Magnesium Oxide Nanoparticles for the Adsorption of Pentavalent Arsenic from Water: Effects of Calcination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Calcination of Magnesium Oxide Nanoparticles
2.3. Characterization of MgO-650 °C Adsorbents
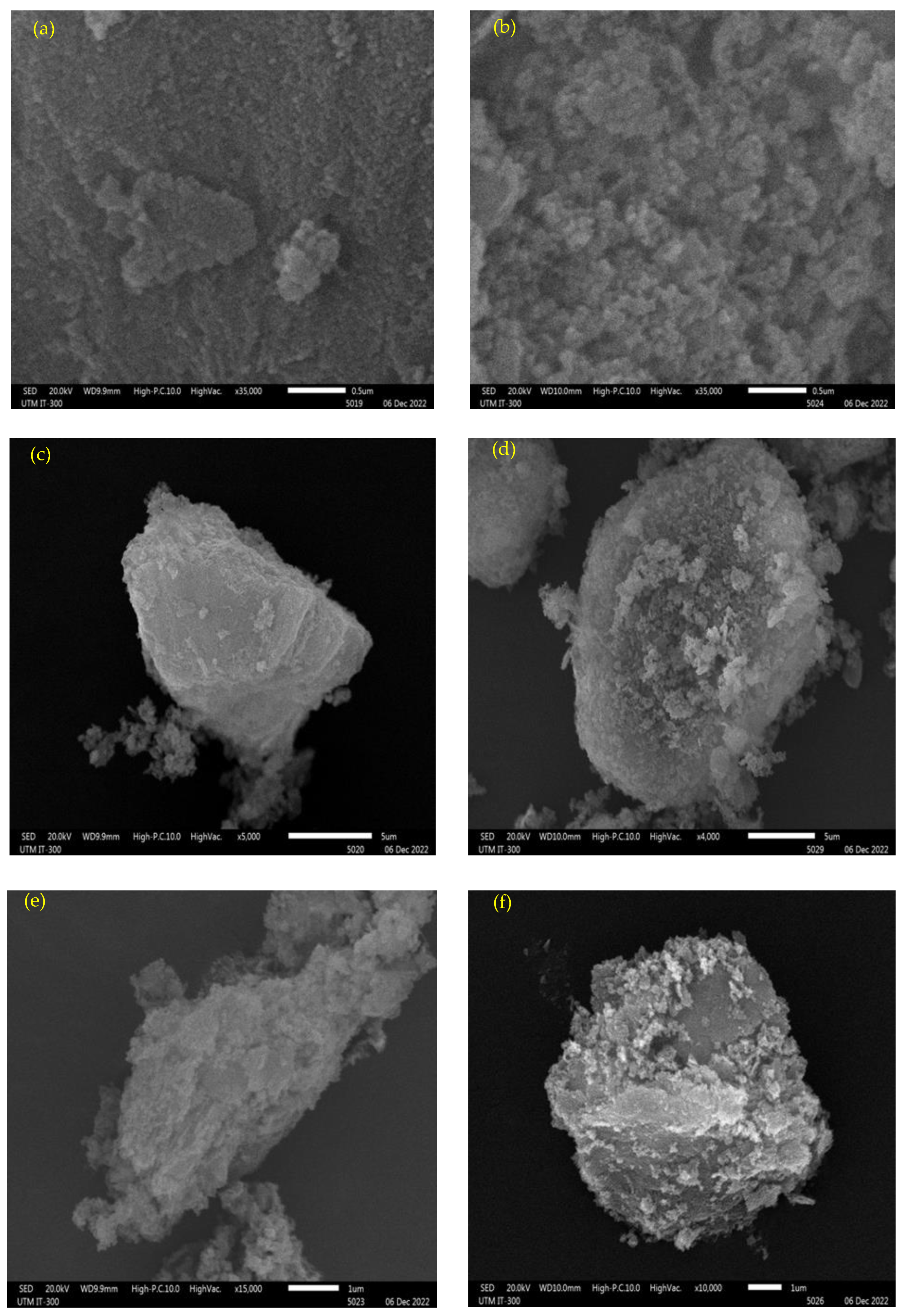
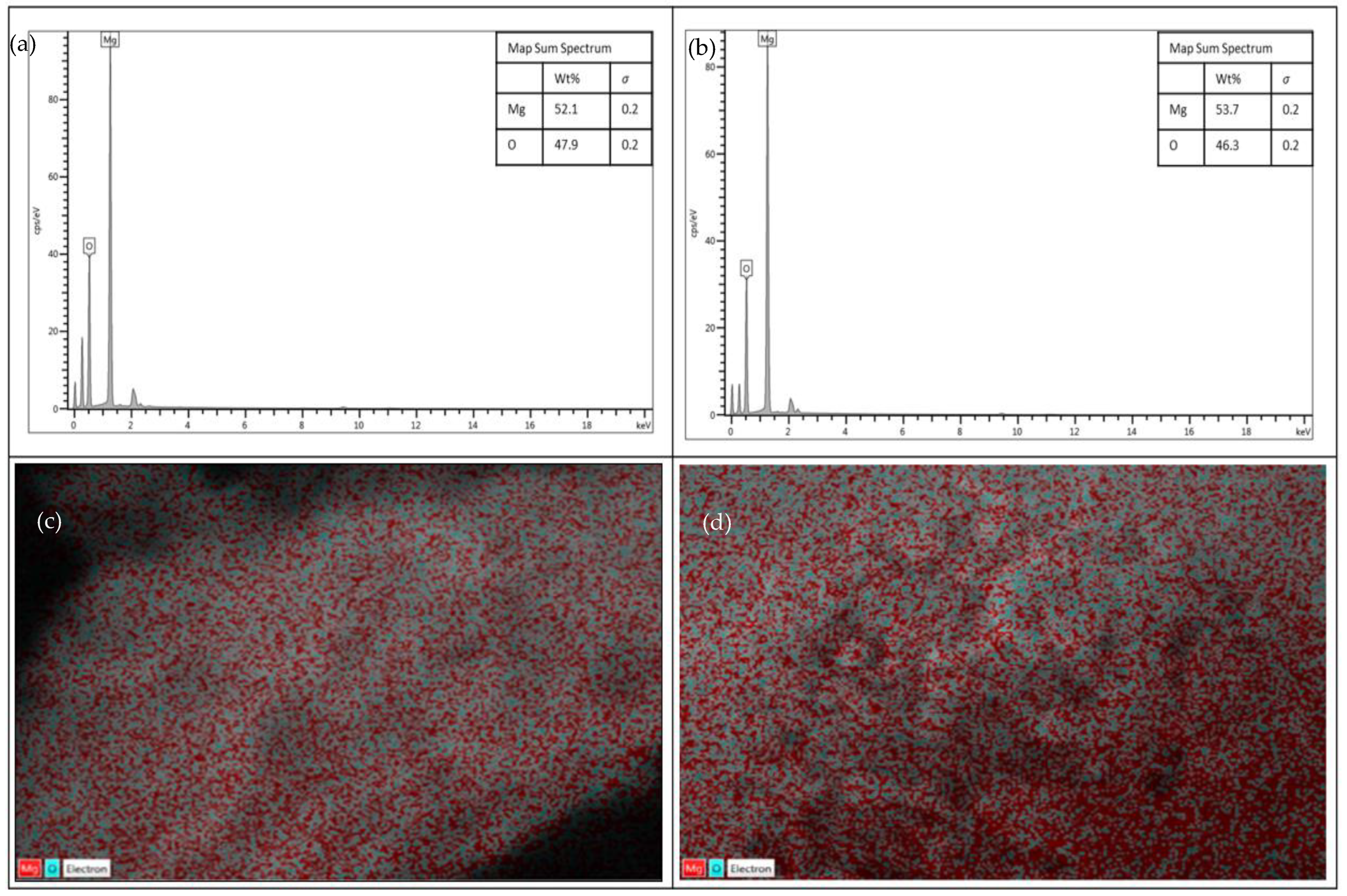
2.3.1. Morphology Analysis
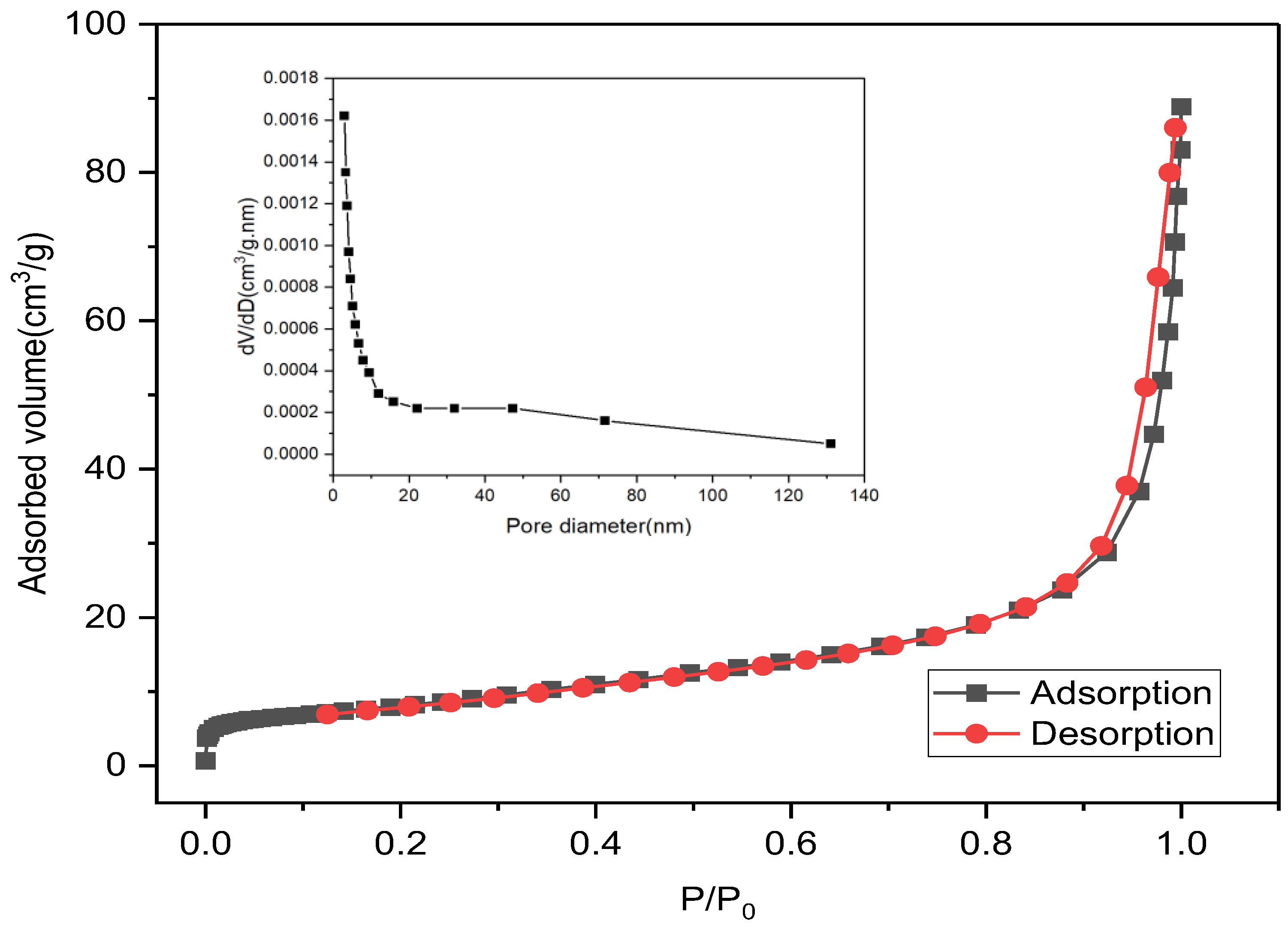
2.3.2. Measurement of Total Active Surface Area and Crystallite Size
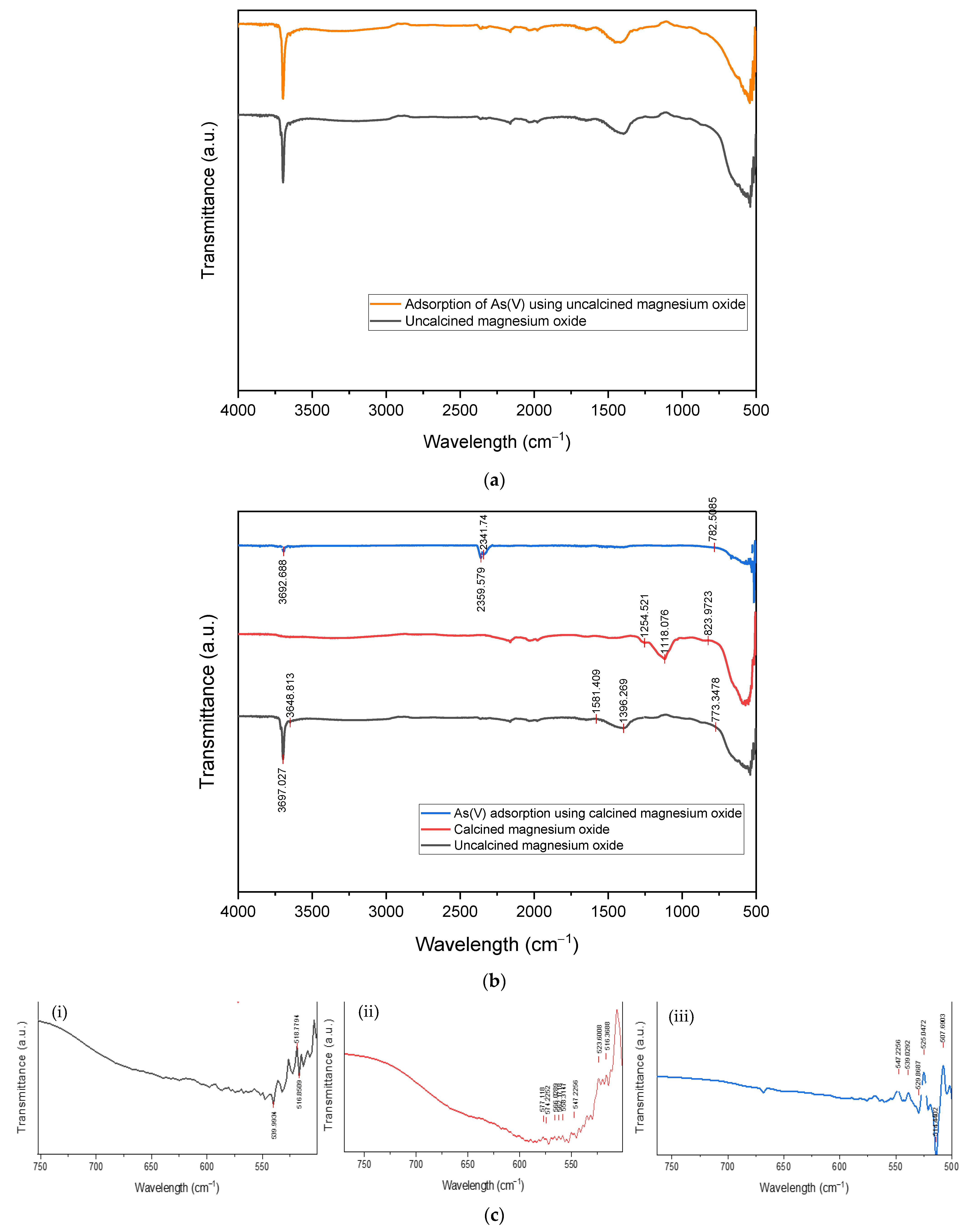
2.3.3. Detection of Functional Groups
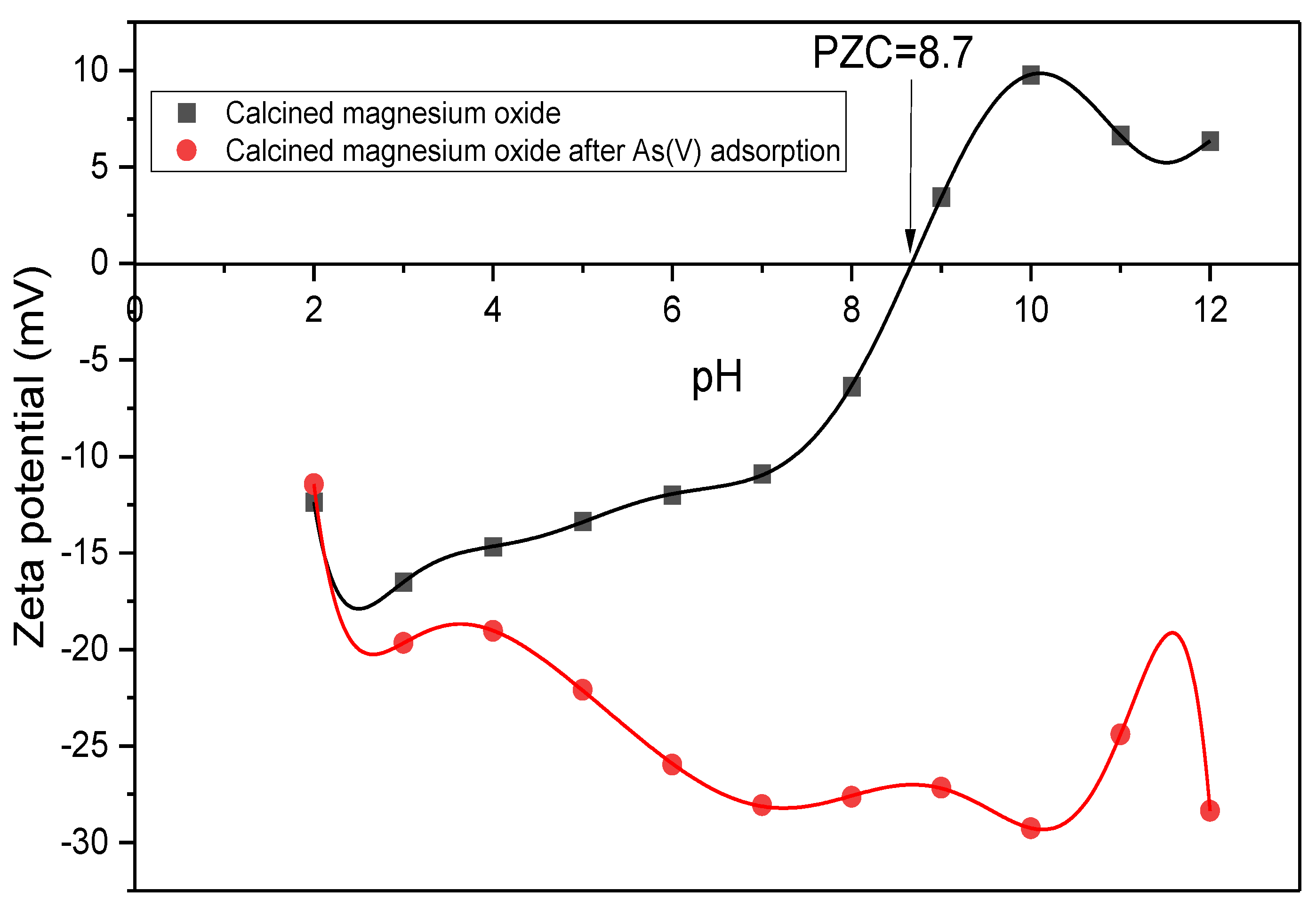
2.3.4. Zeta Potential Measurement
2.4. Batch Adsorption Experiments
2.4.1. Preparation of Arsenate Stock Solution
2.4.2. Measurement of Arsenate Concentration
2.4.3. Kinetics
2.4.4. Isotherm
2.4.5. PH Effect
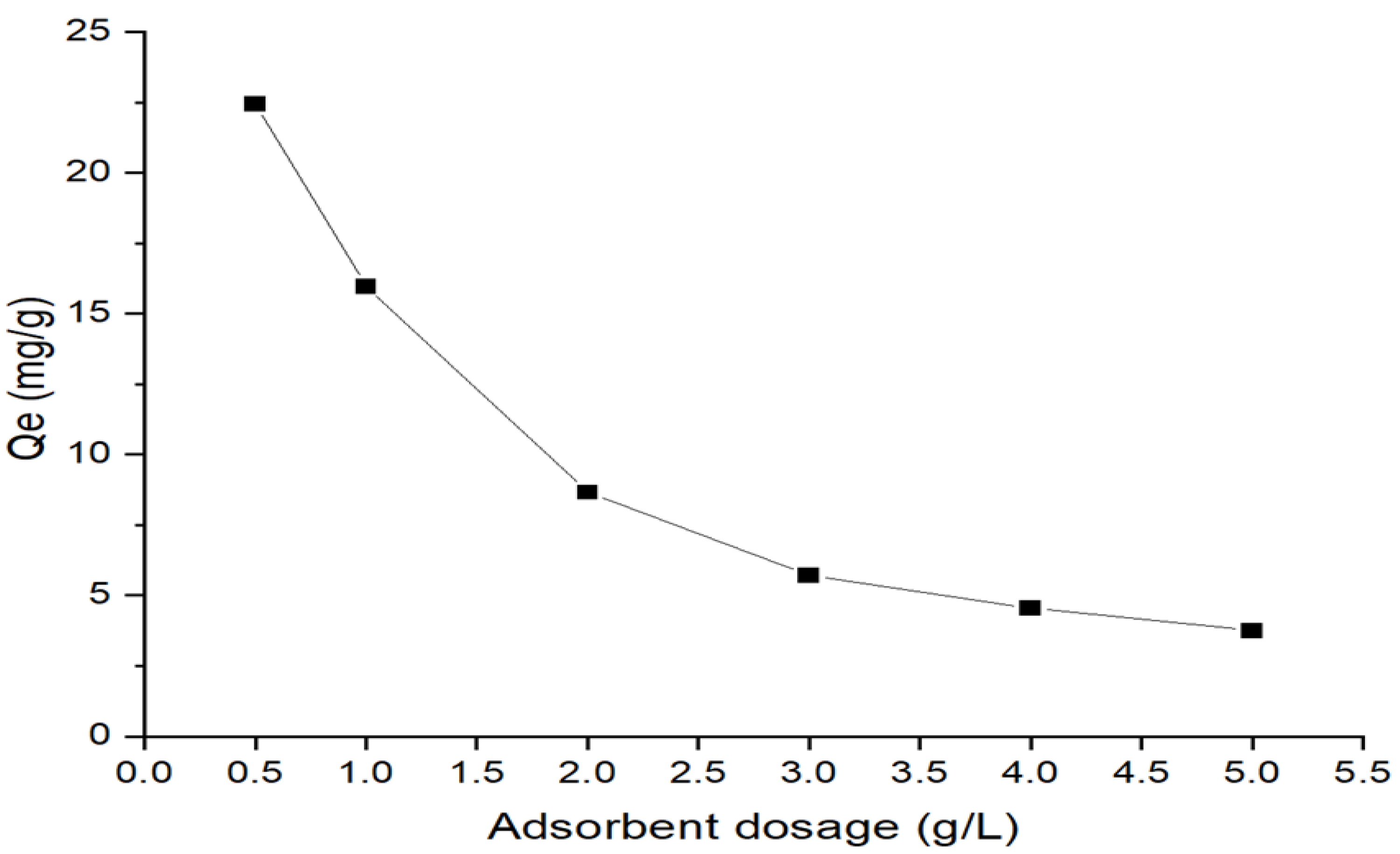
2.4.6. Adsorbent Dosage Effect
2.4.7. Data Analysis
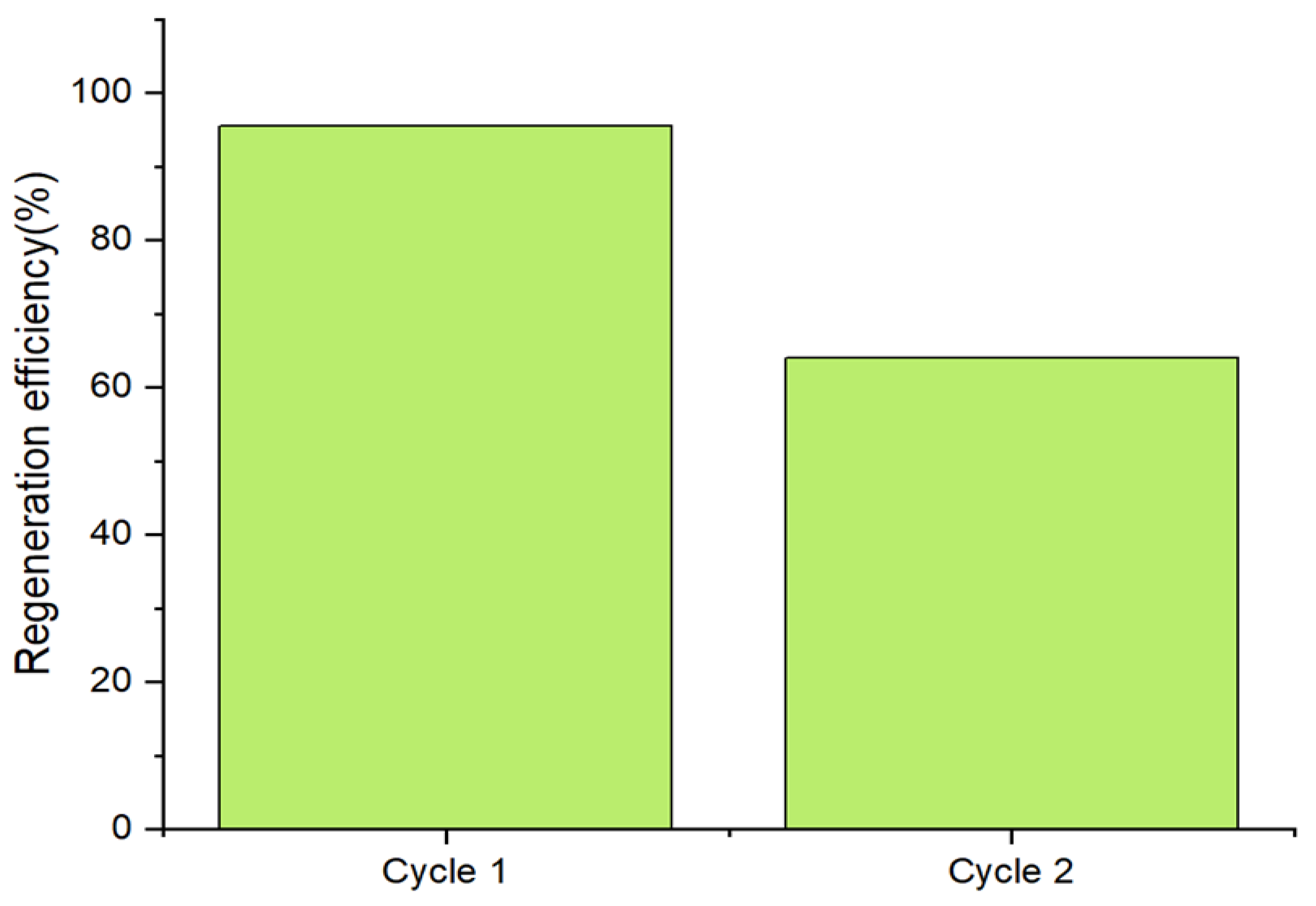
2.4.8. Regeneration Study of Calcined Magnesium Oxide Adsorbents
3. Results
3.1. Characterization of Calcined Magnesium Oxide Nanoparticles in Comparison with the Uncalcined
3.1.1. Surface Morphology and Textural Behavior
3.1.2. Surface Functionality
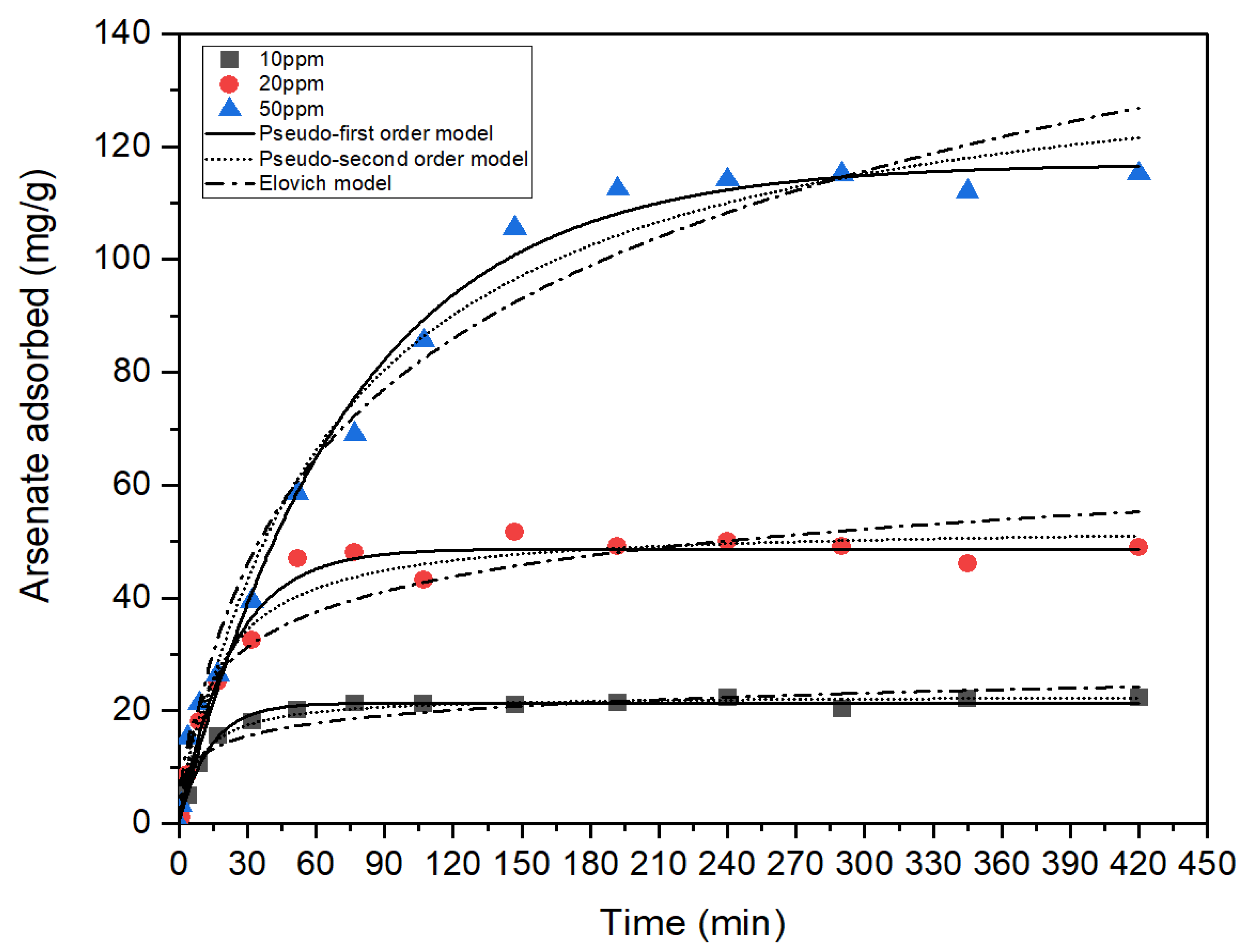
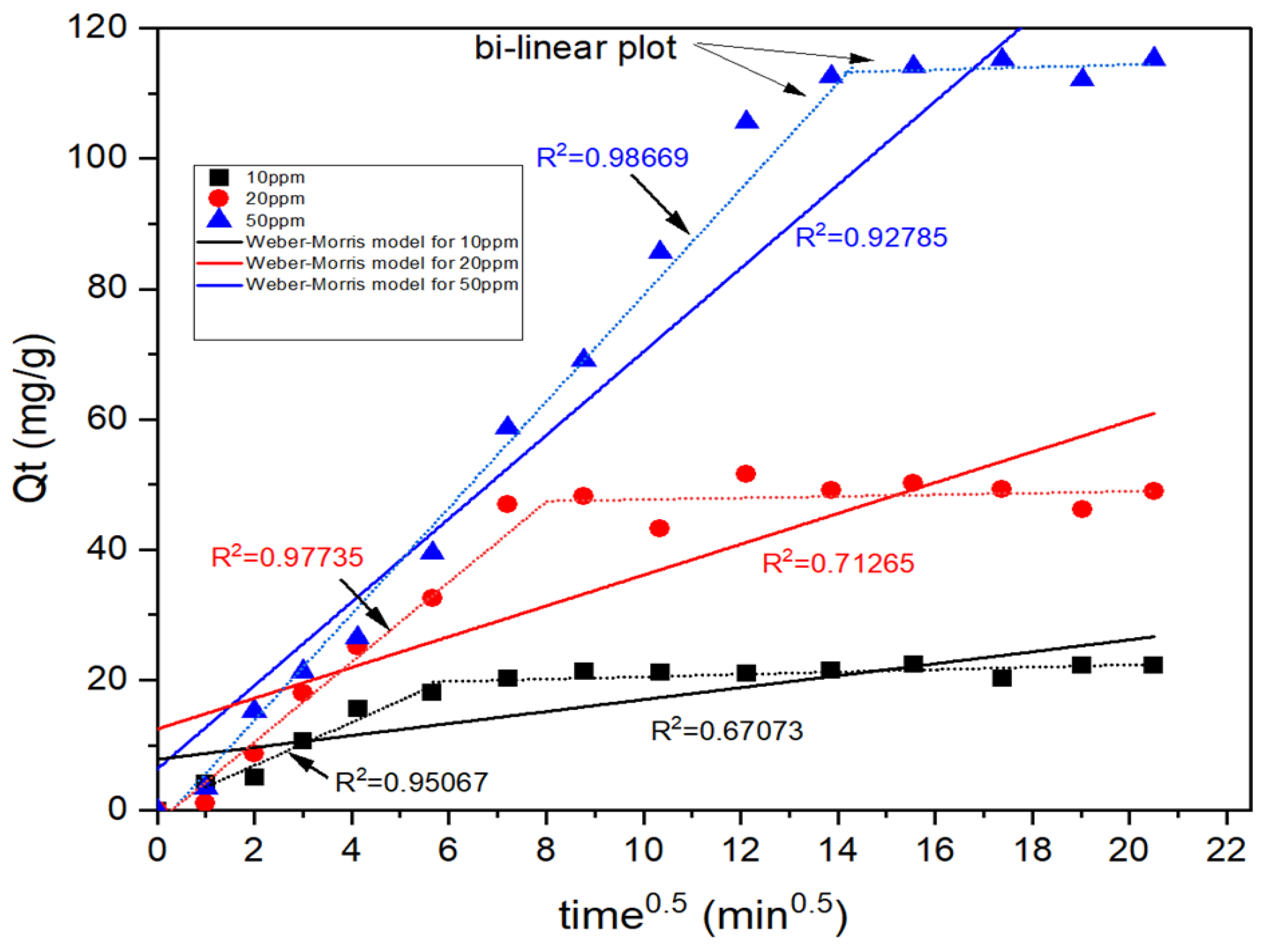
3.2. Adsorption Kinetics
3.3. Adsorption Isotherm
3.4. Effects of PH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagchi, S. Arsenic threat reaching global dimensions. CMAJ 2007, 177, 1344–1345. [Google Scholar] [CrossRef]
- Kulik-Kupka, K.; Koszowska, A.; Brończyk-Puzoń, A.; Nowak, J.; Gwizdek, K.; Zubelewicz-Szkodzińska, B. Arsenic—Poison or medicine? Med. Pract. 2016, 67, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.T.; Barr, R.; Browning, M.; Diao, Z.; Friedlander, E.; Harper, E.M.; Henly, C.; Kavlak, G.; Kwatra, S.; Jun, C.; et al. Criticality of the Geological Copper Family. Environ. Sci. Technol. 2012, 46, 1071–1078. [Google Scholar] [CrossRef]
- Mackenzie, F.T.; Lamtzy, R.J.; Petorson, V. Global trace metal cycle and predictions. J. Int. Assoc. Math. Geol. 1979, 6, 99–142. [Google Scholar] [CrossRef]
- Park, D.; Yang, H.; Jeong, J.; Ha, K.; Choi, S.; Kim, C.; Yoon, C.; Park, D.; Paek, D. A comprehensive review of arsenic levels in the semiconductor manufacturing industry. Ann. Occup. Hyg. 2010, 54, 869–879. [Google Scholar]
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259–284. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U.; Shikha. Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.L.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289–299. [Google Scholar] [CrossRef]
- Neppolian, B.; Celik, E.; Choi, H. Photochemical Oxidation of Arsenic(III) to Arsenic(V) using Peroxydisulfate Ions as an Oxidizing Agent. Environ. Sci. Technol. 2008, 42, 6179–6184. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I.; Jekel, M. Kinetics of Bacterial As(III) Oxidation and Subsequent As(V) Removal by Sorption onto Biogenic Manganese Oxides during Groundwater Treatment. Ind. Eng. Chem. Res. 2004, 43, 486–493. [Google Scholar] [CrossRef]
- Yang, H.; Sun, W.; Ge, H.; Yao, R. The oxidation of As(III) in groundwater using biological manganese removal filtration columns. Environ. Technol. 2015, 36, 2732–2739. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef]
- Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalination 2016, 7, 387–419. [Google Scholar]
- Genç, H.; Tjell, J.C.; McConchie, D.; Schuiling, O. Adsorption of arsenate from water using neutralized red mud. J. Colloid Interface Sci. 2003, 264, 327–334. [Google Scholar] [CrossRef]
- Farrell, J.; Chaudhary, B.K. Understanding Arsenate Reaction Kinetics with Ferric Hydroxides. Environ. Sci. Technol. 2013, 47, 8342–8347. [Google Scholar] [CrossRef]
- Gupta, A.; Chauhan, V.S.; Sankararamakrishnan, N. Preparation and evaluation of iron–chitosan composites for removal of As(III) and As(V) from arsenic contaminated real life groundwater. Water Res. 2009, 43, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.; Gao, S.; Shang, J.K. Exceptional As(III) Sorption Capacity by Highly Porous Magnesium Oxide Nanoflakes Made from Hydrothermal Synthesis. J. Am. Ceram. Soc. 2011, 94, 217–223. [Google Scholar] [CrossRef]
- Schiller, J.E.; Tallman, D.N.; Khalafalla, S.E. Mineral processing water treatment using magnesium oxide. Environ. Prog. 1984, 3, 136–141. [Google Scholar] [CrossRef]
- Hu, J.; Song, Z.; Chen, L.; Yang, H.; Li, J.; Richards, R. Adsorption Properties of MgO(111) Nanoplates for the Dye Pollutants from Wastewater. J. Chem. Eng. Data 2010, 55, 3742–3748. [Google Scholar] [CrossRef]
- Guo, L.; Lei, R.; Zhang, T.C.; Du, D.; Zhan, W. Insight into the role and mechanism of polysaccharide in polymorphous magnesium oxide nanoparticle synthesis for arsenate removal. Chemosphere 2022, 296, 133878. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, D.; Xia, F.; Tan, J.Z.Y.; Huang, P.-P.; Song, W.-G.; Nursam, N.M.; Caruso, R.A. Extremely high arsenic removal capacity for mesoporous aluminium magnesium oxide composites. Environ. Sci. Nano 2016, 3, 94–106. [Google Scholar] [CrossRef]
- Rodríguez-Flores, L.; Marmolejo, Y.; Perez, F.; Castañeda, A.; Sierra-Zenteno, A.; Flores, K.; Cadena, L. Adsorption of arsenic in dacitic tuff pretreated with magnesium oxide. Water Sci. Technol. Water Supply 2015, 15, 181. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Shang, H.; Wei, X.; Zhang, S.; Chen, J.; Ren, Z.J. Carbon transmission of CO2 activated nano-MgO carbon composites enhances phosphate immobilization. J. Mater. Chem. A 2018, 6, 3705–3713. [Google Scholar] [CrossRef]
- Sasaki, K.; Fukumoto, N.; Moriyama, S.; Yu, Q.; Hirajima, T. Chemical regeneration of magnesium oxide used as a sorbent for fluoride. Sep. Purif. Technol. 2012, 98, 24–30. [Google Scholar] [CrossRef]
- Fukuda, H.; Tsuchiya, K.; Toba, Y.; Eguchi, M.; Tokoro, C. Rapid boron removal from wastewater using low-crystalline magnesium oxide. J. Environ. Chem. Eng. 2020, 8, 104171. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Feng, X.; Han, X.; Bai, Z.; Zhang, Z. Calcination temperature-dependent surface structure and physicochemical properties of magnesium oxide. RSC Adv. 2015, 5, 86102–86112. [Google Scholar] [CrossRef]
- Johnson, M.; Wang, J.; Li, Z.; Lew, C.; Yan, Y. Effect of calcination and polycrystallinity on mechanical properties of nanoporous MFI zeolites. Mater. Sci. Eng. A 2007, 456, 58–63. [Google Scholar] [CrossRef]
- Díaz-Parralejo, A.; Macías-García, A.; Ortiz, A.L.; Cuerda-Correa, E.M. Effect of calcination temperature on the textural properties of 3mol% yttria-stabilized zirconia powders. J. Non-Cryst. Solids 2010, 356, 175–178. [Google Scholar] [CrossRef]
- José, N.; Ahmed, H.; Miguel, B.; Luís, E.; Jorge, B. Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review. Materials 2020, 13, 4752. [Google Scholar]
- Zhang, B.; Peng, J.; Zhang, L.; Ju, S. Optimization of Preparation for Magnesium Oxide by Calcination from Basic Magnesium Carbonate Using Response Surface Methodology. In Magnesium Technology 2012; Mathaudhu, S.N., Sillekens, W.H., Neelameggham, N.R., Hort, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 75–79. [Google Scholar]
- Mahmood, T.; Din, S.U.; Naeem, A.; Mustafa, S.; Waseem, M.; Hamayun, M. Adsorption of arsenate from aqueous solution on binary mixed oxide of iron and silicon. Chem. Eng. J. 2012, 192, 90–98. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Rane, V.H.; Gadre, R.V. Influence of Precursors Used in Preparation of MgO on Its Surface Properties and Catalytic Activity in Oxidative Coupling of Methane. J. Catal. 1994, 145, 300–311. [Google Scholar] [CrossRef]
- Gravogl, G.; Knoll, C.; Welch, J.; Artner, W.; Freiberger, N.; Nilica, R.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Werner, A.; et al. Cycle Stability and Hydration Behavior of Magnesium Oxide and Its Dependence on the Precursor-Related Particle Morphology. Nanomaterials 2018, 8, 795. [Google Scholar] [CrossRef]
- Eubank, W.R. Calcination Studies of Magnesium Oxides. J. Am. Ceram. Soc. 1951, 34, 225–229. [Google Scholar] [CrossRef]
- Baig, S.A.; Sheng, T.; Sun, C.; Xue, X.; Tan, L.; Xu, X. Arsenic Removal from Aqueous Solutions Using Fe3O4-HBC Composite: Effect of Calcination on Adsorbents Performance. PLoS ONE 2014, 9, e100704. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Andra, S.; Kommineni, N.; Balu, S.K.; Bulusu, R.; Boseila, A.A.; Akamo, D.O.; Ahmad, Z.; Khan, F.S.; Rahman, M.H.; et al. Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials 2022, 15, 5392. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Jin, J.; Fu, J.; Yang, M.; Li, F. Anchoring Al- and/or Mg-oxides to magnetic biochars for Co-uptake of arsenate and fluoride from water. J. Environ. Manag. 2021, 293, 112898. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Hwang, M.-J.; Shim, W.G. Synthesis of Mg–Al layered double hydroxides-functionalized hydrochar composite via an in situ one-pot hydrothermal method for arsenate and phosphate removal: Structural characterization and adsorption performance. Chem. Eng. J. 2021, 420, 129775. [Google Scholar] [CrossRef]
- Li, R.; Zhan, W.; Song, Y.; Lan, J.; Guo, L.; Zhang, T.C.; Du, D. Template-free synthesis of an eco-friendly flower-like Mg/Al/Fe-CLDH for efficient arsenate removal from aqueous solutions. Sep. Purif. Technol. 2022, 282, 120011. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Zheng, B.; Zhang, Z.; Yang, L.; Yang, X. Fabrication of Mg doped magnetite nanoparticles by recycling of titanium slag and their application of arsenate adsorption. J. Clean. Prod. 2020, 252, 119599. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Chen, R.; Yang, C.; Xiang, L.; Yi, M. A vacuum calcination route to high-surface-area MgO nanoplates for superior arsenate adsorption and catalytic properties. Vacuum 2018, 158, 231–235. [Google Scholar] [CrossRef]
- Song, Y.; Huang, P.; Li, H.; Li, R.; Zhan, W.; Du, Y.; Ma, M.; Lan, J.; Zhang, T.C.; Du, D. Uptake of arsenic(V) using iron and magnesium functionalized highly ordered mesoporous MCM-41 (Fe/Mg-MCM-41) as an effective adsorbent. Sci. Total Environ. 2022, 833, 154858. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, C.; Wang, J.; Zhang, X.; Tu, J. Chapter 9—Layered hydroxides as electrocatalysts for water splitting. In Metal Oxides and Related Solids for Electrocatalytic Water Splitting; Qi, J., Korotcenkov, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 241–272. [Google Scholar]
- Rand, B. Calcination. In Concise Encyclopedia of Advanced Ceramic Materials; Brook, R.J., Ed.; Pergamon: Oxford, UK, 1991; pp. 49–51. [Google Scholar]
- Singh, S.; Singh, B.K. 2—Nanomaterials aspects for photocatalysis. In Nanostructured Materials for Visible Light Photocatalysis; Nayak, A.K., Sahu, N.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–46. [Google Scholar]
- Zhang, W.; Lan, Y.; Ma, M.; Chai, S.; Zuo, Q.; Kim, K.-H.; Gao, Y. A novel chitosan–vanadium-titanium-magnetite composite as a superior adsorbent for organic dyes in wastewater. Environ. Int. 2020, 142, 105798. [Google Scholar] [CrossRef]
- Vinila, V.S.; Isac, J. Chapter 14—Synthesis and structural studies of superconducting perovskite GdBa2Ca3Cu4O10.5+δ nanosystems. In Design, Fabrication, and Characterization of Multifunctional Nanomaterials; Thomas, S., Kalarikkal, N., Abraham, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 319–341. [Google Scholar]
- Khaleel, W.A.; Sadeq, S.A.; Alani, I.A.M.; Ahmed, M.H.M. Magnesium oxide (MgO) thin film as saturable absorber for passively mode locked erbium-doped fiber laser. Opt. Laser Technol. 2019, 115, 331–336. [Google Scholar] [CrossRef]
- Sutradhar, N.; Sinhamahapatra, A.; Pahari, S.K.; Pal, P.; Bajaj, H.C.; Mukhopadhyay, I.; Panda, A.B. Controlled Synthesis of Different Morphologies of MgO and Their Use as Solid Base Catalysts. J. Phys. Chem. C 2011, 115, 12308–12316. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Kumar, R.T.; Kennedy, L.J.; Vijaya, J.J. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J. Alloys Compd. 2011, 509, 9809–9815. [Google Scholar] [CrossRef]
- Zahir, M.; Rahman, M.; Irshad, K.; Mominur, M. Shape-Stabilized Phase Change Materials for Solar Energy Storage: MgO and Mg(OH)2 Mixed with Polyethylene Glycol. Nanomaterials 2019, 9, 1173. [Google Scholar] [CrossRef]
- Joghee, S.; Pradheesh, G.; Vincent, A.; Hong, S.I. Ecofriendly Biosynthesis of Zinc Oxide and Magnesium Oxide Particles from Medicinal Plant Pisonia grandis R.Br. Leaf Extract and Their Antimicrobial Activity. BioNanoScience 2019, 9, 141–154. [Google Scholar] [CrossRef]
- Targan, Ş.; Tirtom, V.N. Arsenic removal from aqueous system using modified chestnut shell. Desalination Water Treat. 2014, 56, 1029–1036. [Google Scholar] [CrossRef]
- Irem, S.; Islam, E.; Khan, Q.; Haq, A.; Hashmat, A. Adsorption of arsenic from drinking water using natural orange waste: Kinetics and fluidized bed column studies. Water Sci. Technol. Water Supply 2017, 17, ws2017009. [Google Scholar] [CrossRef]
- Chinthakuntla, D.; Rao, K.; Chakra, C.H.; Kandregula, G. MgO Nanoparticles Prepared by Microwave-Irradiation Technique and Its Seed Germination Application. Nano Trends J. Nanotechnol. Its Appl. 2016, 18, 10–17. [Google Scholar]
- Hu, S.; Yan, W.; Duan, J. Polymerization of silicate on TiO2 and its influence on arsenate adsorption: An ATR-FTIR study. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 180–186. [Google Scholar] [CrossRef]
- Min, X.; Li, Y.; Ke, Y.; Shi, M.; Chai, L.; Xue, K. Fe-FeS 2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal. Water Sci. Technol. 2017, 76, wst2017204. [Google Scholar] [CrossRef] [PubMed]
- Kandiban, M.; Vigneshwaran, P.; Potheher, I. Synthesis and Characterization of Mgo Nanoparticles for Photocatalytic Applications. In Proceedings of the National Conference on Advances in Crystal Growwth and Nanotechnology, Kottayam, India, 15–16 January 2015. [Google Scholar]
- Temesgen, F.; Gabbiye, N.; Sahu, O. Biosorption of reactive red dye (RRD) on activated surface of banana and orange peels: Economical alternative for textile effluent. Surf. Interfaces 2018, 12, 151–159. [Google Scholar] [CrossRef]
- Chavan, V.D.; Kothavale, V.P.; Sahoo, S.C.; Kollu, P.; Dongale, T.D.; Patil, P.S.; Patil, P.B. Adsorption and kinetic behavior of Cu(II) ions from aqueous solution on DMSA functionalized magnetic nanoparticles. Phys. B Condens. Matter 2019, 571, 273–279. [Google Scholar] [CrossRef]
- Pattanaik, M.; Bhaumik, S.K. Adsorption behaviour of polyvinyl pyrrolidone on oxide surfaces. Mater. Lett. 2000, 44, 352–360. [Google Scholar] [CrossRef]
- Xie, X.; Gao, L. Effect of crystal structure on adsorption behaviors of nanosized TiO2 for heavy-metal cations. Curr. Appl. Phys. 2009, 9 (Suppl. S3), S185–S188. [Google Scholar] [CrossRef]
- Owings, S.M.; Luther, G.W.; Taillefert, M. Development of a rate law for arsenite oxidation by manganese oxides. Geochim. Cosmochim. Acta 2019, 250, 251–267. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Shim, W.-G.; Kim, Y.; Kim, G.; Choi, C.; Kang, S.O.; Cho, D.W. Dye adsorption mechanisms in TiO2 films, and their effects on the photodynamic and photovoltaic properties in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2015, 17, 21974–21981. [Google Scholar] [CrossRef] [PubMed]
- Wadie, A.; Al-Khawaja, E. Removal of cadmium Cd(ii) and silver Ag(i) from aqueous solutions by nano activated alumina. Part i: Batch adsorption experiments. MATEC Web Conf. 2018, 162, 05021. [Google Scholar] [CrossRef]
- McKay, G.; Blair, H.S.; Gardner, J.R. Adsorption of dyes on chitin. I. Equilibrium studies. J. Appl. Polym. Sci. 1982, 27, 3043–3057. [Google Scholar] [CrossRef]
- Goswami, A.; Raul, P.K.; Purkait, M.K. Arsenic adsorption using copper (II) oxide nanoparticles. Chem. Eng. Res. Des. 2012, 90, 1387–1396. [Google Scholar] [CrossRef]
- Walsh, K.; Mayer, S.; Rehmann, D.; Hofmann, T.; Glas, K. Equilibrium data and its analysis with the Freundlich model in the adsorption of arsenic(V) on granular ferric hydroxide. Sep. Purif. Technol. 2020, 243, 116704. [Google Scholar] [CrossRef]
- Chaudhary, B.K.; Farrell, J. Understanding Regeneration of Arsenate-Loaded Ferric Hydroxide-Based Adsorbents. Environ. Eng. Sci. 2015, 32, 353–360. [Google Scholar] [CrossRef] [PubMed]



| Adsorbent | Experimental Conditions | (mg/g) | Reference |
|---|---|---|---|
| Magnesium-aluminium anchored on magnetic biochars | pH 5, T = 10 °C | 34.45 | [38] |
| Polymorphous magnesium oxide nanoparticles | - | 101 | [20] |
| Mg–Al-layered double hydroxides-functionalized hydro-char composite (single component system) | - | 56.299 | [39] |
| Mg–Al-layered double hydroxides-functionalized hydro-char composite (binary-component system) | - | 16.222 | [39] |
| Mg/Al/Fe-CLDH | pH = 2–11 | 70.7 | [40] |
| Mg-doped magnetite nanoparticles by recycling of titanium slag | pH = 8–11, T = 25 °C, concentration = 10 mg/L | 33.71 | [41] |
| MgO nanoplates through the vacuum calcination route | Concentration = 40 mg/L | 481 | [42] |
| Magnesium functionalized highly ordered mesoporous Fe/Mg4-MCM-41 (magnesium accounts for 4%) | Adsorbent dosage = 0.5 g/L, concentration= 10–60 mg/L, pH = 3 | 71.53 | [43] |
| MgO-650 °C | pH = 7, concentration = 50 mg/L, Contact time = 7 h, adsorbent dosage = 0.5 g/L | = 115.27 = 131.93 | This study |
| Sample | (m2g−1) | PSD (nm) | (cm3g−1) | Phase |
|---|---|---|---|---|
| Uncalcined | 72.02 | 39.323 | 0.0717 | Mesoporous |
| Calcined | 55.72 | 45.907 | 0.0535 | Mesoporous |
| MgO-650 °C | Uncalcined MgO | ||||
|---|---|---|---|---|---|
| 2θ (°) | FWHM (°) | D (nm) | 2θ (°) | FWHM (°) | D (nm) |
| 24.51 | 0.15 | 56.99 | 24.56 | 0.16 | 53.52 |
| 32.54 | 0.40 | 21.71 | 25.11 | 0.19 | 45.57 |
| 36.85 | 0.28 | 31.11 | 32.81 | 0.32 | 27.00 |
| 42.85 | 0.32 | 28.17 | 36.82 | 0.22 | 39.18 |
| 50.37 | 0.18 | 52.20 | 37.84 | 0.97 | 9.05 |
| 62.22 | 0.36 | 27.24 | 42.85 | 0.31 | 28.49 |
| 74.58 | 0.33 | 31.47 | 50.50 | 0.73 | 12.62 |
| 78.53 | 0.38 | 28.53 | 58.70 | 0.47 | 20.27 |
| First-Order Model | ||||
|---|---|---|---|---|
| exp | ||||
| mg/L | mg/g | mg/g | min−1 | - |
| 10 | 22.32 | 21.39 | 0.07 | 0.9819 |
| 20 | 48.99 | 48.71 | 0.04 | 0.9831 |
| 50 | 115.27 | 116.96 | 0.01 | 0.9896 |
| Second-Order Model | ||||
| exp | ||||
| mg/L | mg/g | mg/g | min−1 | - |
| 10 | 22.32 | 22.79 | 0.0048 | 0.9818 |
| 20 | 48.99 | 53.00 | 0.0012 | 0.9724 |
| 50 | 115.27 | 141.38 | 1.0359 | 0.9850 |
| Experimental | Weber-Morris Model | Elovich Model | |||||
|---|---|---|---|---|---|---|---|
| α | β | ||||||
| mg/L | mg/g | - | mg/g·min | - | g/mg·h | g/mg | - |
| 10 | 22.32 | 7.806 | 0.917 | 0.6707 | 11.667 | 0.3006 | 0.9295 |
| 20 | 48.99 | 12.456 | 2.363 | 0.7127 | 8.728 | 0.1082 | 0.9221 |
| 50 | 115.27 | 6.293 | 6.402 | 0.9279 | 3.214 | 0.0291 | 0.9758 |
| Langmuir | mg/g) | 131.93 |
| (L/mg) | 6.40 | |
| 0.8545 | ||
| Freundlich | n (dimensionless) | 5.062 |
| (L/g) | 112.78 | |
| 0.9980 |
| 1 | 10 | 20 | 30 | 40 | 50 | |
|---|---|---|---|---|---|---|
| RL | 0.135 | 0.015 | 0.008 | 0.005 | 0.004 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehanathan, S.; Jaafar, J.; Nasir, A.M.; Ismail, A.F.; Matsuura, T.; Othman, M.H.D.; Rahman, M.A.; Yusof, N. Magnesium Oxide Nanoparticles for the Adsorption of Pentavalent Arsenic from Water: Effects of Calcination. Membranes 2023, 13, 475. https://doi.org/10.3390/membranes13050475
Mehanathan S, Jaafar J, Nasir AM, Ismail AF, Matsuura T, Othman MHD, Rahman MA, Yusof N. Magnesium Oxide Nanoparticles for the Adsorption of Pentavalent Arsenic from Water: Effects of Calcination. Membranes. 2023; 13(5):475. https://doi.org/10.3390/membranes13050475
Chicago/Turabian StyleMehanathan, Shaymala, Juhana Jaafar, Atikah Mohd Nasir, Ahmad Fauzi Ismail, Takeshi Matsuura, Mohd Hafiz Dzarfan Othman, Mukhlis A. Rahman, and Norhaniza Yusof. 2023. "Magnesium Oxide Nanoparticles for the Adsorption of Pentavalent Arsenic from Water: Effects of Calcination" Membranes 13, no. 5: 475. https://doi.org/10.3390/membranes13050475
APA StyleMehanathan, S., Jaafar, J., Nasir, A. M., Ismail, A. F., Matsuura, T., Othman, M. H. D., Rahman, M. A., & Yusof, N. (2023). Magnesium Oxide Nanoparticles for the Adsorption of Pentavalent Arsenic from Water: Effects of Calcination. Membranes, 13(5), 475. https://doi.org/10.3390/membranes13050475









