Abstract
It is now generally accepted that the role of bile acids in the organism is not limited to their participation in the process of food digestion. Indeed, bile acids are signaling molecules and being amphiphilic compounds, are also capable of modifying the properties of cell membranes and their organelles. This review is devoted to the analysis of data on the interaction of bile acids with biological and artificial membranes, in particular, their protonophore and ionophore effects. The effects of bile acids were analyzed depending on their physicochemical properties: namely the structure of their molecules, indicators of the hydrophobic–hydrophilic balance, and the critical micelle concentration. Particular attention is paid to the interaction of bile acids with the powerhouse of cells, the mitochondria. It is of note that bile acids, in addition to their protonophore and ionophore actions, can also induce Ca2+-dependent nonspecific permeability of the inner mitochondrial membrane. We consider the unique action of ursodeoxycholic acid as an inducer of potassium conductivity of the inner mitochondrial membrane. We also discuss a possible relationship between this K+ ionophore action of ursodeoxycholic acid and its therapeutic effects.
Keywords:
bile acids; membranes; mitochondria; liver; protonophore; ionophore; nonspecific permeability 1. Introduction
Bile acids and their salts form the basis of bile in higher vertebrates and humans. As part of bile, they play an important role in the process of digestion of food; their main physiological function is to emulsify bile lipids, in particular cholesterol. Emulsification of lipids by bile acids facilitates the absorption of fat-soluble vitamins and calcium in the intestine, and the digestion of triglycerols. In addition, bile excretion is necessary to eliminate toxins, carcinogens, as well as drugs and their metabolites. Apart from cholesterol, other endogenous compounds and metabolic products, such as bilirubin and hormones, are also excreted along with bile (see reviews [1,2]).
Bile acids are well known to be effective antimicrobial agents preventing the growth of bacteria in the small intestine [1,3,4]. Currently, the role of bile acids as signaling molecules regulating various metabolic pathways in cells is also being considered (see reviews [2,5,6,7,8,9]).
Primary bile acids—chenodeoxycholic (CDCA) and cholic (CA)—are synthesized from cholesterol in parenchymal cells (hepatocytes) of the liver of mammals and humans. In rodents, due to alternative hydroxylation, the formation of other primary bile acids is also possible, in particular, α-, β-, and γ-muricholic acids. Secondary bile acids are formed from these bile acids in the intestine with the participation of bacteria—lithocholic acid (LCA)-from CDCA, deoxycholic acid (DCA)-from CA. Additionally, in the intestine, ursodeoxycholic acid (UDCA) is formed from CDCA by epimeration of the hydroxyl group at the seventh carbon atom of the steroid nucleus (transfer of the hydroxyl group from the α-surface to the β-surface). A full description of the primary bile acid synthesis pathways is beyond the scope of this review and is covered in detail in the excellent review articles [2,5,8,10,11,12]. Prior to secretion, bile acids are conjugated with taurine or glycine [10,11,13].
Bile acids are considered as endogenous detergents. In particular, when interacting with artificial and biological membranes in concentrations exceeding their critical micelle concentration, bile acids cause profound disturbances in their structure and function, up to their permeabilization and lysis. It is of note that hydrophobic bile acids are most effective in this case [14,15,16,17]. The protonophore [18,19] and ionophore [20,21,22] effects of bile acids are also known (see the next section for more details).
In cholestasis caused by a blockage of the outflow of bile from the liver, the cells of this organ and the blood show first of all the accumulation of primary bile acids-CDCA and CA [23,24]. At concentrations characteristic of cholestasis, hydrophobic bile acids can solubilize lipids of cell membranes of hepatocytes leading to their damage and, in particular, to the release of γ-glutamyl transpeptidase from cells, whose increase in serum levels is a diagnostic sign of cholestasis [25]. In the course of chronic cholestasis, there is a violation of calcium homeostasis in hepatocytes and the release of calcium ions from the main depots—the endoplasmic reticulum and mitochondria. In this case, monohydroxy bile acids primarily release Ca2+ from the endoplasmic reticulum in the liver [26].
It is well known that hydrophobic bile acids, both primary CDCA and secondary LCA and DCA, as well as their glycine and taurine conjugates, have pronounced cytotoxicity [27,28,29]. LCA has been shown to selectively cause cell death in some malignant neoplasms [27,30]. Unlike other bile acids, UDCA is considered as a drug in the treatment of liver diseases and some other pathologies [31,32,33].
Several types of cell death are known, differing both in morphological features and in biochemical mechanisms. However, in all cases, the central role in these processes is assigned to mitochondria, which integrate many intracellular signaling pathways leading to cell death [28,34,35,36]. It is now generally accepted that one of the links in cell death associated with mitochondria is the opening of a Ca2+-dependent mitochondrial permeability transition pore (MPT-pore) in the inner membrane of these organelles for ions and hydrophilic substances, whose mass does not exceed 1.5 kDa, along their concentration gradient (see reviews [37,38,39,40,41]).
Bile acids have a variety of effects on the mitochondria of vital mammalian organs; they inhibit electron transport along the respiratory chain, induce a decrease on state 3 respiration, the respiratory control ratio, and the membrane potential and cause the induction of the MPT-pore [42,43,44,45,46,47,48,49,50].
This review is devoted to the analysis of literature data on the interaction of bile acids with mitochondria associated with their protonophore and ionophore effects, as well as the induction of the Ca2+-dependent MPT-pore. Particular attention is paid to the analysis of the effects of bile acids depending on their physicochemical properties: the structure of their molecules, indicators of the hydrophobic–hydrophilic balance, and the critical micelle concentration.
2. Physico-Chemical Characteristics of Bile Acids
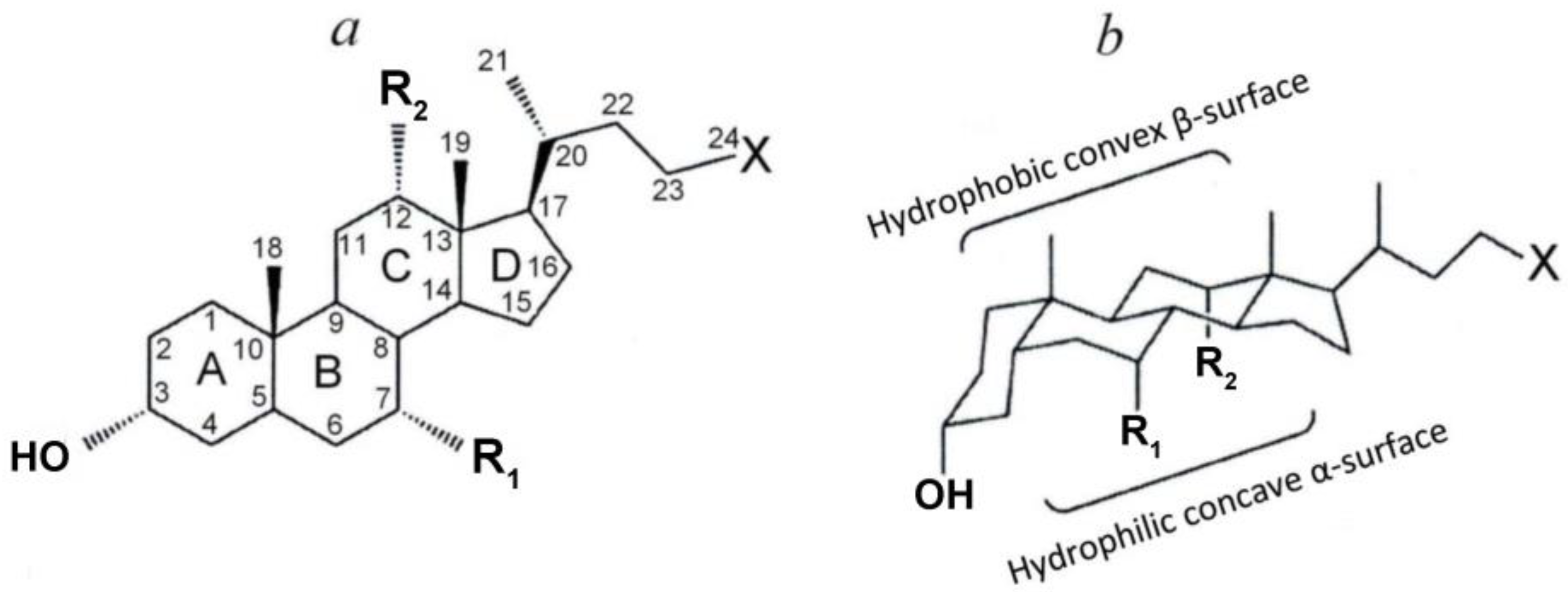
Bile acids are a family of amphiphilic molecules containing a hydrophilic carboxyl group and a hydrophobic steroid structure (steroid core) with a different number of hydroxyl groups. The steroid core is considered as a flat structure with two surfaces depending on the localization of the hydroxyl groups—a more hydrophobic convex β-surface and a more hydrophilic concave α-surface (Figure 1). The whole variety of bile acids (Figure 1 and Table 1) is due to the different number and location of the hydroxyl groups of the steroid core, as well as their conjugation with taurine or glycine [1,11,13,16,51,52,53].

Figure 1.
(a) Planar representation of the general molecular structure of bile acids. Letters (A, B, C, D), indicate the rings of the steroid skeleton, numbers—the carbon atoms, labels Ri—the functional groups (hydroxyl groups or hydrogen atoms), and X—carboxyl group (non-conjugated bile acids) or their glycine and taurine conjugates. (b) Chair representation of the general molecular structure of BAs [1] with modifications.

Table 1.
Physico-chemical properties of the bile acids.
Free (non-conjugated) bile acids are weak acids (pKa of the carboxyl group in aqueous solution is 5.0). Conjugated bile acids are stronger acids, in particular, the pKa values for glycine conjugates in an aqueous solution are 3.9 for tauric ones—in the range from −1.5 to 1.5 [1,55,56,57]. Therefore, at physiological pH values (about 7.4), bile acids dissociate to form their salts. Bile salts are more water soluble than bile acids [52]. The pKa values of the bile acids carboxyl group on the membrane surface is 2.5 units higher than in an aqueous solution [58]. Further, in all cases, we use the term “bile acids”.
Bile acids, being amphiphilic compounds, like other similar amphiphilic compounds, are detergents. However, bile acids differ significantly from classical detergents [13,16,59,60]. In classical amphiphiles, in particular lipids, the hydrophilic head consists of relatively small polar or charged groups, and the hydrophobic tails are often long, flexible, and non-polar hydrocarbon chains. Bile salts show other features. The polar hydroxyl groups are oriented towards the concave side of the rigid steroid ring system, which thus becomes hydrophilic, while the convex side is hydrophobic [1,13,16,59,60]. Thus, bile salts have a face structure with hydrophobic and hydrophilic sides or, depending on the position and orientation of the hydroxyl groups, a hydrophilic ‘edge’ only. Consequently, the hydrophilic and hydrophobic domains are not as clearly separated as is typical for classical amphiphiles. In addition, the hydrophobic tails of common amphiphiles inside the micellar core are liquid-like, while the steroid ring system is very rigid [1,13,16,59,60]. The hydrophobic–hydrophilic balance of bile acids as well as other amphiphilic compounds can be quantified as the partition coefficient between the lipid and aqueous phases (P or logP) (Table 1). The lower the value of this coefficient, the less hydrophobic and, therefore, more hydrophilic is the bile acid. As can be seen from the table, the values of this coefficient differ significantly depending on the method of their determination. However, depending on the structure of the bile acid molecule (the number of hydroxyl groups), the values of these coefficients show that monohydroxyl LCA is the most hydrophobic, dihydroxyl DCA, CDCA, and UDCA are less hydrophobic, and trihydroxyl cholic acid is the least hydrophobic. As follows from the data in the table, the hydrophobicity of glycine and taurine conjugates is significantly less than the corresponding non-conjugated acids.
Like amphiphilic compounds, bile acids are capable of self-organization with the formation of micelles when their concentration in water is increased to a certain level [13,52,53,61]. This extreme concentration of bile acids is called the critical micelle concentration (CMC) [13,52,53,60]. CMC values for bile acids and their glycine and taurine conjugates are shown in Table 1. Bile acids can form either primary or secondary micelles. Primary bile acid micelles have aggregation numbers from 2 to 10 and are formed via hydrophobic interactions, while secondary micelles (aggregation numbers 10–100) are formed via hydrogen bonding interactions of the primary micellar structures [13,59,60]. The pH at which CMC formation occurs is called the critical micellar pH at which the solubility increases markedly [52]. The CMC of trihydroxy bile acids is greater than the CMC of dihydroxy bile acids, and this value in turn is greater than the CMC of monohydroxy LCA (Table 1). The high CMC of trihydroxy bile acids is ascribed to their higher solubility in water [52,59]. It has been reported that conjugation with glycine or taurine slightly lowers the CMC of bile acids (Table 1) [53]. The orientation of hydroxy substituents also influences the CMC values and the changing of a hydroxy substituent from α- to β-configuration increases the CMC values. Moreover, addition of Na+ to a total concentration of 0.15 M lowers the CMC [53]. A more detailed consideration of the structure and kinetics of micelle formation by bile acids is beyond the scope of this review and is covered in detail in the original articles [61,62,63,64] and excellent review articles [1,13,59,60,65,66,67].
3. Effect of Bile Acids on the Permeability of Biological and Artificial Phospholipid Membranes for Protons and Ions
3.1. Protonophore Action of Bile Acids
As mentioned above, free (non-conjugated) bile acids are weak acids. A large number of weak organic acids are known to be effective protonophore uncouplers of oxidative phosphorylation in animal mitochondria [68,69,70,71]. Among them, the mechanism of the uncoupling action of classical protonophore uncouplers (2,4-dinitrophenol or DNP, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, or FCCP, etc.) has been well studied. These uncouplers, by increasing the proton conductivity of the inner mitochondrial membrane and thus dissipating the proton motive force (Δp), stimulate respiration and reduce the efficiency of oxidative ATP synthesis (reduce the ADP/O and respiratory control ratios) [72,73]. Protonophore uncouplers also have the ability to inhibit the production of reactive oxygen species by mitochondria [72,74,75,76]. These and similar compounds are able to cross the phospholipid membrane in both protonated and anionic form due to the presence of a delocalized negative charge. Thus, they carry out cyclic transport of protons through the membrane to a compartment with a lower concentration [68,71,72,74]. In recent years, interest in protonophore uncouplers has increased significantly due to their possible use as pharmacological agents [69,70,71,76,77].
Perhaps the most fully studied natural protonophore uncouplers of oxidative phosphorylation are free monocarboxylic fatty acids [68,75,76,77,78,79,80]. It has been established that carrier proteins of the inner mitochondrial membrane that carry out the exchange transport of ADP to ATP (ADP/ATP-antiporter) are involved in the uncoupling effect of monocarboxylic fatty acids in mitochondria of vital organs of mammals (heart, liver, kidneys, skeletal muscles) [72,78,80,81,82,83]. The specific inhibitor of this carrier, carboxyatractylate, significantly reduces the uncoupling effect of fatty acids. In liver mitochondria, in addition to the ADP/ATP antiporter, the carrier that exchanges aspartate for glutamate (aspartate/glutamate antiporter) also participates in the uncoupling action of fatty acids [72,83]. It is assumed that the participation of ADP/ATP- and aspartate/glutamate antiporters in the uncoupling action of fatty acids consists in the transfer of the fatty acid anion from the inner monolayer of the membrane to the outer one, where these anions are protonated and move in the opposite direction without the participation of proteins by the flip-flop mechanism, releasing protons to the matrix [72,78,84].
The protonophore effect of bile acids was studied both on model membrane systems [19] and on isolated mitochondria [42,45,85]. Hamilton’s laboratory [19] studied the permeability of membranes for fatty and bile acids in experiments with liposomes loaded with the fluorescent pH indicator pyranine. It was found that after the addition of bile acids—CA, DCA, CDCA—to a suspension of liposomes, their neutral molecules are able to move from the outer monolayer of the membrane to the inner one (flip-flop). After reaching the inner monolayer, some of the bile acid molecules donate protons to the buffer containing pyranine, which is accompanied by a decrease in pH from 7.4 to 7.1 [19]. It has been shown that the ability of bile acids to carry protons across the membrane of liposomes depends on the hydrophobicity of their molecules, since the most hydrophilic CA is less effective among the bile acids listed above. At the same time, taurine conjugates of all three bile acids, being strong acids, are ineffective [19].
Non-conjugated bile acids (LCA, CDCA, DCA) are considered as effective uncouplers of oxidative phosphorylation [42]. These bile acids have been shown to stimulate respiration in state 4 and reduce respiratory control and membrane potential (Δψ) of succinate-fueled liver mitochondria. The most hydrophobic LCA showed the strongest effect. The less hydrophobic chenodeoxycholic and deoxycholic acids were less effective. It should be noted that the hydrophobicity of UDCA is the same as that of CDCA and DCA (Table 1), but its effectiveness as an uncoupler of oxidative phosphorylation is significantly inferior to these two bile acids [42]. Hydrophilic taurine and glycine conjugates of these bile acids were significantly less effective [42] despite their lower CMC (Table 1). The authors of this work assessed the protonophore action of bile acids by the intensity of mitochondrial swelling in an isotonic ammonium nitrate solution. It is known that the induction of proton conductivity with simultaneous transport of NH3 and NO3− leads to the accumulation of osmotically active NH4+, NO3− ions in the matrix and, as a result, swelling of mitochondria [86]. This method allows the determination of the presence of proton transport from the intermembrane space to the matrix, as is typical for passive leakage of protons through the inner membrane of energized mitochondria. At the same time, it is known that the transport of such anions as NO3−, SCN−, etc. through the inner mitochondrial membrane is carried out with the participation of an anion channel, whose activity can be affected by hydrophobic compounds [87]. Therefore, the swelling of mitochondria induced by bile acids observed by Rolo and colleagues [42] cannot be considered as evidence of their protonophore action.
Previously, the laboratory of V.P. Skulachev [85] found that CA, like palmitic acid and dodecyl sulfate, stimulates respiration and H+ conduction of the inner membrane of liver mitochondria, and this effect is suppressed by the ADP/ATP antiporter inhibitor carboxyatractylate. The authors of this work evaluated the protonophore action of these compounds in experiments on de-energized mitochondria by recording the ΔpH dissipation rate (difference in H+ concentrations on the inner mitochondrial membrane) using a pH meter after the rapid addition of a certain amount of hydrochloric acid. In this case, the addition of CA led to an increase in the rate of H+ transport into the mitochondrial matrix, as is typical for protonophore uncouplers [85]. The data obtained were considered as evidence that the ADP/ATP antiporter is involved in the uncoupling action of bile acid [84]. Supporting this suggestion is the fact that bile acids can interact directly with the ADP/ATP antiporter, as shown in proteoliposomes [88].
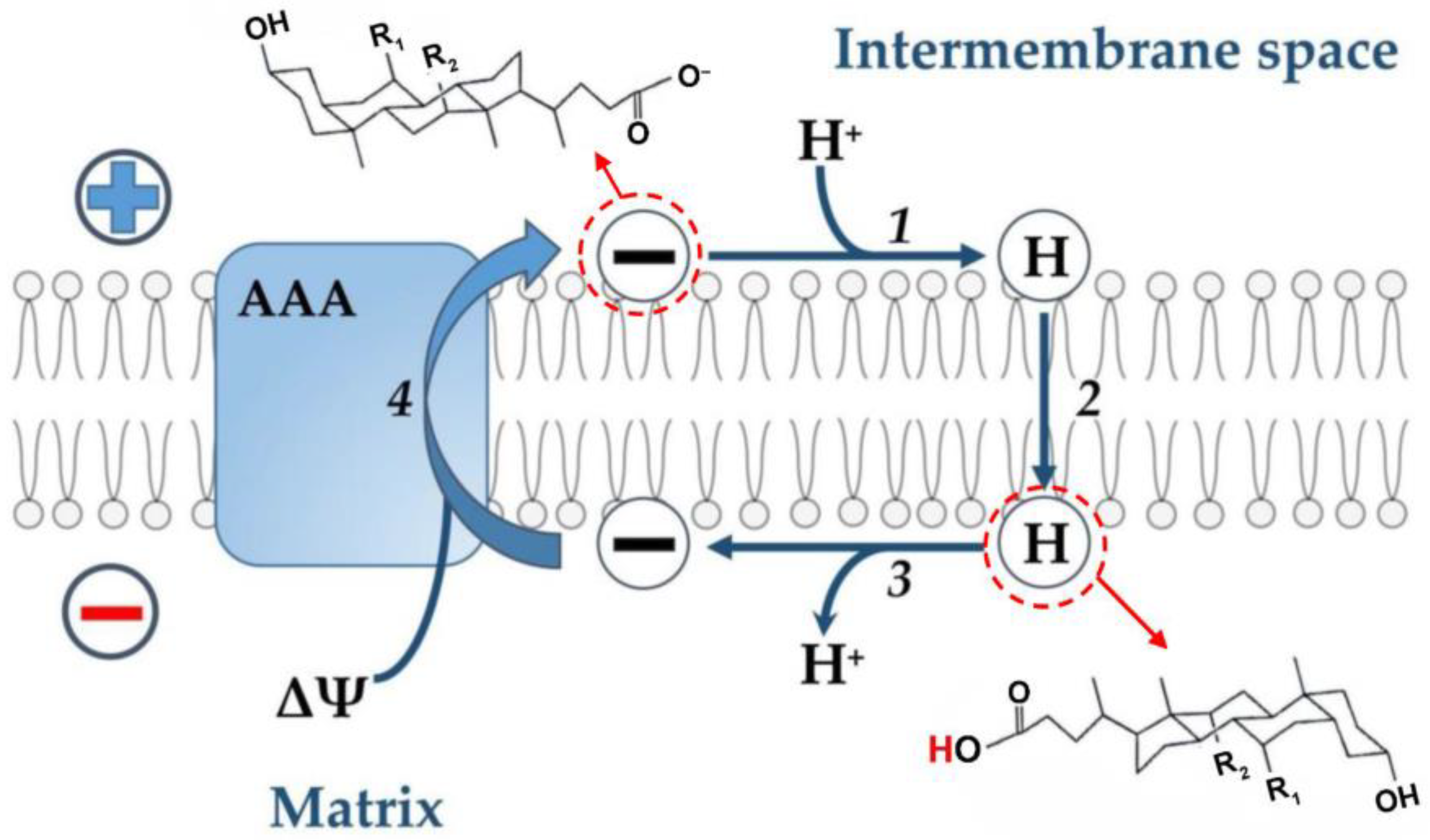
Based on these data and by analogy with the uncoupling effect of fatty acids (see above), the following hypothetical scheme can be proposed (Figure 2). It is assumed that free (non-conjugated) anions are protonated on the outer surface of the inner mitochondrial membrane, since their pKa on the membrane surface is 2.5 units higher than in an aqueous solution [58] and, therefore, its value is 7.5. Further, neutral molecules of bile acids are transported through the phospholipid bilayer to the opposite side of the membrane by the flip-flop mechanism, followed by the release of a proton into the mitochondrial matrix. The transport of the bile acid anion in the opposite direction is carried out with the assistance of the ADP/ATP antiporter. At the same time, it cannot be ruled out that the effects of bile acids described above may be due to damage to the inner mitochondrial membrane, as was suggested for the nonionic detergent Triton X-100 and the cationic detergent cetyltrimethylammonium bromide [85].

Figure 2.
Hypothetical scheme illustrating the transport of protons by bile acid across the inner membrane of liver mitochondria with the participation of the ADP/ATP-antiporter (AAA). According to this scheme, the bile acid anion (symbol (−)) is protonated on the outer surface of the inner membrane (step 1). The neutral bile acid molecule (symbol (H)) is transported across the phospholipid bilayer to the opposite side of the membrane by the flip-flop mechanism (step 2). This stage is fast [19] and does not require any carrier. During this stage, protons are transported from a more acidic compartment (membrane space) to a more alkaline one (matrix). On the inner surface of the inner membrane, a neutral bile acid molecule releases a proton into the matrix (deprotonates) to form a bile acid anion (step 3). The bile acid anion moves in the opposite direction with the participation of the ADP/ATP-antiporter (step 4). This step 4 is electrogenic since energy in the form of ΔΨ is expended to move the anion.
3.2. Ionophore Action of Bile Acids
It is well known that many bile acids have a relatively high affinity for Ca2+ [62,63,65,89,90,91]. High concentrations of Ca2+ (2.5–5 mM) and bile acids (2–5 mM) are able to form micellar aggregates, gels, and precipitates in aqueous solutions [62,63,65]. One should note the recent paper by Du et al., which demonstrates that bile acids can aggregate at a concentration much lower than CMC, when specific conditions are created, such as the interactions with polyelectrolytes [92]
In experiments on model lipid membranes (black lipid membrane), it is well established that free (non-conjugated) bile acids—CA and DCA—are capable of transporting divalent metal ions (Ba2+, Ca2+, Sr2+, Mg2+, and Mn2+) [20]. Similar results were obtained in the study of the effect of bile acids on the permeability of liposomes to divalent metal ions [21,22]. Thus, these bile acids can be considered as ionophores capable of transporting divalent metal ions, including calcium ions, directly through the phospholipid bilayer. Taurine conjugates of bile acids also have calcium ionophore activity [89]. It has been shown that bile acids increase calcium transport into red blood cells [93] and pig jejunal brush border membranes [94] while increasing Ca2+ concentration in kidney cell lines [95] and hepatocytes [96]. The ionophoric properties of bile acids are related to the hydrophobicity of the steroid nucleus. Thus, the ability of bile acids to raise Ca2+ concentration increases with increasing hydrophobicity [96]. At the same time, this action of bile acids may be associated with their effect on special carriers that carry out transmembrane Ca2+ transport [97].
Currently, several structures are known that transport Ca2+ in mitochondria. Among them, the most important are the mitochondrial Ca2+ uniporter (MCU), Ca2+/H+ antiporter (Letm1), Ca2+/Na+ antiporter (NCLX), and others (see reviews [40,98]. The effect of bile acids as inducers of Ca2+ release from the matrix was studied in experiments on isolated rat liver mitochondria [47]. In these studies, mitochondria in the presence of cyclosporin A (a Ca2+-dependent pore blocker, see below) were fueled by succinate, loaded with Ca2+, and deenergized with malonate after addition of ruthenium red, a calcium uniporter inhibitor. It has been shown that under these conditions, bile acids—LCA, HDCA, CA, and UDCA—induce the release of Ca2+ from the mitochondrial matrix. The release of these ions was not associated with damage to the inner membrane of mitochondria by bile acids, as it is accompanied by the generation of Δψ—the formation of a diffusion potential. It was suggested that by ejecting Ca2+ from the matrix, bile acids transport H+ in the opposite direction, i.e., carry out electrically neutral Ca2+/2H+ exchange [47].
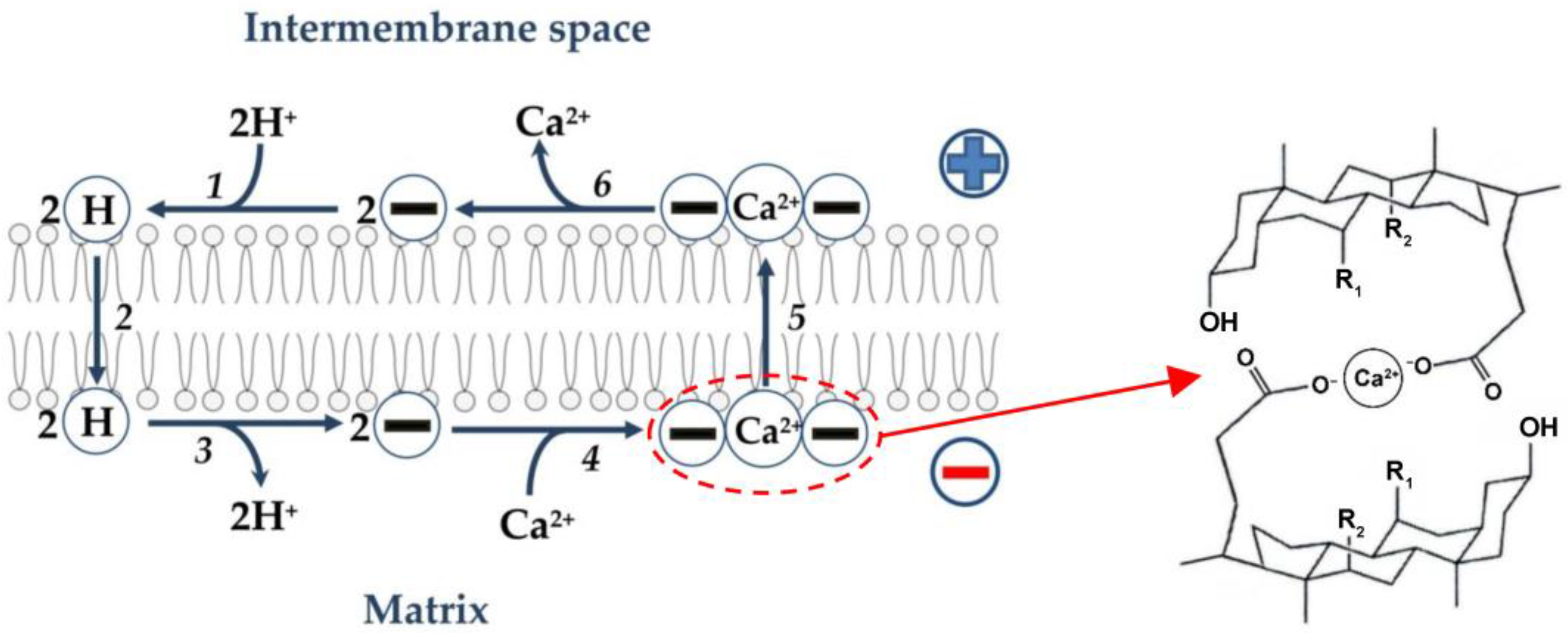
As noted above, bile acids are relatively flat and rigid molecules with a polar and a hydrophobic face [1,13,16,60]. A complex can be envisioned that would consist of two such molecules, with Ca2+ sequestered between the two hydrophilic surfaces [89]. It has been suggested that bile acids are likely to function as mobile (in contrast to channel-forming) Ca2+ ionophores, with the 2:1 bile acid/Ca2+ complex as the possible transport intermediate [22,89]. Based on the foregoing, the following hypothetical scheme can be assumed (Figure 3). As noted above (see also Figure 2), anions of free (non-conjugated) bile acids are protonated on the outer surface of the inner mitochondrial membrane. Further, neutral molecules of bile acids are transported through the phospholipid bilayer to the opposite side of the membrane by the flip-flop mechanism, followed by the release of protons into the mitochondrial matrix. It can be assumed that a neutral complex of calcium cation with two anions of bile acids is formed on the outer surface of the inner membrane, which diffuses to the opposite side of the membrane. However, it cannot be ruled out that bile acids directly affect the Ca2+/H+ antiporter, thus stimulating Ca2+/2H+ exchange.

Figure 3.
Hypothetical scheme illustrating Ca2+ transport by bile acids directly across the phospholipid bilayer of the inner membrane of liver mitochondria. In this scheme, the first three stages are similar to the first three stages of proton transport by bile acids, as shown in Figure 2. Two bile acid anions (symbol 2(−)) are protonated on the outer surface of the inner membrane (step 1). Two neutral bile acid molecules (symbol 2(H)) are transported across the phospholipid bilayer to the opposite side of the membrane by the flip-flop mechanism (step 2). Two neutral bile acid molecules release two protons into the matrix (deprotonate) on the inner surface of the inner membrane to form two bile acid anions (step 3). These anions interact with Ca2+ to form an electrically neutral complex (symbol (−)(Ca2+)(−)) (step 4). This complex (a hypothetical structure is depicted on the right side [89]) moves through the phospholipid bilayer of the inner membrane to its outer surface (step 5), where this complex decomposes with the release of Ca2+ into the intermembrane space and the formation of two bile acid ions (step 6).
3.3. Bile Acids as Inducers of the Ca2+-Dependent Cyclosporine A-Sensitive Pore in the Inner Mitochondrial Membrane
As noted in the introduction, one of the links in cell death associated with mitochondria is the opening of a Ca2+-dependent MPT-pore in the inner membrane of these organelles for ions and hydrophilic substances, whose mass does not exceed 1.5 kDa along their concentration gradient. A highly selective inhibitor of this pore is cyclosporin A (CsA) which completely inhibits MPT-pore opening at submicromolar concentrations (see reviews [36,37,38,39,40,41]). The opening of the MPT-pore promotes rapid transfer of protons into mitochondria leading to depolarization of the inner membrane, uncoupling of oxidative phosphorylation, and, simultaneously, rapid release of Ca2+ from the matrix. In this case, water with dissolved low molecular weight substances rushes into the mitochondria due to colloid osmotic pressure leading to high-amplitude swelling of the organelles. Swelling of mitochondria leads to a decrease in the light scattering through the suspension, and this can be registered as a decrease in the optical density of the mitochondrial suspension [99,100]. Thus, registration of a decrease in the optical density of a suspension of mitochondria associated with the swelling of these organelles is one of the main methods for determining the pore induction [42,46,47,48,88,99,100,101].
The process of closing–opening of the CsA-sensitive pore is regulated by a number of physiological modulators. Among low molecular weight pore modulators, inorganic phosphate (Pi) occupies a special place. It is well known that Pi increases the sensitivity of mitochondria to Ca2+ as a pore inductor. Pi has also been found to enhance the effect of CsA as a pore blocker [102,103,104,105,106].
It should be noted that a number of studies allows the consideration of the mitochondrial pore as a mechanism for the release of Ca2+ from organelles [107,108]. The question is being discussed of whether the non-selectivity of the mitochondrial pore is an important feature that allows the rapid and efficient release of Ca2+ from the matrix of organelles, which suggests the physiological role of this system (see reviews [40,109]).
LCA, DCA, HDCA, UDCA, as well as their glycine and taurine conjugates, are able to induce pore opening, which is inhibited by CsA in mitochondria isolated from the liver and loaded with Ca2+ [42,46,47,48,88,102,110]. In these experiments, the concentration of Ca2+ did not exceed 50 µM, and the concentration of bile acids was less than CMC by more than an order of magnitude (see Table 1). It is of note that the action of these bile acids as inducers of the mitochondrial CsA-sensitive pore is not associated with the modulation of the effect of inorganic phosphate as a pore inducer [46]. The effectiveness of bile acids as inducers of the Ca2+-dependent CsA-sensitive pore depends on the hydrophobicity of their molecules. The most hydrophobic LCA is the most effective, the less hydrophobic DCA and HDCA are less effective, and the more hydrophilic CA is significantly less effective [42,46]. At the same time, UDCA, being as hydrophobic as DCA and CDCA (Table 1), is significantly inferior to them in terms of efficiency as an inducer of the Ca2+-dependent CsA-sensitive pore [42,46]. Glycine and taurine conjugates of these bile acids are significantly less effective as inducers of the mitochondrial CsA-sensitive pore [42,102] despite their lower CMC (Table 1).
It was found that ruthenium red by inhibiting Ca2+ transport into the mitochondrial matrix is able to reduce the effect of bile acids as inducers of the CsA-sensitive mitochondrial pore [47]. Obviously, this is possible only if, as mentioned above, these bile acids are able to effectively induce the release of Ca2+ from the matrix without violating the integrity of the inner membrane, while ruthenium red prevents the return of these ions to the matrix. It is noted that other amphiphilic compounds do not have such an effect, in particular the free fatty acids: palmitic and α,ω-hexadecanedioic (Unpublished data of E. Khoroshavina). Thus, under conditions of reduced activity of the calcium uniporter, the release of Ca2+ from the matrix induced by bile acids may be one of the mechanisms that reduce the effectiveness of their action as inducers of the Ca2+-dependent CsA-sensitive pore in mitochondria.
Unlike other studied bile acids, the effects of UDCA associated with the induction of the permeability of the inner membrane (swelling of mitochondria, a drop in Δψ, and the release of Ca2+ from the matrix) in the presence of potassium chloride in the incubation medium, but without Pi, are also observed in the presence of CsA [46]. Obviously, these effects of UDCA, in contrast to the effects of other studied bile acids, are due to a different spatial orientation of the hydroxyl group at the seventh carbon atom of the steroid nucleus—the β-position instead of α-position, as in CDCA (Table 1). We suggest that the induction of CsA-insensitive inner membrane permeability by UDCA is associated with the activation of electrophoretic transport of K+ into the matrix of Ca2+-loaded mitochondria. This is known to be accompanied by their swelling and decrease in Δψ (see reviews [111,112]). Thus, UDCA can be considered as a K+ ionophore. At the same time, the involvement of other potassium ion transport systems, in particular Ca2+-activated K+ channels, cannot be ruled out [112].
4. Conclusions
Currently, bile acids are largely attracting attention as signaling molecules involved in the regulation of various pathways of cell metabolism by interacting with special receptors on membranes (see reviews [2,5,6,7,8,9]). Bile acids, being amphiphilic compounds, are also able to directly interact with the lipid component of membranes. Interacting with membranes, bile acids can significantly change their structure and functions (detergent action) [14,15,16,17], as well as induce transmembrane transport of protons (protonophore action) [18,19] and divalent ions (ionophore action) [20,21,22]. At the same time, further studies are needed to elucidate the mechanisms of the protonophore and ionophore effects of bile acids in the mitochondria of animals and humans. It is necessary to elucidate the dependence of these effects of bile acids on their physicochemical properties: the degree of hydrophobicity, the critical micelle concentration, the number and orientation of the hydroxyl groups of the steroid nucleus. The foregoing fully applies to the study of the mechanism of action of bile acids as inducers of Ca2+-dependent CsA-sensitive permeabilization of the inner mitochondrial membrane. In particular, a promising direction seems to be research aimed at elucidating the relationship between the Ca2+-ionophore effect of bile acids and their action as potential modulators of nonspecific permeability of the inner mitochondrial membrane and, as a consequence, cell death. As noted above, protonophore uncouplers are able to suppress the formation of reactive oxygen species in mitochondria [70,74,75,76]. It is not known whether free (non-conjugated) bile acids have a similar effect. Unlike other bile acids, UDCA is used as a drug in the treatment of liver diseases and some other pathologies [31,32,33]. Studies on intact mitochondria have shown that UDCA can be considered as a K+-ionophore [46]. It is known that various pharmacological agents that activate the transport of potassium ions into the mitochondrial matrix have a cytoprotective effect [113,114]. A promising line of research seems to be elucidation of the relationship between the K+-ionophore action of UDCA on mitochondria and its therapeutic effects.
Author Contributions
Conceptualization, V.N.S.; writing—original draft preparation, V.N.S., E.I.K., E.K.P., M.V.D. and A.A.S.; writing—review and editing, V.N.S. and M.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- di Gregorio, M.C.; Cautela, J.; Galantini, L. Physiology and physical chemistry of bile acids. Int. J. Mol. Sci. 2021, 22, 1780. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Shirokova, E.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Sinitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; et al. A recent ten-year perspective: Bile acid metabolism and signaling. Molecules 2022, 27, 1983. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Urdaneta, V.; Casadesús, J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- Zhou, H.; Hylemon, P.B. Bile acids are nutrient signaling hormones. Steroids 2014, 86, 62–68. [Google Scholar] [CrossRef]
- Grant, S.M.; DeMorrow, S. Bile acid signaling in neurodegenerative and neurological disorders. Int. J. Mol. Sci. 2020, 21, 5982. [Google Scholar] [CrossRef]
- Zagoskin, P.P.; Erlykina, E.I. Bile acids as a new type of steroid hormones regulating nonspecific energy expenditure of the body (Review). Sovrem. Tekhnologii Med. 2021, 12, 114–127. [Google Scholar] [CrossRef]
- Way, G.W.; Jackson, K.G.; Muscu, S.R.; Zhou, H. Key signaling in alcohol-associated liver disease: The role of bile acids. Cells 2022, 11, 1374. [Google Scholar] [CrossRef]
- Weng, Z.B.; Chen, Y.R.; Lv, J.T.; Wang, M.X.; Chen, Z.Y.; Zhou, W.; Shen, X.C.; Zhan, L.B.; Wang, F. A review of bile acid metabolism and signaling in cognitive dysfunction-related diseases. Oxid. Med. Cell. Longev. 2022, 2022, 4289383. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid metabolism in liver pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Li, J.; Dawson, P.A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta Mol. Basis. Dis. 2019, 1865, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, M.; Xu, W.; Yu, S. Research progress of bile acids in cancer. Front. Oncol. 2022, 11, 77825. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-enhancing effects of bile salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef] [PubMed]
- Schölmerich, J.; Becher, M.S.; Schmidt, K.; Schubert, R.; Kremer, B.; Feldhaus, S.; Gerok, W. Influence of hydroxylation and conjugation of bile salts on their membrane-damaging properties—Studies on isolated hepatocytes and lipid membrane vesicles. Hepatology 1984, 4, 661–666. [Google Scholar] [CrossRef]
- O’Connor, C.J.; Wallace, R.G.; Iwamoto, K.; Taguchi, T.; Sunamoto, J. Bile salt damage of egg phosphatidyl choline liposomes. Biochim. Biophys. Acta 1985, 817, 95–102. [Google Scholar] [CrossRef]
- Garidel, P.; Hildebrand, A.; Knauf, K.; Blume, A. Membranolytic activity of bile salts: Influence of biological membrane properties and composition. Molecules 2007, 12, 2292–2326. [Google Scholar] [CrossRef]
- Camilleri, M. Bile acid detergency: Permeability, inflammation, and effects of sulfation. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, 480–488. [Google Scholar] [CrossRef]
- Zhao, D.; Hirst, B.H. Prostaglandin protects against bile salt induced increases in proton permeation of duodenal brush border membrane. Gut. 1991, 32, 645–648. [Google Scholar] [CrossRef]
- Kamp, F.; Hamilton, J.A. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry 1993, 32, 11074–11086. [Google Scholar] [CrossRef]
- Abramson, J.J.; Shamoo, A.E. Anionic detergents as divalent cation ionophores across black lipid membranes. J. Membr. Biol. 1979, 50, 241–455. [Google Scholar] [CrossRef]
- Hunt, G.R.; Jawaharlal, K. A 1H-NMR investigation of the mechanism for the ionophore activity of the bile salts in phospholipid vesicular membranes and the effect of cholesterol. Biochim. Biophys. Acta 1980, 601, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Oelberg, D.G.; Wang, L.B.; Sackman, J.W.; Adcock, E.W.; Lester, R.; Dubinsky, W.P. Bile salt-induced calcium fluxes in artificial phospholipid vesicles. Biochim. Biophys. Acta 1988, 937, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Arduini, A.; Serviddio, G.; Escobar, J.; Tormos, A.M.; Bellanti, F.; Vina, J.; Monsalve, M.; Sastre, J. Mitochondrial biogenesis fails in secondary biliary cirrhosis in rats leading to mitochondrial DNA depletion and deletions. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Arduini, A.; Serviddio, G.; Tormos, A.M.; Monsalve, M.; Sastre, J. Mitochondrial dysfunction in cholestatic liver diseases. Front. Biosci. 2012, 4, 2233–2252. [Google Scholar] [CrossRef]
- Monte, M.J.; Marin, J.J.; Antelo, A.; Vazquez-Tato, J. Bile acids: Chemistry, physiology, and pathophysiology. World J. Gastroenterol. 2009, 15, 804–816. [Google Scholar] [CrossRef]
- Combettes, L.; Dumont, M.; Berthon, B.; Erlinger, S.; Claret, M. Release of calcium from the endoplasmic reticulum by bile acids in rat liver cells. J. Biol. Chem. 1988, 26, 2299–2303. [Google Scholar] [CrossRef]
- Goldberg, A.A.; Beach, A.; Davies, G.F.; Harkness, T.A.; Leblanc, A.; Titorenko, V.I. Lithocholic bile acid selectively kills neuroblastoma cells, while sparing normal neuronal cells. Oncotarget 2011, 10, 761–782. [Google Scholar] [CrossRef]
- Malhi, H.; Guicciardi, M.E.; Gores, G.L. Hepatocyte death: A clear and present danger. Physiol. Rev. 2010, 90, 165–1194. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Li, F.; Xie, Y.; Farhood, A.; Fickert, P.; Trauner, M.; Jaeschke, H. Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol. Lett. 2014, 228, 56–66. [Google Scholar] [CrossRef]
- Luu, T.H.; Bard, J.M.; Carbonnelle, D.; Chaillou, C.; Huvelin, J.M.; Bobin-Dubigeon, C.; Nazih, H. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell. Oncol. 2018, 41, 13–24. [Google Scholar] [CrossRef]
- Guarino, M.P.; Cocca, S.; Altomare, A.; Emerenziani, S.; Cicala, M. Ursodeoxycholic acid therapy in gallbladder disease, a story not yet completed. World J. Gastroenterol. 2013, 19, 5029–5034. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.J.; Steer, C.J.; Lajczak-McGinley, N.K. Ursodeoxycholic acid: A promising therapeutic target for inflammatory bowel diseases? Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Picard, E.; Guégan, J.; Jaworski, T.; Parenti, L.; Delaunay, K.; Naud, M.C.; Berdugo, M.; Boatright, J.H.; Behar-Cohen, F. Comparative analysis of urso-and tauroursodeoxycholic acid neuroprotective effects on retinal degeneration models. Pharmaceuticals 2022, 15, 334. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Bauer, T.M.; Murphy, E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res. 2020, 126, 280–293. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Ramaccini, D.; Morciano, G.; Pedriali, G.; Kahsay, A.E.; Bouhamida, E.; Giorgi, C.; Wieckowski, M.R.; Pinton, P. Physiopathology of the permeability transition pore: Molecular mechanisms in human pathology. Biomolecules 2020, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell. Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Dubinin, M.V. Diabetes mellitus, mitochondrial dysfunction and Ca2+-dependent permeability transition pore. Int. J. Mol. Sci. 2020, 21, 6559. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Dubinin, M.V.; Belosludtseva, N.V.; Mironova, G.D. Mitochondrial Ca2+ transport: Mechanisms, molecular structures, and role in cells. Biochemistry 2019, 84, 593–607. [Google Scholar] [CrossRef]
- Bernardi, P.; Carraro, M.; Lippe, G. The mitochondrial permeability transition: Recent progress and open questions. FEBS J. 2022, 289, 7051–7074. [Google Scholar] [CrossRef]
- Rolo, A.P.; Oliveira, P.J.; Moreno, A.J.; Palmeira, C.M. Bile acids affect liver mitochondrial bioenergetics: Possible relevance for cholestasis therapy. Toxicol. Sci. 2000, 57, 177–185. [Google Scholar] [CrossRef]
- Ferreira, M.; Coxito, P.M.; Sardão, V.A.; Palmeira, C.M.; Oliveira, P.J. Bile acids are toxic for isolated cardiac mitochondria: A possible cause for hepatic-derived cardiomyopathies? Cardiovasc. Toxicol. 2005, 5, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Castro, R.E.; Pinto, S.N.; Coutinho, A.; Lucas, S.D.; Moreira, R.; Rodrigues, C.M.P.; Prieto, M.; Fernandes, F. Deoxycholic acid modulates cell death signaling through changes in mitochondrial membrane properties. J. Lipid. Res. 2015, 56, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Khoroshavina, E.I.; Dubinin, M.V.; Samartsev, V.N. The effects of bile acids on the liver mitochondria in the presence and absence of Ca2+. FEBS J. 2015, 282 (Suppl. S1), 100. [Google Scholar]
- Khoroshavina, E.I.; Dubinin, M.V.; Samartsev, V.N. Ursodeoxycholic acid, in contrast to other bile acids, induces Ca2+-dependent cyclosporin a-insensitive permeability transition in liver mitochondria in the presence of potassium chloride. Biochem. Mosc. Suppl. Ser. A 2016, 10, 287–293. [Google Scholar] [CrossRef]
- Khoroshavina, E.I.; Dubinin, M.V.; Khokhlov, A.V.; Samartsev, V.N. Bile acid-induced Ca2+ efflux from liver mitochondria as a factor preventing the formation of mitochondrial pores. Biochem. Mosc. Suppl. Ser. A 2018, 12, 128–136. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Khoroshavina, E.I.; Samartsev, V.N. Lithocholic acid induces two different calcium-dependent inner membrane permeability systems in liver mitochondria. Biochem. Mosc. Suppl. Ser. A 2017, 11, 231–236. [Google Scholar] [CrossRef]
- Penman, S.L.; Sharma, P.; Aerts, H.; Park, B.K.; Weaver, R.J.; Chadwick, A.E. Differential toxic effects of bile acid mixtures in isolated mitochondria and physiologically relevant HepaRG cells. Toxicol. Vitr. 2019, 61, 104595. [Google Scholar] [CrossRef]
- Abrigo, J.; Olguín, H.; Gutierrez, D.; Tacchi, F.; Arrese, M.; Cabrera, D.; Valero-Breton, M.; Elorza, A.A.; Simon, F.; Cabello-Verrugio, C. Bile acids induce alterations in mitochondrial function in skeletal muscle fibers. Antioxidants 2022, 11, 1706. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Carey, M.C. The hydrophobic-hydrophilic balance of bile salts.Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J. Lipid. Res. 1982, 23, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Roda, A. Physicochemical properties of bile acids and their relationship to biological properties: An overview of the problem. J. Lipid. Res. 1984, 25, 1477–1489. [Google Scholar] [CrossRef]
- Roda, A.; Minutello, A.; Angellotti, M.A.; Fini, A. Bile acid structure-activity relationship: Evaluation of bile acid lipophilicity using 1-octanol/water partition coefficient and reverse phase HPLC. J. Lipid. Res. 1990, 31, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Roda, A. Chemical properties of bile acids. IV. Acidity constants of glycine-conjugated bile acids. J. Lipid. Res. 1987, 28, 755–759. [Google Scholar] [CrossRef]
- Heuman, D.M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid. Res. 1989, 30, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, A.; Först, G.; Tauber, P.; Schubert, R. Membrane/water partition coefficients of bile salts determined using laurdan as a fluorescent probe. Biophys. J. 2016, 111, 1714–1723. [Google Scholar] [CrossRef][Green Version]
- Neves, M.C.; Filipe, H.A.L.; Reis, R.L.; Prates Ramalho, J.P.; Coreta-Gomes, F.; Moreno, M.J.; Loura, L.M.S. Interaction of bile salts with lipid bilayers: An atomistic molecular dynamics study. Front. Physiol. 2019, 10, 393. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maitra, U. Chemistry and biology of bile acids. Curr. Sci. 2004, 87, 1666–1683. [Google Scholar]
- Madenci, D.; Egelhaaf, S.U. Self-assembly in aqueous bile salt solutions. Curr. Opin. Colloid. Interface Sci. 2010, 15, 109–115. [Google Scholar] [CrossRef]
- Chen, M.; Grätzel, M.; Thomas, J.K. Kinetic studies in bile acid micelles. J. Am. Chem. Soc. 1975, 97, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.J.; Hofmann, A.F.; Ton-Nu, H.T.; Schteingart, C.D.; Mysels, K.J. Solubility of calcium salts of unconjugated and conjugated natural bile acids. J. Lipid. Res. 1992, 33, 635–646. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Galantini, L.; Gavuzzo, E.; Giglio, E.; Mazza, F. Calcium ion binding to bile salts. Langmuir 1997, 13, 3090–3095. [Google Scholar] [CrossRef]
- Schryver, S.B. Some investigations on the phenomena of “clot” formations. Part I—On the clotting of milk. Proc. R. Soc. B Biol. Sci. 1913, 86, 460–481. [Google Scholar]
- Hofmann, A.F.; Mysels, K.J. Bile acid solubility and precipitation in vitro and in vivo: The role of conjugation, pH, and Ca2+ ions. J. Lipid Res. 1992, 33, 617–626. [Google Scholar] [CrossRef]
- di Gregorio, M.C.; Travaglini, L.; Del Giudice, A.; Cautela, J.; Pavel, N.V.; Galantini, L. Bile salts: Natural surfactants and precursors of a broad family of complex amphiphiles. Langmuir 2019, 35, 6803–6821. [Google Scholar] [CrossRef]
- Jover, A.; Fraga, F.; Meijide, F.; Vázquez Tato, J.; Cautela, J.; Del Giudice, A.; di Gregorio, M.C. Revealing the complex self-assembly behaviour of sodium deoxycholate in aqueous solution. J. Colloid Interface Sci. 2021, 604, 415–428. [Google Scholar] [CrossRef]
- Terada, H. Uncouplers of oxidative phosphorylation. Environ. Health Perspect. 1990, 87, 213–218. [Google Scholar] [CrossRef]
- Goedeke, L.; Shulman, G.I. Therapeutic potential of mitochondrial uncouplers for the treatment of metabolic associated fatty liver disease and NASH. Mol. Metab. 2021, 46, 101178. [Google Scholar] [CrossRef]
- Shrestha, R.; Johnson, E.; Byrne, F.L. Exploring the therapeutic potential of mitochondrial uncouplers in cancer. Mol. Metab. 2021, 46, 101222. [Google Scholar] [CrossRef]
- Kotova, E.A.; Antonenko, Y.N. Fifty Years of research on protonophores: Mitochondrial uncoupling as a basis for therapeutic action. Acta Nat. 2022, 14, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. Uncoupling: New approaches to an old problem of bioenergetics. Biochim. Biophys. Acta 1998, 1363, 100–124. [Google Scholar] [CrossRef] [PubMed]
- Samartsev, V.N.; Semenova, A.A.; Dubinin, M.V. A comparative study of the action of protonophoreuncouplers and decoupling agents as inducers of free respiration in mitochondria in states 3 and 4: Theoretical and Experimental Approaches. Cell Biochem. Biophys. 2020, 78, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P.; Bogachev, A.V.; Kasparinsky, F.O. Principles of Bioenergetics; Springer: Berlin/Heidelberg, Germany, 2013; p. 436. [Google Scholar]
- Semenova, A.A.; Samartsev, V.N.; Pavlova, S.I.; Dubinin, M.V. ω-Hydroxypalmitic and α,ω-hexadecanedioic acids as activators of free respiration and inhibitors of H2O2 generation in liver mitochondria. Biochem. Mosc. Suppl. Ser. A 2020, 14, 24–33. [Google Scholar] [CrossRef]
- Zorov, D.B.; Andrianova, N.V.; Babenko, V.A.; Pevzner, I.B.; Popkov, V.A.; Zorov, S.D.; Zorova, L.D.; Plotnikov, E.Y.; Sukhikh, G.T.; Silachev, D.N. Neuroprotective potential of mild uncoupling in mitochondria. Pros and cons. Brain Sci. 2021, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.G. 2,4-Dinitrophenol as medicine. Cells 2019, 8, 280. [Google Scholar] [CrossRef]
- Severin, F.F.; Severina, I.I.; Antonenko, Y.N.; Rokitskaya, T.I.; Cherepanov, D.A.; Mokhova, E.N.; Vyssokikh, M.Y.; Pustovidko, A.V.; Markova, O.V.; Yaguzhinsky, L.S.; et al. Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore. Proc. Natl. Acad. Sci. USA 2010, 107, 663–668. [Google Scholar] [CrossRef]
- Samartsev, V.N.; Marchik, E.I.; Shamagulova, L.V. Free fatty acids as inducers and regulators of uncoupling of oxidative phosphorylation in liver mitochondria with participation of ADP/ATP and aspartate/glutamate antiporter. Biochemistry 2011, 76, 217–224. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Chouchani, E.T.; Kazak, L.; Angelin, A.; Fedorenko, A.; Long, J.Z.; Vidoni, S.; Garrity, R.; Cho, J.; Terada, N.; et al. H+ transport is an integral function of the mitochondrial ADP/ATP carrier. Nature 2019, 7766, 515–520. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Bondareva, T.O.; Dedukhova, V.I.; Mokhova, E.N.; Skulachev, V.P.; Tsofina, L.M.; Volkov, N.I.; Vygodina, T.V. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur. J. Biochem. 1989, 182, 585–592. [Google Scholar] [CrossRef]
- Schönfeld, P. Does the function of adenine nucleotide translocase in fatty acid uncoupling depend on the type mitochondria? FEBS Lett. 1990, 264, 246–248. [Google Scholar] [CrossRef]
- Samartsev, V.N.; Smirnov, A.V.; Zeldi, I.P.; Markova, O.V.; Mokhova, E.N.; Skulachev, V.P. Involved of aspartate/glutamate antiporter in fatty acid-induced uncoupling of liver mitochondria. Biochim. Biophys. Acta 1997, 1339, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. Fatty acid circuit as physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991, 294, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Brustovetsky, N.N.; Dedukhova, V.I.; Egorova, M.V.; Mokhova, E.N.; Skulachev, V.P. Inhibitors of the ATP/ADP antiporter suppress stimulation of mitochondrial respiration and H+ permeability by palmitate and anionic detergents. FEBS Lett. 1990, 272, 187–189. [Google Scholar] [CrossRef]
- Brierley, G.P.; Jurkowitz, M.; Scott, K.M.; Merola, A.J. Ion transport by heart mitochondria. XX. Factors affecting passive osmotic swelling of isolated mitochondria. J. Biol. Chem. 1970, 245, 5404–5411. [Google Scholar] [CrossRef]
- Ponnalagu, D.; Singh, H. Anion channels of mitochondria. Handb. Exp. Pharmacol. 2017, 240, 71–101. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Schmitt, S.; Wimmer, R.; Aichler, M.; Eisenhofer, S.; Lichtmannegger, J.; Eberhagen, C.; Artmann, R.; Tookos, F.; Walch, A.; et al. Progressive stages of mitochondrial destruction caused by cell toxic bile salts. Biochim. Biophys. Acta 2013, 1828, 2121–2133. [Google Scholar] [CrossRef]
- Zimniak, P.; Little, J.M.; Radominska, A.; Oelberg, D.G.; Anwer, M.S.; Lester, R. Taurine-conjugated bile acids act as Ca2+ ionophores. Biochemistry 1991, 30, 8598–8604. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.M.; Leonard, M.R.; Batta, A.K.; Carey, M.C. Calcium affinity for biliary lipid aggregates in model biles: Complementary importance of bile salts and lecithin. Gastroenterology 1994, 107, 831–846. [Google Scholar] [CrossRef]
- Oelberg, D.G.; Dubinsky, W.P.; Adcock, E.W.; Lester, R. Calcium binding by lithocholic acid derivatives. Am. J. Physiol. 1984, 247 Pt 1, G112–G115. [Google Scholar] [CrossRef]
- Du, G.; Belić, D.; Del Giudice, A.; Alfredsson, V.; Carnerup, A.M.; Zhu, K.; Nyström, B.; Wang, Y.; Galantini, L.; Schillén, K. Condensed supramolecular helices: The twisted sisters of DNA. Angew. Chem. Int. Ed. 2022, 61, e202113279. [Google Scholar] [CrossRef] [PubMed]
- Oelberg, D.G.; Dubinsky, W.P.; Sackman, J.W.; Wang, L.B.; Adcock, E.W.; Lester, R. Bile salts induce calcium uptake in vitro by human erythrocytes. Hepatology 1987, 7, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Maenz, D.D.; Forsyth, G.W. Ricinoleate and deoxycholate are calcium ionophores in jejunal brush border vesicles. J. Membr. Biol. 1982, 70, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Montrose, M.H.; Lester, R.; Zimniak, P.; Anwer, M.S.; Murer, H. Bile acids increase cellular free calcium in cultured kidney cells (LLC-PK). Pflugers Arch. 1988, 412, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.S.; Little, J.M.; Oelberg, D.G.; Zimniak, P.; Lester, R. Effect of bile acids on calcium efflux from isolated rat hepatocytes and perfused rat livers. Proc. Soc. Exp. Biol. Med. 1989, 191, 147–152. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, X.; Liu, Q.; Wu, C.; Wang, Q.; Long, Z.; Li, L. Hydrophobic bile acids relax rat detrusor contraction via inhibiting the opening of the Na⁺/Ca²⁺ exchanger. Sci. Rep. 2016, 6, 21358. [Google Scholar] [CrossRef]
- De Stefani, D.; Rizzuto, R.; Pozzan, T. Enjoy the trip: Calcium in mitochondria back and forth. Annu. Rev. Biochem. 2016, 85, 161–192. [Google Scholar] [CrossRef]
- Petronilli, V.; Cola, C.; Massari, S.; Colonna, R.; Bernardi, P. Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J. Biol. Chem. 1993, 268, 21939–21945. [Google Scholar] [CrossRef]
- Sultan, A.; Sokolove, P. Palmitic acid opens anovel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 2001, 386, 31–51. [Google Scholar] [CrossRef]
- Mironova, G.D.; Belosludtsev, K.N.; Belosludtseva, N.V.; Gritsenko, E.N.; Khodorov, B.I.; Saris, N.E. Mitochondrial Ca2+ cycle mediated by the palmitate-activated cyclosporine A-insensitive pore. J. Bioenerg. Biomembr. 2007, 39, 167–174. [Google Scholar] [CrossRef]
- Rolo, A.P.; Oliveira, P.J.; Moreno, A.J.; Palmeira, C.M. Chenodeoxycholate induction of mitochondrial permeability transition pore is associated with increased membrane fluidity and cytochrome c release: Protective role of carvedilol. Mitochondrion 2003, 2, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Chavez, E.; Moreno-Sanchez, R.; Zazueta, C.; Rodriguez, J.S.; Bravo, C.; Reyes-Vivas, H. On the protection by inorganic phosphate of calcium-induced membrane permeability transition. J. Bioenerg. Biomembr. 1997, 29, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Basso, E.; Petronilli, V.; Forte, M.A.; Bernardi, P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 2008, 283, 26307–26311. [Google Scholar] [CrossRef] [PubMed]
- McGee, A.M.; Baines, C.P. Phosphate is not an absolute requirement for the inhibitory effects of cyclosporine A or cyclophilin D deletion on mitochondrial permeability transition. J. Biochem. 2012, 443, 185–191. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Samartsev, V.N.; Starinets, V.S.; Khoroshavina, E.I.; Belosludtsev, K.N. Induction of the Ca2+-dependent permeability transition in liver mitochondria by α,ω-hexadecanedioic acid is blocked by inorganic phosphate in the presence of cyclosporin A. Biochem. Mosc. Suppl. Ser. A 2019, 13, 58–66. [Google Scholar] [CrossRef]
- Elrod, J.W.; Wong, R.; Mishra, S.; Vagnozzi, R.J.; Sakthievel, B.; Goonasekera, S.A.; Karch, J.; Gabel, S.; Farber, J.; Force, T.; et al. Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Investig. 2010, 120, 3680–3687. [Google Scholar] [CrossRef]
- Barsukova, A.; Komarov, A.; Hajnoczky, G.; Bernardi, P.; Bourdette, D.; Forte, M. Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons. Eur. J. Neurosci. 2011, 33, 831–842. [Google Scholar] [CrossRef]
- Mironova, G.D.; Pavlov, E.V. Mitochondrial cyclosporine A-independent palmitate/Ca2+-induced permeability transition pore (PA-mPT Pore) and its role in mitochondrial function and protection against calcium overload and glutamate toxicity. Cells 2021, 10, 125. [Google Scholar] [CrossRef]
- Gores, G.J.; Miyoshi, H.; Botla, R.; Aguilar, H.I.; Bronk, S.F. Induction of the mitochondrial permeability transition as a mechanism of liver injury during cholestasis: A potential role for mitochondrial proteases. Biochim. Biophys. Acta 1998, 1366, 167–175. [Google Scholar] [CrossRef]
- Bernardi, P. Mitochondrial transport of cations: Channels, exchengers, and permeability transition. Physiol. Rev. 1999, 79, 1127–1155. [Google Scholar] [CrossRef]
- Szabo, I.; Zoratti, M. Mitochondrial channels: Ion fluxes and more. Physiol. Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef] [PubMed]
- Krylova, I.B.; Selina, E.N.; Bulion, V.V.; Rodionova, O.M.; Evdokimova, N.R.; Belosludtseva, N.V.; Shigaeva, M.I.; Mironova, G.D. Uridine treatment prevents myocardial injury in rat models of acute ischemia and ischemia/reperfusion by activating the mitochondrial ATP-dependent potassium channel. Sci. Rep. 2021, 11, 16999. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Starinets, V.S.; Belosludtseva, N.V.; Mikheeva, I.B.; Chelyadnikova, Y.A.; Penkina, D.K.; Vedernikov, A.A.; Belosludtsev, K.N. The effect of uridine on the state of skeletal muscles and the functioning of mitochondria in Duchenne dystrophy. Int. J. Mol. Sci. 2022, 23, 10660. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).