Fabrication of Photo-Responsive Mesh Membrane with Surface-Engineered Wettability for Oil–Water Separation and Photocatalytic Degradation of Organic Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
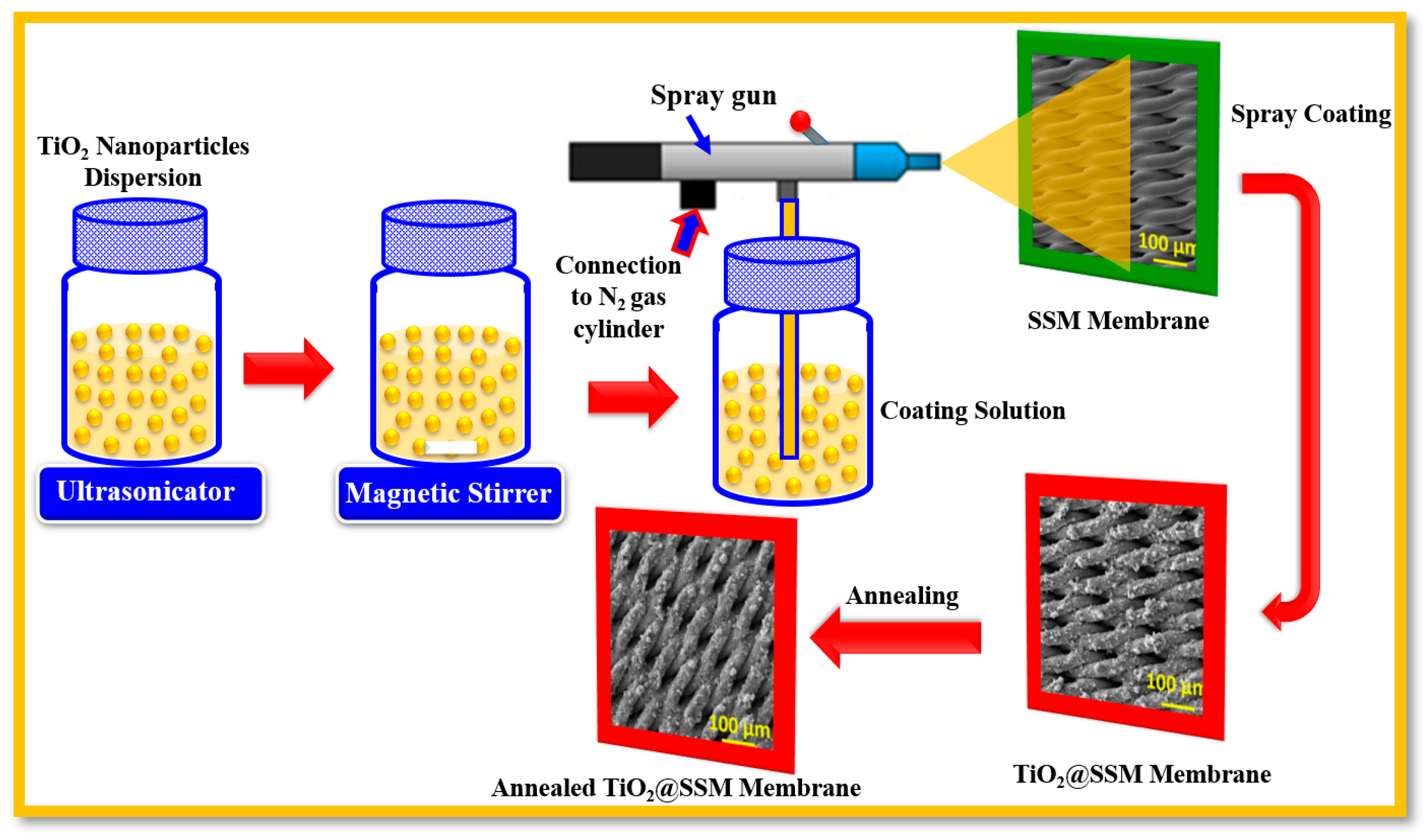
2.2. Fabrication of TiO2 Nanoparticles-Coated SSM Membrane
2.3. Characterization
2.4. Oil–Water Separation
2.5. Photocatalytic Dye Degradation
3. Results and Discussion
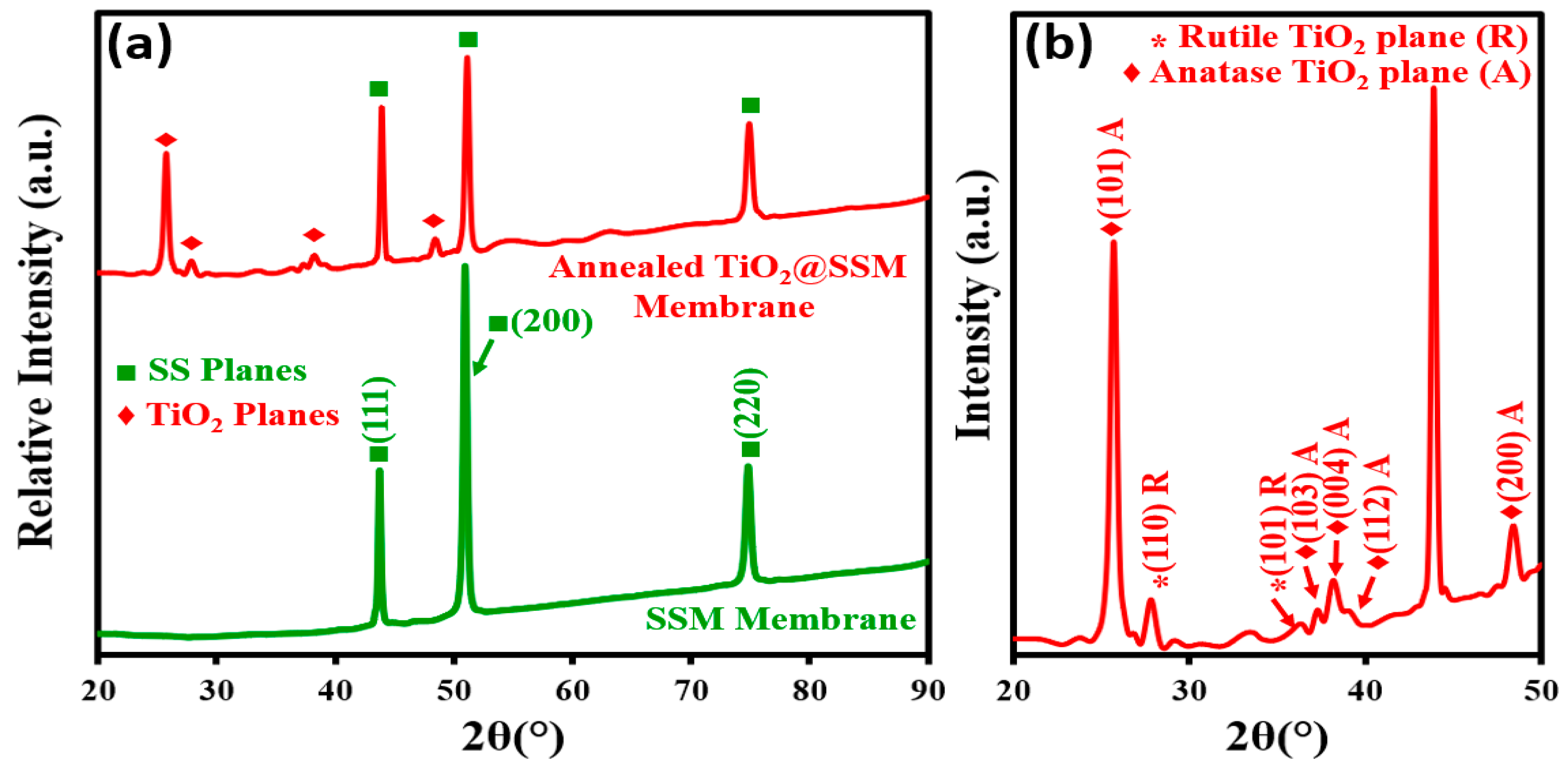
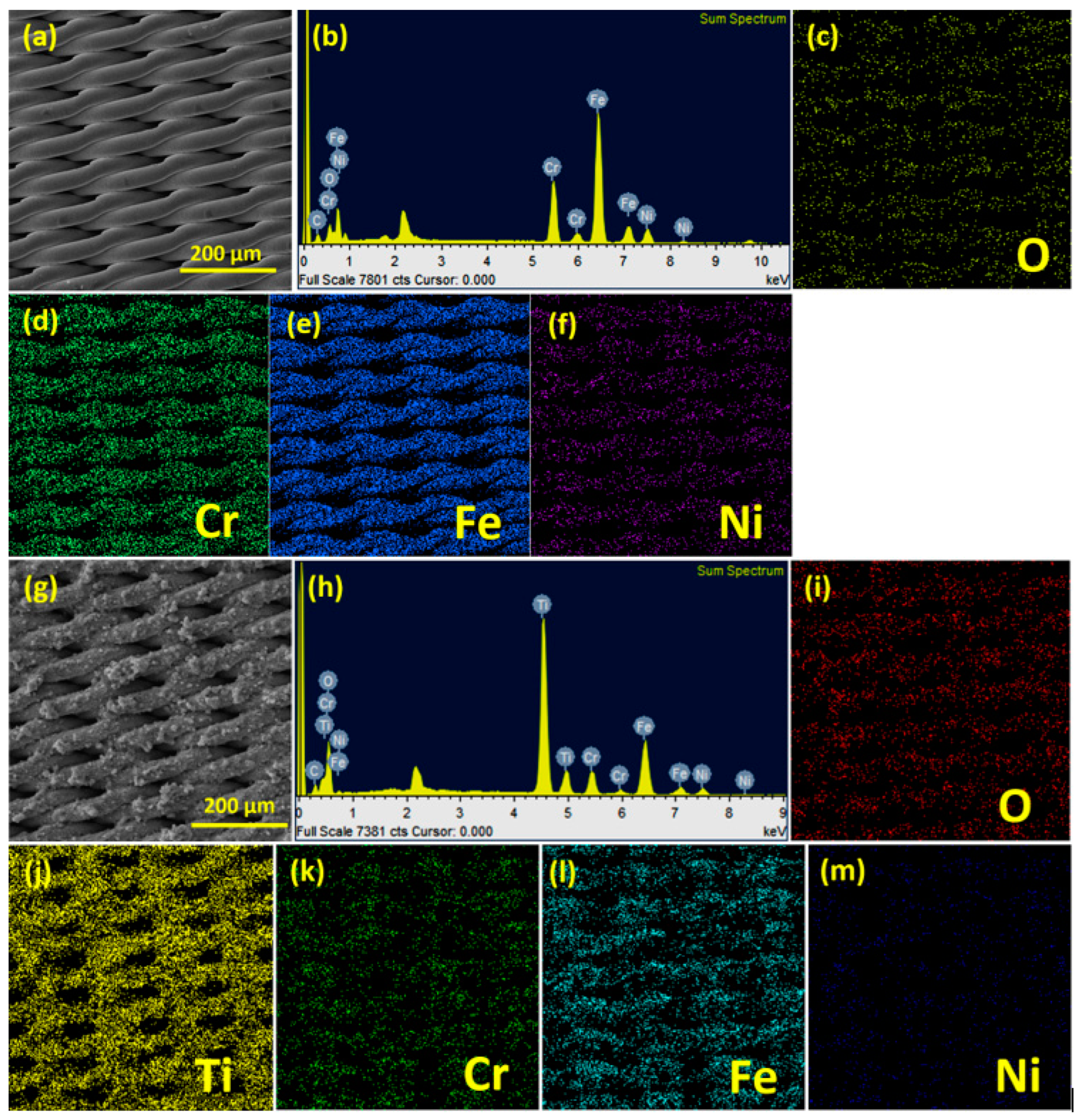
3.1. X-ray Diffraction and Morphological Analysis
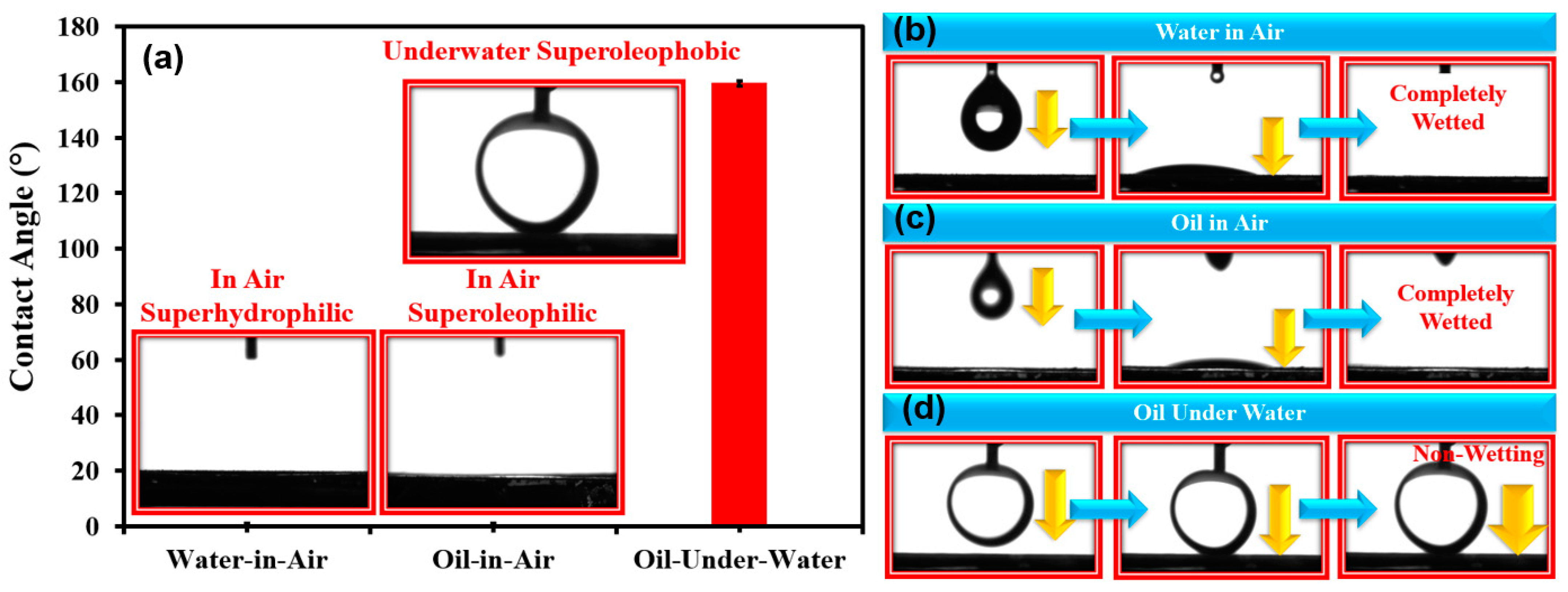
3.2. Surface Wettability of TiO2 Nanoparticles-Coated SSM
3.3. Oil–Water Separation Performance
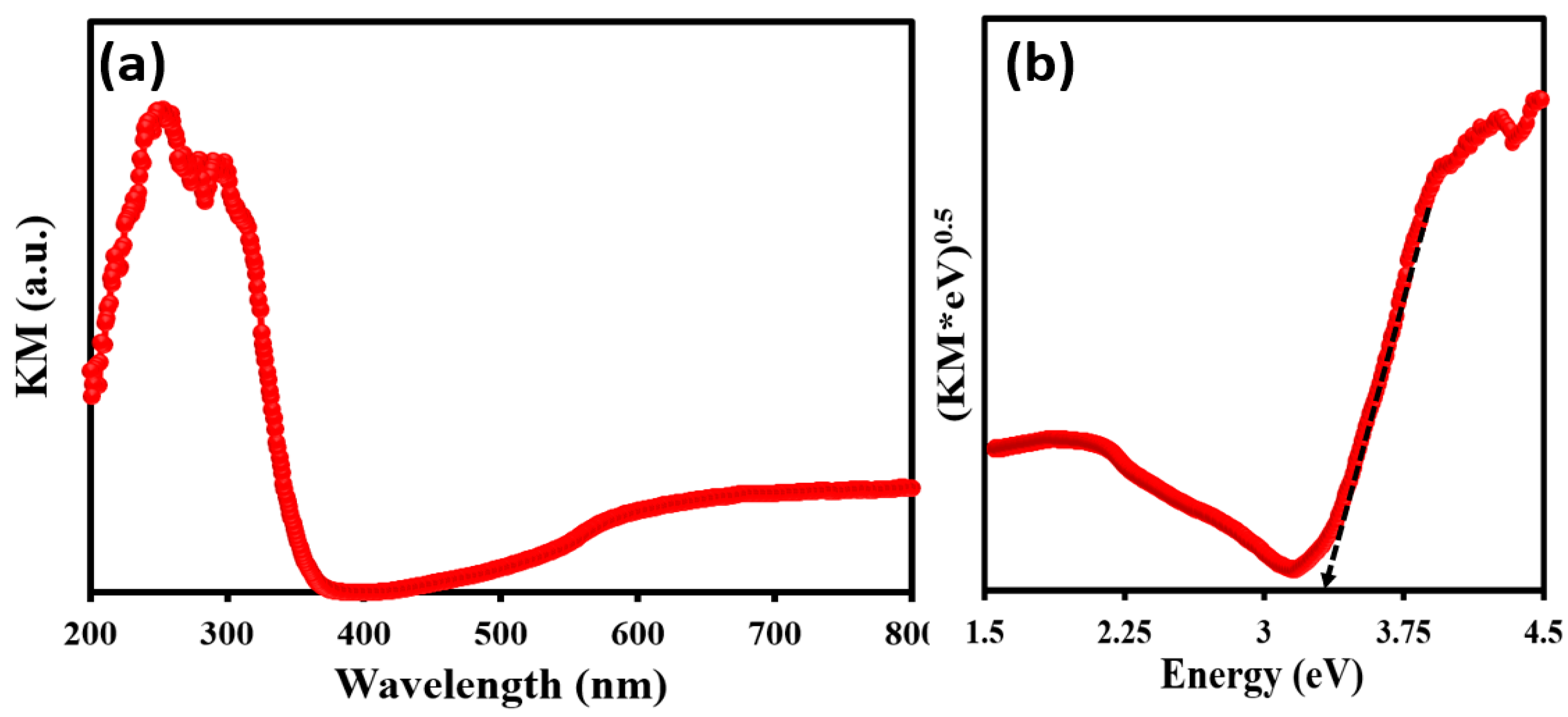
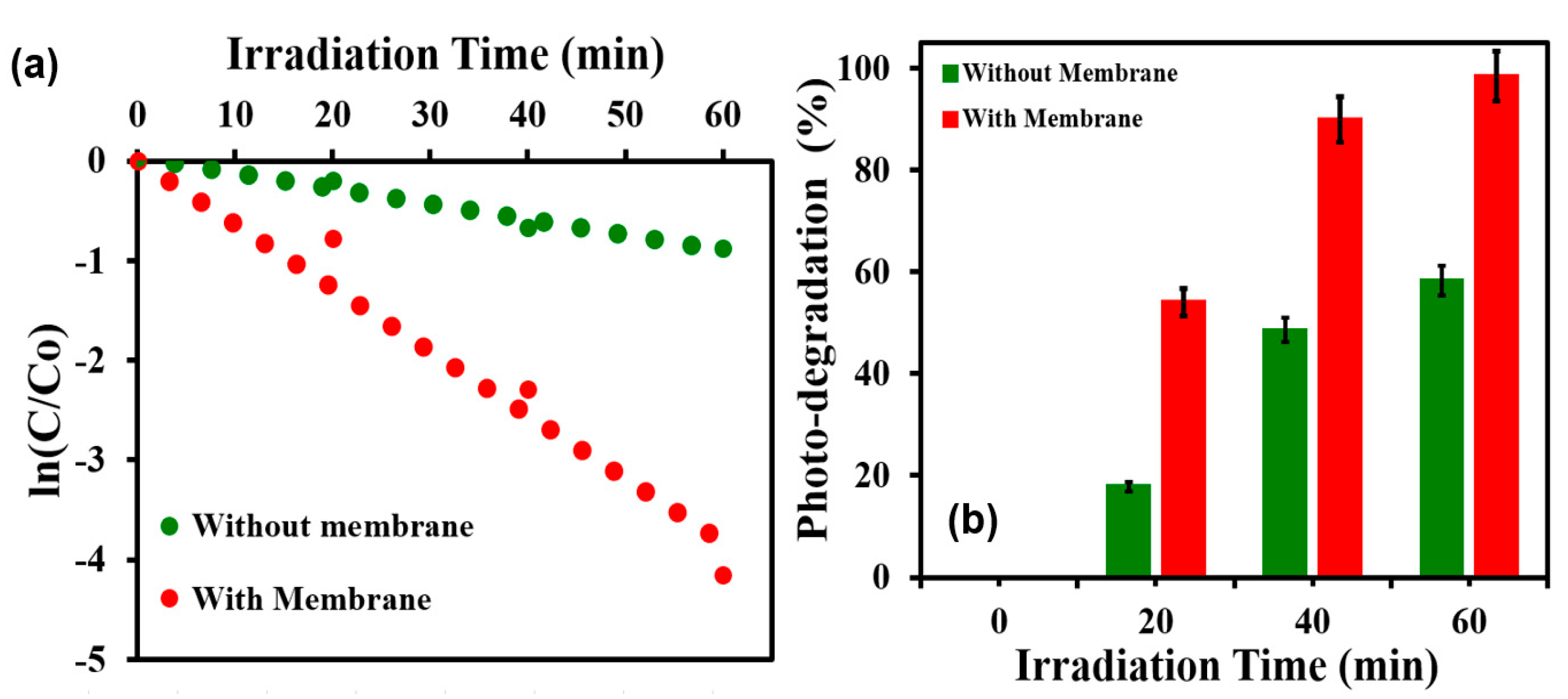
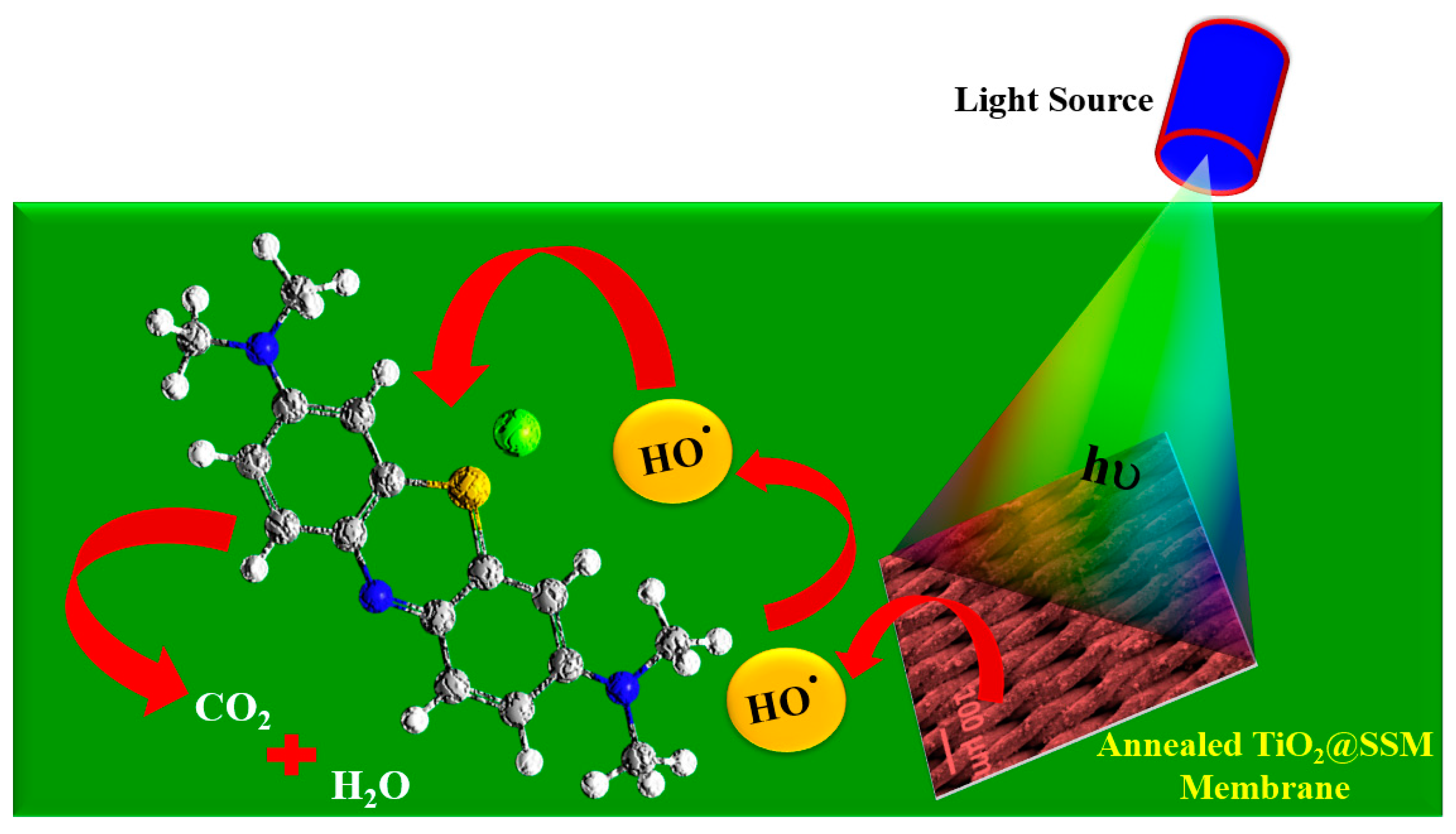
3.4. Photocatalytic Performance of TiO2@SSM Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hien, E. Real Impact of Oil Tankers as Opposed to Other Sources of Oil in the Marine Environment. World Maritime University Dissertations. 2000. Available online: https://commons.wmu.se/all_dissertations/403 (accessed on 1 January 2020).
- Rogowska, J.; Namieśnik, J. Environmental implications of oil spills from shipping accidents. Rev. Environ. Contam. Toxicol. 2010, 206, 95–114. [Google Scholar]
- Akpan, E. Environmental consequences of oil spills on marine habitats and the mitigating measures—The niger delta perspective. J. Geosci. Environ. Prot. 2022, 10, 191–203. [Google Scholar] [CrossRef]
- Chang, S.; Stone, J.; Demes, K.; Piscitelli, M. Consequences of oil spills: A review and framework for informing planning. Ecol. Soc. 2014, 19, 26. [Google Scholar] [CrossRef]
- Alsalem, F.; Thiemann, T. Produced Water from Oil and Gas Exploration—Problems, Solutions and Opportunities. J. Water Resour. Prot. 2022, 14, 142. [Google Scholar]
- Baig, U.; Faizan, M.; Waheed, A. A review on super-wettable porous membranes and materials based on bio-polymeric chitosan for oil-water separation. Adv. Colloid Interface Sci. 2022, 303, 102635. [Google Scholar] [CrossRef] [PubMed]
- Baig, U.; Faizan, M.; Sajid, M. Multifunctional membranes with super-wetting characteristics for oil-water separation and removal of hazardous environmental pollutants from water: A review. Adv. Colloid Interface Sci. 2020, 285, 102276. [Google Scholar] [CrossRef] [PubMed]
- Baig, U.; Faizan, M.; Dastageer, M. Polyimide based super-wettable membranes/materials for high performance oil/water mixture and emulsion separation: A review. Adv. Colloid Interface Sci. 2021, 297, 102525. [Google Scholar] [CrossRef]
- Baig, U.; Gondal, M.; Dastageer, M.; Khalil, A.; Zubair, S. Photo-catalytic deactivation of hazardous sulfate reducing bacteria using palladium nanoparticles decorated silicon carbide: A comparative study with pure silicon carbide nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 187, 113–119. [Google Scholar] [CrossRef]
- Gondal, M.; Dastageer, M.; Khalil, A.; Rashid, S.; Baig, U. Photo-catalytic deactivation of sulfate reducing bacteria–a comparative study with different catalysts and the preeminence of Pd-loaded WO3 nanoparticles. RSC Adv. 2015, 5, 51399–51406. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Sajid, M. Semiconducting graphitic carbon nitride integrated membranes for sustainable production of clean water: A review. Chemosphere 2021, 282, 130898. [Google Scholar] [CrossRef]
- Baig, U.; Gondal, M.; Dastageer, M. Oil-water separation using surface engineered superhydrophobic and superoleophilic membrane for the production of clean water. J. Water Process. Eng. 2022, 45, 102473. [Google Scholar] [CrossRef]
- Baig, U.; Gondal, M.; Dastageer, M. Customization of surface wettability of nano-SiO2 by coating Trimethoxy(vinyl)silane modifier for oil-water separation: Fabrication of metal-based functional superwetting nanomaterial, characterizations and performance evaluation. Chemosphere 2022, 308, 136405. [Google Scholar] [CrossRef] [PubMed]
- Baig, U.; Gondal, M.; Dastageer, M.; Falath, W. Rapid fabrication of textured membrane with super-wettability using simple spray-coating of Pd-doped WO3 nanoparticles for efficient oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125643. [Google Scholar] [CrossRef]
- Baig, U.; Dastageer, M.; Gondal, M. Fabrication of Super-wettable mesh membrane and their application in efficient gravity driven oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 660, 130793. [Google Scholar] [CrossRef]
- Matin, A.; Baig, U.; Gondal, M.; Akhtar, S.; Zubair, S. Superhydrophobic and superoleophilic surfaces prepared by spray-coating of facile synthesized cerium(IV)oxide nanoparticles for efficient oil/water separation. Appl. Surf. Sci. 2018, 462, 95–104. [Google Scholar] [CrossRef]
- Matin, A.; Baig, U.; Gondal, M.; Akhtar, S.; Zubair, S. Facile fabrication of superhydrophobic/superoleophilic microporous membranes by spray-coating ytterbium oxide particles for efficient oil-water separation. J. Mem. Sci. 2018, 548, 390–397. [Google Scholar] [CrossRef]
- Gondal, M.; Sadullah, M.; Qahtan, T.; Dastageer, M.; Baig, U. Fabrication and wettability study of WO3 coated photocatalytic membrane for oil-water separation: A comparative study with ZnO coated membrane. Sci. Rep. 2017, 7, 1686. [Google Scholar] [CrossRef]
- He, H.; Zhang, T.; Ouyang, L.; Yuan, S. Superwetting and photocatalytic Ag2O/TiO2@CuC2O4 nanocomposite-coated mesh membranes for oil/water separation and soluble dye removal. Mater. Today Chem. 2022, 23, 100717. [Google Scholar] [CrossRef]
- Song, S.; Yang, H.; Zhou, C.; Cheng, J.; Jiang, Z.; Lu, Z.; Miao, J. Underwater superoleophobic mesh based on BiVO4 nanoparticles with sunlight-driven self-cleaning property for oil/water separation. Chem. Eng. J. 2017, 320, 342–351. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Zhang, L.; Jiang, B.; Yang, X.; Yang, N.; Peng, F.; Xu, M.; Xiao, X. Dual-functional mesh with Zn-Ni-Co LDHs@NiMoO4 heterojunction nanoarrays for highly efficient oil/water separation and photocatalytic degradation. Sep. Purif. Technol. 2021, 259, 118116. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Y.; Li, H.; Zeng, X.; Pi, P.; Wen, X.; Xu, S.; Cheng, J. A self-cleaning titanium mesh with underwater superoleophobicity for oil/water separation and aqueous pollutant degradation. Surf. Coat. Technol. 2017, 313, 55–62. [Google Scholar] [CrossRef]




| Material Used | Wetting Behavior | Contact Angle | Oil–Water Separation Efficiency (%) | Photocatalytic Activity (%) | Refs. |
|---|---|---|---|---|---|
| Ag2O/TiO2@CuC2O4 nanocomposite-coated mesh | Superhydrophilic and underwater superoleophobic | WCA (in-air) = ~0° OCA (under water) = ~150° | ~95% | ~94% degradation of MB dye in 60 min | [19] |
| BiVO4-coated mesh | Superhydrophilic and underwater superoleophobic | WCA (in-air) = ~0° OCA (under water) = ~159° | ~98.6% | ~85% degradation of MB dye in 200 min | [20] |
| Zn-Ni-Co LDHs@NiMoO4-coated mesh | Superhydrophilic and underwater superoleophobic | WCA (in-air) = ~0° OCA (under water) = ~164.9° | ~99% | ~93.95% degradation of MB dye in 80 min | [21] |
| W, N-co-doped-TiO2 nanobelts (WNTNBs)-coated mesh | Superhydrophilic and underwater superoleophobic | WCA (in-air) = ~0° OCA (under water) = ~150° | ~99.5% | ~94.3% degradation of MB dye in 180 min | [22] |
| TiO2@SSM membrane | Superhydrophilic and underwater superoleophobic | WCA (in-air) = ~0° OCA (under water) = ≥160° | 99% | 98.43% degradation of MB dye in 60 min | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, U.; Dastageer, M.A. Fabrication of Photo-Responsive Mesh Membrane with Surface-Engineered Wettability for Oil–Water Separation and Photocatalytic Degradation of Organic Pollutants. Membranes 2023, 13, 302. https://doi.org/10.3390/membranes13030302
Baig U, Dastageer MA. Fabrication of Photo-Responsive Mesh Membrane with Surface-Engineered Wettability for Oil–Water Separation and Photocatalytic Degradation of Organic Pollutants. Membranes. 2023; 13(3):302. https://doi.org/10.3390/membranes13030302
Chicago/Turabian StyleBaig, Umair, and Mohamed A. Dastageer. 2023. "Fabrication of Photo-Responsive Mesh Membrane with Surface-Engineered Wettability for Oil–Water Separation and Photocatalytic Degradation of Organic Pollutants" Membranes 13, no. 3: 302. https://doi.org/10.3390/membranes13030302
APA StyleBaig, U., & Dastageer, M. A. (2023). Fabrication of Photo-Responsive Mesh Membrane with Surface-Engineered Wettability for Oil–Water Separation and Photocatalytic Degradation of Organic Pollutants. Membranes, 13(3), 302. https://doi.org/10.3390/membranes13030302






