The Meeting of Micropeptides with Major Ca2+ Pumps in Inner Membranes—Consideration of a New Player, SERCA1b
Abstract
1. Introduction
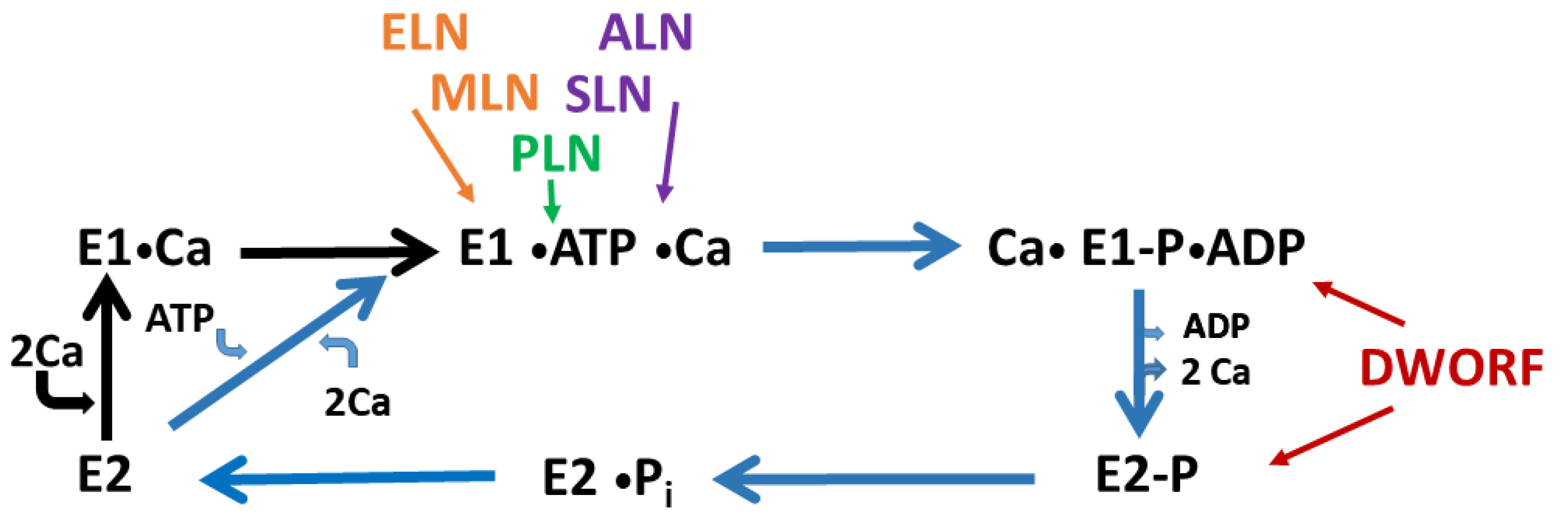
2. The Sarco/Endoplasmic Reticulum Ca2+ ATPase
3. Phospholamban
4. Sarcolipin
5. Novel Transmembrane Micropeptides
5.1. Myoregulin

5.2. DWORF
5.3. Other Regulins
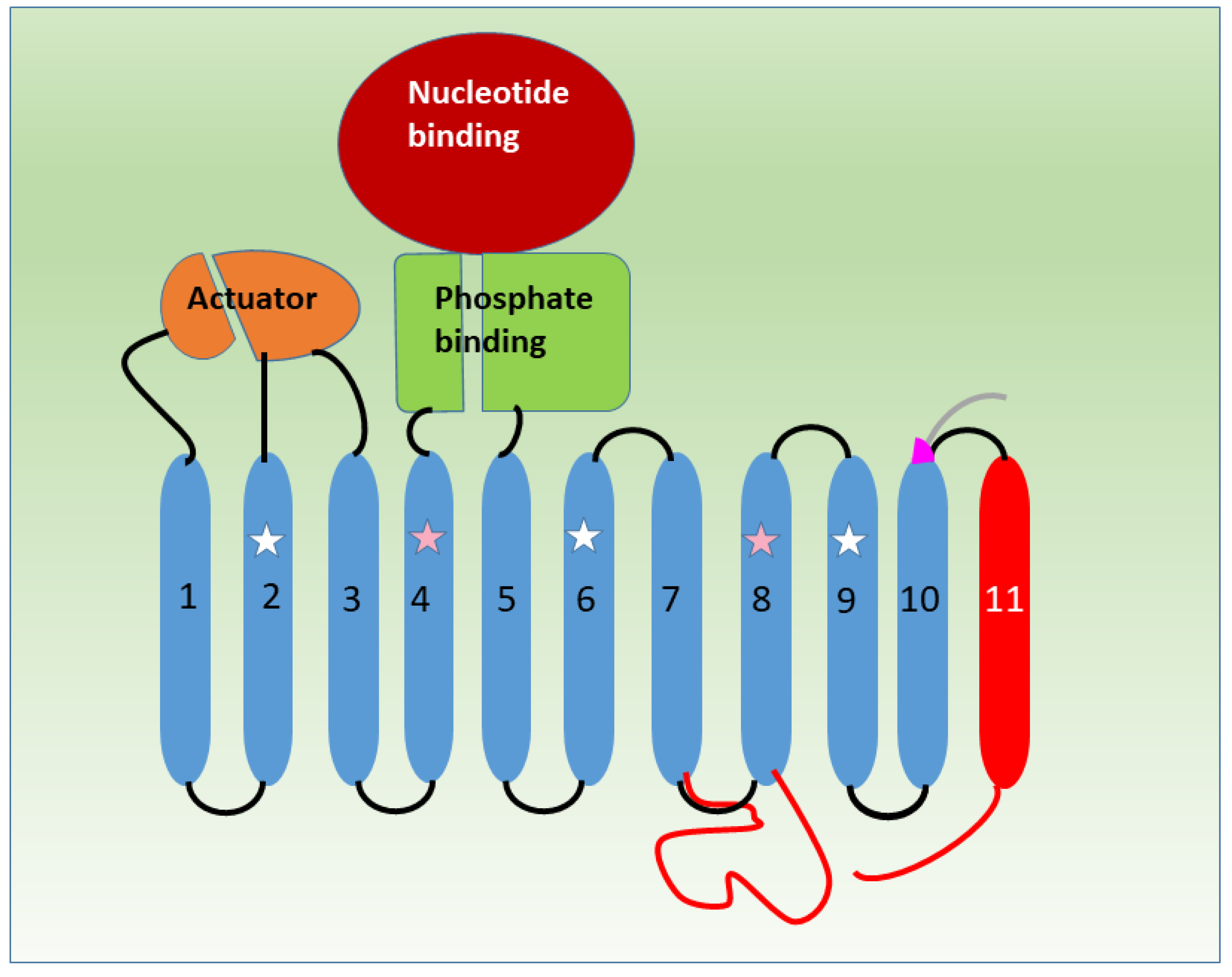
6. SERCA2b: Providing a Tail to Catch a Problem?
7. SERCA1b: A Grey Eminence
| SERCA | SERCA1a | SERCA1b | SERCA2a | SERCA2b | SERCA3 | |
|---|---|---|---|---|---|---|
| Regulin | ||||||
| PLN | mouse, rat, rabbit and pig atria; rabbit and pig EDL [51]; mouse soleus; human vastus l. [107] | NA | mouse, rat, rabbit and pig ventricle and atria; rabbit and pig soleus and EDL [51]; mouse soleus; human vastus l. [107] | NA/Ub | NA | |
| SLN | mouse and rat atria; rabbit and pig soleus; rabbit and pig EDL [51]; human vastus l. [107]; mouse soleus and diaphragm [108] | NA | rabbit and pig soleus and EDL [51]; C2C12 cells [106]; human vastus l. [107]; mouse soleus and diaphragm [108] | NA/Ub | NA | |
| DWORF | NA | NA | mouse heart, soleus and diaphragm [67] | NA/Ub | NA | |
| MLN * | mouse EDL and soleus [62,94] | C2C12 cells ** [62,93] | mouse soleus and EDL [62,93]; C2C12 cells ** [62,106] | NA/Ub | NA | |
| ELN * | NA | C2C12 cells ** [75,94] | C2C12 cells ** [75,106] | intestine and liver in mouse embryo [75]/Ub | bronchus, dorsal aorta, epithelium of trachea, intestine, liver, lung, pancreas and urothelium in mouse embryo [75] | |
| ALN * | NA/Ub? | NA/Ub? | NA/Ub? | bladder, bronchus, epidermal epithelium, heart, intestine, liver and salivary gland in mouse embryo [75] | intestine and liver in mouse embryo [75] | |
8. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berrid ge., M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Krebs, J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Makarewich, C.A. The hidden world of membrane microproteins. Exp. Cell Res. 2020, 388, 111853. [Google Scholar] [CrossRef] [PubMed]
- Doroudgar, S.; Glembotski, C.C. New concepts of endoplasmic reticulum function in the heart: Programmed to conserve. J. Mol. Cell. Cardiol. 2013, 55, 85–91. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA control of cell death and survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Fransen, P.; Chen, J.; Vangheluwe, P.; Guns, P.J. Contractile Behavior of Mouse Aorta Depends on SERCA2 Isoform Distribution: Effects of Replacing SERCA2a by SERCA2b. Front. Physiol. 2020, 11, 282. [Google Scholar] [CrossRef]
- Bonilauri, B.; Dallagiovanna, B. Microproteins in skeletal muscle: Hidden keys in muscle physiology. J. Cachexia Sarcopenia Muscle 2022, 13, 100–113. [Google Scholar] [CrossRef]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.E.; Young, H.S. The SarcoEndoplasmic Reticulum Calcium ATPase. In Membrane Protein Complexes: Structure and Function, Subcell Biochem; Harris, J.R., Boekema, E.J., Eds.; Springer: Singapore, 2018; Volume 87, pp. 229–258. [Google Scholar]
- Moller, J.V.; Olesen, C.; Winther, A.M.; Nissen, P. The sarcoplasmic Ca2+-ATPase: Design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 2010, 43, 501–566. [Google Scholar] [CrossRef]
- Chami, M.; Gozuacik, D.; Lagorce, D.; Brini, M.; Falson, P.; Peaucellier, G.; Pinton, P.; Lecoeur, H.; Gougeon, M.L.; le Maire, M.; et al. SERCA1 truncated proteins unable to pump calcium reduce the endoplasmic reticulum calcium concentration and induce apoptosis. J. Cell Biol. 2001, 153, 1301–1314. [Google Scholar] [CrossRef]
- Vandecaetsbeek, I.; Vangheluwe, P.; Raeymaekers, L.; Wuytack, F.; Vanoevelen, J. The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb. Perspect. Biol. 2011, 3, a004184. [Google Scholar] [CrossRef] [PubMed]
- Dyla, M.; Terry, D.; Kjaergaard, M.; Sørensen, T.L.; Lauwring Andersen, J.; Andersen, J.P.; Rohde Knudsen, C.; Altman, R.B.; Nissen, P.; Blanchard, S.C. Dynamics of P-type ATPase transport revealed by single-molecule FRET. Nature 2017, 551, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ropero, J.M.; Arroyo-Urea, S.; Neubrand, V.E.; Martín-Oliva, D.; Marín-Teva, J.L.; Cuadros, M.A.; Vangheluwe, P.; Navascués, J.; Mata, A.M.; Sepúlveda, M.R. The endoplasmic reticulum Ca2+-ATPase SERCA2b is upregulated in activated microglia and its inhibition causes opposite effects on migration and phagocytosis. Glia 2021, 69, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Dally, S.; Bredoux, R.; Corvazier, E.; Andersen, J.P.; Clausen, J.D.; Dode, L.; Fanchaouy, M.; Gelebart, P.; Monceau, V.; Del Monte, F.; et al. Ca2+-ATPases in non-failing and failing heart: Evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+-ATPase 2 isoform (SERCA2c). Biochem. J. 2006, 395, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Dally, S.; Corvazier, E.; Bredoux, R.; Bobe, R.; Enouf, J. Multiple and diverse coexpression, location, and regulation of additional SERCA2 and SERCA3 isoforms in nonfailing and failing human heart. J. Mol. Cell Cardiol. 2010, 48, 633–644. [Google Scholar] [CrossRef]
- Papp, B.; Launay, S.; Gélébart, P.; Arbabian, A.; Enyedi, A.; Brouland, J.P.; Carosella, E.D.; Adle-Biassette, H. Endoplasmic Reticulum Calcium Pumps and Tumor Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 3351. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef]
- Zádor, E.; Kósa, M. Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: Cham, Switzerland, 2018; pp. 4836–4841. [Google Scholar]
- Toyoshima, C. Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch. Biochem. Biophys. 2008, 476, 3–11. [Google Scholar] [CrossRef]
- Toyoshima, C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 2009, 1793, 941–946. [Google Scholar] [CrossRef]
- Sørensen, T.L.; Møller, J.V.; Nissen, P. Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science 2004, 304, 1672–1675. [Google Scholar] [CrossRef]
- Olesen, C.; Sørensen, T.L.; Nielsen, R.C.; Møller, J.V.; Nissen, P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science 2004, 306, 2251–2255. [Google Scholar] [CrossRef]
- Olesen, C.; Picard, M.; Winther, A.M.; Gyrup, C.; Morth, J.P.; Oxvig, C.; Møller, J.V.; Nissen, P. The structural basis of calcium transport by the calcium pump. Nature 2007, 450, 1036–1042. [Google Scholar] [CrossRef]
- Sugita, Y.; Miyashita, N.; Ikeguchi, M.; Kidera, A.; Toyoshima, C. Protonation of the acidic residues in the transmembrane cation-binding sites of the ca(2+) pump. J. Am. Chem. Soc. 2005, 127, 6150–6151. [Google Scholar] [CrossRef]
- Kobayashi, C.; Matsunaga, Y.; Jung, J.; Sugita, Y. Structural and energetic analysis of metastable intermediate states in the E1P-E2P transition of Ca2+-ATPase. Proc. Natl. Acad. Sci. USA 2021, 118, e2105507118. [Google Scholar] [CrossRef]
- Winther, A.M.; Bublitz, M.; Karlsen, J.L.; Moller, J.V.; Hansen, J.B.; Nissen, P.; Buch-Pedersen, M.J. The sarcolipin-bound calcium pump stabilizes calcium sites exposed to the cytoplasm. Nature 2013, 495, 265–269. [Google Scholar] [CrossRef]
- Toyoshima, C.; Iwasawa, S.; Ogawa, H.; Hirata, A.; Tsueda, J.; Inesi, G. Crystal structures of the calcium pump and sarcolipin in the Mg2+-bound E1 state. Nature 2013, 495, 260–264. [Google Scholar] [CrossRef]
- Akin, B.L.; Hurley, T.D.; Chen, Z.; Jones, L.R. The structural basis for phospholamban inhibition of the calcium pump in sarcoplasmic reticulum. J. Biol. Chem. 2013, 288, 30181–30191. [Google Scholar] [CrossRef]
- Reddy, L.G.; Jones, L.R.; Pace, R.C.; Stokes, D.L. Purified, reconstituted cardiac Ca2+-ATPase is regulated by phospholamban but not by direct phosphorylation with Ca2+/calmodulindependent protein kinase. J. Biol. Chem. 1996, 271, 14964–14970. [Google Scholar] [CrossRef]
- Zhao, Y.; Ogawa, H.; Yonekura, S.; Mitsuhashi, H.; Mitsuhashi, S.; Nishino, I.; Toyoshima, C.; Ishiura, S. Functional analysis of SERCA1b, a highly expressed SERCA1 variant in myotonic dystrophy type 1 muscle. Biochim. Biophys. Acta 2015, 1852, 2042–2047. [Google Scholar] [CrossRef]
- Lytton, J.; Westlin, M.; Burk, S.E.; Shull, G.E.; MacLennan, D.H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992, 267, 14483–14489. [Google Scholar] [CrossRef]
- Dode, L.; Vilsen, B.; Van Baelen, K.; Wuytack, F.; Clausen, J.D.; Andersen, J.P. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 3 isoforms by steady-state and transient kinetic analyses. J. Biol. Chem. 2002, 277, 45579–45591. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, P.C.; Kargacin, M.E.; Deans, J.P.; Lytton, J. Determination of apparent calcium affinity for endogenously expressed human sarco(endo)plasmic reticulum calcium-ATPase isoform SERCA. Am. J. Physiol. Cell Physiol. 2009, 296, C1105–C1114. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Kurzydlowski, K.; Tada, M.; MacLennan, D.H. Identification of regions in the Ca(2+)-ATPase of sarcoplasmic reticulum that affect functional association with phospholamban. J. Biol. Chem. 1993, 268, 2809–2815. [Google Scholar] [CrossRef]
- Toyofuko, T.; Kurzydlowski, K.; Tada, M.; MacLennan, D.H. Amino acids Glu2 to Ile18 in the cytoplasmic domain of phospholamban are essential for functional association with the Ca-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 1994, 269, 3088–3094. [Google Scholar] [CrossRef]
- Kirchberber, M.A.; Tada, M.; Katz, A.M. Phospholamban: A regulatory protein of the cardiac sarcoplasmic reticulum. Recent Adv. Stud. Card. Struct. Metab. 1975, 5, 103–115. [Google Scholar]
- Katz, A.M. Discovery of phospholamban. A personal history. Ann. N. Y. Acad. Sci. 1998, 853, 9–19. [Google Scholar] [CrossRef]
- Luo, W.; Grupp, I.L.; Harrer, J.; Ponniah, S.; Grupp, G.; Duffy, J.J.; Doetschman, T.; Kranias, E.G. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ. Res. 1994, 75, 401–409. [Google Scholar] [CrossRef]
- Simmerman, H.K.; Collins, J.H.; Theibert, J.L.; Wegener, A.D.; Jones, L.R. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J. Biol. Chem. 1986, 261, 13333–13341. [Google Scholar] [CrossRef]
- Trieber, C.A.; Afara, M.; Young, H.S. Effects of phospholamban transmembrane mutants on the calcium affinity, maximal activity, and cooperativity of the sarcoplasmic reticulum calcium pump. Biochemistry 2009, 48, 9287–9296. [Google Scholar] [CrossRef]
- Kimura, Y.; Kurzydlowski, K.; Tada, M.; MacLennan, D.H. Phospholamban inhibitory function is activated by depolymerization. J. Biol. Chem. 1997, 272, 15061–15064. [Google Scholar] [CrossRef]
- Autry, J.; Jones, L. Functional co-expression of the canine cardiac Ca2+ pump and phospholamban in Spodoptera frugiperda (Sf21) cells reveals new insights on ATPase regulation. J. Biol. Chem. 1997, 272, 15872–15880. [Google Scholar] [CrossRef]
- Chu, G.; Li, L.; Sato, Y.; Harrer, J.M.; Kadambi, V.J.; Hoit, B.D.; Bers, D.M.; Kranias, E.G. Pentameric assembly of phospholamban facilitates inhibition of cardiac function in vivo. J. Biol. Chem. 1998, 273, 33674–33680. [Google Scholar] [CrossRef]
- Asahi, M.; McKenna, E.; Kurzydlowski, K.; Tada, M.; MacLennan, D. Physical interactions between phospholamban and sarco(endo)plasmic reticulum Ca2+-ATPases are dissociated by elevated Ca2+, but not by phospholamban phosphorylation, vanadate, or thapsigargin, and are enhanced by ATP. J. Biol. Chem. 2000, 275, 15034–15038. [Google Scholar] [CrossRef]
- Tada, M.; Inui, M.; Yamada, M.; Kadoma, M.; Kuzuya, T.; Abe, H.; Kakiuchi, S. Effects of phospholamban phosphorylation catalyzed by adenosine 3′:5′-monophosphate- and calmodulindependent protein kinases on calcium transport ATPase of cardiac sarcoplasmic reticulum. J. Mol. Cell Cardiol. 1983, 15, 335–346. [Google Scholar] [CrossRef]
- Mattiazzi, A.; Kranias, E.G. The role of CaMKII regulation of phospholamban activity in heart disease. Front. Pharmacol. 2014, 5, 5. [Google Scholar] [CrossRef]
- MacDougall, L.K.; Jones, L.R.; Cohen, P. Identification of the major protein phosphatases in mammalian cardiac muscle which dephosphorylate phospholamban. Eur. J. Biochem. 1991, 196, 725–734. [Google Scholar] [CrossRef]
- Schmitt, J.P.; Kamisago, M.; Asahi, M.; Li, G.H.; Ahmad, F.; Mende, U.; Kranias, E.G.; MacLennan, D.H.; Seidman, J.G.; Seidman, C.E. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 2003, 299, 1410–1413. [Google Scholar] [CrossRef]
- Lai, P.; Nikolaev, V.O.; De Jong, K.A. Understanding the Role of SERCA2a Microdomain Remodeling in Heart Failure Induced by Obesity and Type 2 Diabetes. J. Cardiovasc. Dev. Dis. 2022, 9, 163. [Google Scholar] [CrossRef]
- Vangheluwe, P.; Schuermans, M.; Zádor, E.; Waelkens, E.; Raeymaekers, L.; Wuytack, F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem. J. 2005, 389, 151–159. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, D.H.; Asahi, M.; Tupling, A.R. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann. N. Y. Acad. Sci. 2003, 986, 472–480. [Google Scholar] [CrossRef]
- Babu, G.J.; Bhupathy, P.; Carnes, C.A.; Billman, G.E.; Periasamy, M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J. Mol. Cell. Cardiol. 2007, 43, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Goonasekera, S.A.; Molkentin, J.D.; et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Shaikh, S.A.; Sopariwala, D.H.; Bal, N.C.; Periasamy, M. Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J. Biol. Chem. 2013, 288, 6881–6889. [Google Scholar] [CrossRef]
- Gorski, P.A.; Glaves, J.P.; Vangheluwe, P.; Young, H.S. Sarco(endo)plasmic reticulum calcium ATPase (SERCA) inhibition by sarcolipin is encoded in its luminal tail. J. Biol. Chem. 2013, 288, 8456–8467. [Google Scholar] [CrossRef] [PubMed]
- Trieber, C.A.; Douglas, J.L.; Afara, M.; Young, H.S. The effects of mutation on the regulatory properties of phospholamban in co-reconstituted membranes. Biochemistry 2005, 44, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P.J.; Juracic, E.S.; Fajardo, V.A.; Tupling, A.R. Role of SERCA and sarcolipin in adaptive muscle remodeling. Am. J. Physiol. Cell Physiol. 2022, 322, C382–C394. [Google Scholar] [CrossRef] [PubMed]
- Bhupathy, P.; Babu, G.J.; Ito, M.; Periasamy, M. Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J. Mol. Cell Cardiol. 2009, 47, 723–729. [Google Scholar] [CrossRef]
- Gramolini, A.O.; Trivieri, M.G.; Oudit, G.Y.; Kislinger, T.; Li, W.; Patel, M.M.; Emili, A.; Kranias, E.G.; Backx, P.H.; Maclennan, D.H. Cardiac-specific overexpression of sarcolipin in phospholamban null mice impairs myocyte function that is restored by phosphorylation. Proc. Natl. Acad. Sci. USA 2006, 103, 2446–2451. [Google Scholar] [CrossRef]
- Andrews, S.J.; Rothnagel, J.A. Emerging evidence for functional peptides encoded by short open reading frames. Nat. Rev. Genet. 2014, 15, 193–204, Erratum in Nat. Rev. Genet. 2014, 15, 286. [Google Scholar] [CrossRef]
- Anderson, D.M.; Anderson, K.M.; Chang, C.L.; Makarewich, C.A.; Nelson, B.R.; McAnally, J.R.; Kasaragod, P.; Shelton, J.M.; Liou, J.; Bassel-Duby, R.; et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef]
- Magny, E.G.; Pueyo, J.I.; Pearl, F.M.; Cespedes, M.A.; Niven, J.E.; Bishop, S.A.; Couso, J.P. Conserved regulation of cardiac calcium uptake by peptides encoded in small open reading frames. Science 2013, 341, 1116–1120. [Google Scholar] [CrossRef]
- Tupling, A.R.; Bombardier, E.; Gupta, S.C.; Hussain, D.; Vigna, C.; Bloemberg, D.; Quadrilatero, J.; Trivieri, M.G.; Babu, G.J.; Backx, P.H.; et al. Enhanced Ca2+ transport and muscle relaxation in skeletal muscle from sarcolipin-null mice. Am. J. Physiol. Cell Physiol. 2011, 301, C841–C849. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Rietze, B.A.; Chambers, P.J.; Bellissimo, C.; Bombardier, E.; Quadrilatero, J.; Tupling, A.R. Effects of sarcolipin deletion on skeletal muscle adaptive responses to functional overload and unload. Am. J. Physiol. Cell Physiol. 2017, 313, C154–C161. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.; Bak, J.J.; Primeau, J.O.; Fisher, M.E.; Espinoza-Fonseca, L.M.; Lemieux, M.J.; Young, H.S. Nothing Regular about the Regulins: Distinct Functional Properties of SERCA Transmembrane Peptide Regulatory Subunits. Int. J. Mol. Sci. 2021, 22, 8891. [Google Scholar] [CrossRef]
- Nelson, B.R.; Makarewich, C.A.; Anderson, D.M.; Winders, B.R.; Troupes, C.D.; Wu, F.; Reese, A.L.; McAnally, J.R.; Chen, X.; Kavalali, E.T.; et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 2016, 351, 271–275. [Google Scholar] [CrossRef]
- Fisher, M.E.; Bovo, E.; Aguayo-Ortiz, R.; Cho, E.E.; Pribadi, M.P.; Dalton, M.P.; Rathod, N.; Lemieux, M.J.; Espinoza-Fonseca, L.M.; Robia, S.L.; et al. Dwarf open reading frame (DWORF) is a direct activator of the sarcoplasmic reticulum calcium pump SERCA. Elife 2021, 10, e65545. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yuen, S.L.; Stroik, D.R.; Kleinboehl, E.; Cornea, R.L.; Thomas, D.D. The transmembrane peptide DWORF activates SERCA2a via dual mechanisms. J. Biol. Chem. 2021, 296, 100412. [Google Scholar] [CrossRef] [PubMed]
- Cleary, S.R.; Fang, X.; Cho, E.E.; Pribadi, M.P.; Seflova, J.; Beach, J.R.; Kekenes-Huskey, P.M.; Robia, S.L. Inhibitory and stimulatory micropeptides preferentially bind to different conformations of the cardiac calcium pump. J. Biol. Chem. 2022, 298, 102060. [Google Scholar] [CrossRef] [PubMed]
- Reddy, U.V.; Weber, D.K.; Wang, S.; Larsen, E.K.; Gopinath, T.; De Simone, A.; Robia, S.; Veglia, G. A kink in DWORF helical structure controls the activation of the sarcoplasmic reticulum Ca2+-ATPase. Structure 2022, 30, 360–370. [Google Scholar] [CrossRef]
- Mbikou, P.; Rademaker, M.T.; Charles, C.J.; Richards, M.A.; Pemberton, C.J. Cardiovascular effects of DWORF (dwarf open reading frame) peptide in normal and ischaemia/reperfused isolated rat hearts. Peptides 2020, 124, 170192. [Google Scholar] [CrossRef]
- Makarewich, C.A.; Munir, A.Z.; Schiattarella, G.G.; Bezprozvannaya, S.; Raguimova, O.N.; Cho, E.E.; Vidal, A.H.; Robia, S.L.; Bassel-Duby, R.; Olson, E.N. The DWORF micropeptide enhances contractility and prevents heart failure in a mouse model of dilated cardiomyopathy. Elife 2018, 7, e38319. [Google Scholar] [CrossRef] [PubMed]
- Makarewich, C.A.; Bezprozvannaya, S.; Gibson, A.M.; Bassel-Duby, R.; Olson, E.N. Gene Therapy with the DWORF Micropeptide Attenuates Cardiomyopathy in Mice. Circ. Res. 2020, 127, 1340–1342. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Makarewich, C.A.; Anderson, K.M.; Shelton, J.M.; Bezprozvannaya, S.; Bassel-Duby, R.; Olson, E.N. Wide-spread control of calcium signaling by a family of SERCA-inhibiting micropeptides. Sci. Signal. 2016, 9, ra119. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.R.; Dalton, M.P.; Cho, E.E.; Pribadi, M.P.; Zak, T.J.; Šeflová, J.; Makarewich, C.A.; Olson, E.N.; Robia, S.L. Newly Discovered Micropeptide Regulators of SERCA Form Oligomers but Bind to the Pump as Monomers. J. Mol. Biol. 2019, 431, 4429–4443. [Google Scholar] [CrossRef]
- Glaves, J.P.; Primeau, J.O.; Gorski, P.A.; Espinoza-Fonseca, L.M.; Lemieux, M.J.; Young, H.S. Interaction of a Sarcolipin Pentamer and Monomer with the Sarcoplasmic Reticulum Calcium Pump, SERCA. Biophys. J. 2020, 118, 518–531. [Google Scholar] [CrossRef]
- Glaves, J.P.; Primeau, J.O.; Espinoza-Fonseca, L.M.; Lemieux, M.J.; Young, H.S. The Phospholamban Pentamer Alters Function of the Sarcoplasmic Reticulum Calcium Pump SERCA. Biophys. J. 2019, 116, 633–647. [Google Scholar] [CrossRef]
- Alford, R.F.; Smolin, N.; Young, H.S.; Gray, J.J.; Robia, S.L. Protein docking and steered molecular dynamics suggest alternative phospholamban-binding sites on the SERCA calcium transporter. J. Biol. Chem. 2020, 295, 11262–11274. [Google Scholar] [CrossRef]
- Lytton, J.; MacLennan, D.H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J. Biol. Chem. 1988, 263, 15024–15031. [Google Scholar] [CrossRef]
- Verboomen, H.; Wuytack, F.; Van den Bosch, L.; Mertens, L.; Casteels, R. The functional importance of the extreme C-terminal tail in the gene 2 organellar Ca(2+)-transport ATPase (SERCA2a/b). Biochem. J. 1994, 303, 979–984. [Google Scholar] [CrossRef]
- Dode, L.; Andersen, J.P.; Leslie, N.; Dhitavat, J.; Vilsen, B.; Hovnanian, A. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J. Biol. Chem. 2003, 278, 47877–47889. [Google Scholar] [CrossRef]
- Vandecaetsbeek, I.; Trekels, M.; De Maeyer, M.; Ceulemans, H.; Lescrinier, E.; Raeymaekers, L.; Wuytack, F.; Vangheluwe, P. Structural basis for the high Ca2+ affinity of the ubiquitous SERCA2b Ca2+ pump. Proc. Natl. Acad. Sci. USA 2009, 106, 18533–18538. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.D.; Vandecaetsbeek, I.; Wuytack, F.; Vangheluwe, P.; Andersen, J.P. Distinct roles of the C-terminal 11th transmembrane helix and luminal extension in the partial reactions determining the high Ca2+ affinity of sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2b (SERCA2b). J. Biol. Chem. 2012, 287, 39460–39469. [Google Scholar] [CrossRef] [PubMed]
- Gorski, P.A.; Trieber, C.A.; Larivière, E.; Schuermans, M.; Wuytack, F.; Young, H.S.; Vangheluwe, P. Transmembrane helix 11 is a genuine regulator of the endoplasmic reticulum Ca2+ pump and acts as a functional parallel of β-subunit on α-Na+,K+-ATPase. J. Biol. Chem. 2012, 287, 19876–19885. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sakuta, N.; Watanabe, S.; Zhang, Y.; Yoshikaie, K.; Tanaka, Y.; Ushioda, R.; Kato, Y.; Takagi, J.; Tsukazaki, T.; et al. Structural Basis of Sarco/Endoplasmic Reticulum Ca2+-ATPase 2b Regulation via Transmembrane Helix Interplay. Cell Rep. 2019, 27, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Inoue, M.; Tsutsumi, A.; Watanabe, S.; Nishizawa, T.; Nagata, K.; Kikkawa, M.; Inaba, K. Cryo-EM structures of SERCA2b reveal the mechanism of regulation by the luminal extension tail. Sci. Adv. 2020, 6, eabb0147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Watanabe, S.; Tsutsumi, A.; Kadokura, H.; Kikkawa, M.; Inaba, K. Cryo-EM analysis provides new mechanistic insight into ATP binding to Ca2+-ATPase SERCA2b. EMBO J. 2021, 40, e108482. [Google Scholar] [CrossRef]
- Zhang, Y.; Inaba, K. Structural basis of the conformational and functional regulation of human SERCA2b, the ubiquitous endoplasmic reticulum calcium pump. Bioessay 2022, 44, e2200052. [Google Scholar] [CrossRef]
- Sitsel, A.; De Raeymaecker, J.; Drachmann, N.D.; Derua, R.; Smaardijk, S.; Andersen, J.L.; Vandecaetsbeek, I.; Chen, J.; De Maeyer, M.; Waelkens, E.; et al. Structures of the heart specific SERCA2a Ca2+-ATPase. EMBO J. 2019, 38, e100020. [Google Scholar] [CrossRef]
- Kabashima, Y.; Ogawa, H.; Nakajima, R.; Toyoshima, C. What ATP binding does to the Ca2+ pump and how nonproductive phosphoryl transfer is prevented in the absence of Ca2+. Proc. Natl. Acad. Sci. USA 2020, 117, 18448–18458. [Google Scholar] [CrossRef]
- Brandl, C.J.; deLeon, S.; Martin, D.R.; MacLennan, D.H. Adult forms of the Ca2+ ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J. Biol. Chem. 1987, 262, 3768–3774. [Google Scholar] [CrossRef]
- Zádor, E.; Kósa, M. The neonatal sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA1b): A neglected pump in scope. Pflug. Arch. 2015, 467, 1395–1401. [Google Scholar] [CrossRef]
- Zádor, E.; Vangheluwe, P.; Wuytack, F. The expression of the neonatal sarcoplasmic reticulum Ca2+ pump (SERCA1b) hints to a role in muscle growth and development. Cell Calcium 2007, 41, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Wuytack, F.; Zádor, E. The effect of passive movement on denervated soleus highlights a differential nerve control on SERCA and MyHC isoforms. J. Histochem. Cytochem. 2008, 56, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Mendler, L.; Szakonyi, G.; Zádor, E.; Görbe, A.; Dux, L.; Wuytack, F. Expression of sarcoplasmic/endoplasmic reticulum Ca2+ ATPases in the rat extensor digitorum longus (EDL) muscle regenerating from notexin-induced necrosis. J. Muscle Res. Cell Motil. 1998, 19, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Zádor, E.; Mendler, L.; Ver Heyen, M.; Dux, L.; Wuytack, F. Changes in mRNA levels of the sarcoplasmic/endoplasmic-reticulum Ca(2+)-ATPase isoforms in the rat soleus muscle regenerating from notexin-induced necrosis. Biochem. J. 1996, 320, 107–113. [Google Scholar] [CrossRef]
- Guglielmi, V.; Oosterhof, A.; Voermans, N.C.; Cardani, R.; Molenaar, J.P.; van Kuppevelt, T.H.; Meola, G.; van Engelen, B.G.; Tomelleri, G.; Vattemi, G. Characterization of sarcoplasmic reticulum Ca(2+) ATPase pumps in muscle of patients with myotonic dystrophy and with hypothyroid myopathy. Neuromuscul. Disord. 2016, 26, 378–385. [Google Scholar] [CrossRef]
- Kósa, M.; Brinyiczki, K.; van Damme, P.; Goemans, N.; Hancsák, K.; Mendler, L.; Zádor, E. The neonatal sarcoplasmic reticulum Ca2+-ATPase gives a clue to development and pathology in human muscles. J. Muscle Res. Cell Motil. 2015, 36, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.; Fodor, J.; Vincze, J.; Oláh, T.; Juhász, T.; Zákány, R.; Csernoch, L.; Zádor, E. The Effect of SERCA1b Silencing on the Differentiation and Calcium Homeostasis of C2C12 Skeletal Muscle Cells. PLoS ONE 2015, 10, e0123583. [Google Scholar] [CrossRef]
- Fodor, J.; Gomba-Tóth, A.; Oláh, T.; Almássy, J.; Zádor, E.; Csernoch, L. Follistatin treatment suppresses SERCA1b levels independently of other players of calcium homeostasis in C2C12 myotubes. J. Muscle Res. Cell Motil. 2017, 38, 215–229. [Google Scholar] [CrossRef]
- Maruyama, K.; MacLennan, D.H. Mutation of aspartic acid-351, lysine-352, and lysine-515 alters the Ca2+ transport activity of the Ca2+-ATPase expressed in COS-1 cells. Proc. Natl. Acad. Sci. USA 1988, 85, 3314–3318. [Google Scholar] [CrossRef]
- De Meis, L. Brown adipose tissue Ca2+-ATPase: Uncoupled ATP hydrolysis and thermogenic activity. J. Biol. Chem. 2003, 278, 41856–41861. [Google Scholar] [CrossRef] [PubMed]
- Toustrup-Jensen, M.S.; Holm, R.; Einholm, A.P.; Schack, V.R.; Morth, J.P.; Nissen, P.; Andersen, J.P.; Vilsen, B. The C terminus of Na+, K+-ATPase controls Na+ affinity on both sides of the membrane through Arg935. J. Biol. Chem. 2009, 284, 18715–18725. [Google Scholar] [CrossRef]
- Seth, M.; Li, T.; Graham, V.; Burch, J.; Finch, E.; Stiber, J.A.; Rosenberg, P.B. Dynamic regulation of sarcoplasmic reticulum Ca(2+) stores by stromal interaction molecule 1 and sarcolipin during muscle differentiation. Dev. Dyn. 2012, 241, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Bombardier, E.; Vigna, C.; Devji, T.; Bloemberg, D.; Gamu, D.; Gramolini, A.O.; Quadrilatero, J.; Tupling, A.R. Co-expression of SERCA isoforms, phospholamban and sarcolipin in human skeletal muscle fibers. PLoS ONE 2013, 8, e84304. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Smith, I.C.; Bombardier, E.; Chambers, P.J.; Quadrilatero, J.; Tupling, A.R. Diaphragm assessment in mice overexpressing phospholamban in slow-twitch type I muscle fibers. Brain Behav. 2016, 6, e00470. [Google Scholar] [CrossRef]
- Chen, Q.N.; Fan, Z.; Lyu, A.K.; Wu, J.; Guo, A.; Yang, Y.F.; Chen, J.L.; Xiao, Q. Effect of sarcolipin-mediated cell transdifferentiation in sarcopenia-associated skeletal muscle fibrosis. Exp. Cell Res. 2020, 389, 111890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zádor, E. The Meeting of Micropeptides with Major Ca2+ Pumps in Inner Membranes—Consideration of a New Player, SERCA1b. Membranes 2023, 13, 274. https://doi.org/10.3390/membranes13030274
Zádor E. The Meeting of Micropeptides with Major Ca2+ Pumps in Inner Membranes—Consideration of a New Player, SERCA1b. Membranes. 2023; 13(3):274. https://doi.org/10.3390/membranes13030274
Chicago/Turabian StyleZádor, Ernő. 2023. "The Meeting of Micropeptides with Major Ca2+ Pumps in Inner Membranes—Consideration of a New Player, SERCA1b" Membranes 13, no. 3: 274. https://doi.org/10.3390/membranes13030274
APA StyleZádor, E. (2023). The Meeting of Micropeptides with Major Ca2+ Pumps in Inner Membranes—Consideration of a New Player, SERCA1b. Membranes, 13(3), 274. https://doi.org/10.3390/membranes13030274






