The Influence of Polycation and Counter-Anion Nature on the Properties of Poly(ionic liquid)-Based Membranes for CO2 Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Poly(vinylbenzyl chloride) (pVBCl) Synthesis
2.3. Poly(vinylbenzylpyridinium chloride) (pVBPyCl) and Poly(vinylbenzylmethylimidazolium chloride) (pVBmimCl) Synthesis
2.4. Anion Exchange Reaction
2.5. Conductometric Titration
2.6. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.7. Gel Permeation Chromatography (GPC)
2.8. Attenuated Total Reflectance Fourier Transforms Infrared Spectroscopy (ATR-FTIR)
2.9. Density of Polymers
2.10. Membrane Preparation
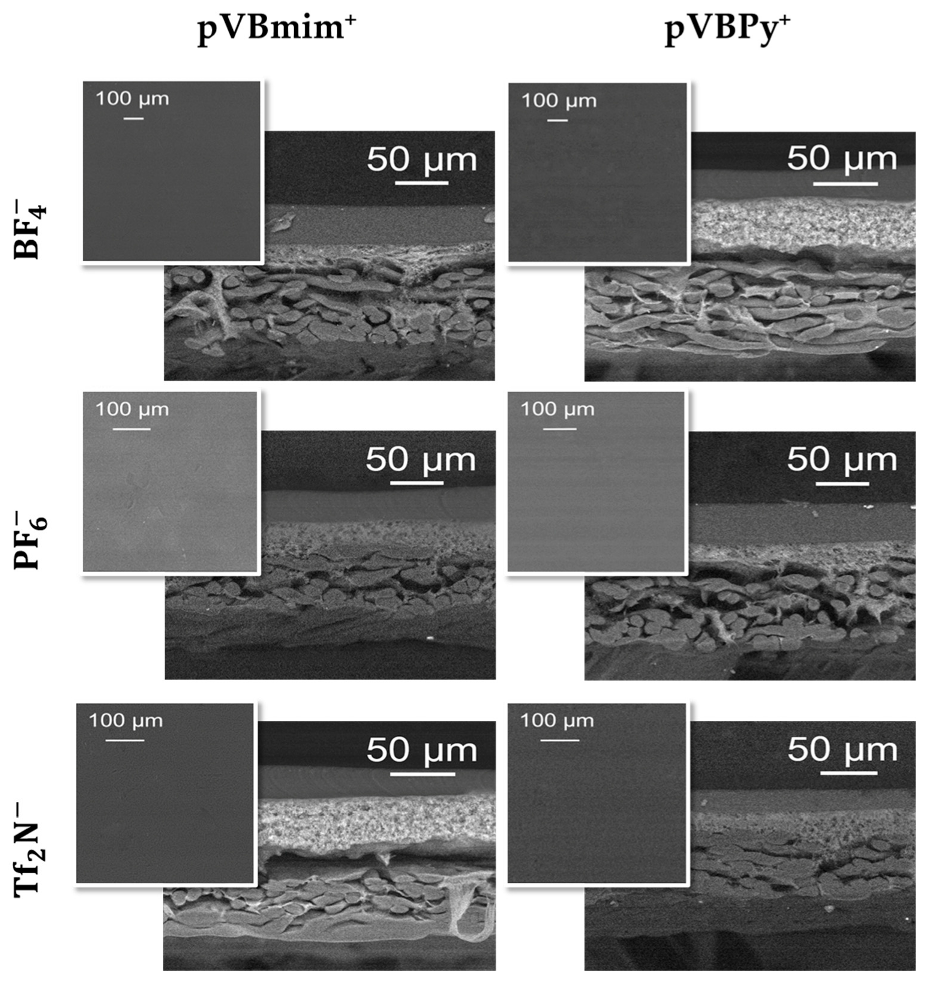
2.11. Scanning Electron Microscopy (SEM)
2.12. Wettability Measurements and Surface Free Energy Calculation
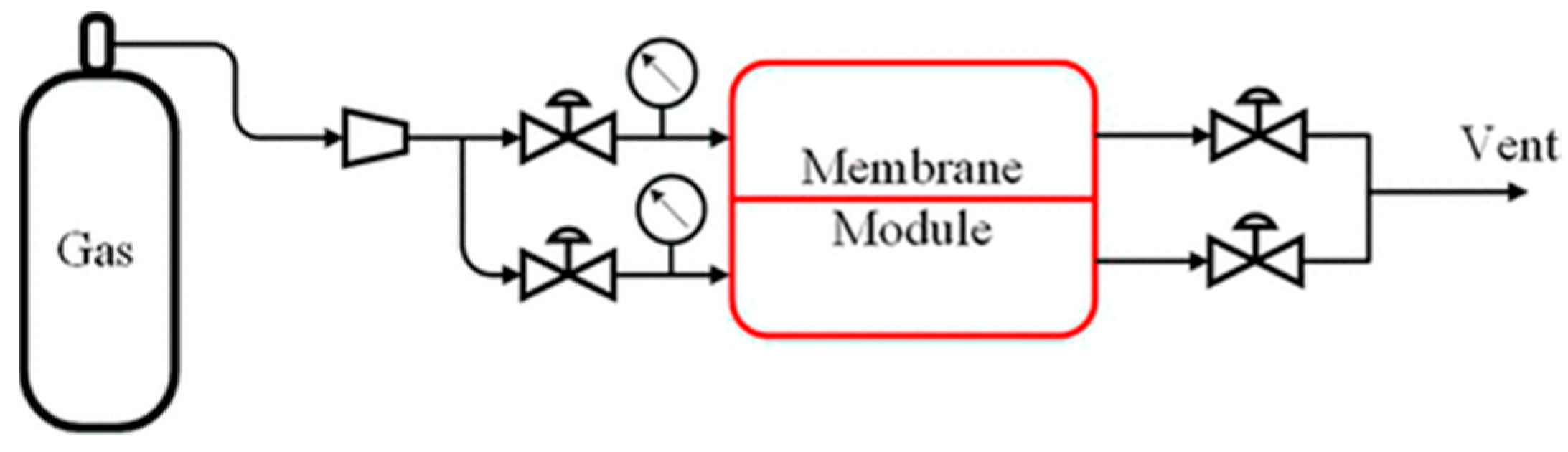
2.13. Gas Permeation Tests
3. Results and Discussion
3.1. PILs Identification and Properties
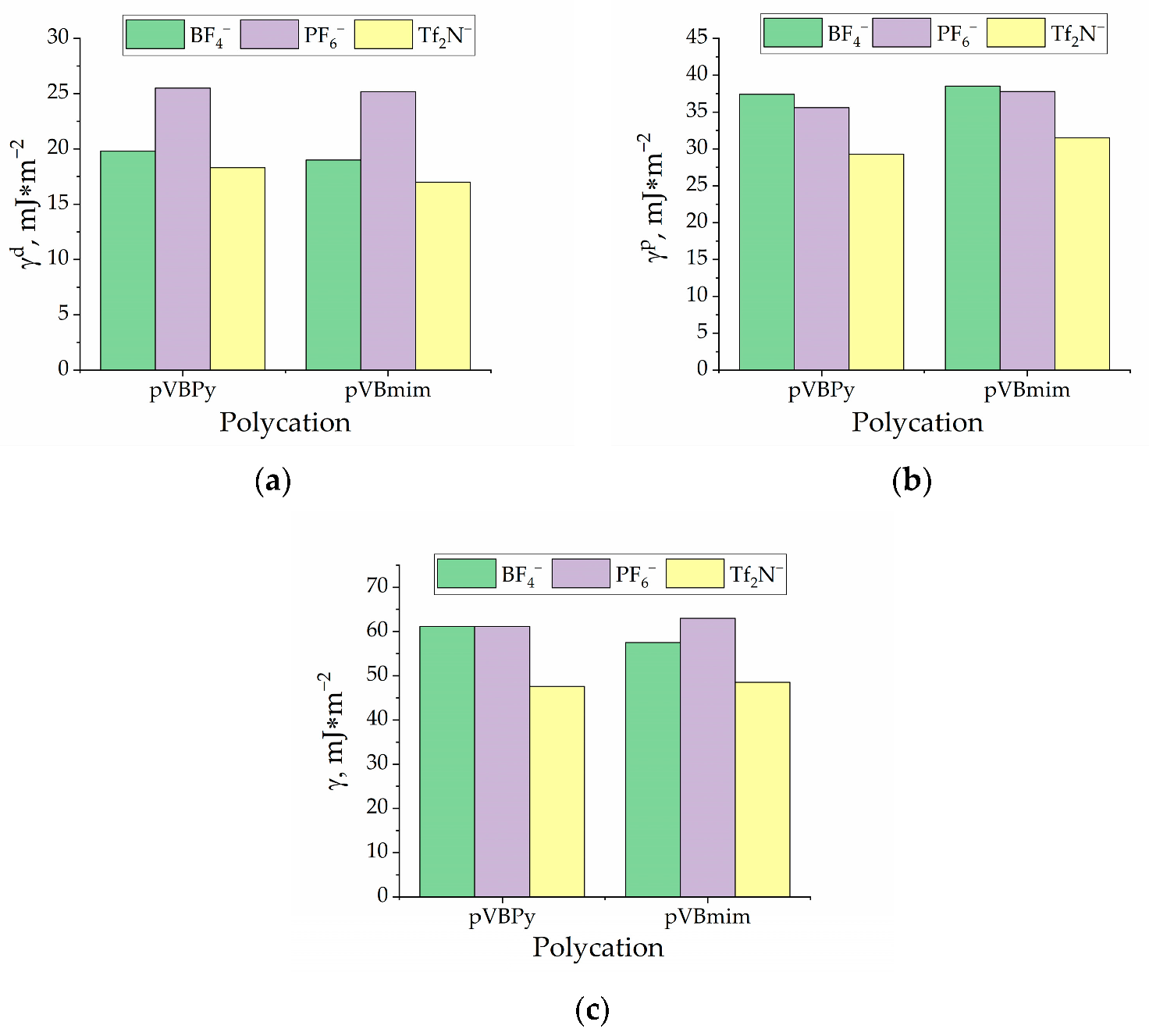
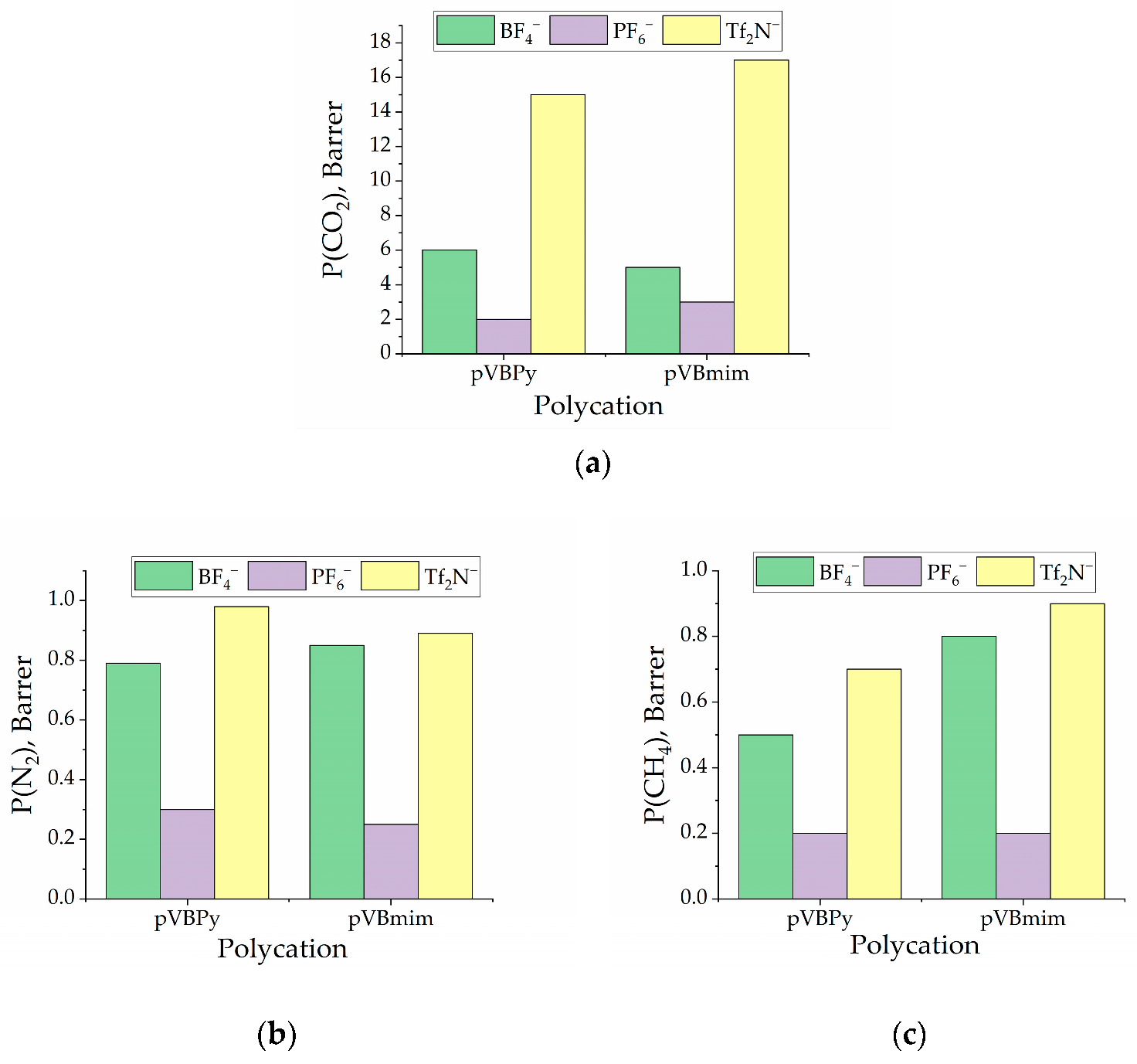
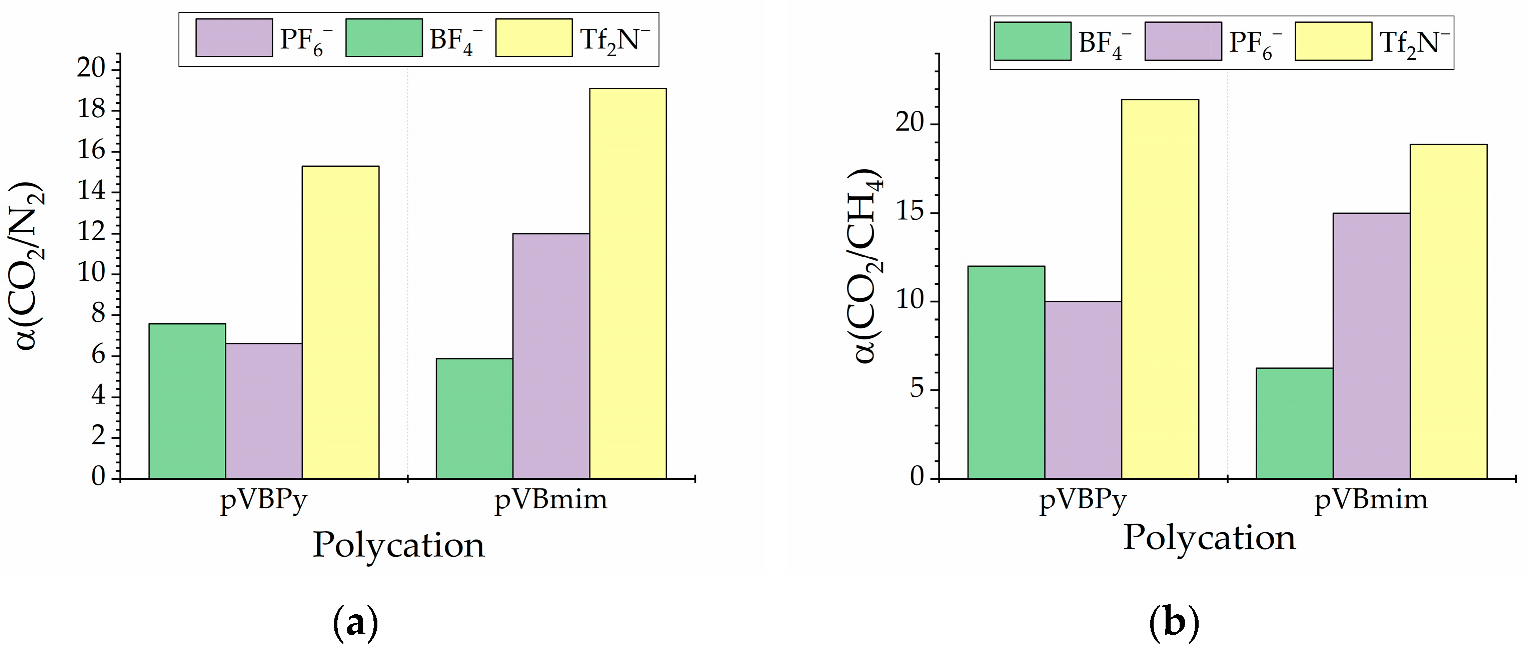
3.2. Membrane Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.T.; Wang, K.; Sueyoshi, T.; Wang, D.D. ESG: Research Progress and Future Prospects. Sustainability 2021, 13, 11663. [Google Scholar] [CrossRef]
- Martin-Gil, V.; Ahmad, M.Z.; Castro-Muñoz, R.; Fila, V. Economic Framework of Membrane Technologies for Natural Gas Applications. Sep. Purif. Rev. 2018, 48, 298–324. [Google Scholar] [CrossRef]
- Sazanova, T.S.; Otvagina, K.V.; Vorotyntsev, I.V. The Contributions of Supramolecular Organization to Mechanical Properties of Chitosan and Chitosan Copolymers with Synthetic Polymers According to Atomic Force Microscopy. Polym. Test. 2018, 68, 350–358. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Jia, Y. Membrane Technology: Past, Present and Future. In Membrane and Desalination Technologies; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–45. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- George, G.; Bhoria, N.; Alhallaq, S.; Abdala, A.; Mittal, V. Polymer Membranes for Acid Gas Removal from Natural Gas. Sep. Purif. Technol. 2016, 158, 333–356. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Polymeric Membranes for CO2 Separation and Capture. J. Membr. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Friess, K.; Izák, P.; Kárászová, M.; Pasichnyk, M.; Lanč, M.; Nikolaeva, D.; Luis, P.; Jansen, J.C. A Review on Ionic Liquid Gas Separation Membranes. Membranes 2021, 11, 97. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green Processing Using Ionic Liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Kazarina, O.V.; Agieienko, V.N.; Nagrimanov, R.N.; Atlaskina, M.E.; Petukhov, A.N.; Moskvichev, A.A.; Nyuchev, A.V.; Barykin, A.V.; Vorotyntsev, I.V. A Rational Synthetic Approach for Producing Quaternary Ammonium Halides and Physical Properties of the Room Temperature Ionic Liquids Obtained by This Way. J. Mol. Liq. 2021, 344, 117925. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Yanbikov, N.R.; Atlaskin, A.A.; Trubyanov, M.M.; Mechergui, A.; Otvagina, K.V.; Razov, E.N.; Mochalova, A.E.; Vorotyntsev, I.V. Acidic Gases Separation from Gas Mixtures on the Supported Ionic Liquid Membranes Providing the Facilitated and Solution-Diffusion Transport Mechanisms. Membranes 2019, 9, 9. [Google Scholar] [CrossRef]
- Akhmetshina, A.A.; Davletbaeva, I.M.; Grebenschikova, E.S.; Sazanova, T.S.; Petukhov, A.N.; Atlaskin, A.A.; Razov, E.N.; Zaripov, I.I.; Martins, C.F.; Neves, L.A.; et al. The Effect of Microporous Polymeric Support Modification on Surface and Gas Transport Properties of Supported Ionic Liquid Membranes. Membranes 2015, 6, 4. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Gumerova, O.R.; Atlaskin, A.A.; Petukhov, A.N.; Sazanova, T.S.; Yanbikov, N.R.; Nyuchev, A.V.; Razov, E.N.; Vorotyntsev, I.V. Permeability and Selectivity of Acid Gases in Supported Conventional and Novel Imidazolium-Based Ionic Liquid Membranes. Sep. Purif. Technol. 2017, 176, 92–106. [Google Scholar] [CrossRef]
- Mechergui, A.; Akhmetshina, A.I.; Kazarina, O.V.; Atlaskina, M.E.; Petukhov, A.N.; Vorotyntsev, I.V. Acidic Gases Solubility in Bis(2-Ethylhexyl) Sulfosuccinate Based Ionic Liquids Using the Predictive Thermodynamic Model. Membranes 2020, 10, 429. [Google Scholar] [CrossRef]
- Sazanova, T.S.; Akhmetshina, A.I.; Petukhov, A.N.; Vorotyntsev, A.V.; Suvorov, S.S.; Barysheva, A.V.; Mechergui, A.; Nyuchev, A.V.; Kazarina, O.V.; Stepakova, A.N.; et al. The Cation Effect on the Free Volume and the Solubility of H2S and CO2 in Ionic Liquids Based on Bis(2-Ethylhexyl) Sulfosuccinate Anion. Membranes 2023, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Atlaskin, A.A.; Kryuchkov, S.S.; Smorodin, K.A.; Markov, A.N.; Kazarina, O.V.; Zarubin, D.M.; Atlaskina, M.E.; Vorotyntsev, A.V.; Nyuchev, A.V.; Petukhov, A.N.; et al. Towards the Potential of Trihexyltetradecylphosphonium Indazolide with Aprotic Heterocyclic Ionic Liquid as an Efficient Absorbent for Membrane-Assisted Gas Absorption Technique for Acid Gas Removal Applications. Sep. Purif. Technol. 2021, 257, 117835. [Google Scholar] [CrossRef]
- Otvagina, K.V.; Mochalova, A.E.; Sazanova, T.S.; Petukhov, A.N.; Moskvichev, A.A.; Vorotyntsev, A.V.; Afonso, C.A.M.; Vorotyntsev, I.V. Preparation and Characterization of Facilitated Transport Membranes Composed of Chitosan-Styrene and Chitosan-Acrylonitrile Copolymers Modified by Methylimidazolium Based Ionic Liquids for CO2 Separation from CH4 and N2. Membranes 2016, 6, 31. [Google Scholar] [CrossRef]
- Duczinski, R.; Bernard, F.; Rojas, M.; Duarte, E.; Chaban, V.; Vecchia, F.D.; Menezes, S.; Einloft, S. Waste Derived MCMRH- Supported IL for CO2/CH4 Separation. J. Nat. Gas Sci. Eng. 2018, 54, 54–64. [Google Scholar] [CrossRef]
- Nisar, M.; Bernard, F.L.; Duarte, E.; Chaban, V.V.; Einloft, S. New Polysulfone Microcapsules Containing Metal Oxides and ([BMIM][NTf2]) Ionic Liquid for CO2 Capture. J. Environ. Chem. Eng. 2021, 9, 104781. [Google Scholar] [CrossRef]
- Solangi, N.H.; Anjum, A.; Tanjung, F.A.; Mazari, S.A.; Mubarak, N.M. A Review of Recent Trends and Emerging Perspectives of Ionic Liquid Membranes for CO2 Separation. J. Environ. Chem. Eng. 2021, 9, 105860. [Google Scholar] [CrossRef]
- Zhou, X.; Weber, J.; Yuan, J. Poly(Ionic Liquid)s: Platform for CO2 Capture and Catalysis. Curr. Opin. Green Sustain. Chem. 2019, 16, 39–46. [Google Scholar] [CrossRef]
- Zunita, M.; Hastuti, R.; Alamsyah, A.; Kadja, G.T.M.; Khoiruddin, K.; Kurnia, K.A.; Yuliarto, B.; Wenten, I.G. Polyionic Liquid Membrane: Recent Development and Perspective. J. Ind. Eng. Chem. 2022, 113, 96–123. [Google Scholar] [CrossRef]
- Durga, G.; Kalra, P.; Kumar Verma, V.; Wangdi, K.; Mishra, A. Ionic Liquids: From a Solvent for Polymeric Reactions to the Monomers for Poly(Ionic Liquids). J. Mol. Liq. 2021, 335, 116540. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(Ionic Liquid) Latexes Prepared by Dispersion Polymerization of Ionic Liquid Monomers. Macromolecules 2011, 44, 744–750. [Google Scholar] [CrossRef]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and Performance of Polymerizable Room-Temperature Ionic Liquids as Gas Separation Membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gouveia, A.S.L.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric Ionic Liquid-Based Membranes: Influence of Polycation Variation on Gas Transport and CO2 Selectivity Properties. J. Membr. Sci. 2015, 486, 40–48. [Google Scholar] [CrossRef]
- Atlaskina, M.E.; Kazarina, O.V.; Mochalova, A.E.; Vorotyntsev, I.V. Synthesis of Monomeric Ionic Liquids Based on 4-Vinylbenzyl Chloride as Precursors of a Material for the Selective Layer of Gas Separation Membranes. Membr. Membr. Technol. 2021, 3, 36–42. [Google Scholar] [CrossRef]
- Bernard, F.L.; Rodrigues, D.M.; Polesso, B.B.; Chaban, V.V.; Seferin, M.; Vecchia, F.D.; Einloft, S. Development of inexpensive cellulose-based sorbents for carbon dioxide. Braz. J. Chem. Eng. 2019, 36, 511–521. [Google Scholar] [CrossRef]
- Tomé, L.C.; Aboudzadeh, M.A.; Rebelo, L.P.N.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric Ionic Liquids with Mixtures of Counter-Anions: A New Straightforward Strategy for Designing Pyrrolidinium-Based CO2 Separation Membranes. J. Mater. Chem. A 2013, 1, 10403–10411. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Morozova, S.M.; Lozinskaya, E.I.; Vlasov, P.S.; Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M.; Vygodskii, Y.S. Turning into Poly(Ionic Liquid)s as a Tool for Polyimide Modification: Synthesis, Characterization and CO2 Separation Properties. Polym. Chem. 2016, 7, 580–591. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Pyrrolidinium-Based Polymeric Ionic Liquid Materials: New Perspectives for CO2 Separation Membranes. J. Memb. Sci. 2013, 428, 260–266. [Google Scholar] [CrossRef]
- Tomé, L.C.; Isik, M.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Novel Pyrrolidinium-Based Polymeric Ionic Liquids with Cyano Counter-Anions: High Performance Membrane Materials for Post-Combustion CO2 Separation. J. Membr. Sci. 2015, 483, 155–165. [Google Scholar] [CrossRef]
- Camper, D.; Bara, J.; Koval, C.; Noble, R. Bulk-Fluid Solubility and Membrane Feasibility of Rmim-Based Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2006, 45, 6279–6283. [Google Scholar] [CrossRef]
- Ravula, S.; O’harra, K.E.; Watson, K.A.; Bara, J.E. Poly(Ionic Liquid)s with Dicationic Pendants as Gas Separation Membranes. Membranes 2022, 12, 264. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Ventaja, L.; Tomé, L.C.; Marrucho, I.M. Towards Biohydrogen Separation Using Poly(Ionic Liquid)/Ionic Liquid Composite Membranes. Membranes 2018, 8, 124. [Google Scholar] [CrossRef]
- Mazzei, I.R.; Nikolaeva, D.; Fuoco, A.; Loïs, S.; Fantini, S.; Monteleone, M.; Esposito, E.; Ashtiani, S.J.; Lanč, M.; Vopička, O.; et al. Poly[3-Ethyl-1-Vinyl-Imidazolium] Diethyl Phosphate/Pebax® 1657 Composite Membranes and Their Gas Separation Performance. Membranes 2020, 10, 224. [Google Scholar] [CrossRef]
- Morozova, S.M.; Lozinskaya, E.I.; Sardon, H.; Suárez-García, F.; Vlasov, P.S.; Vaudemont, R.; Vygodskii, Y.S.; Shaplov, A.S. Ionic Polyureas—A Novel Subclass of Poly(Ionic Liquid)s for CO2 Capture. Membranes 2020, 10, 240. [Google Scholar] [CrossRef]
- Teodoro, R.M.; Tomé, L.C.; Mantione, D.; Mecerreyes, D.; Marrucho, I.M. Mixing Poly(Ionic Liquid)s and Ionic Liquids with Different Cyano Anions: Membrane Forming Ability and CO2/N2 Separation Properties. J. Membr. Sci. 2018, 552, 341–348. [Google Scholar] [CrossRef]
- Cowan, M.G.; Gin, D.L.; Noble, R.D. Poly(Ionic Liquid)/Ionic Liquid Ion-Gels with High “Free” Ionic Liquid Content: Platform Membrane Materials for CO2/Light Gas Separations. Acc. Chem. Res. 2016, 49, 724–732. [Google Scholar] [CrossRef]
- Tomé, L.C.; Guerreiro, D.C.; Teodoro, R.M.; Alves, V.D.; Marrucho, I.M. Effect of Polymer Molecular Weight on the Physical Properties and CO2/N2 Separation of Pyrrolidinium-Based Poly(Ionic Liquid) Membranes. J. Membr. Sci. 2018, 549, 267–274. [Google Scholar] [CrossRef]
- Ogihara, W.; Washiro, S.; Nakajima, H.; Ohno, H. Effect of Cation Structure on the Electrochemical and Thermal Properties of Ion Conductive Polymers Obtained from Polymerizable Ionic Liquids. Electrochim. Acta 2006, 51, 2614–2619. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Kumbharkar, S.C.; Kharul, U.K. Polymeric Ionic Liquids (PILs): Effect of Anion Variation on Their CO2 Sorption. J. Memb. Sci. 2012, 389, 305–315. [Google Scholar] [CrossRef]
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The Design of Polymeric Ionic Liquids for the Preparation of Functional Materials. Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Bara, J.E.; Gabriel, C.J.; Hatakeyama, E.S.; Carlisle, T.K.; Lessmann, S.; Noble, R.D.; Gin, D.L. Improving CO2 Selectivity in Polymerized Room-Temperature Ionic Liquid Gas Separation Membranes through Incorporation of Polar Substituents. J. Membr. Sci. 2008, 321, 3–7. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sun, W.; Plancher, H.; Radosz, M.; Shen, Y. Poly(Ionic Liquid)s: A New Material with Enhanced and Fast CO2 Absorption. Chem. Commun. 2005, 3325–3327. [Google Scholar] [CrossRef]
- Carlisle, T.K.; Wiesenauer, E.F.; Nicodemus, G.D.; Gin, D.L.; Noble, R.D. Ideal CO2/Light Gas Separation Performance of Poly(Vinylimidazolium) Membranes and Poly(Vinylimidazolium)-Ionic Liquid Composite Films. Ind. Eng. Chem. Res. 2013, 52, 1023–1032. [Google Scholar] [CrossRef]
- Tang, J.; Shen, Y.; Radosz, M.; Sun, W. Isothermal Carbon Dioxide Sorption in Poly(Ionic Liquid)S. Ind. Eng. Chem. Res. 2009, 48, 9113–9118. [Google Scholar] [CrossRef]
- Tang, H.; Tang, J.; Ding, S.; Radosz, M.; Shen, Y. Atom Transfer Radical Polymerization of Styrenic Ionic Liquid Monomers and Carbon Dioxide Absorption of the Polymerized Ionic Liquids. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1432–1443. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Sheridan, E.; Sandru, M.; Genua, A.; Tanczyk, M.; Jaschik, M.; Warmuzinski, K.; Jansen, J.C.; Vankelecom, I.F.J. Poly(Vinylbenzyl Chloride)-Based Poly(Ionic Liquids) as Membranes for CO2 Capture from Flue Gas. J. Mater. Chem. A 2017, 5, 19808–19818. [Google Scholar] [CrossRef]
- Atlaskina, M.E.; Atlaskin, A.A.; Kazarina, O.V.; Petukhov, A.N.; Zarubin, D.M.; Nyuchev, A.V.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Synthesis and Comprehensive Study of Quaternary-Ammonium-Based Sorbents for Natural Gas Sweetening. Environments 2021, 8, 134. [Google Scholar] [CrossRef]
- Andreev, G.A.; Hartmanoá, M. Flotation Method of Precise Density Measurements. Phys. Status Solidi 1989, 116, 457–468. [Google Scholar] [CrossRef]
- Otvagina, K.V.; Penkova, A.V.; Dmitrenko, M.E.; Kuzminova, A.I.; Sazanova, T.S.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Novel Composite Membranes Based on Chitosan Copolymers with Polyacrylonitrile and Polystyrene: Physicochemical Properties and Application for Pervaporation Dehydration of Tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef]
- Sazanova, T.S.; Otvagina, K.V.; Kryuchkov, S.S.; Zarubin, D.M.; Fukina, D.G.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Revealing the Surface Effect on Gas Transport and Mechanical Properties in Nonporous Polymeric Membranes in Terms of Surface Free Energy. Langmuir 2020, 36, 12911–12921. [Google Scholar] [CrossRef]
- Navarro, P.; García, J.; Rodríguez, F.; Carvalho, P.J.; Coutinho, J.A.P. Impact of Water on the [C4C1im][Ac] Ability for the CO2/CH4 Separation. J. CO2 Util. 2019, 31, 115–123. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Xu, Y.; Zhang, Y.; Li, Y.; Wang, Y.; Wei, J.; Chu, T. Thermodynamic and Kinetic Effects of Quaternary Ammonium and Phosphonium Ionic Liquids on CO2Hydrate Formation. ACS Omega 2022, 8, 1191–1205. [Google Scholar] [CrossRef]
- Yeadon, D.J.; Jacquemin, J.; Plechkova, N.V.; Maréchal, M.; Seddon, K.R.; Yeadon, D.J.; Jacquemin, J.; Plechkova, N.V.; Seddon, K.R. Induced Protic Behaviour in Aprotonic Ionic Liquids by Anion Basicity for Efficient Carbon Dioxide Capture. ChemPhysChem 2020, 21, 1369–1374. [Google Scholar] [CrossRef]
- Zhao, W.; He, G.; Zhang, L.; Ju, J.; Dou, H.; Nie, F.; Li, C.; Liu, H. Effect of Water in Ionic Liquid on the Separation Performance of Supported Ionic Liquid Membrane for CO2/N2. J. Membr. Sci. 2010, 350, 279–285. [Google Scholar] [CrossRef]
- Neves, L.A.; Afonso, C.; Coelhoso, I.M.; Crespo, J.G. Integrated CO2 Capture and Enzymatic Bioconversion in Supported Ionic Liquid Membranes. Sep. Purif. Technol. 2012, 97, 34–41. [Google Scholar] [CrossRef]
- Iskra, A.; Gentleman, A.S.; Kartouzian, A.; Kent, M.J.; Sharp, A.P.; Mackenzie, S.R. Infrared Spectroscopy of Gas-Phase M+(CO2)n (M = Co, Rh, Ir) Ion-Molecule Complexes. J. Phys. Chem. A 2017, 121, 133–140. [Google Scholar] [CrossRef]
- Isokoski, K.; Poteet, C.A.; Linnartz, H. Highly Resolved Infrared Spectra of Pure CO2 Ice (15–75 K). Astron. Astrophys. 2013, 555, A85. [Google Scholar] [CrossRef]
- Bara, J.E.; Hatakeyama, E.S.; Gin, D.L.; Noble, R.D. Improving CO2 Permeability in Polymerized Room-Temperature Ionic Liquid Gas Separation Membranes through the Formation of a Solid Composite with a Room-Temperature Ionic Liquid. Polym. Adv. Technol. 2008, 19, 1415–1420. [Google Scholar] [CrossRef]
- Bogdanova, Y.G.; Dolzhikov, V.D. Relationship between Energy Characteristics of Surface of Polymeric Membranes and Their Transport Properties. Russ. J. Appl. Chem. 2018, 91, 1311–1321. [Google Scholar] [CrossRef]



| PIL Formula | P a (CO2), Barrer d | P (N2), Barrer | P (CH4), Barrer | A b (CO2/N2) | α (CO2/CH4) | References |
|---|---|---|---|---|---|---|
| p[VBimOEG1 *][Tf2N] | 16 ± 1 | 0.39 ± 0.02 | 0.48 ± 0.01 | 41 | 33 | [46] |
| P[VBimOEG2 *][Tf2N] | 22 ± 1 | 0.50 ± 0.01 | 0.74 ± 0.02 | 44 | 29 | |
| p[VBimC3CN][Tf2N] | 4.1 ± 0.1 | 0.11 ± 0.01 | 0.11 ± 0.01 | 37 | 37 | |
| p[VBimC5CN][Tf2N] | 8.2 ± 0.3 | 0.21 ± 0.01 | 0.28 ± 0.02 | 40 | 30 | |
| p[VB(CH2CH2O)2CH3im][Tf2N] | 22 ± 1 | _ | _ | 44 | 29 | |
| p[VBmim][Tf2N] | 9.2 ± 0.5 | 0.29 ± 0.01 | 0.24 ± 0.01 | 32 | 39 | [27] |
| p[VBbim][Tf2N] | 20 ± 1 | 0.67 ± 0.02 | 0.91 ± 0.06 | 30 | 22 | |
| p[VBC6im][Tf2N] | 32 ± 1 | 1.4 ± 0.1 | 2.3 ± 0.1 | 28 | 17 | |
| PIL Formula | c, GPU e | , GPU b | α ** (CO2/N2) | |||
| p[VBTMA][Tf2N] | 132.0 ± 44.0 GPU | 5.0 ± 2.0 GPU | _ | 27.0 ± 1.3 | _ | [51] |
| p[VBHEDMA][Tf2N] | 109.0 ± 0.5 GPU | 2.6 ± 0.5 GPU | _ | 41.6 ± 0.6 | _ | |
| p[VBMP][Tf2N] | 1334.0 ± 263.8 GPU | 78.0 ± 15.5 GPU | _ | 17.2 ± 0.1 | _ |
| Test Liquid | |||
|---|---|---|---|
| Water | 19.9 | 52.2 | 72.1 |
| Glycerol | 37.0 | 26.4 | 63.4 |
| Diiodomethane | 49.5 | 1.3 | 50.8 |
| Polymer | Molecular Weight | Polydispersity | |
|---|---|---|---|
| Mn, kDa | Mw, kDa | ||
| pVBCl | 131 | 228 | 2.5 |
| Polymer | FD, % | ExD, % | The Amount of IL Monomer Units per 1 g of PIL, mol/g |
|---|---|---|---|
| pVBPyCl | 91 | - | 0.0045 |
| pVBPyBF4 | - | 86.5 | 0.0030 |
| pVBPyPF6 | - | 88.5 | 0.0023 |
| pVBPyTf2N | - | 97 | 0.0025 |
| pVBmimCl | 85 | - | 0.0041 |
| pVBmimBF4 | - | 67.5 | 0.0022 |
| pVBmimPF6 | - | 67 | 0.0016 |
| pVBmimTf2N | - | 90.5 | 0.0021 |
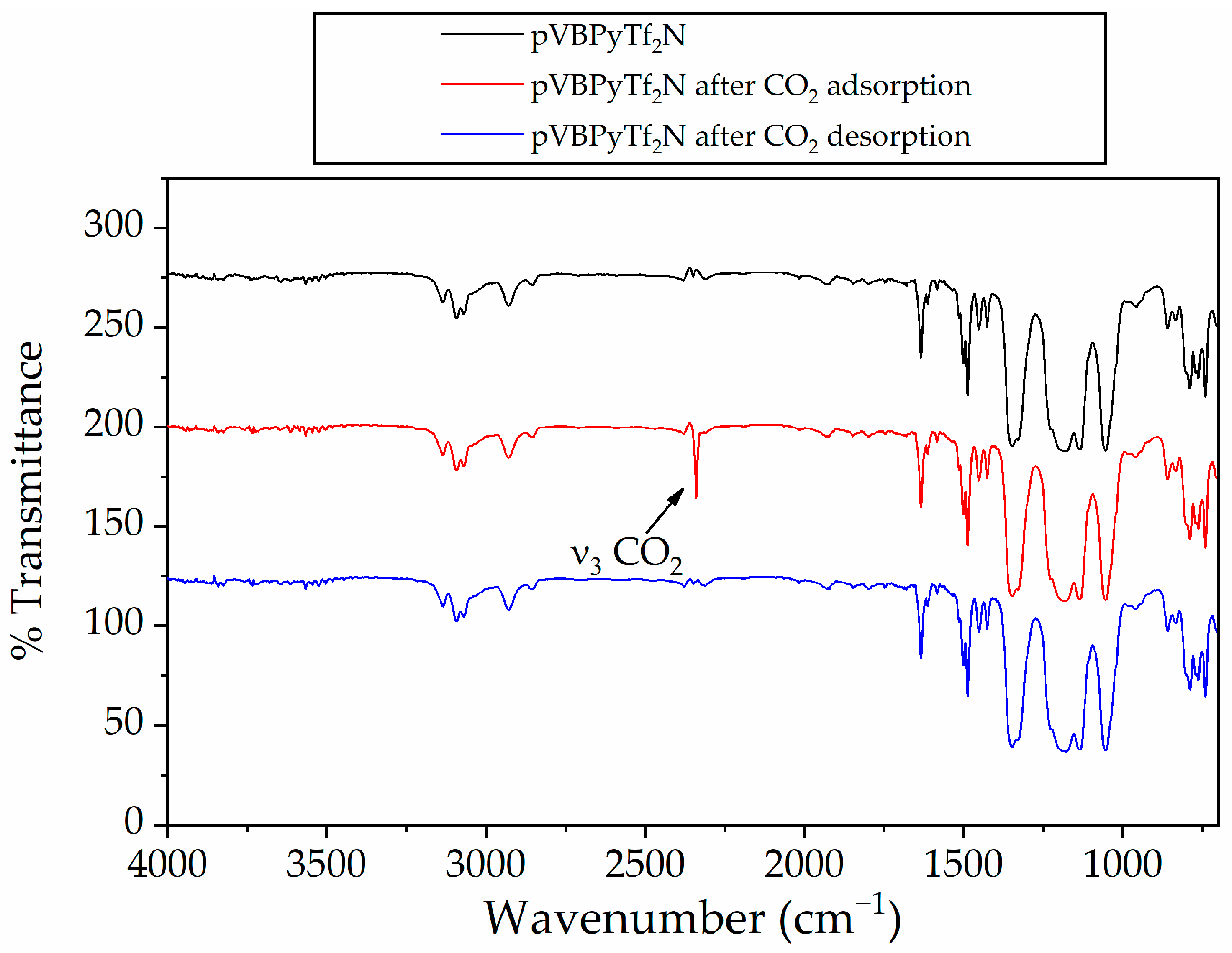
| Polymer | ν3 CO2, cm−1 |
|---|---|
| pVBmimCl | 2335 |
| pVBmimBF4 | 2339 |
| pVBmimPF6 | 2341 |
| pVBmimTf2N | 2339 |
| pVBPyCl | 2333 |
| pVBPyBF4 | 2339 |
| pVBPyPF6 | 2339 |
| pVBPyTf2N | 2339 |
| Reference | ν3 CO2, cm−1 |
| Gas [61] | 2349 |
| Crystalline solid, 77 K [62] | 2344 |
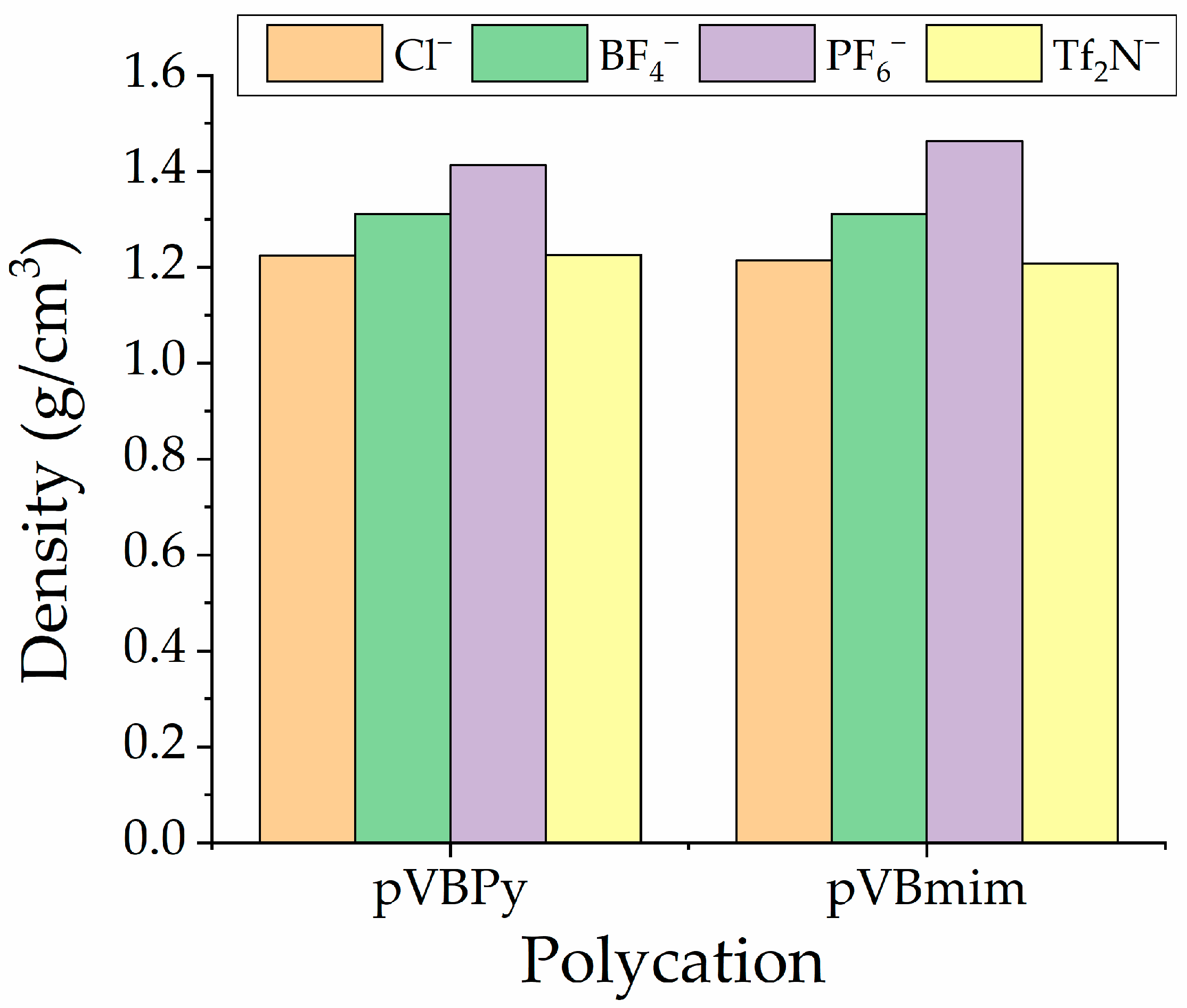
| PIL Name | Density, g/cm3 | Surface Free Energy, | P(N2), Barrer | P(CO2), Barrer | P(CH4), Barrer | α (CO2/N2) | α (CO2/CH4) | ||
|---|---|---|---|---|---|---|---|---|---|
| pVBmimCl | 1.2140 | - * | - | - | - | - | - | - | - |
| pVBmimBF4 | 1.3115 | 19 | 38.5 | 57.5 | 0.8 | 5 | 0.8 | 5.88 | 6.25 |
| pVBmimPF6 | 1.4635 | 25.2 | 37.8 | 62.9 | 0.2 | 3 | 0.2 | 12 | 15 |
| pVBmimTf2N | 1.2076 | 17 | 31.5 | 48.5 | 0.9 | 17 | 0.9 | 19.1 | 18.88 |
| pVBPyCl | 1.2240 | - | - | - | - | - | - | - | - |
| pVBPyBF4 | 1.3111 | 19.8 | 37.4 | 57.5 | 0.8 | 6 | 0.5 | 7.6 | 12 |
| pVBPyPF6 | 1.4132 | 25.5 | 35.6 | 62.9 | 0.3 | 2 | 0.2 | 6.6 | 10 |
| pVBPyTf2N | 1.2258 | 18.3 | 29.3 | 48.5 | 1 | 15 | 0.7 | 15.3 | 21.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otvagina, K.V.; Maslov, A.A.; Fukina, D.G.; Petukhov, A.N.; Malysheva, Y.B.; Vorotyntsev, A.V.; Sazanova, T.S.; Atlaskin, A.A.; Kapinos, A.A.; Barysheva, A.V.; et al. The Influence of Polycation and Counter-Anion Nature on the Properties of Poly(ionic liquid)-Based Membranes for CO2 Separation. Membranes 2023, 13, 539. https://doi.org/10.3390/membranes13060539
Otvagina KV, Maslov AA, Fukina DG, Petukhov AN, Malysheva YB, Vorotyntsev AV, Sazanova TS, Atlaskin AA, Kapinos AA, Barysheva AV, et al. The Influence of Polycation and Counter-Anion Nature on the Properties of Poly(ionic liquid)-Based Membranes for CO2 Separation. Membranes. 2023; 13(6):539. https://doi.org/10.3390/membranes13060539
Chicago/Turabian StyleOtvagina, Ksenia V., Alexey A. Maslov, Diana G. Fukina, Anton N. Petukhov, Yulia B. Malysheva, Andrey V. Vorotyntsev, Tatyana S. Sazanova, Artem A. Atlaskin, Alexander A. Kapinos, Alexandra V. Barysheva, and et al. 2023. "The Influence of Polycation and Counter-Anion Nature on the Properties of Poly(ionic liquid)-Based Membranes for CO2 Separation" Membranes 13, no. 6: 539. https://doi.org/10.3390/membranes13060539
APA StyleOtvagina, K. V., Maslov, A. A., Fukina, D. G., Petukhov, A. N., Malysheva, Y. B., Vorotyntsev, A. V., Sazanova, T. S., Atlaskin, A. A., Kapinos, A. A., Barysheva, A. V., Suvorov, S. S., Zanozin, I. D., Dokin, E. S., Vorotyntsev, I. V., & Kazarina, O. V. (2023). The Influence of Polycation and Counter-Anion Nature on the Properties of Poly(ionic liquid)-Based Membranes for CO2 Separation. Membranes, 13(6), 539. https://doi.org/10.3390/membranes13060539








