A.K.; Frank, R.N.; Stevens, M.J. Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes 2003, 52, 864–871. [Google Scholar] [CrossRef]
- Drel, V.R.; Pacher, P.; Stevens, M.J.; Obrosova, I.G. Aldose reductase inhibition counteracts nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells. Free Radic. Biol. Med. 2006, 40, 1454–1465. [Google Scholar] [CrossRef]
- Sun, W.; Oates, P.J.; Coutcher, J.B.; Gerhardinger, C.; Lorenzi, M. A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes 2006, 55, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, S.; Hao, X.; Qin, X.; Parveen, S.; Yang, S.; Ma, B.; Zhu, C. Pyridothiadiazine derivatives as aldose reductase inhibitors having antioxidant activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 126–130. [Google Scholar] [CrossRef]
- Hao, X.; Han, Z.; Li, Y.; Li, C.; Wang, X.; Zhang, X.; Yang, Q.; Ma, B.; Zhu, C. Synthesis and structure-activity relationship studies of phenolic hydroxyl derivatives based on quinoxalinone as aldose reductase inhibitors with antioxidant activity. Bioorg. Med. Chem. Lett. 2017, 27, 887–892. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Zhang, X.; Fan, Z.; Liu, W.; Lei, Y.; Zhu, C.; Ma, B. Dihydrobenzoxazinone derivatives as aldose reductase inhibitors with antioxidant activity. Bioorg. Med. Chem. 2020, 28, 115699. [Google Scholar] [CrossRef]
- Hao, X.; Qi, G.; Ma, H.; Zhu, C.; Han, Z. Novel 2-phenoxypyrido[3,2-b]pyrazin-3(4H)-one derivatives as potent and selective aldose reductase inhibitors with antioxidant activity. J. Enzym. Inhib. Med. Chem. 2019, 34, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Maradesha, T.; Patil, S.M.; Al-Mutairi, K.A.; Ramu, R.; Madhunapantula, S.V.; Alqadi, T. Inhibitory Effect of Polyphenols from the Whole Green Jackfruit Flour against α-Glucosidase, α-Amylase, Aldose Reductase and Glycation at Multiple Stages and Their Interaction: Inhibition Kinetics and Molecular Simulations. Molecules 2022, 7, 1888. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Tokita, M.; Uda, K.; Ishikawa, C.; Satoh, M. Synthesis and in vitro evaluation of botryllazine B analogues as a new class of inhibitor against human aldose reductase. Tetrahedron 2009, 65, 3019–3026. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Ramana, K.V.; Chandra, D.; Srivastava, S.; Bhatnagar, A. Regulation of aldose reductase and the polyol pathway activity by nitric oxide. Chem. Biol. Interact. 2003, 143–144, 333–340. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faingold, I.I.; Soldatova, Y.V.; Poletaeva, D.A.; Klimanova, E.N.; Sanina, N.A. Influence of Nitrosyl Iron Complex with Thiosulfate Ligands on Therapeutically Important Targets Related to Type 2 Diabetes Mellitus. Membranes 2023, 13, 615. https://doi.org/10.3390/membranes13070615
Faingold II, Soldatova YV, Poletaeva DA, Klimanova EN, Sanina NA. Influence of Nitrosyl Iron Complex with Thiosulfate Ligands on Therapeutically Important Targets Related to Type 2 Diabetes Mellitus. Membranes. 2023; 13(7):615. https://doi.org/10.3390/membranes13070615
Chicago/Turabian StyleFaingold, Irina I., Yuliya V. Soldatova, Darya A. Poletaeva, Elena N. Klimanova, and Nataliya A. Sanina. 2023. "Influence of Nitrosyl Iron Complex with Thiosulfate Ligands on Therapeutically Important Targets Related to Type 2 Diabetes Mellitus" Membranes 13, no. 7: 615. https://doi.org/10.3390/membranes13070615
APA StyleFaingold, I. I., Soldatova, Y. V., Poletaeva, D. A., Klimanova, E. N., & Sanina, N. A. (2023). Influence of Nitrosyl Iron Complex with Thiosulfate Ligands on Therapeutically Important Targets Related to Type 2 Diabetes Mellitus. Membranes, 13(7), 615. https://doi.org/10.3390/membranes13070615




