Influence of Humidity and Heating Rate on the Continuous ZIF Coating during Hydrothermal Growth
Abstract
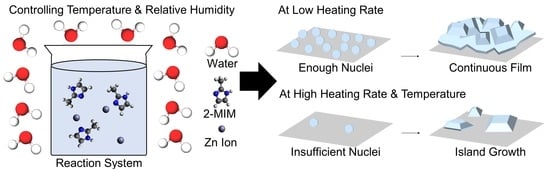
1. Introduction
2. Materials and Methods
2.1. Materials
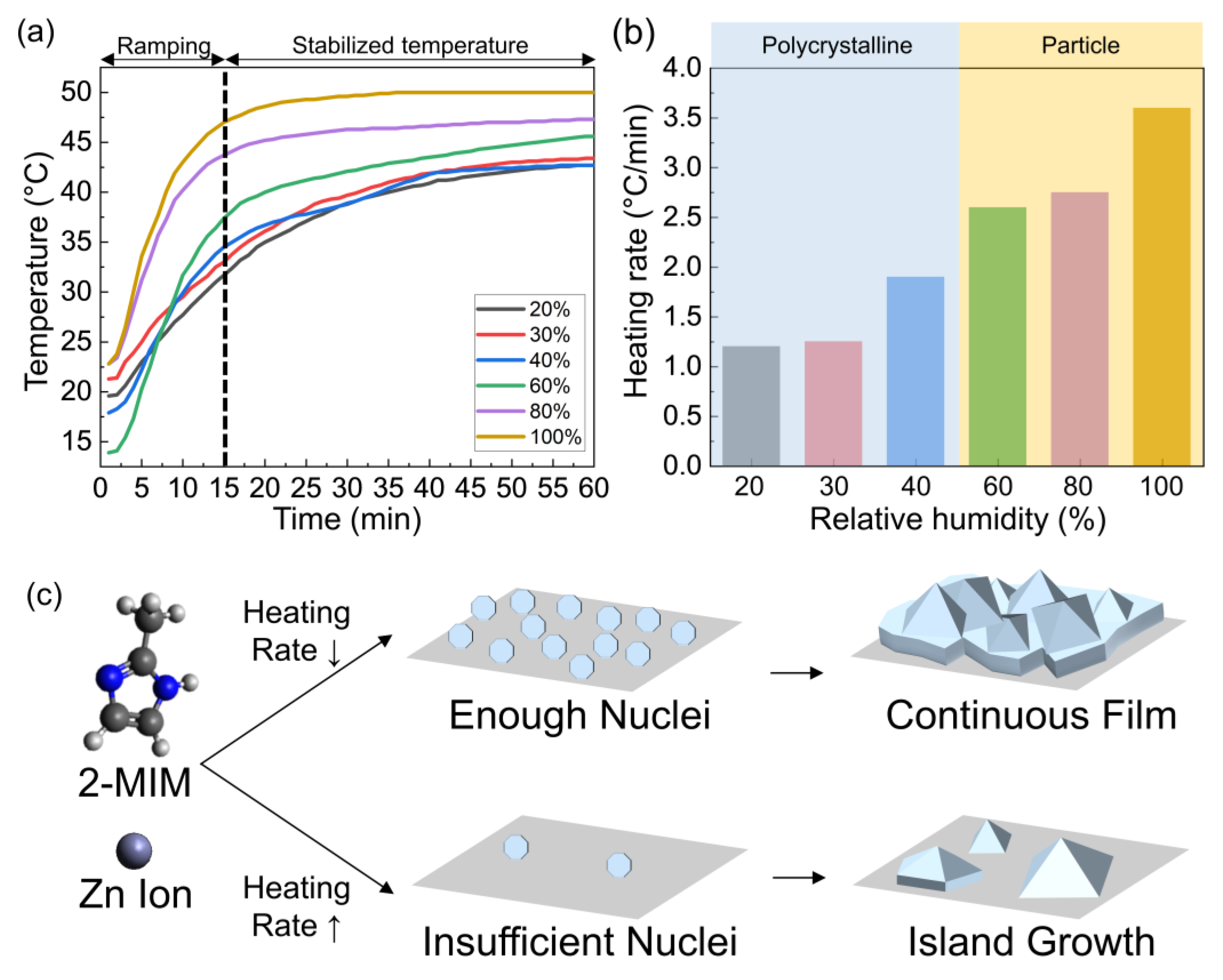
2.2. Synthesis of ZIF-8 Layer with Different Humidity and Temperature
2.3. Characterization
2.4. Measuring the Size Distribution of ZIF-8 Crystal Grain Size
3. Results and Discussions
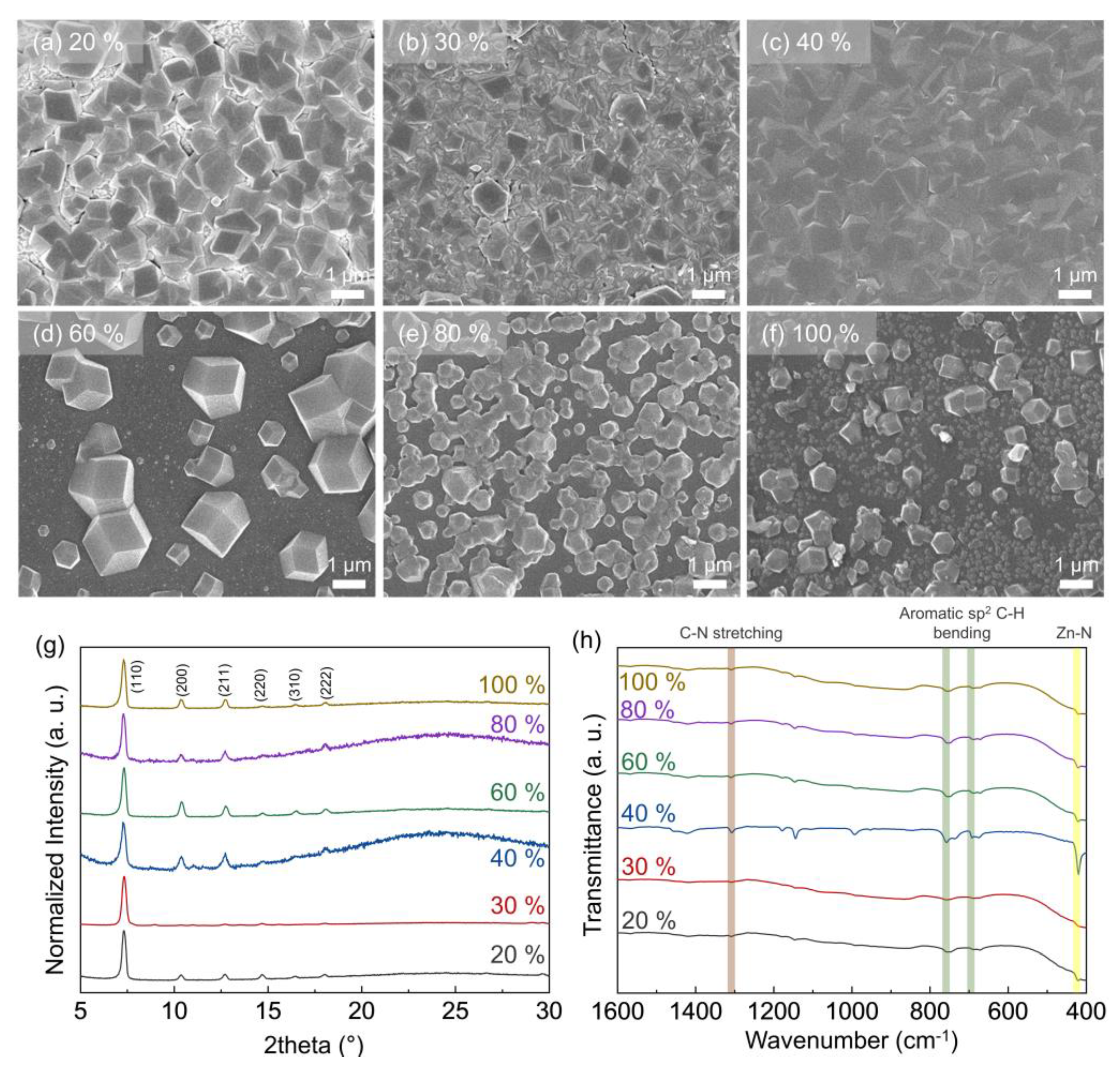
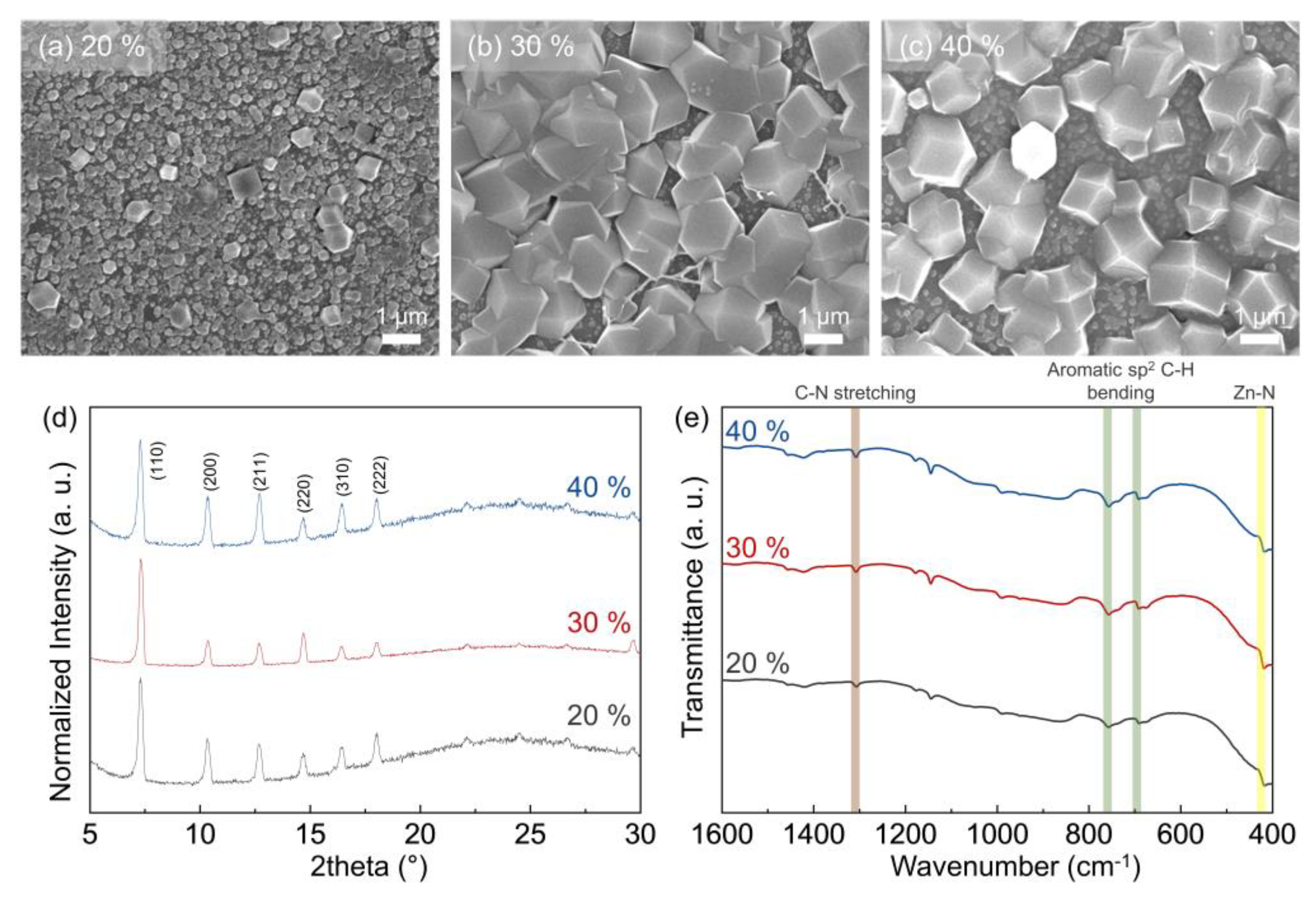
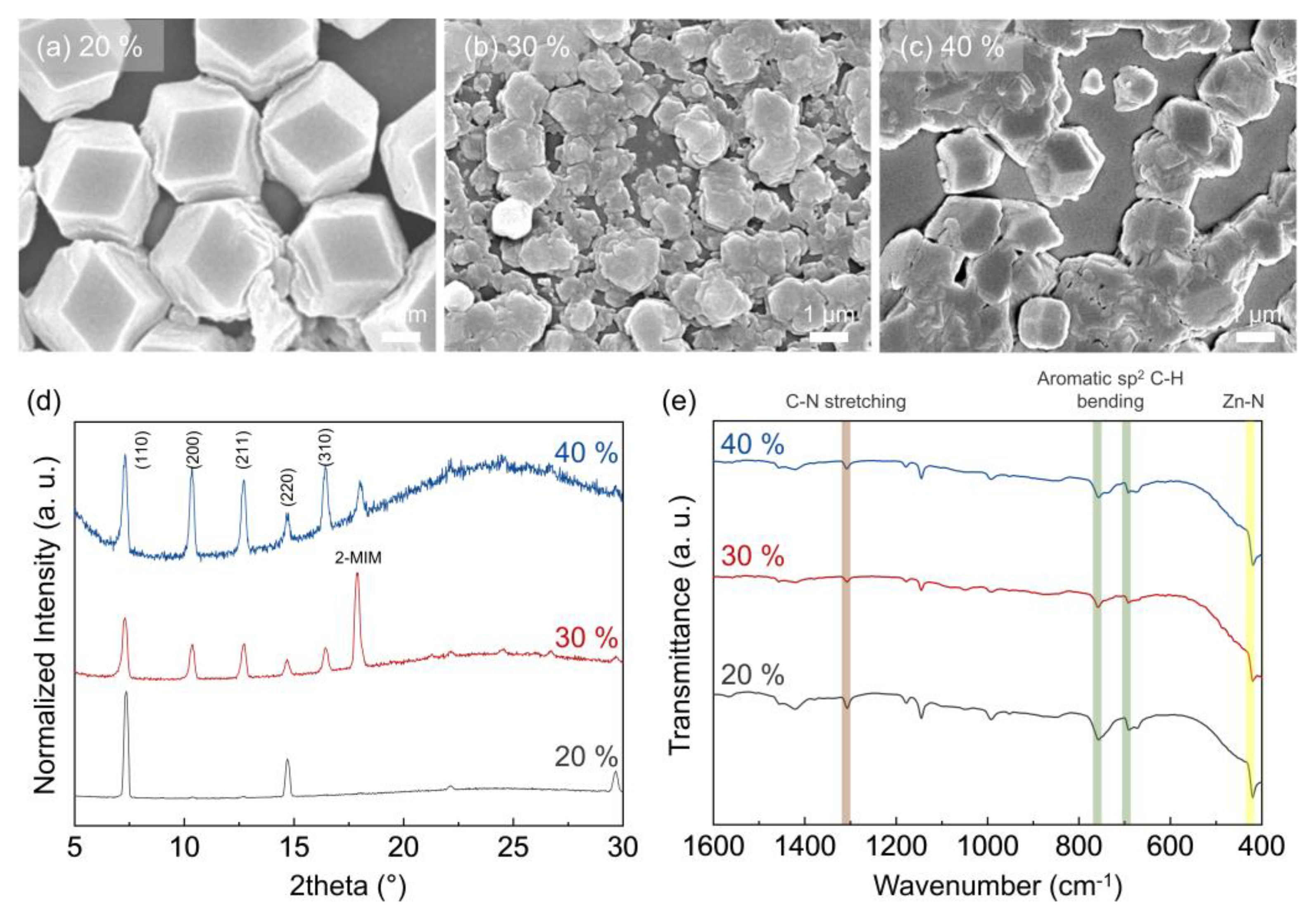
3.1. Morphology of ZIF-8 Crystals Depending on Chamber Temperature and Relative Humidity
3.2. Particle and Grain Size Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Dong, X.; Colombo, V.; Wang, Q.; Liu, Y.; Liu, W.; Wang, X.L.; Huang, X.Y.; Proserpio, D.M.; Sironi, A.; et al. Tailor-Made Microporous Metal–Organic Frameworks for the Full Separation of Propane from Propylene Through Selective Size Exclusion. Adv. Mater. 2018, 30, 1805088. [Google Scholar] [CrossRef]
- Cui, W.G.; Hu, T.L.; Bu, X.H. Metal–Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2019, 32, 1806445. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, J. Designer Metal–Organic Frameworks for Size-Exclusion-Based Hydrocarbon Separations: Progress and Challenges. Adv. Mater. 2020, 32, 2002603. [Google Scholar] [CrossRef]
- Li, J.; Bhatt, P.M.; Li, J.; Eddaoudi, M.; Liu, Y. Recent Progress on Microfine Design of Metal–Organic Frameworks: Structure Regulation and Gas Sorption and Separation. Adv. Mater. 2020, 32, 2002563. [Google Scholar] [CrossRef] [PubMed]
- Krokidas, P.; Moncho, S.; Brothers, E.N.; Castier, M.; Economou, I.G. Tailoring the gas separation efficiency of metal organic framework ZIF-8 through metal substitution: A computational study. Phys. Chem. Chem. Phys. 2018, 20, 4879–4892. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Hong, S.J.; Kim, Y.J.; Choi, S.E.; Choi, Y.; Kim, J.H.; Kang, J.; Kwon, O.; Eum, K.; Han, B.; et al. Pore Tuning of Metal-Organic Framework Membrane Anchored on Graphene-Oxide Nanoribbon. Adv. Funct. Mater. 2021, 31, 2011146. [Google Scholar] [CrossRef]
- Choi, E.; Choi, J.I.; Kim, Y.J.; Kim, Y.J.; Eum, K.; Choi, Y.; Kwon, O.; Kim, M.; Choi, W.; Ji, H.; et al. Graphene Nanoribbon Hybridization of Zeolitic Imidazolate Framework Membranes for Intrinsic Molecular Separation. Angew. Chem. Int. Ed. 2022, 61, e202214269. [Google Scholar] [CrossRef]
- Fan, W.; Ying, Y.; Peh, S.B.; Yuan, H.; Yang, Z.; Yuan, Y.D.; Shi, D.; Yu, X.; Kang, C.; Zhao, D. Multivariate Polycrystalline Metal–Organic Framework Membranes for CO2/CH4 Separation. J. Am. Chem. Soc. 2021, 143, 17716–17723. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kwon, H.T.; Jeong, H.K. High-Flux Zeolitic Imidazolate Framework Membranes for Propylene/Propane Separation by Postsynthetic Linker Exchange. Angew. Chem. Int. Ed. 2017, 57, 156–161. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.-F.; Yao, J.; Wang, H. Contra-diffusion synthesis of metal-organic framework separation membranes: A review. Sep. Pur. Technol. 2022, 300, 121837. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Z.Y.; Zhu, P.W.; Mohamed, A.R.; Chai, S.-P. A well inter-grown ZIF-8 membrane synthesized via two-step hydrothermal synthesis on coarse α-Al2O3 support. Mater. Lett. 2014, 129, 162–165. [Google Scholar] [CrossRef]
- Eum, K.; Rownaghi, A.; Choi, D.; Bhave, R.R.; Jones, C.W.; Nair, S. Fluidic Processing of High-Performance ZIF-8 Membranes on Polymeric Hollow Fibers: Mechanistic Insights and Microstructure Control. Adv. Funct. Mater. 2016, 26, 5011–5018. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Lyu, L.; Hou, Q.; Caro, J.; Wang, H. Flexible Polypropylene-Supported ZIF-8 Membranes for Highly Efficient Propene/Propane Separation. J. Am. Chem. Soc. 2020, 142, 20915–20919. [Google Scholar] [CrossRef]
- Neelakanda, P.; Barankova, E.; Peinemann, K.-V. Polymer supported ZIF-8 membranes by conversion of sputtered zinc oxide layers. Microporous Mesoporous Mater. 2016, 220, 215–219. [Google Scholar] [CrossRef]
- Valadez Sánchez, E.P.; Gliemann, H.; Haas-Santo, K.; Wöll, C.; Dittmeyer, R. ZIF-8 SURMOF Membranes Synthesized by Au-Assisted Liquid Phase Epitaxy for Application in Gas Separation. Chem. Ing. Tech. 2016, 88, 1798–1805. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K. In Situ Synthesis of Thin Zeolitic–Imidazolate Framework ZIF-8 Membranes Exhibiting Exceptionally High Propylene/Propane Separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Barkova, M.I.; Kustov, L.M.; Syrtsova, D.A.; Efimova, E.A.; Teplyakov, V.V. In situ synthesis of novel ZIF-8 membranes on polymeric and inorganic supports. J. Mater. Chem. A 2015, 3, 7469–7476. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Pan, J.H.; Steinbach, F.; Caro, J. In situ synthesis of MOF membranes on ZnAl-CO3 LDH buffer layer-modified substrates. J. Am. Chem. Soc. 2014, 136, 14353–14356. [Google Scholar] [CrossRef]
- Shah, M.; Kwon, H.T.; Tran, V.; Sachdeva, S.; Jeong, H.-K. One step in situ synthesis of supported zeolitic imidazolate framework ZIF-8 membranes: Role of sodium formate. Microporous Mesoporous Mater. 2013, 165, 63–69. [Google Scholar] [CrossRef]
- He, M.; Yao, J.; Li, L.; Zhong, Z.; Chen, F.; Wang, H. Aqueous solution synthesis of ZIF-8 films on a porous nylon substrate by contra-diffusion method. Microporous Mesoporous Mater. 2013, 179, 10–16. [Google Scholar] [CrossRef]
- Ramu, G.; Lee, M.; Jeong, H.-K. Effects of zinc salts on the microstructure and performance of zeolitic-imidazolate framework ZIF-8 membranes for propylene/propane separation. Microporous Mesoporous Mater. 2018, 259, 155–162. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohsaki, S.; Hanafusa, T.; Takada, K.; Tanaka, H.; Mae, K.; Miyahara, M.T. Synthesis of zeolitic imidazolate framework-8 particles of controlled sizes, shapes, and gate adsorption characteristics using a central collision-type microreactor. Chem. Eng. J. 2017, 313, 724–733. [Google Scholar] [CrossRef]
- Yao, J.; Li, L.; Benjamin Wong, W.H.; Tan, C.; Dong, D.; Wang, H. Formation of ZIF-8 membranes and crystals in a diluted aqueous solution. Mater. Chem. Phys. 2013, 139, 1003–1008. [Google Scholar] [CrossRef]
- Bustamante, E.L.; Fernández, J.L.; Zamaro, J.M. Influence of the solvent in the synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals at room temperature. J. Colloid Interface Sci. 2014, 424, 37–43. [Google Scholar] [CrossRef]
- Nagai, N.; Hashimoto, H. FT-IR-ATR study of depth profile of SiO2 ultra-thin films. Appl. Surf. Sci. 2001, 172, 307–311. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, Y. A Simple Step toward Enhancing Hydrothermal Stability of ZIF-8. ACS Omega 2019, 4, 19905–19912. [Google Scholar] [CrossRef]
- Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal Growth of ZIF-8, ZIF-67, and Their Mixed-Metal Derivatives. J. Am. Chem. Soc. 2018, 140, 1812–1823. [Google Scholar] [CrossRef]
- Wu, R.; Fan, T.; Chen, J.; Li, Y. Synthetic Factors Affecting the Scalable Production of Zeolitic Imidazolate Frameworks. ACS Sustain. Chem. Eng. 2019, 7, 3632–3646. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, Q.; Luan, C. The Thermodynamic Relations between the Melting Point and the Size of Crystals. J. Colloid Interface Sci. 2001, 243, 388–390. [Google Scholar] [CrossRef]
- Choi, E.; Lee, J.; Kim, Y.-J.; Kim, H.; Kim, M.; Hong, J.; Kang, Y.C.; Koo, C.M.; Kim, D.W.; Kim, S.J. Enhanced Stability of Ti3C2Tx MXene Enabled by Continuous ZIF-8 Coating. Carbon 2022, 191, 593–599. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, M.; Choi, E.; Bae, J.H.; Yoo, S.; Won, J.C.; Kim, Y.H.; Shin, J.H.; Lee, J.S.; Kim, D.W. High-aspect Ratio Zeolite Framework (ZIF) Nanoplates for Hydrocarbon Separation Membranes. Sci. Adv. 2022, 8, eabl6841. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.; Lee, C.-H.; Kim, D.W. Influence of Humidity and Heating Rate on the Continuous ZIF Coating during Hydrothermal Growth. Membranes 2023, 13, 414. https://doi.org/10.3390/membranes13040414
Choi E, Lee C-H, Kim DW. Influence of Humidity and Heating Rate on the Continuous ZIF Coating during Hydrothermal Growth. Membranes. 2023; 13(4):414. https://doi.org/10.3390/membranes13040414
Chicago/Turabian StyleChoi, Eunji, Choong-Hoo Lee, and Dae Woo Kim. 2023. "Influence of Humidity and Heating Rate on the Continuous ZIF Coating during Hydrothermal Growth" Membranes 13, no. 4: 414. https://doi.org/10.3390/membranes13040414
APA StyleChoi, E., Lee, C.-H., & Kim, D. W. (2023). Influence of Humidity and Heating Rate on the Continuous ZIF Coating during Hydrothermal Growth. Membranes, 13(4), 414. https://doi.org/10.3390/membranes13040414