The Immunosuppressive Niche Established with a Curcumin-Loaded Electrospun Nanofibrous Membrane Promotes Cartilage Regeneration in Immunocompetent Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Cur/PLGA Nanofibrous Membrane
2.2. Characterization of the Cur/PLGA Nanofibrous Membrane
2.3. Chondrocyte and RAW264.7 Macrophage Culturing
2.4. Evaluation of the Cytocompatibility of the Cur/PLGA Nanofibrous Membrane
2.5. Evaluation of In Vitro Anti-Inflammatory Effect
2.6. In Vitro Cartilage Regeneration
2.7. Subcutaneous Implantation
2.8. Statistical Analysis
3. Results
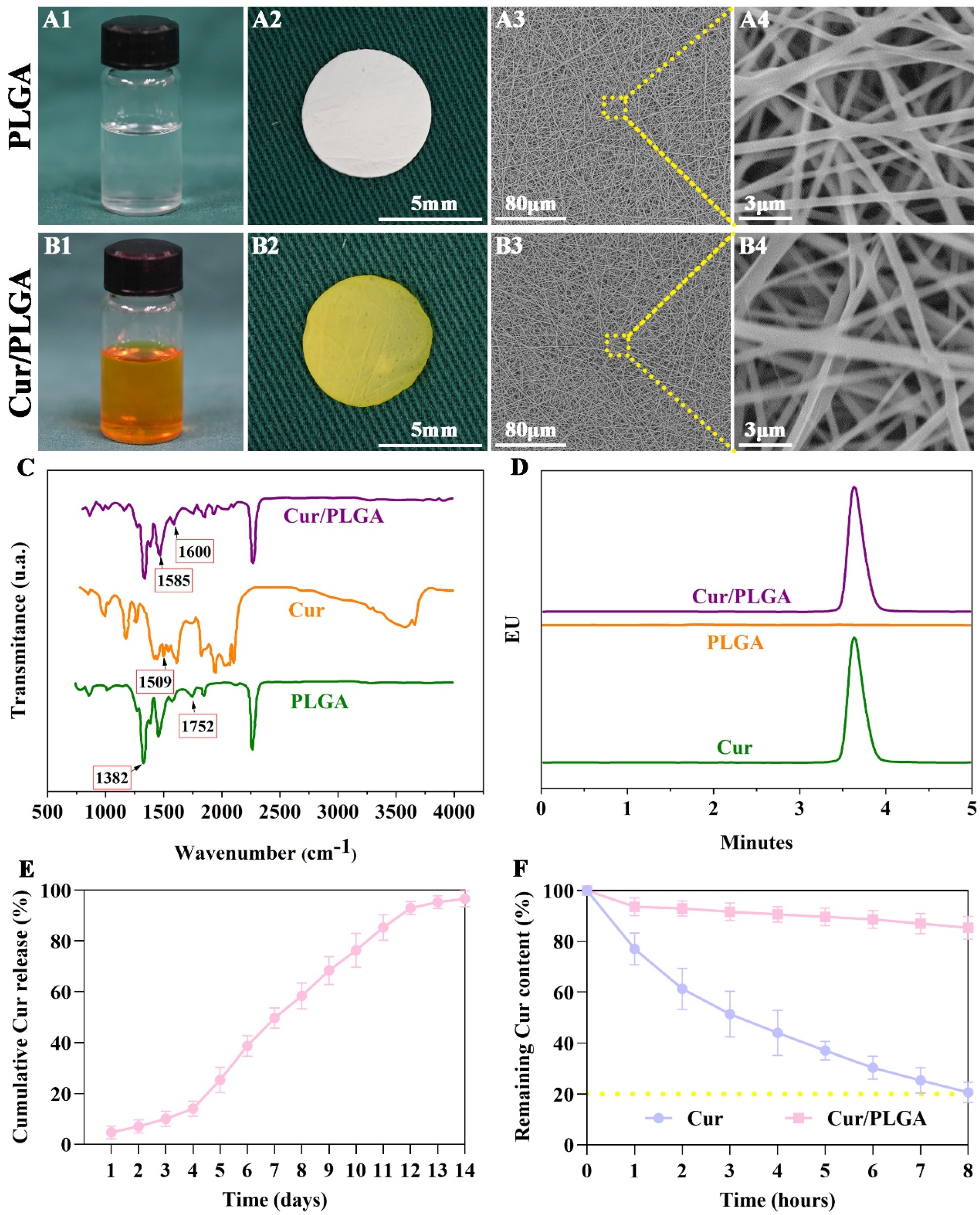
3.1. The Cur/PLGA Nanofibrous Membrane Was Successfully Developed with Sustained Release Kinetics and Satisfactory Stability of Cur
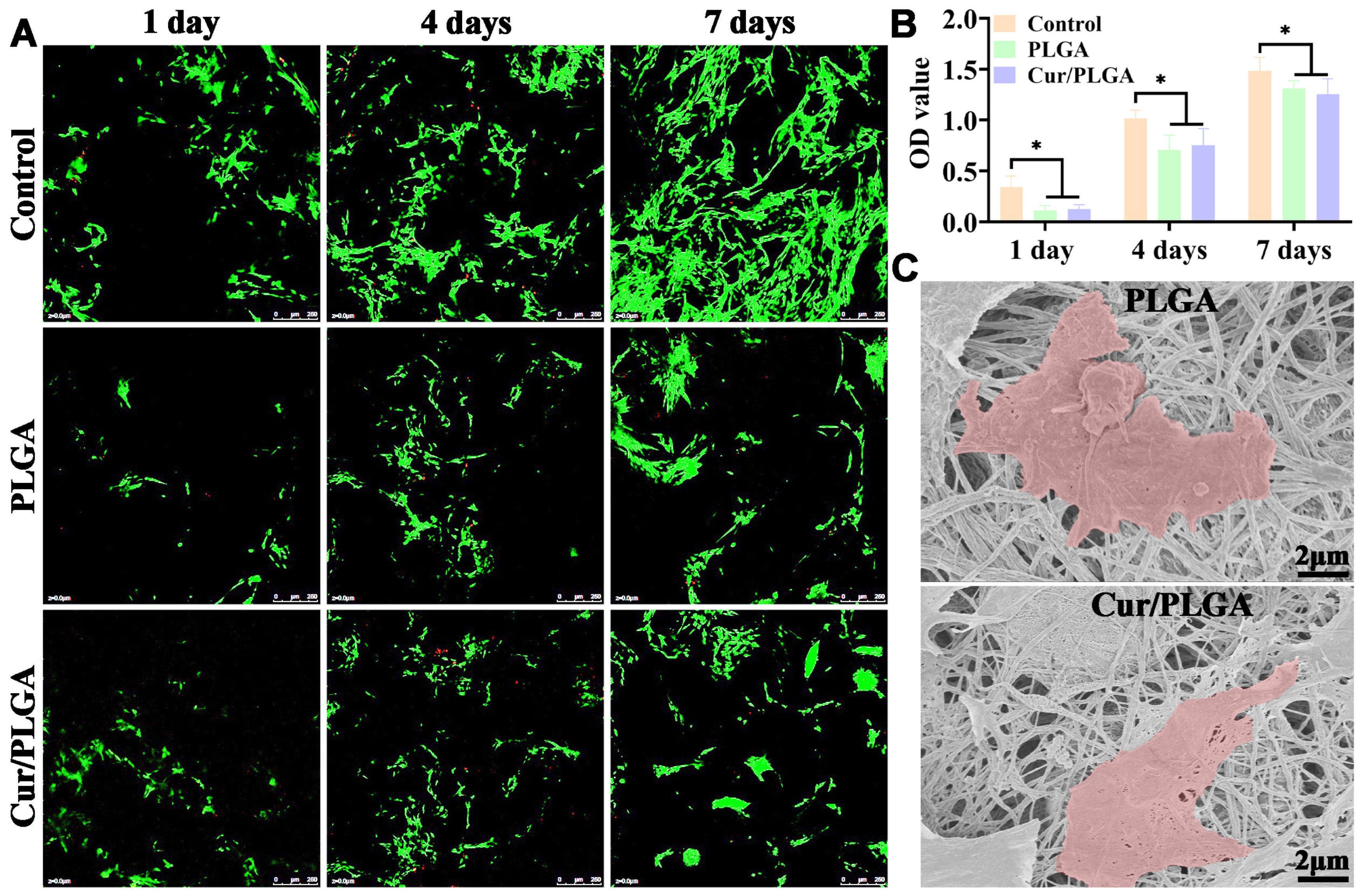
3.2. The Synthesized Cur/PLGA Nanofibrous Membrane Is Cytocompatible
3.3. The Cur/PLGA Nanofibrous Membrane Exerted a Satisfactory Anti-Inflammatory Effect In Vitro
3.4. The Successful Generation of Cartilage Tissue In Vitro
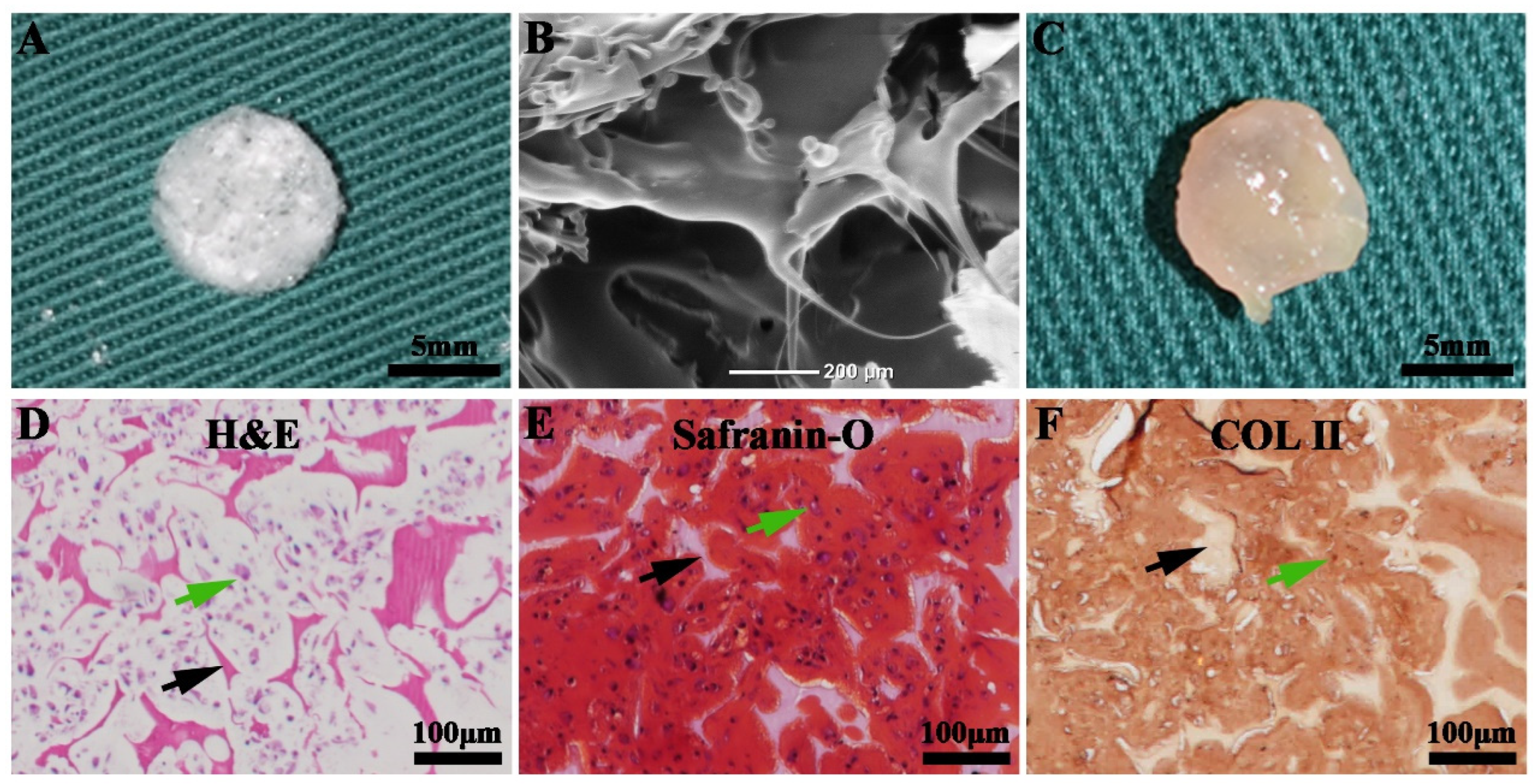
3.5. Enhanced Cartilage Regeneration via Subcutaneous Implantation of Nanofibrous-Membrane-Packaged Cartilage Tissue
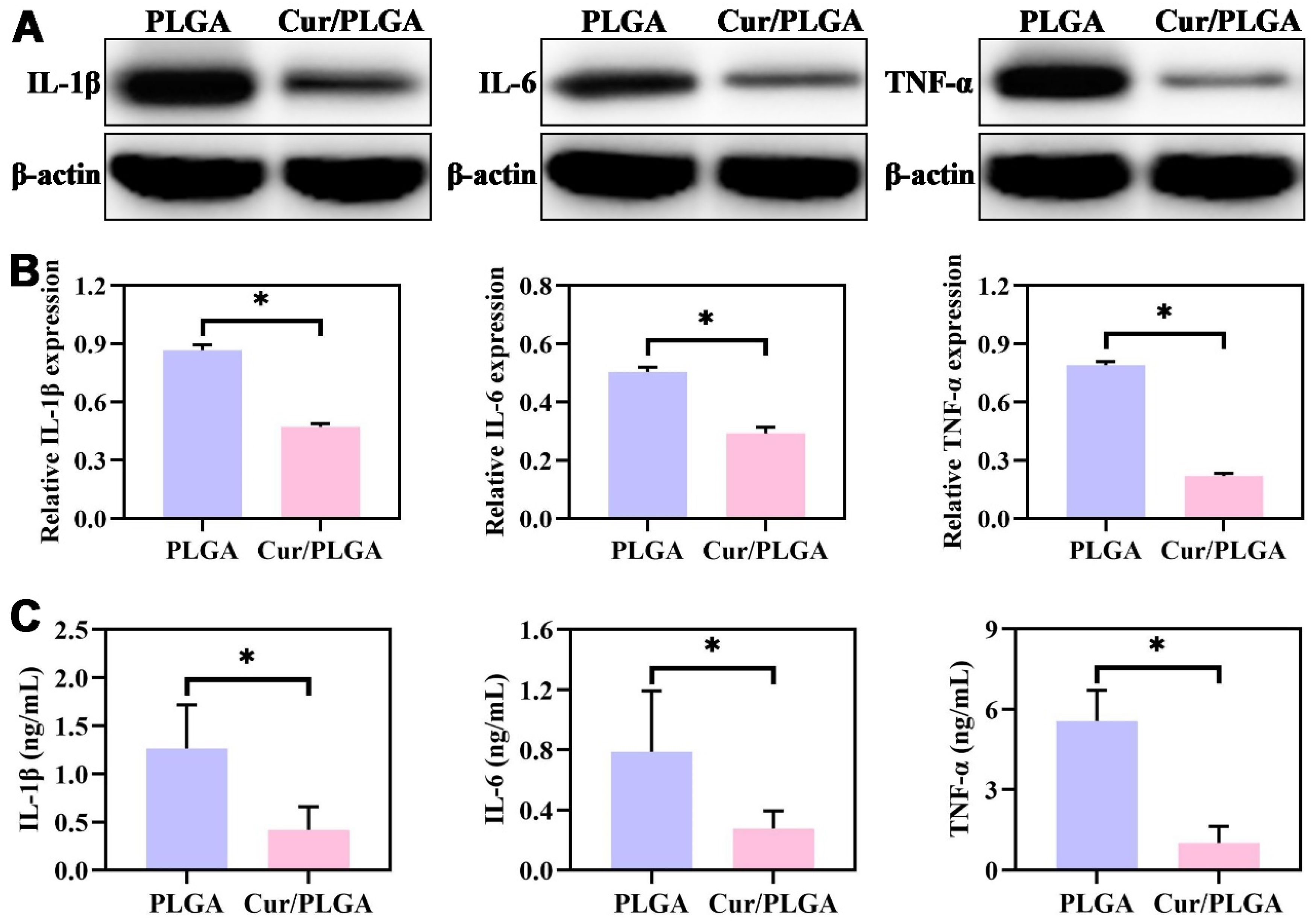
3.6. The Cur/PLGA Nanofibrous Membrane Exhibited a Favorable Anti-Inflammatory Effect In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Guo, Y.F.; Li, Y.Q.; Huo, Y.Y.; She, Y.L.; Li, H.; Jia, Z.H.; Jiang, G.N.; Zhou, G.D.; You, Z.W.; et al. Biomimetic Trachea Regeneration Using a Modular Ring Strategy Based on Poly(Sebacoyl Diglyceride)/Polycaprolactone for Segmental Trachea Defect Repair. Adv. Funct. Mater. 2020, 30, 2004276. [Google Scholar] [CrossRef]
- Cao, R.F.; Xu, Y.; Xu, Y.; Brand, D.D.; Zhou, G.D.; Xiao, K.Y.; Xia, H.T.; Czernuszka, J.T. Development of Tri-Layered Biomimetic Atelocollagen Scaffolds with Interfaces for Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2022, 11, 2101643. [Google Scholar] [CrossRef] [PubMed]
- Davachi, S.M.; Haramshahi, S.M.A.; Akhavirad, S.A.; Bahrami, N.; Hassanzadeh, S.; Ezzatpour, S.; Hassanzadeh, N.; Malekzadeh Kebria, M.; Khanmohammadi, M.; Bagher, Z. Development of chitosan/hyaluronic acid hydrogel scaffolds via enzymatic reaction for cartilage tissue engineering. Mater. Today Commun. 2022, 30, 103230. [Google Scholar] [CrossRef]
- Lin, L.Q.; Xu, Y.W.; Li, Y.Q.; Gong, X.D.; Wei, M.; Zhang, W.; Zhang, X.; Xu, Y. Nanofibrous Wharton’s jelly scaffold in combination with adipose-derived stem cells for cartilage engineering. Mater. Design 2020, 186, 108216. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In Vitro Regeneration of Patient-specific Ear-shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. EBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhao, S.; Jian, Y.; Shao, X.; Han, D.; Zhang, F.; Liang, C.; Liu, W.; Fan, J.; Yang, Z.; et al. Icariin-loaded hydrogel with concurrent chondrogenesis and anti-inflammatory properties for promoting cartilage regeneration in a large animal model. Front. Cell Dev. Biol. 2022, 10, 1011260. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef]
- Fu, S.; Meng, H.; Inamdar, S.; Das, B.; Gupta, H.; Wang, W.; Thompson, C.L.; Knight, M.M. Activation of TRPV4 by mechanical, osmotic or pharmaceutical stimulation is anti-inflammatory blocking IL-1beta mediated articular cartilage matrix destruction. Osteoarthr. Cartil. 2021, 29, 89–99. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, T.; Yi, B.; Wang, X.; Tang, H.; Liu, W.; Zhang, Y. Electrospun acid-neutralizing fibers for the amelioration of inflammatory response. Acta Biomater. 2019, 97, 200–215. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhou, G.; Liu, Y.; Cao, Y. Biological Evaluation of Acellular Cartilaginous and Dermal Matrixes as Tissue Engineering Scaffolds for Cartilage Regeneration. Front. Cell Dev. Biol. 2020, 8, 624337. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, P.; Ci, Z.; Zhang, W.; Liu, Y.; Jiang, H.; Zhou, G. Immune-Inflammatory Responses of an Acellular Cartilage Matrix Biomimetic Scaffold in a Xenotransplantation Goat Model for Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 667161. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yin, Z.; Liu, Y.; Feng, S.; Liu, Y.; Lu, F.; Xu, Y.; Min, P.; Hou, M.; Li, K.; et al. Regeneration of trachea graft with cartilage support, vascularization, and epithelization. Acta Biomater. 2019, 89, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Yin, Z.Q.; Luo, X.S.; Liu, W.; Zhang, W.J.; Zhang, Z.Y.; Cao, Y.L.; Liu, Y.; Zhou, G.D. Prolonged in vitro precultivation alleviates post-implantation inflammation and promotes stable subcutaneous cartilage formation in a goat model. Biomed. Mater. 2017, 12, 015006. [Google Scholar] [CrossRef]
- Yang, M.L.; Sun, W.Y.; Wang, L.; Tang, H.; Xu, X.; Yang, L.W.; Ni, J.J.; Zheng, K.E.; Jiang, X.; Xu, W.W.; et al. Curcumin loaded polycaprolactone scaffold capable of anti-inflammation to enhance tracheal cartilage regeneration. Mater. Des. 2022, 224, 111299. [Google Scholar] [CrossRef]
- Gao, E.; Wang, P.; Chen, F.; Xu, Y.; Wang, Q.; Chen, H.; Jiang, G.; Zhou, G.; Li, D.; Liu, Y.; et al. Skin-derived epithelial lining facilitates orthotopic tracheal transplantation by protecting the tracheal cartilage and inhibiting granulation hyperplasia. Biomater. Adv. 2022, 139, 213037. [Google Scholar] [CrossRef]
- Vegas, A.J.; Veiseh, O.; Gurtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Xun, X.; Zhang, W.; Xu, Y.; Gu, D. Three-Dimensional Porous Scaffolds with Biomimetic Microarchitecture and Bioactivity for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 36359–36370. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, T.; Wang, Q.; Liu, J. Anti-inflammatory effect of combined tetramethylpyrazine, resveratrol and curcumin in vivo. BMC Complement. Altern. Med. 2017, 17, 233. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 316, 359–380. [Google Scholar] [CrossRef]
- Kozina, N.D.; Blokhin, A.N.; Kirila, T.U.; Razina, A.B.; Bursian, A.E.; Kurlykin, M.P.; Filippov, A.P.; Tenkovtsev, A.V. Synthesis of novel star-shaped calix [4]arene-based poly(2-oxazoline)s and study of their complexation with curcumin. Mater. Today Commun. 2023, 34, 105403. [Google Scholar] [CrossRef]
- Chen, W.M.; Xu, Y.; Li, Y.Q.; Jia, L.T.; Mo, X.M.; Jiang, G.N.; Zhou, G.D. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem. Eng. J. 2020, 382, 122986. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Yin, Z.; He, A.; Lin, M.; Jiang, G.; Song, X.; Hu, X.; Liu, Y.; Wang, J.; et al. Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique. Acta Biomater. 2017, 58, 113–121. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Y.; Yi, B.; Wang, X.; Tang, H.; Chen, C.; Zhang, Y. Engineering a Highly Biomimetic Chitosan-Based Cartilage Scaffold by Using Short Fibers and a Cartilage-Decellularized Matrix. Biomacromolecules 2021, 22, 2284–2297. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, J.; Zhu, X.; Cao, R.; Song, N.; Liu, M.; Liu, X.; Zhu, J.; Pan, F.; Qin, L.; et al. Biomimetic Trachea Engineering via a Modular Ring Strategy Based on Bone-Marrow Stem Cells and Atelocollagen for Use in Extensive Tracheal Reconstruction. Adv. Mater. 2022, 34, e2106755. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Duan, H.; Li, Y.Q.; She, Y.L.; Zhu, J.J.; Zhou, G.D.; Jiang, G.N.; Yang, Y. Nanofibrillar Decellularized Wharton’s Jelly Matrix for Segmental Tracheal Repair. Adv. Funct. Mater. 2020, 30, 1910067. [Google Scholar] [CrossRef]
- Gao, E.; Wang, Y.; Wang, P.; Wang, Q.; Wei, Y.; Song, D.; Xu, H.; Ding, J.; Xu, Y.; Xia, H.; et al. C-Shaped Cartilage Development Using Wharton’s Jelly-Derived Hydrogels to Assemble a Highly Biomimetic Neotrachea for use in Circumferential Tracheal Reconstruction. Adv. Funct. Mater. 2023, 2212830. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, G.; Liu, W.; Zhang, W.J.; Cen, L.; Cui, L.; Cao, Y. In vitro precultivation alleviates post-implantation inflammation and enhances development of tissue-engineered tubular cartilage. Biomed. Mater. 2009, 4, 025006. [Google Scholar] [CrossRef]
- Gao, E.; Li, G.; Cao, R.F.; Xia, H.T.; Xu, Y.; Jiang, G.N.; Xiao, K.Y.; Chen, J.; Chen, R.; Duan, L. Bionic tracheal tissue regeneration using a ring-shaped scaffold comprised of decellularized cartilaginous matrix and silk fibroin. Compos. Part B-Eng. 2022, 229, 109470. [Google Scholar] [CrossRef]
- Xu, Y.W.; Xu, Y.; Bi, B.; Hou, M.J.; Yao, L.; Du, Q.R.; He, A.J.; Liu, Y.; Miao, C.L.; Liang, X.Q.; et al. A moldable thermosensitive hydroxypropyl chitin hydrogel for 3D cartilage regeneration in vitro and in vivo. Acta Biomater. 2020, 108, 87–96. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Hua, Y.; Zhu, X.; Wang, Y.; Duan, L.; Zhu, L.; Jiang, G.; Xia, H.; She, Y.; et al. Photocrosslinked natural hydrogel composed of hyaluronic acid and gelatin enhances cartilage regeneration of decellularized trachea matrix. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111628. [Google Scholar] [CrossRef]
- Yamawaki, T.; Fujihara, Y.; Harata, M.; Takato, T.; Hikita, A.; Hoshi, K. Electron microscopic observation of human auricular chondrocytes transplanted into peritoneal cavity of nude mice for cartilage regeneration. Regen. Ther. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Okubo, R.; Asawa, Y.; Watanabe, M.; Nagata, S.; Nio, M.; Takato, T.; Hikita, A.; Hoshi, K. Proliferation medium in three-dimensional culture of auricular chondrocytes promotes effective cartilage regeneration in vivo. Regen. Ther. 2019, 11, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of Inflammatory Reaction in Health and Disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, Y.; Xu, X.; Zhu, J.; Chen, L.; Xu, Y.; Yang, Y.; Song, N. Bevacizumab-Laden Nanofibers Simulating an Antiangiogenic Niche to Improve the Submuscular Stability of Stem Cell Engineered Cartilage. Small 2022, 18, e2201874. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. Curcumin May (Not) Defy Science. ACS Med. Chem. Lett. 2017, 8, 467–470. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Franzone, F.; Nebbioso, M.; Pergolizzi, T.; Attanasio, G.; Musacchio, A.; Greco, A.; Limoli, P.G.; Artico, M.; Spandidos, D.A.; Taurone, S.; et al. Anti-inflammatory role of curcumin in retinal disorders (Review). Exp. Ther. Med. 2021, 22, 790. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, F.; Borran, S.; Ashrafizadeh, M.; Zarrabi, A.; Pourhanifeh, M.H.; Khaksary Mahabady, M.; Sahebkar, A.; Mirzaei, H. Curcumin and inflammatory bowel diseases: From in vitro studies to clinical trials. Mol. Immunol. 2021, 130, 20–30. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cai, R.; Li, J.; Wu, X. The Immunosuppressive Niche Established with a Curcumin-Loaded Electrospun Nanofibrous Membrane Promotes Cartilage Regeneration in Immunocompetent Animals. Membranes 2023, 13, 335. https://doi.org/10.3390/membranes13030335
Zhang Y, Cai R, Li J, Wu X. The Immunosuppressive Niche Established with a Curcumin-Loaded Electrospun Nanofibrous Membrane Promotes Cartilage Regeneration in Immunocompetent Animals. Membranes. 2023; 13(3):335. https://doi.org/10.3390/membranes13030335
Chicago/Turabian StyleZhang, Yu, Renzhong Cai, Jun Li, and Xu Wu. 2023. "The Immunosuppressive Niche Established with a Curcumin-Loaded Electrospun Nanofibrous Membrane Promotes Cartilage Regeneration in Immunocompetent Animals" Membranes 13, no. 3: 335. https://doi.org/10.3390/membranes13030335
APA StyleZhang, Y., Cai, R., Li, J., & Wu, X. (2023). The Immunosuppressive Niche Established with a Curcumin-Loaded Electrospun Nanofibrous Membrane Promotes Cartilage Regeneration in Immunocompetent Animals. Membranes, 13(3), 335. https://doi.org/10.3390/membranes13030335




