1. Introduction
Molecular sieve zeolite crystals can be embedded in polymer membranes to enhance selectivity and permeability in molecular and ionic separations [
1]. As schematically shown in
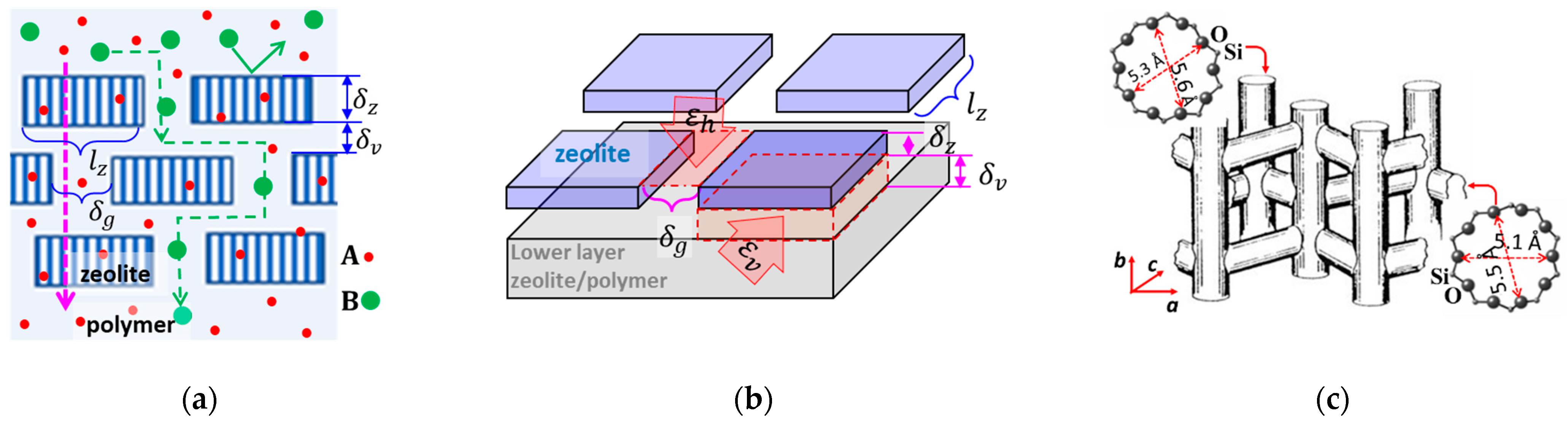
Figure 1a,b, the embedded discrete zeolite crystals allow penetration for molecules smaller than the zeolitic pore size while rejecting those larger than the pore size. The larger molecules or ions rejected by the zeolitic pores can penetrate through the membrane only by diffusing along the tortuous inter-crystal pathways at a reduced rate that improves the transport selectivity towards the smaller molecules. The selectivity of the composite membranes depends on the spaces between the embedded zeolite crystals and the individual crystal area and aspect ratio
, (where
and
are characteristic side length and thickness of a slab-shaped crystal, respectively). The selectivity of the composite membrane can be enhanced by reducing the inter-crystal spaces (i.e.,
and/or
) and increasing
when the zeolitic channels are oriented along the thickness.
Figure 1c illustrates the 3-dimensional channel system in the MFI-type zeolite, which comprises near-cylindrical straight channels along the
b-axis interconnected with sinusoidal channels running in the a–c plane [
2]. Because of the slightly bigger opening (dia. ~0.56 nm) and shorter length of the straight channels, an MFI zeolite membrane with thickness oriented in the
b-axis direction thus provides higher fluxes than those with other crystal orientations.
The 2-dimensional (2D) zeolite nanosheets (ZNs) with large
and nanometre
in the
b-axis direction offer the opportunity to construct zeolite-polymer composite membranes with high selectivity and low transport resistances. As depicted by
Figure 1b, for a membrane with monolayer ZNs, the separation selectivity depends on the width (
) and porosity (
) of horizontal inter-ZN gaps in the membrane surface. The molecular or ion sieving selectivity of such monolayer ZN membranes can be maximized by converting the discrete ZNs into an intergrown polycrystalline film via secondary growth that leads to
and
[
3].
However, the formation of an intergrown monolayer ZN membrane requires smooth inorganic substrates to avoid misalignments of the ZN layer and allow zeolite activation after the secondary growth that is impractical for polymer substrates. A ZN-based membrane on more affordable and amendable polymer supports without secondary growth for horizontal inter-ZN gap elimination is practically desirable. Recently, multilayered MFI-type ZN-laminated membranes (ZNLM) on porous polymer films have been demonstrated to offer high selectivity and fluxes in molecular separations [
4,
5]. As schematically shown in
Figure 1a,b, in the multilayered ZNLMs, the
is inevitably much larger than the
between overlapping ZNs; hence, the membrane selectivity is mainly determined by the width (
) and porosity (
) of inter-ZN spaces in thickness direction. The
may be estimated by
The polymer-supported ultrathin ZNLM layers without binders [
4] or with minimal polymer binders between ZNs [
5] could minimize
to substantially improve the selectivity and flux compared with conventional composite membranes of polymers embedded with isotropic zeolite crystals. Recently, the MFI-type ZNs have been attracting growing interest for membrane development because of the potential to achieve high performances in many impactful petrochemical purification and ion separation processes permitted by its 10-membered ring medium pore size and extraordinary structural stability [
6,
7,
8].
In the literature, single crystal MFI ZNs have been synthesized by a silicalite nanoparticle-seeded secondary growth method using the diquaternary bis-1,5(tripropyl ammonium) pentamethylene diiodide (dC
5) as a structure-directing agent (SDA) [
6,
7]. The as-synthesized ZNs are impermeable because the zeolitic pores are occupied by the large SDA molecules. These SDA molecules are most effectively and conveniently removed by calcination in air at >450 °C. However, the high-temperature calcination process inevitably causes aggregation of the ZNs, which are difficult to breakup or redisperse. Thus, synthesis of ZNLMs on polymer substrates, which requires suspensions of well-dispersed open-pore ZNs, has been challenging with limited progresses reported so far.
To avoid the solid-state irreversible aggregation, Zhang et al. [
4] activated pure-silica MFI (i.e., silicalite) ZNs (
~1.0 um,
~3.2 nm, and
~312) by multiple cycles of treatment in piranha solution (H
2SO
4:H
2O
2 volume ratio of 3:1) to decompose the C
22H
45–N
+(CH
3)
2–C
6H
12–N
+(CH
3)
2-C
6H
13](Br
2) (C
22-6-6) SDA. This liquid phase activation process provided well-dispersed open-pore ZN suspensions. The preactivated ZN suspension was used for coating binder-free ZNLMs on porous polybenzimidazole supports by vacuum filtration. The binder-free ZNLM had minimized
to reduce the less selective inter-ZN transport, and that in turn achieved the molecular sieving effect between
n-butane and
i-butane. However, the binder-free multilayered ZNLM is likely to lack structural stability in liquid phase, especially in electrolyte solutions where the layered ZNs could disintegrate and redisperse upon surface solvation or ionization.
In a previous work, we synthesized aluminum-containing MFI (i.e., ZSM-5) ZNLM on porous polyvinylidene fluoride (PVDF) support using a minimal amount of PVDF as binder to prevent the multilayered ZNs from dissociation in concentrated slat solutions [
5]. The suspension of open-pore ZSM-5 ZNs was obtained after calcination by multiple cycles of sonicated dispersion and sedimentation and centrifugation to remove the ZN agglomerates. The individual ZSM-5 ZNs had
~0.85 um,
~7.0–7.5 nm, and
~113. The ZNLM exhibited nearly perfect slat rejection (>99%) and high water flux (>6 L/h‧m
2) during pervaporation desalination of brines with total dissolved salts of ~22 wt.%. The efficient ion rejection was achieved because the nanometer-scale
effectively limited the inter-ZN salt migration under fast water transport rates through the ZNs. However, this ZNLM was unable to exhibit the molecular sieving effect for
n-/
i-butane because its
was enlarged by the inserted binder polymer chains compared with that of the binder-free ZNMLs [
4].
More recently, the MFI-type ZNs were used to fabricate a composite membrane by embedding well-aligned ZNs in a Nafion
® matrix film [
8]. This ZN-embedded Nafion membrane exhibited improved proton-to-vanadyl ion selectivity because of the ion sieving effect of the MFI pores without increasing the electric resistance due to the nanometer thickness of ZNs [
8,
9]. This ZN-Nafion mixed matrix membrane was demonstrated as an ion separator to significantly improve the efficiency of the vanadium redox flow battery (VRFB) compared with the pure Nafion membrane. However, the use of expensive ionic polymers, especially the environmentally problematic sulfonated tetrafluoroethylene-based polymer (e.g., Nafion
®), is practically unfavorable. On the other hand, studies on the fabrication and ion conduction behavior for ZNLMs coated on ubiquitous nonionic polymer supports are so far very limited. In the present work, we investigate the MFI ZN activation by different methods for coating ultrathin ZNLMs on the nonionic macroporous PVDF and Nafion
® films, respectively. The synthesized ZNLMs are studied for proton-selective ion conduction in electrolyte solutions.
2. Experimental
2.1. Chemicals and Materials
The chemicals and materials used in this work included tetraethyl orthosilicate (TEOS, >99%, Sigma-Aldrich, St. Louis, MO, USA), tetrapropylammonium hydroxide (TPAOH, 1 M aqueous, Sigma-Aldrich, USA), sodium aluminate (50–56 wt.% as Al2O3, Sigma-Aldrich, St. Louis, USA), sulfuric acid (95–98%, Sigma-Aldrich, St. Louis, USA), vanadium(IV) sulfate oxide hydrate (99.9%, Alfa Aesar, Haverhill, MA, USA), magnesium sulfate (≥99.5%, Sigma-Aldrich), hydrogen peroxide (30%, Fisher Scientific, Waltham, MA, USA), Nafion ® perfluorinated resin solution (20%, Sigma-Aldrich, St. Louis, USA), ethanol (>99.5%, Sigma-Aldrich, USA), methanol (>99.8%, Sigma-Aldrich, St. Louis, USA), N,N-dimethylformamide (DMF, 99.8%, Sigma-Aldrich, St. Louis, USA), dimethyl sulfoxide (DMSO, 99.8%, Sigma-Aldrich, St. Louis, USA), hydrophilic surface PDVF film (average pore diameter dp of 0.22 µm and porosity of >80%, TISCH Scientific, Fremont, CA, USA), hydrophilic surface PVDF film (dp~0.45 µm and ~80%, TISCH Scientific, CA, USA), and deionized water.
2.2. Material Characterizations
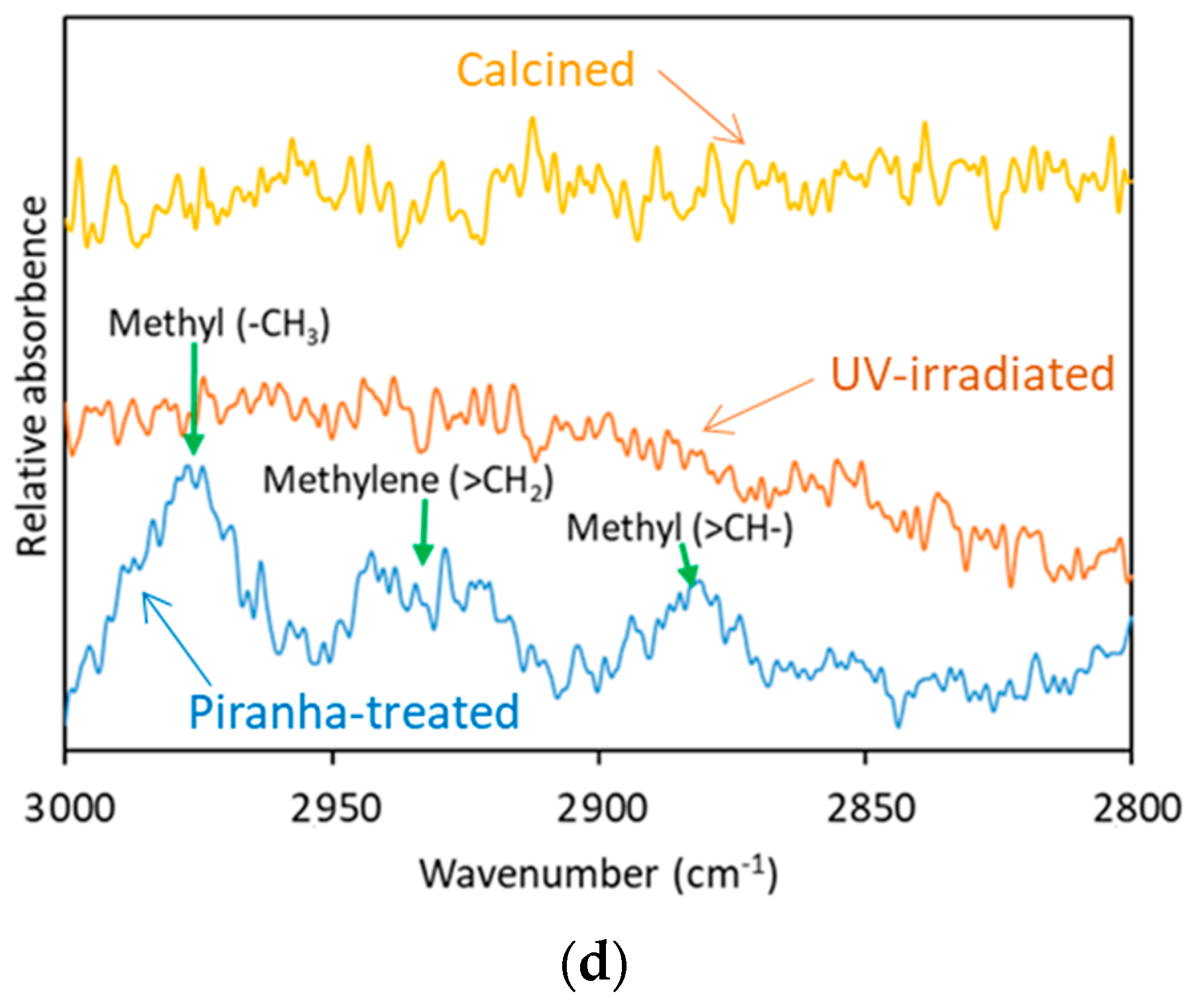
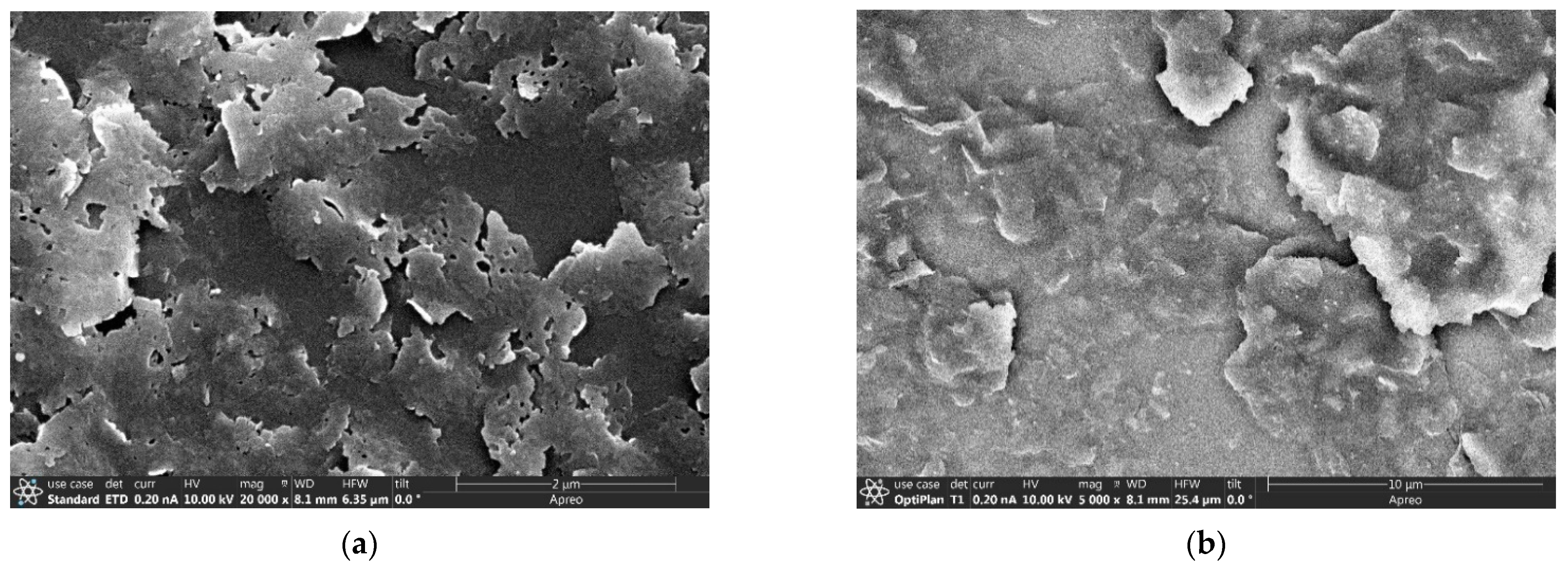
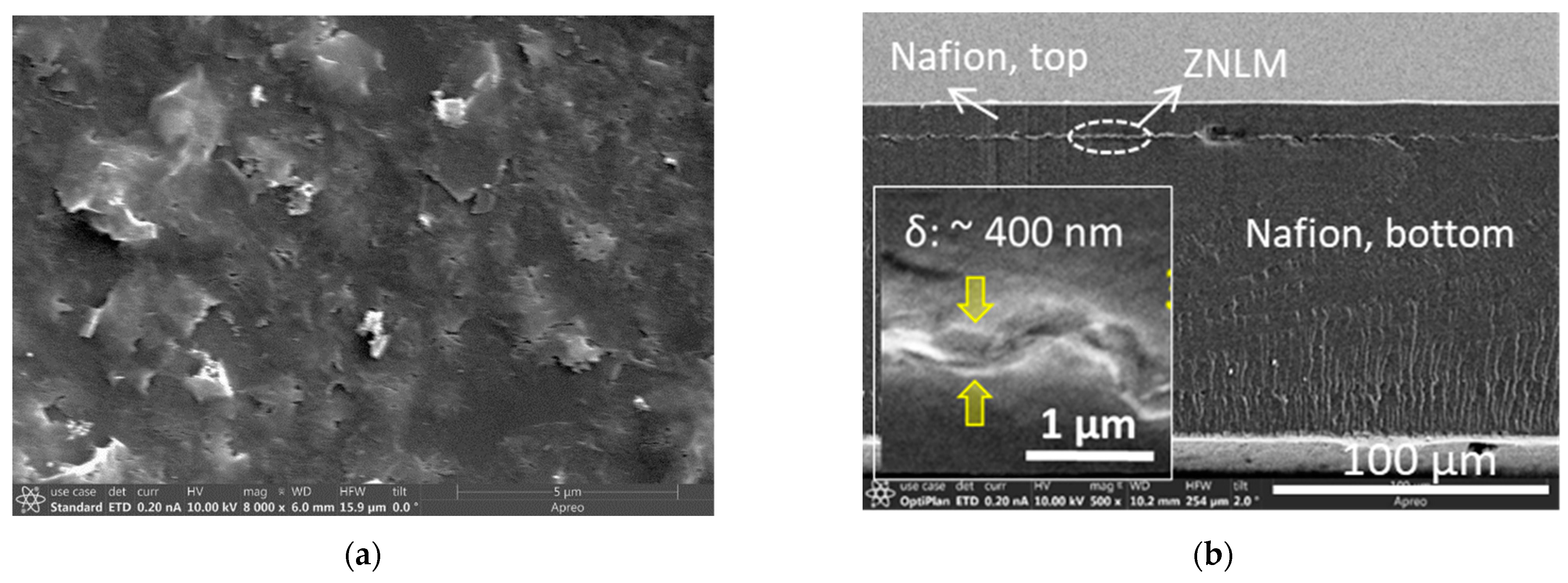
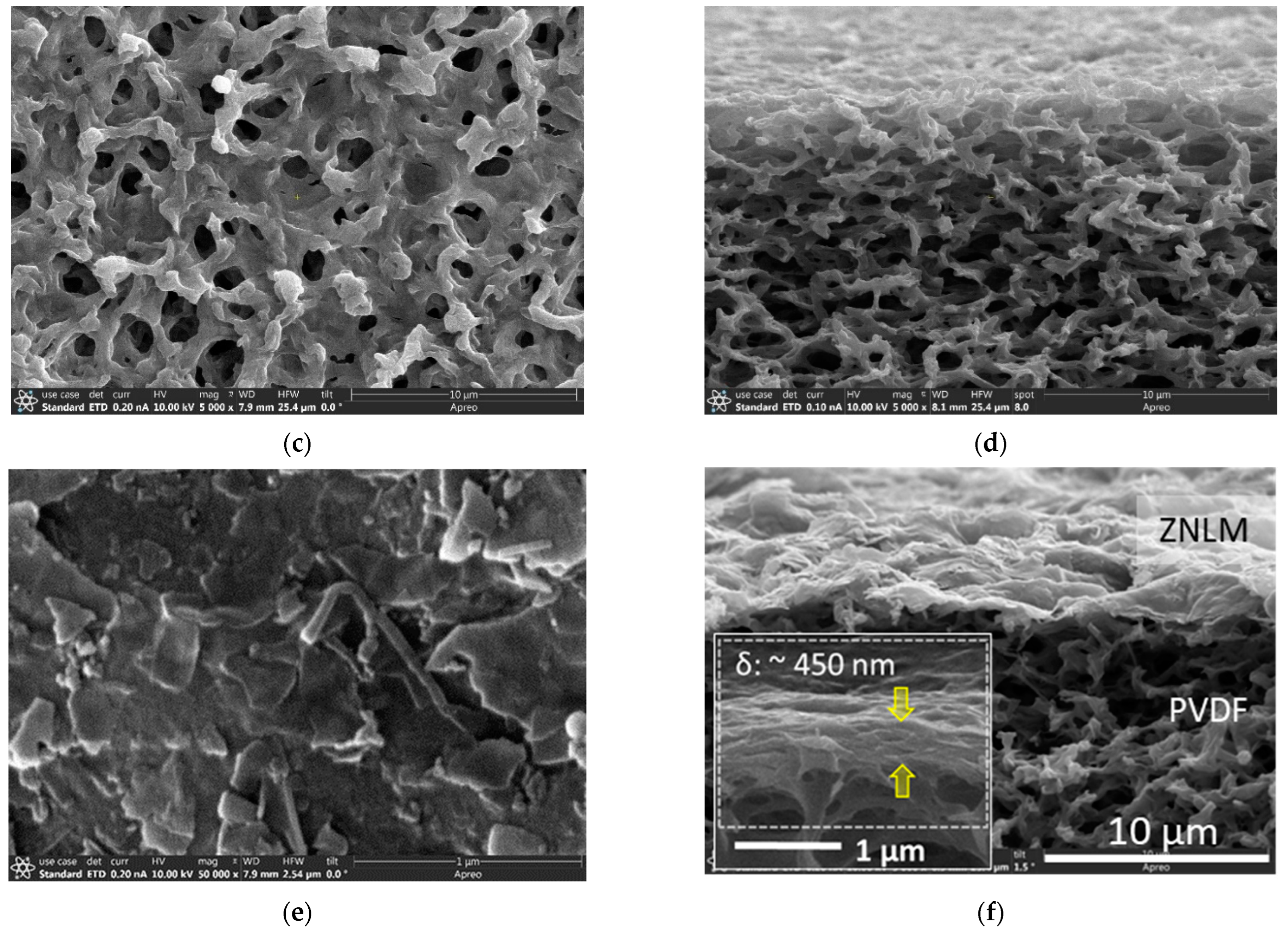
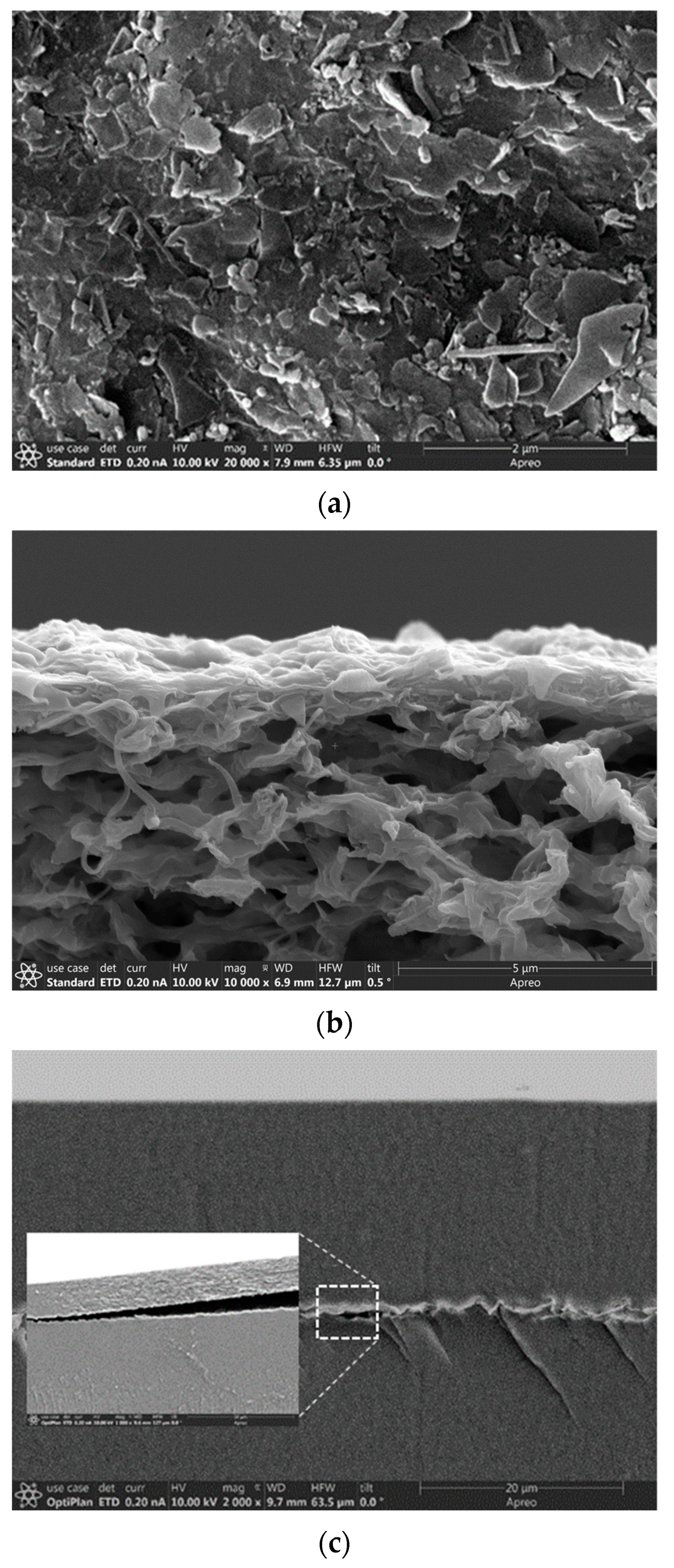
The morphological properties of the zeolite materials, i.e., nanoparticles, ZNs, and thin films, were examined by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) using a FEI Scios DualBeam microscope equipped with Ametek Octane Super EDAX. The N2 adsorption and desorption BET analysis was performed at 77 K by a Micromeritics ASAP 2020 unit. The zeolite films activated by UV irradiation were also characterized by attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR, Thermo Scientific Nicolet iS50 FT-IR spectrometer) to verify the removal of organic SDA molecules from the zeolite channels.
2.3. ZSM-5 ZN Synthesis
The ZSM-5 ZNs were synthesized by the uninterrupted two-stage secondary growth process using the silicalite nanoparticle seeds and dC
5 SDA. The dC
5 was synthesized in-house via the reported method of exhaustive alkylation of 1,5-diaminopentane with 1-iodopropane [
6,
7]. The silicalite nanoparticle seeds were prepared by a procedure detailed in the same publications, which involved a two-step in situ crystallization process using a starting solution with a molar composition of 10 SiO
2 + 2.4 TPAOH + 0.87 NaOH + 114 H
2O [
5]. This solution was obtained by mixing 0.16 g water, 0.127 g NaOH, 8.93 g of 1.0 M TPAOH aqueous solution, and 2.5 g silicic acid in a Teflon flask. The solution was stirred at room temperature overnight and then reacted in a closed Teflon autoclave at 50 °C for 6 days under static condition. After the 6-day reaction, the liquid phase of the mixture was collected by a GHP syringe filter (pore size 0.45 μm). The recovered clear solution was subsequently reacted in a Teflon autoclave at 100 °C for 3 days. The produced silicalite nanoparticles were recovered by centrifugation and washed with DI water until the suspension reached neutral pH (~7).
The synthesis of ZSM-5 ZNs was accomplished by the silicalite nanoparticle (SNP)-seeded secondary growth method described in our previous publications [
5,
7]. The hydrothermal reactions of silicalite nanoparticle-seeded ZN growth were conducted at 140 °C and autogenous pressure. The reactions were conducted in a custom-made Teflon-lined autoclave, which allowed injection of additional reactant to the closed autoclave without interrupting the reaction process [
7]. The entire hydrothermal reaction had a duration of 4 days, including a first stage of 3.5-day reaction in an aluminum-free precursor with molar ratios of 80 TEOS: 3.75 dC
5: 20 KOH: 9500 H
2O and a continued second stage of 0.5-day reaction after injecting a controlled amount of 1 M NaAlO
2 solution. Before the hydrothermal reaction, the aluminum-free precursor solution was aged at room temperature for 16 h under an Ar purging flow (50 mL/min) for reducing the ethanol content. The number of silicalite nanoparticle seeds added into the precursor was 1/200 of the TEOS on the basis of Si mass. The amount of injected NaAlO
2 was calculated such that the Si/Al atomic ratio of the whole mixture reached 50.
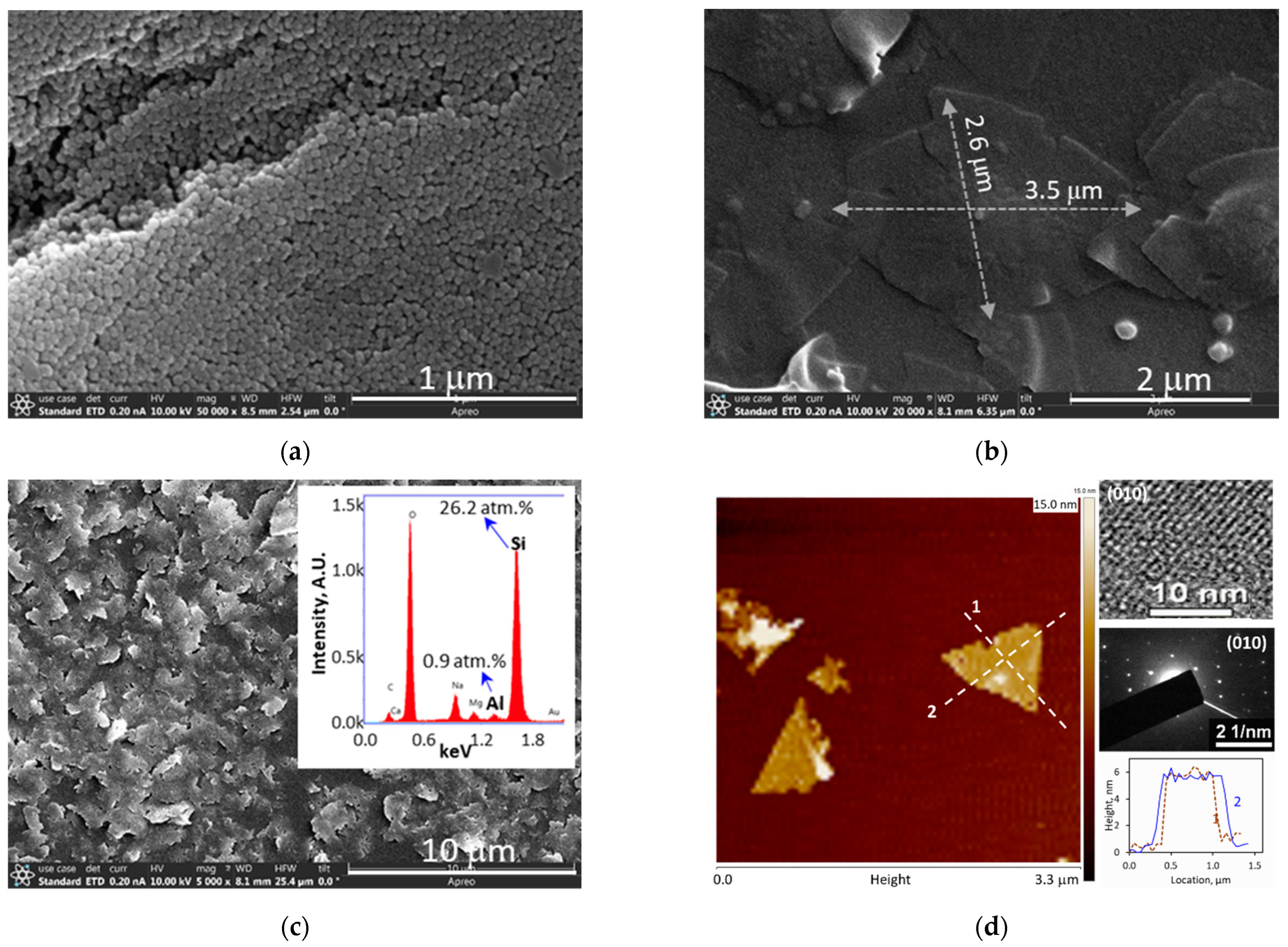
After reaction, the autoclave was quenched to room temperature in tap water flow and the solid products were recovered by centrifugation and filtration. The solid products were immersed in a solution of 0.1 M KOH + 1 M KCl (pH~13) for 1 week to dissolve unreacted amorphous silica materials and then rinsed by DI water. The as-synthesized ZN crystals were treated with intensive sonication for 2 h in liquid water or ethanol (EtOH) filled with ϕ4 mm ZrO2 milling beads. These milling beads were used to mechanically breakup the originally rhombus-shaped ZN crystals of which each contained a seed-evolved thick core encircled by a flat ZN. The flat ZN flakes dissociated from the uniformly thin sheets encircling the center cores were then separated from the large debris of the core part by centrifugation. The mass of the separated flat ZNs was approximately 10% of the total rhombus crystals.
2.4. ZN Activation
The removal of dC
5 SDA from the ZSM-5 ZNs was investigated by different techniques, including high-temperature calcination, UV irradiation in air, and piranha solution treatment. The UV irradiation method was demonstrated to be effective for decomposing the diquaternary [C
22H
45-N
+(CH
3)
2-C
6H
12-N
+(CH
3)
2-C
6H
13](Br
2) (C
22-6-6Br
2) SDA in air to activate multilayered ZNs under dry conditions [
10]. The piranha solution with an H
2SO
4/H
2O
2 volumetric ratio of 3:1 was also reported for oxidation removal of the diquaternary ammonium C
22-6-6 SDA from silicalite ZNs that led to open-pore ZNs in a liquid-dispersed state [
4].
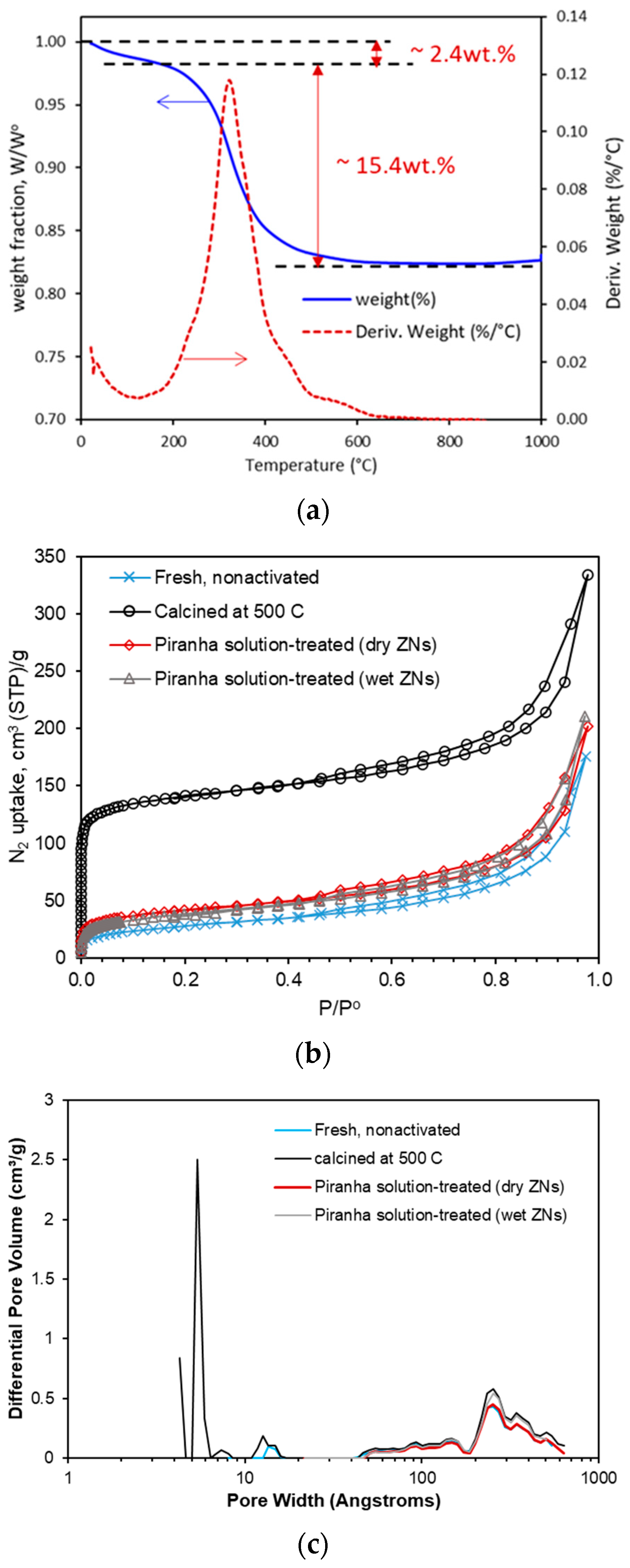
For thermal activation, the dry ZNs were randomly spread out over the crucible bottom and then calcined in air at 500 °C for 6 h. The effectiveness of dC5 SDA removal by high-temperature calcination is confirmed by thermogravimetric analysis (TGA) in air flow. The thermally activated ZNs were then redispersed by intensive sonication for extended periods in liquid media of which the pH value was adjusted by HNO3 or KOH/NaOH solutions. The as-synthesized ZNs were also directly dried and fired without removing the amorphous residuals and subsequently dispersed in the 0.1 KOH + 1.0 KCl solution. This uncleaned sample was to test the use of amorphous silica existing between individual ZNs for assisting with redispersion of calcined ZNs. This was attempted based on the assumption that amorphous silica could be dissolved in the KOH solution to help dissociate the aggregated ZNs. All calcined ZNs were redispersed into liquid solvents to form suspension for ZNLM coating on polymers.
In the UV irradiation method, a thin ZN film was deposited on the hydrophobic PVDF film (d
p~0.22 µm) from a suspension containing 0.02 wt.% ZNs in a solvent of 80 wt.% water and 20 wt.% ethanol. The film was obtained by vacuum filtration of 3 mL of suspension over a 9.1 cm
2 disc-shaped porous PDVF film. The procedure of UV activation of the ZN film was similar to the literature-reported method for activating SSZ-13 membranes [
11]. The ZN film was placed to face the UV lamp (Mineralight
® R-52G) at a distance of 3 mm from the UV bulb. The UV irradiation activation used a typical duration of 6 days. The removal of dC
5 SDA was examined by EDS and FTIR analyses. These elemental analyses were performed for ZN film coated on an alumina disc (d
p~0.1 μm) to avoid interferences of the targeted elements from the PVDF support. The ZN film on alumina was obtained by 10 s of dip-coating in a suspension containing 0.2 wt.% ZNs and activated by the UV irradiation process with varied duration.
The piranha solution treatment used the basic procedure reported by Zhang et al. [
4], which was effective for oxidizing and removal of the diquaternary ammonium SDA (C
22H
45–N
+(CH
3)
2–C
6H
12–N
+(CH
3)
2-C
6H
13)(Br
2) from the nonionic silicalite ZNs. The treatment process included the following operations: (i) 0.1 g of dry zeolite nanosheets was first mixed with 6 mL of H
2SO
4 (95–98%) solution followed with a 5 min sonication treatment; (ii) 2 mL of hydrogen peroxide (H
2O
2, 30%) was added to the suspension and subsequently allowed to react at 80 °C overnight with the reactor mouth loosely covered; (iii) the ZNs were separated from the suspension by centrifugation after overnight reaction; and (iv) the ZNs were redispersed into the piranha solution and the steps mentioned above were repeated 4 times. The thus-treated ZNs were then separated and rinsed with DI water and dried for characterizations. The piranha solution activation process mentioned above was also conducted for wet ZNs separated directly after the hydrothermal synthesis. The piranha solution treatment of the wet ZN samples was to test the possibility of improving zeolitic pore accessibility to the oxidizing H
2O
2 by avoiding ZN aggregation in dry states. The results, however, showed no differences from the treatment of dry ZNs.
2.5. Fabrication of ZNLMs on Polymers
Two types of ZN-polymer composite membranes were fabricated for evaluating proton-selective ion conduction in aqueous solutions. These included a ZNLM formed by sandwiching a UV-activated continuous ZN film between two Nafion® layers, denoted as ZNLM-Nafion hereafter, and a Nafion-free ZNLM coated on a macropore (dp~0.45 μm) hydrophilic PVDF film, denoted as ZNLM-PVDF hereafter. The ZNLM-PVDF was fabricated by filtration coating from a ZN suspension prepared by fractionation and redispersion of the thermally activated ZNs.
2.5.1. ZNLM-Nafion Fabrication
After the UV activation, the entire multilayered ZN film detached from the hydrophobic PVDF (d
p~0.22 μm) because of the lack of bonding between the polymer and ZN surface and shrinkage of the activated ZN layer. Recast Nafion films made in-house from the Nafion
® solution were used for fabrication of ZNLM membranes in a Nafion/ZNLM/Nafion sandwich structure. The Nafion film casting process was the same as that reported in reference [
12]. First, the 20 wt.% Nafion solution was mixed with methanol, ethanol, and N,N-dimethylformamide at room temperature under stirring. The volumetric ratio of the components of the cast solution were 1.25 (20% Nafion solution); 0.75 (methanol, 99.8%); 0.85 (ethanol, 99.5%); and 3.0 (DMF, 99.8%). A total of ~24 mL of this final solution was placed into a Teflon petri dish (diameter of 3 inches) and dried by evaporating the solvents in a vacuum oven at 80 °C for 20 h under a pressure of 23.7 kPa. The dried film was further treated for 4 h at 120 °C under the same vacuum pressure. This resulted in a 110 μm thick dry Nafion film that was then brushed with the 20 wt. % Nafion solution on one side. The fully wetted surface was immediately pressed on the UV-activated ZN film loosely covered on the PVDF film. The ZN film was entirely attached to the Nafion film by pressing with a flat Teflon plate. The free side of the ZNLM surface was subsequently brushed with the 20 wt.% Nafion solution to strengthen the adhesion and form an ~10 μm thick Nafion film on the top. The ZN film sandwiched by the Nafion layers, i.e., the ZNLM-Nafion, was then dried at 40 °C in air followed by curing at 120 °C for 4 h under a vacuum pressure of ~23.7 kPa.
2.5.2. ZNLM-PVDF Fabrication
The ZNLM on the porous PVDF film was coated by vacuum filtration of a controlled amount of ZN suspension following a procedure described in our previous publication [
5]. The suspension for membrane coating contained a minimal amount of PVDF (0.06 wt.%) dissolved in the DMSO/EtOH (1:2 ratio) mixed solvent, which was used as binder to physically attach and retain the ZNLM film on the porous PVDF substrate (d
p~0.45 μm).
The key to formation of a uniform and pinhole-free thin ZNLM is the high-degree dispersion state of the thermally activated ZSM-5 ZNs. To achieve this, the calcined ZNs were sonicated in 0.1 M KOH + 1.0 M KCl (pH~13) solution and 0.01 M HCl + 1.0 KCl solutions (pH~2–3) alternately for one week. The ZNs were filtered and redispersed in a water + EtOH mixture with a water/EtOH weight ratio of 80/20. The suspension was stirred overnight and peptized with 1 M HNO3 solution at pH of 3–4. Sedimentation and centrifugation were used to remove the aggregated ZNs. The fast-settling large ZN agglomerates in the bottom portion and the smallest debris remaining in the top section of the liquid column were removed. The remaining suspension contained relatively uniform and large-sized ZNs, which was ~10% of the initially retrieved ZNs. Therefore, the overall yield of open-pore ZNs by the thermal activation method was only ~1% from the initially synthesized rhombus crystals. The dispersion and aggregate removal were repeated until particle settling was not observed over 30 min under static condition. It should be noted that the ZN dispersibility did not change appreciably when suspension pH was reduced from ~7, where the ZN zeta potential (ζ) was −49.57 ± 1.79 mV, to 3–4, where ζ was −12.05 ± 1.81 mV.
These well-dispersed ZNs were recovered by a syringe filter and redispersed into EtOH. This suspension of ZNs in EtOH was then reformulated into the composition for coating ZNLM on the porous PVDF by vacuum filtration [
5]. The final ZN suspension for membrane coating contained 0.02 wt.% ZN + 0.06 wt.% dissolved PVDF in an EtOH/DMSO solution mixed at an EtOH: DMSO weight ratio of 2:1. The continuous ZN-laminated layer was obtained by total filtration of 3 mL suspension over the 9.1 cm
2 PVDF surface. The resultant PVDF-supported ZNLM, i.e., ZNLM-PVDF, was dried at 80 °C for 3 h under vacuum pressure of ~1.5 kPa followed by a 3 h curing process at 120 °C under an absolute pressure of ~23.7 kPa.
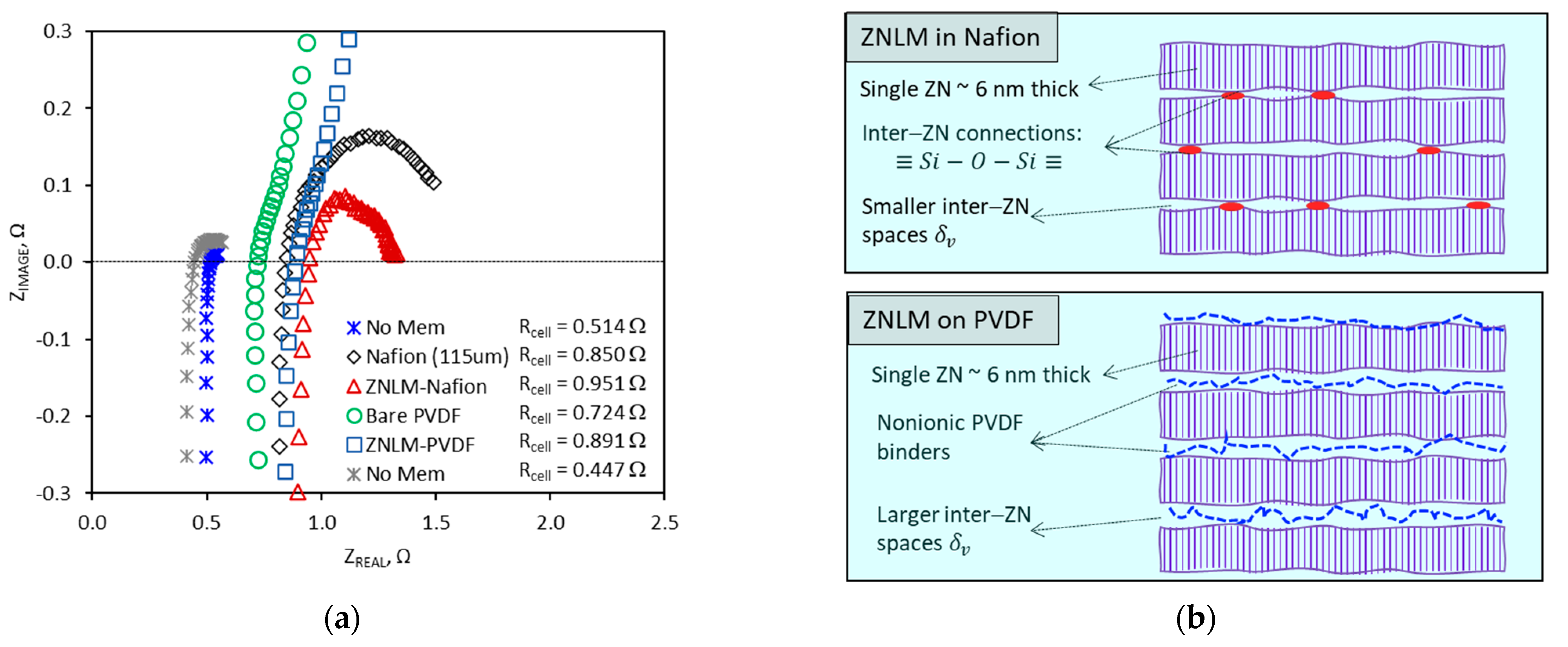
2.6. Proton Conduction in Aqueous Solutions
The ZSM-5 ZNLMs supported on the ionic Nafion films and nonionic macropore PVDF substrate were investigated for ion diffusion and proton-selective ion conduction in aqueous electrolyte solutions containing proton and vanadyl cations (VO2+) that are relevant to the aqueous all-vanadium redox flow battery (VRFB).
Proton/vanadyl ion diffusion. The measurements of ion permeation rates were performed by a procedure described in our previous work [
9,
12]. The membrane was mounted in a Teflon permeation cell and the solutions on the two sides were continuously circulated for effectively mixing solutions in the two compartments and minimizing concentration polarization at the membrane surfaces. The membrane was fully soaked with DI water when mounted before circulating the solutions to start the measurement. The feed side was circulated with 10 mL of 4/7 M VO
2+ sulfate solution in 4/7 M H
2SO
4, and the permeate side was circulated with 20 mL of 1 M MgSO
4 solution. The 1 M MgSO
4 solution was used in the permeate side to balance the osmotic pressure and ionic strength between the two sides. The pH value and VO
2+ concentration in the MgSO
4 solution were monitored as a function of time using a pH meter (Thermo Scientific Orion 320) and a UV/vis spectrometer, respectively.
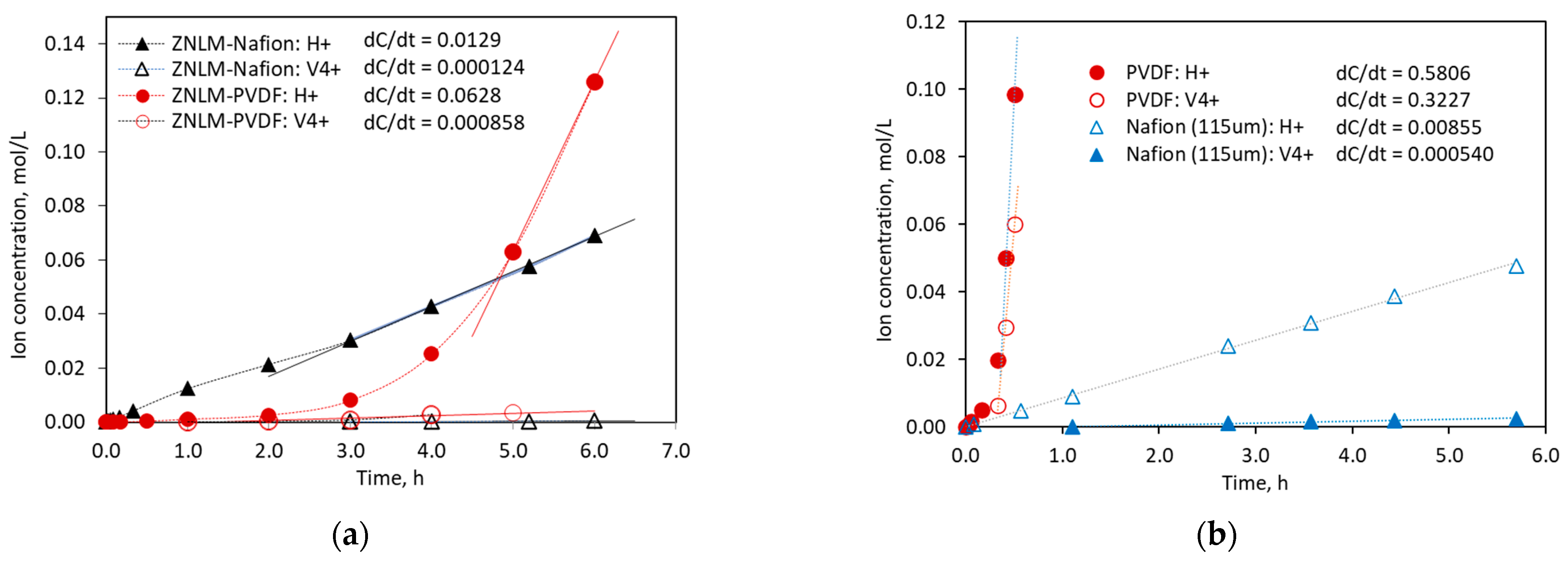
The ion fluxes
(
H
+ and VO
4+; mol/cm
2‧h) were estimated from the time-dependency of their concentrations (
) in the permeate side tank in the linear region, i.e., the slope
) when
was far smaller than the feed concentrations (
),
where the balancing solution volume in the permeate side was
= 20 mL. The permeation selectivity
is defined as the ratio of proton flux (
) to vanadyl ion flux (
), and the actual transport selectivity (
) can be obtained through normalization of the
by the ratio of driving force, which is (
) when
,
Proton conduction. The proton conduction of the membrane was evaluated through the measurement of area specific electric resistance (ASR) by electrochemical impedance spectroscopy (EIS). The measurement was performed by a cell of typical flow battery structure where the membrane was sandwiched between two compressed carbon felt electrodes to form a membrane-electrode assembly (MEA). The active area of the mounted membrane was 2.0 cm
2. This MEA was then held between two dense graphite discs of which the outer surfaces were attached to two copper sheet terminals by silver adhesive. The complete apparatus was described in our previous reports, which included the single cell, a Reference-600™ Potentiostat (Gamry Instruments Inc., Warminster, PA, USA), and two electrolyte tanks under circulation by tube pumps [
9,
12]. The EIS measurements were conducted when both sides of the membrane were circulated with a 2 M H
2SO
4 solution.