Abstract
Developing photothermal solar driven membrane distillation (PSDMD) is of great importance in providing fresh water for remote off-grid regions. The production of freshwater through the PSDMD is driven by the temperature difference between feed and distillate sides created via the addition of efficient photothermal nanostructures. Here we proposed nickel sulfides and nickel tellurium nanoparticles (NPs) to be loaded into the polymeric membrane to enhance its performance. Ag and CuSe NPs are also considered for comparison as they are previously used for membrane distillation (MD). Our theoretical approach showed that all of the considered NPs increased the temperature of the PVDF membrane by around a few degrees. and NPs are the most efficient solar light-to-heat converters compared to NiTe and NPs due to their efficient absorption over the visible range. PVDF membrane loaded with 25% of NiCs NPs and a porosity of 32% produced a transmembrane vapor flux between 22 and 27 L/m2h under a 10-times-amplified sun intensity. Under the same conditions, the PVDF membrane loaded with and Ag NPs produced 15 and 18 L/m2h of vapor flux, respectively. The implantation of NPs through the membrane not only increased its surface temperature but also possessed a high porosity which provided a higher distillation and energy efficiency that reached 58% with NiS NPs. Finally, great agreement between our theoretical model and experimental measurement is obtained.
1. Introduction
Nowadays the world is suffering from water scarcity due to global warming, pollution and water over usage. To overcome this issue, there has been an increasing demand for purifying abundant alternative sources such as seawater or high salinity processed water [1,2,3]. Among purifying technologies, osmosis inverse (RO) [4,5] and thermal desalination [6,7] are considered as promising technologies that turn seawater into freshwater, but at a high cost and with high energy consumption [8]. Recently, membrane distillation (MD), an hybrid membrane thermal system, has been introduced as a sustainable solution for seawater desalination [9,10,11] that distills water at lower temperatures and pressures [12,13]. Despite convincing results and significant importance, the MD efficiency is severely reduced because of the temperature polarization which consists in the reduction of membrane temperature compared to the bulk feed water [14,15,16]. This decreases the cross-membrane vapor pressure difference, hence the membrane flux. However, it has been demonstrated [16,17,18] that heating the feed water at the feed–membrane interface instead of heating the bulk feed stream outside the membrane module can reverse the temperature polarization with an enhancement of the evaporation process. Thus, the heating of the membrane surface can be obtained by the addition of photothermal materials such as plasmonic [16,17,19] or carbonic materials [18,20] to the MD system. In fact, a quite high light-to-heat conversion efficiency was achieved with these materials, leading to the increasing membrane temperature. The addition of photothermal nanostructures not only reverses the membrane temperature polarization, but also opens the way for the development of a new generation of membrane distillation powered directly by solar energy [18,21,22,23,24,25,26,27,28,29,30]. These solar driven membrane distillation (SDMD) systems are designed with physically separated solar thermal collectors and membrane distillation modules [31,32]. Nevertheless, the proposed prototype is difficult to integrate into isolated seats because of its complexity and its high production costs. Recent works have proposed an integrated solar membrane distillation prototype with the membrane distillation modules built directly into the evacuated solar tubes. This was possible via the integration of films containing photothermal nanostructures onto the membrane [18,22]. The role of these nanostructures with highly photothermal heating, induced via solar illumination, is to drive the distillation process without the requirement of heating the input water. Another study has considered a direct contact between the membrane and photothermal nanostructures (SiO2/Au nanoshell, CB NPs, and Fe3O4 NPs) [33,34,35]. The NPs are coated at the membrane surface and demonstrate that thermal energy is transmitted rapidly from the nanoparticles to the polymeric membrane via direct contact. On the other hand, the increasing concentration of coating nanoparticles, which enhances heat production, ends with reducing the vapor flux; many of the base membrane’s pores were blocked by the NPs, which resulted in a decrease in vapor permeability [35]. The best solution is to implant photothermal nanostructures through the polymeric membrane. This strategy will not only simplify the membrane fabrication process but may also improve the membrane hydrophilicity and enlarge the pores in the membrane [36]. Structural investigations demonstrated that NPs, entrapped in the PVDF membrane during the demixing process, were well dispersed throughout the polymeric matrix [16]. However, many implanted NPs either involve expensive composites or absorb solar energy in a limited spectral range. The challenge remains to introduce materials with low cost, low toxicity and proficiency in harvesting the full range of the solar spectrum.
Transition metal chalcogenide (TMCs) NPs have been proposed for use in photothermal therapy (PTT) treatments for cancer [37,38] and antibacterial membranes [39]. More recently, S. Abramovich et al. [40] have shown that embedding NiSe and CoSe nanofillers into the polymeric membrane increases the transmembrane flux by 330% and 690%, respectively. These materials have strong photo absorption characteristics in a wide range of wavelengths and have shown to be less toxic than graphene, suggesting they might have a wide range of water treatment and biomedical applications. TMC nanostructures can also degrade toxic environmental contaminants into nonhazardous products [39,41,42]. An efficient photothermal candidate must exhibit a metal-like character and show like localized plasmonic resonances absorption in particular spectral regions. Depending on their composite elements, TMCs can exist as semiconductors, semi-metals, or topological insulators [39,40,41,42,43,44]. TMC semi-conductors show metal-like absorption but typically operate in the deep UV. Among a variety of semi-metal or topological insulator TMCs, nickel chalcogenide (NiCs) series (Se, S, Te) demonstrate high metallic features due to the high degree of covalency in the nickel–chalcogen bond [43,44]. Further, NiCs are also demonstrated with a high chemical stability and ease of synthesis [37,38,45].
In this regard, the aim of this theoretical study is to push NiC NPs into the pool of desalination to invite more research investigations. Here we propose an innovative PSDMD system made by poly (vinylidenefluoride) (PVDF) materials loaded with nickel sulfides and nickel tellurides nanoparticles (NPs). The manuscript is structured as follows. In section II, we introduce the theoretical approach used to determine the membrane temperature profile and the resulting transmembrane vapor flux. In section III, we firstly discuss the absorption cross section of several types of photothermal NPs to define the most efficient absorber for the solar spectrum. Then we compare our calculation to the experimental measurement for validation. After, we illustrate the profile of membrane temperature under different intensities of solar radiation. We also compare the amount of vapor flux produced by different loaded PVDF membranes. Finally, we treat the impact of increasing the porosity of composite membrane on the performance of PSDMD.
2. Materials and Methods
The schematic design of the PSDMD proposed in this theoretical study is depicted in Figure 1. This system uses a porous hydrophobic membrane that separates a hot feed from a cold distillate. The partial vapor pressure difference between each side of the membrane drives membrane vapor flux, generating pure water upon condensation. The membrane materials are inert hydrophobic polymers including poly (vinylidenefluoride) (PVDF) loaded with NiC NPs.
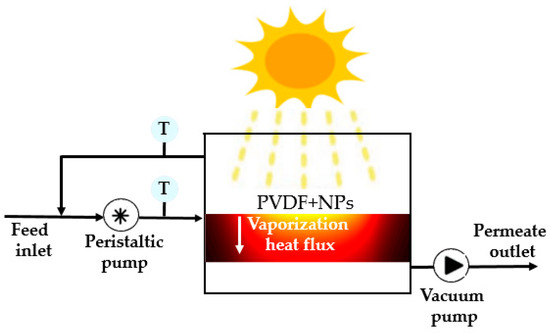
Figure 1.
Schematic design of the solar driven membraneprototype. The NPs increase the temperature of the membrane at its surface thanks to their efficient light-to-heat conversion. The difference in temperature between the two sides of the membrane creates a gradient of vapor concentration. This helps the diffusion of vapor flux through the PVDF membrane and enhances its rate.
The experimental realization of the nanocomposite films, i.e., polymer membrane loaded with NiC NPs, was presented by P. Riddy et al. [46]. Thus, the nanocomposite films were fabricated using a cost-effective solution casting technique by dispersing different contents of NiC NPs in the polymeric matrix. The synthesis of the NiC NPs used a green synthesis approach followed by their incorporation into the polymeric matrix [46]. These results suggest that NiC NPs were dispersed homogeneously in the polymeric matrix. Accordingly, in our theoretical approach, the implanted NPs will be supposed as uniformly distributed in the membrane geometry. The temperature rise distribution of the PVDF membrane by NiC NPs, illuminated via solar irradiation, is obtained by solving the heat flow transfer equation given using:
where is the membrane temperature increase, and are their thermal conductivity and thermal diffusivity, respectively, is the mass density, is the specific heat and is the rate of generated heat density. The thermal conductivity and diffusivity are calculated using an effective medium theory as a function of the membrane porosity ():
where and () are the thermal conductivity (thermal diffusivity) of the polymeric material and the gas phase inside the membrane pores, respectively. The rate of generated heat density will be corrected using an additional term, taking into account the loss of heat via conduction through the membrane wall, defined as [17]:
where is a cooling time constant included to describe the loss of heat inside the PVDF membrane, whatever its form. This term will be deduced from experimental measurement [16]. is regarded as effectively continuous heat production, due to the absorbed energy on NPs and is described by the exponential decrease across the thickness of the PVDF membrane:
where is the number of illuminated particles per unit of volume, is the absorption coefficient of NPs embedded in the PVDF membrane () and is the solar irradiation intensity. is the absorption cross section of individual NPs which depends on its radius and the wavelength of incident light. Regarding the size of the considered NPs (NP radius 30 nm) and their metal-like characteristics, the quantum confinement effect will be negligible and Mie theory will be sufficient for the evaluation of this parameter. More details about this approach can be found in ref [17].
To obtain an analytical solution to Equation (1), we suppose that the implanted NPs are randomly distributed in an effective plat cylinder of radius and thickness H (H ) and that the heat source generation is thermally localized within this region. The radius is estimated using the radius of the irradiated area and H using the membrane thickness. Then, the time-dependant temperature increase in the membrane inside and outside the cylindrical region is solution to the following equations:
Experimental measurement shows that, under continuous excitation sources, the membrane temperature tends to a stationary regime [16]. Then we could deal with the stability of the time dependent solution of equation (1) and suppose the total solution as a sum of a steady state solution and a time dependent perturbation : . Thus, demonstrates a differential equation where the solutions inside and outside the cylinder are the Bessel modified functions of the first and second kind, respectively (see supporting information). Finally, together with the boundary conditions ( and ), the time dependant temperature rise is reduced to:
The obtained solution of Equation 1 defines the temperature difference between THE feed water and membrane layer at finite and . It decreases while increases, inducing a difference in temperature between the membrane’s opposite sides. This leads to a gradient of vapor concentration through the PVDF membrane. The water vapor concentration at any point of the membrane is given using: , where is the ideal gas constant, is the membrane temperature and is the saturation vapor pressure. The gradient of concentration can be approximated using an average value at the top () and the bottom () of the membrane thickness (see supporting information). According to Fick’s first law, the vapor flux through the membrane increases due to the increase in this gradient concentration. Hence, the vapor flux will be expressed as:
where is the water vapor molar mass, and is the effective diffusion coefficient of water for a tortuosity [47] and porosity (). Then the time-dependent transmembrane vapor flux expressed in L/m2 h is given using:
where S is the total membrane area. Another useful parameter that can be calculated to estimate the overall device efficiency is energy efficiency. Energy efficiency () provides a quantitative estimation of the percentage of the heat that is effectively used to promote water evaporation through the membrane. is thus defined as:
where is the evaporation enthalpy of water. and denote the sunlight intensity and the irradiated membrane area, respectively.
3. Results and Discussions
3.1. Nanoparticles Absorption Cross Section
To quantitatively characterize the absorption performance of proposed NPs, the absorption cross section (ABS) will first be discussed. Two parameters are necessary for the identification of solar light efficient absorbers: the amplitude of the absorption cross section and its energy. Figure 2 illustrates the absorption cross sections of different NP materials for a 30 nm NP radius. The absorption cross sections of Ag and NPs are also considered. and NPs show pronounced resonance peaks similar to those observed with Ag NP with an amplitude of nm2 and nm2, respectively. These resonances are observed at 1.45 eV for NP and 3.08 eV for NP. The resonance peak of NP extends over large spectral regions of visible range with an amplitude of nm2. and NPs display extended ABS in a broad spectral range from infrared to ultraviolet. Each of the resonance peaks originated from the negative real part dielectric function in particular spectral regions; absorption resonance meets when the dielectric real part is negative and the imaginary part varies slightly with energy. As presented in Figure S1 (see supplementary materials) e, and show extended deeper regions of negative. These regions cover the infrared spectral range for materials while being extended from infrared to visible with materials and over the visible range with materials. This is not the case for and , where the negative region is so small and meets at a higher energy, which explained the absence of resonance peaks at a lower energy.
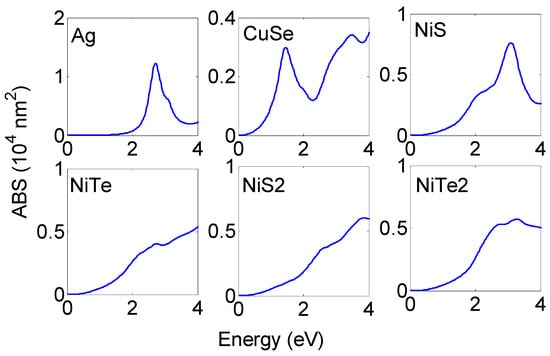
Figure 2.
Absorption cross sections of different NP materials for 30 nm NP radius. and NPs show pronounced resonance peaks. NP resonates over the large spectral region of visible range while and NPs display extended ABS in a broad spectral range from the infrared to ultraviolet.
The ABS resonance peaks can be manipulated by changing some parameters. ABS resonance energy and its amplitude are sensitive to the nanostructure composition, shape, size, and surrounding medium [17]. Increasing the NP radius leads to the enhancement in ABS amplitude and the appearance of new resonance peaks. This is apparent in Figure 3, where we have plotted the ABS distribution of different NPs as a function of the NP radius. As expected, all of the considered NPs show increasing ABS amplitude and rising, new resonance peaks with an increasing radius. Some of the considered NPs are good absorbers for solar light with small sizes while others need large radii for achieving resonance in the visible. For small radii (30 nm), only A,g, and NPs show absorption for the visible while and NPs absorb over the UV. For greater sizes (beyond 30 nm), the ABS resonances of these NPs are more pronounced and cover the visible and infrared regions. Large NP shows efficient absorption for infrared (ABS nm2 for radius = 100 nm) and the rise of broadband resonance over the visible range (NP radius 60 nm).
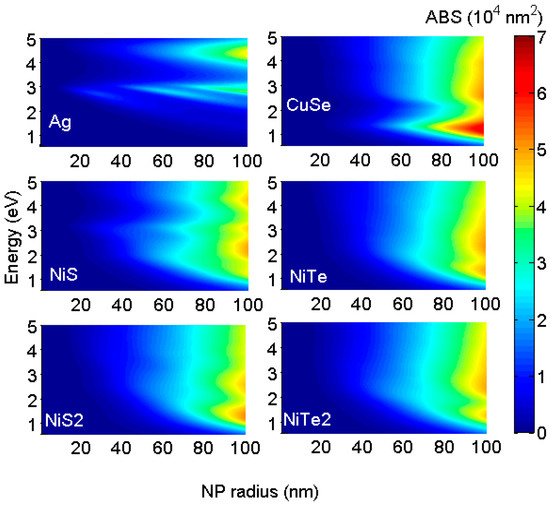
Figure 3.
Distribution of absorption cross sections of different NPs function of energy and NP radius. All considered NPs show increasing ABS amplitude and rising new resonance peaks with increasing radius. Some of the considered NPs are good absorbers for solar light with small sizes while others need large radii for achieving resonance in the visible.
In sum, and with small radii (NP radius 40 nm) will be excellent absorbers of solar radiation. The larger size is needed for and to enhance their absorption of solar radiation. NPs are good absorbers for the infrared with resonance peaks at 1.45 eV; this is consistent with the experimental measurement [39]. This makes NPs a promising candidate for biomedical application.
3.2. Photothermal Membrane Temperature and Vapor Flux
The absorption of different NPs determines the heat source density which dictates the solar photothermal temperature rise and thus the water vapor flux. As discussed in previous works [16,22], the membrane-localized heating via illuminated NPs dominates the total produced heat and induces vaporization of the feed water. This will be confirmed by our simulation for experimental measurements obtained by A. Politano et al. [16] on PVDF membrane loaded with Ag NPs. Figure 4a,b show a comparison between our theoretical calculations and the experimental measurement [16] for the membrane temperature rise and the transmembrane vapor flux to pure water, respectively. The experimental measurement was obtained using a high-pressure UV mercury lamp, with a wavelength of 366 nm, and a viewing angle of 90° is used to irradiate the membrane area of 21.24 cm2 [16]. Three different compositions of Ag NPs are considered: magenta, blue and black curves indicate a 15%, 20% and 25% of Ag NPs composition in the membrane, respectively. The incident light is about 23 kW/m2, respectively. The cooling time is equal to 600 s and is obtained by fitting the measured bulk temperature increase using an exponential function: .
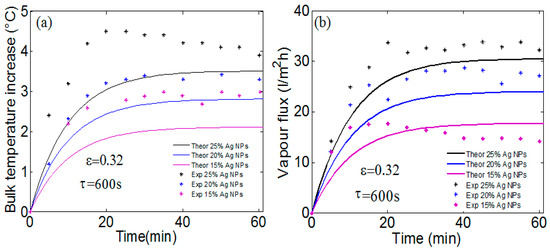
Figure 4.
Theoretical calculations and measurement for (a) the membrane temperature rise and (b) the transmembrane vapor flux to pure water. The cooling time is obtained by fitting the measured bulk temperature increase using an exponential function. PVDF membranes loaded with 25% Ag NPs can produce vapor flux around 11-fold higher than the corresponding values for unloaded membranes.
The temperature rise is calculated at the center of the membrane surface () for an ambient temperature of 21 °C and a porosity of 32%. Great agreement between calculation and measurement for both the temperature rise and transmembrane vapor flux is obtained. The small difference is due to the choice of cooling time, where a greater value is possible and may lead to increasing membrane absorption and a temperature rise. In addition, the contribution of UV lamp heat to the total produced heat has not been considered. One can note that the PVDF membranes loaded with 25% Ag NPs can produce vapor flux by about 30 L/m2 h, which is 10-fold higher than the corresponding values for unloaded membranes [16] Despite their efficient photothermal conversion under UV radiation, Ag NPs will not be of great interest to solar sources where broadband absorption over the visible range is needed.
The accuracy between our theoretical model and the experimental measurement allows us to theoretically examine the membrane temperature rise as a function of implanted NPs. As discussed before, and show large important absorption over the visible range, which can enhance the membrane temperature rise under solar radiation. In addition, the heat production of NPs increases with increasing excitation intensity due to their linear dependence. This is shown in Figure 5, where we present the temperature rise profile of the PVDF membrane under solar radiation () with two different intensities. The solar spectral irradiance is considered at sea level (see supporting information). The PVDF membrane is loaded with 25% of different NPs (including Ag NPs) with a radius of 40 nm. We can see an increase of about 10 times in the PVDF temperature rise while the intensity is amplified. The temperature rise of the PVDF membrane loaded with and NPs reaches 0.25 °C and 2.5 °C (at the membrane surface center) under natural solar sun radiation and its amplified intensity, respectively. A less-heated membrane surface is obtained with and NPs, where an increase of 2 °C is observed with amplified solar radiation.
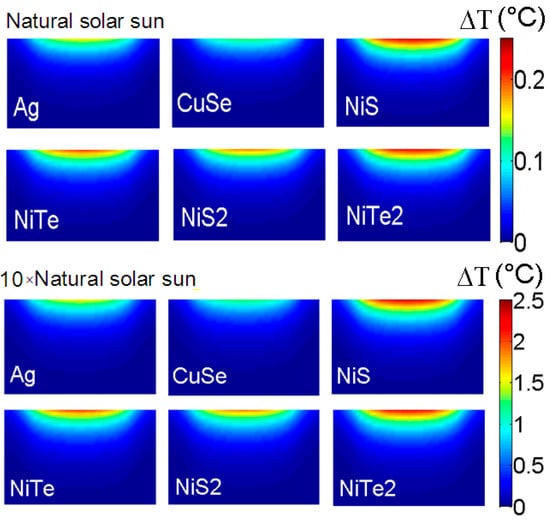
Figure 5.
The temperature rise profile of PVDF membrane under solar radiation () with two different intensities. The temperature rise of PVDF membrane loaded with NiS and NiTe2 NPs reaches 0.25 °C and 2.5 °C (at the membrane surface center) under natural solar sun radiation and its amplified intensity, respectively.
Due to their lower absorption for visible (compared to the NiCs NPs), and Ag NPs produce lower heat under solar radiation where a rise of 1 °C is obtained for amplified intensity. In spite of this, the implantation of NPs through the membrane can stop the penetration of contaminants and hence the deterioration of treated water quality [39]. It is worth noting that for different loaded membranes, the temperature rise is about 0 °C at the bottom of the membrane (distillate side). Hence, the difference in temperature between the two sides will be manipulated by the nature of implanted NPs.
3.3. Membrane Efficiency
The evaluation of membrane efficiency is the key parameter for the technical realization of PSDMD. In a membrane system the contact angle, pore size and vapor flux are critical. However, the implantation of NPs into the polymeric membrane not only increases its temperature and vapor flux but also increases its pore size and contact angle (>110°). As a result, the membrane exhibited much higher resistance to wetting [16,40]. In this regard, the wetting effect will be neglected and only the effects of implanted NPs and porosity on the membrane efficiency will be discussed.
Let us first discuss the effect of implanted NPs on the production of vapor flux. As expected, the transmembrane vapor flux increases with the amplification of solar irradiation intensity and the nature of injected NPs. As shown in Figure 6, after one hour of illumination, the highest transmembrane vapor flux is about 26–27 L/m2h for amplified intensity and 2.7 L/m2h under the natural sun. These values, which are obtained with and NPs, are approximately half more than those obtained with Ag NPs. A lower vapor flux is obtained when the membrane is loaded with 25% of and NPs; it can produce about 22 and 24 L/m2h of vapor flux under an amplified intensity. Despite their weak photothermal effect under solar radiation, the implantation of NPs helps to produce 15 L/m2h of vapor flux.
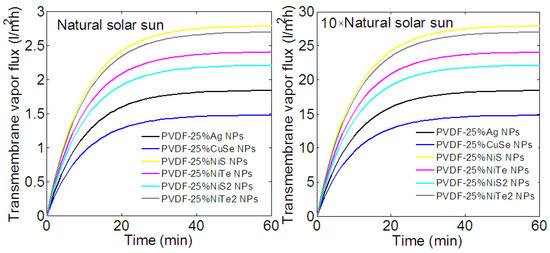
Figure 6.
Time-dependent transmembrane vapor flux of different composite membranes for two solar intensities. After 1 h of illumination the highest transmembrane vapor flux is about 26–27 L/m2h for amplified intensity and 2.7 L/m2h under the natural sun. A membrane loaded with and NPs can produce about 22 and 24 L/m2h of vapor flux.
The increasing transmembrane vapor flux with temperature can be explained if we consider how the vapor concentration (through the membrane) depends on temperature. In a composite photothermal membrane, the temperature is maximal at the feed PVDF membrane interface and decreases along its thickness (see Figure 5). Hence, the feed flow increases its temperature close to the PVDF membrane and its water vapor concentration. The difference in temperature between the hot and cold sides produced a higher flux because of the gradient of vapor concentration along the membrane thickness. With increasing intensity and efficient photothermal NPs, this temperature difference increases, leading to an increasing vapor flux. We can note that the excellent water vapor generation performance of the PVDF-25% NiC NPs composite membrane is associated with the high light absorption range of the NiC NPs over the visible solar spectrum and good photothermal conversion efficiency.
All of the physical parameters (porosity and cooling time) used in the latter simulation are taken from ref. [16], where the PVDF membrane is loaded with Ag NPs. However, the nature of NPs and their sizes affect the porosity, which changes the membrane’s thermal conductivity and diffusivity. In fact, the implantation of NPs through the membrane increases the size of its pores, leading to an increasing porosity and a decreasing loss of heat, i.e., long cooling time. Analyzing these effects through the PVDF membrane is important to enhance the performance of the nickel chalcogenide composite PVDF membrane. We further investigate the transmembrane vapor flux and the energy efficiency under the variation of porosity and cooling time. As discussed in previous experimental measurements, the porosity varies from 27 to 56% with the nature and composition of injected NPs [16,40]. Other works show that the porosity of composite membranes is still as high as 85% [22]. According to these results, the porosity will be varied from 0 to 90%. The variation of transmembrane vapor flux as a function of the porosity and for two cooling times is depicted in Figure 7. As we can see, under solar radiation, a cooling time of 600 s, and the maximum of porosity (about 90%), a PVDF membrane loaded with different NPs produces between 3 and 5.5 L/m2h of vapor flux. This corresponds, as calculated in Equation (9) and indicated in Figure S2 (see supplementary materials), to the energy efficiency of 28 and 52% (these values increase to 4.2 and 8 L/m2h when the cooling time is about 1000 s, supposedly 41 and 80% of energy efficiency. Figure S2 also shows that, for 32% of porosity and a cooling time of 600 s, which corresponds to a PVDF membrane loaded with Ag NPs [16], the energy efficiency is about 26% for the PVDF membrane loaded with 25% of NPs. However, a porosity of about 56% is obtained when the membrane is loaded with NiCs NPs [40], and a higher energy efficiency and long cooling time is thus expected. Then, for a porosity of 56% and a cooling time of 1000 s, Figure S2 shows an energy efficiency of 58% for a membrane loaded with NiS NPs. This result is in good agreement with recent experimental measurements [40].
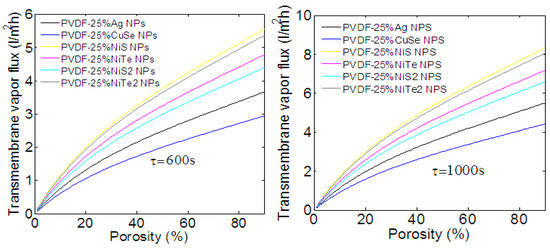
Figure 7.
Transmembrane vapor flux as a function of the porosity. PVDF membrane loaded with different TMCs NPs produces between 3 and 5.5 L/m2h of vapor flux. These values increase to 4.2 and 8 L/m2h when the cooling time is about 1000 s, i.e., lower loss of heat.
The increasing energy efficiency with increasing porosity can be explained via the higher diffusion coefficient and the lower loss of heat. The higher diffusion coefficient helps the diffusion of vapor flux through the membrane. The lower loss of heat, which is translated by a long cooling time, leads to an increasing temperature at the membrane surface. Hence, the difference in temperature between opposite sides of the membrane increases, resulting in an increased vapor flux. We can conclude that the composite membrane possesses a high porosity, which provides a higher distillation and energy efficiency. All of the considered NPs show efficient photothermal conversion in a particular spectral region. However, as the variation of porosity as a function of most of the implanted NPs is not defined, we are not able to define exactly the most efficient photothermal candidate, and thus more experimental investigations are needed.
4. Conclusions
To summarize, we proposed transition metal chalcogenide NPs to be loaded to the hydrophobic membrane to enhance the performance of the solar-driven membrane. Our theoretical investigation has demonstrated that nickel chalcogenide (S and Te) nanoparticles were the most efficient photothermal absorbers of solar radiation. and Ag NPs showed a lower absorption for solar radiation, and are even more effective in the infrared and ultraviolet regions, respectively. All of the considered NPs raised the temperature of the membrane surface compared to the feed temperature. The increasing difference in temperature between the opposite sides of the membrane has driven the production of vapor flux through the membrane, which reached 27 L/m2h with NPs and obtained a smaller heat input. In addition to their photothermal capacities, the implantation of nickel chalcogenide NPs through the PVDF membrane increased its pore size and wetting resistance, leading to a higher energy efficiency. Accordingly, our investigation showed an energy efficiency of 58% for a PVDF loaded with NiS NPs, which is in good agreement with a recent experiment [40]. Finally, we hope that our proposed approach helps other experiments to develop a new generation of photothermal membrane distillation and encourage the use of nickel chalcogenide NPs for such applications. Thanks to their efficient absorption for solar spectra, these NPs can be also used to enhance the absorption of thin film solar cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/membranes13020195/s1, Figure S1: dielectric function of all of considered materials; Figure S2: energy efficiency of different composite membranes at different porosity and cooling times; Figure S3: solar spectral irradiance at sea level; Table S1: different parameters used to calculate the membrane temperature and vapor flux [47,48,49,50,51,52,53,54].
Author Contributions
Conceptualization, D.E. and S.J.; methodology, D.E.; software, D.E. and I.B.A.; validation, D.E. and S.J.; formal analysis, D.E.; investigation, D.E.; resources, D.E.; data curation, D.E. and I.B.A.; writing—original draft preparation, D.E.; writing—review and editing, D.E, and S.J.; visualization, D.E.; supervision, S.J.; project administration, S.J.; funding acquisition, S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting reported results are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by EXTRASEA (Extracting water, minerals and energy from seawater desalination brine) project (2018–2021): ERANETMED3-166.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S.; Zhu, J. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Desalination Freshens Up. Science 2006, 313, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2568. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Al-Shammiri, M.; Safar, M. Multi-effect distillation plants: State of the art. Desalination 1999, 126, 45–59. [Google Scholar] [CrossRef]
- Kiss, A.A.; Flores Landaeta, S.J.; Infante Ferreira, C.A. Towards energy efficient distillation technologies—Making the right choice. Energy 2012, 47, 531–542. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Assad, M.E.; Sayed, E.T.; Soudan, B. Recent progress in the use of renewable energy sources to power water desalination plants. Desalination 2018, 435, 97–113. [Google Scholar] [CrossRef]
- Hogan, P.A.; Fane, A.G.; Morrison, G.L. Desalination by solar heated membrane distillation. Desalination 1991, 81, 81–90. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Review: Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Gryta, M. Effectiveness of Water Desalination by Membrane Distillation Process. Membranes 2012, 2, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.-S. Recent advances in membrane distillation processes. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Schofield, R.; Fane, A.G.; Fell, C.J.D.; Macoun, R. Factors affecting flux in membrane distillation. Desalination 1990, 77, 279–294. [Google Scholar] [CrossRef]
- Politano, A.; Di Profio, G.; Fontananova, E.; Sanna, V.; Cupolillo, A.; Curcio, E. Overcoming temperature polarization in membrane distillation by thermoplasmonic effects activated by Ag nanofillers in polymeric membranes. Desalination 2019, 451, 192–199. [Google Scholar] [CrossRef]
- Elmaghraoui, D.; Politano, A.; Jaziri, S. Photothermal response of plasmonic nanofillers for membrane distillation. J. Chem. Phys. 2020, 152, 114102. [Google Scholar] [CrossRef]
- Santoro, S.; Avci, A.H.; Politano, A.; Curcio, E. The advent of thermoplasmonic membrane distillation. Chem. Soc. Rev. 2022, 51, 6087–6125. [Google Scholar] [CrossRef]
- Dongare, P.D.; Alabastri, A.; Pedersen, S.; Zodrow, K.R.; Hogan, N.J.; Neumann, O.; Wu, J.; Wang, T.; Deshmukh, A.; Elimelech, M.; et al. Nanophotonics-enabled solar membrane distillation for off-grid water purification. Proc. Natl. Acad. Sci. USA 2017, 114, 6936–6941. [Google Scholar] [CrossRef]
- Santoro, S.; Aquino, M.; Seo, D.H.; Laan, T.V.D.; Lee, M.; Yun, J.S.; Park, M.J.; Bendavid, A.; Shon, H.K.; Avci, A.H.; et al. Dimensionally controlled graphene-based surfaces for photothermal membrane crystallization. J. Colloid Interface Sci. 2022, 623, 607–616. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; Zuo, K.; Chen, L.; Yuan, L.; Liang, J.; Li, Q.; Ajayan, P.M.; Zhao, Y.; Lou, J. Bio-derived ultrathin membrane for solar driven water purification. Nano Energy 2019, 60, 567–575. [Google Scholar] [CrossRef]
- Dongare, P.D.; Alabastri, A.; Neumanna, O.; Nordlander, P.; Halas, N.J. Solar thermal desalination as a nonlinear optical process. Proc. Natl. Acad. Sci. USA 2019, 116, 13183–13187. [Google Scholar] [CrossRef]
- Gao, M.; Peh, C.K.; Meng, F.L.; Ho, G.W. Photothermal Membrane Distillation toward Solar Water Production. Small Methods 2021, 5, 2001200. [Google Scholar] [CrossRef]
- Ho, C.D. Solar-Assisted Membrane Distillation. Membranes 2022, 12, 304. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Zhang, Y.; Cong, S.; Guo, F. Experimental Investigation on Floating Solar-Driven Membrane Distillation Desalination Modules. Membranes 2021, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Mustakeem, M.; El-Demellawi, J.K.; Obaid, M.; Ming, F.; Alshareef, H.N.; Ghaffour, N. MXene-Coated Membranes for Autonomous Solar-Driven Desalination. ACS Appl. Mater. Interfaces 2022, 14, 5265–5274. [Google Scholar] [CrossRef]
- Zhang, B.; Wong, P.W.; Guo, J.; Zhou, Y.; Wang, Y.; Sun, J.; Jiang, M.; Wang, Z.; An, A.K. Transforming Ti3C2Tx MXene’s intrinsic hydrophilicity into superhydrophobicity for efficient photothermal membrane desalination. Nat. Commun. 2022, 13, 3315. [Google Scholar] [CrossRef]
- CK, P.P.; Kumaresan, G.; Abraham, R.; Santosh, R.; Velraj, R. Effect of Teflon-Coated PVDF Membrane on the Performance of a Solar-Powered Direct Contact Membrane Distillation System. Sustainability 2022, 14, 6895. [Google Scholar] [CrossRef]
- Shin, D.; Kang, G.; Gupta, P.; Behera, S.; Lee, H.; Urbas, A.M.; Park, W.; Kim, K. Thermoplasmonic and Photothermal Metamaterials for Solar Energy applications. Adv. Opt. Mater. 2018, 6, 1800317. [Google Scholar] [CrossRef]
- Xue, G.; Chen, Q.; Lin, S.; Duan, J.; Yang, P.; Liu, K.; Li, J.; Zhou, J. Highly Efficient Water Harvesting with Optimized Solar Thermal Membrane Distillation Device. Glob. Chall. 2018, 2, 1800001. [Google Scholar] [CrossRef]
- Zhong, W.; Hou, J.; Yang, H.-C.; Chen, V. Superhydrophobic membranes via facile bio-inspired mineralization for vacuum membrane distillation. J. Membr. Sci. 2017, 540, 98–107. [Google Scholar] [CrossRef]
- Li, Q.; Beier, L.J.; Tan, J.; Brown, C.; Lian, B.; Zhong, W.; Wang, Y.; Ji, C.; Dai, P.; Li, T.; et al. An integrated, solar-driven membrane distillation system for water purification and energy generation. Appl. Energy 2019, 237, 534–548. [Google Scholar] [CrossRef]
- Wu, J.; Zodrow, K.R.; Szemraj, P.B.; Li, Q. Photothermal nanocomposite membranes for direct solar membrane distillation. J. Mater. Chem. A 2017, 578, 23712. [Google Scholar] [CrossRef]
- Lia, W.; Chenb, Y.; Yaoc, L.; Rena, X.; Lia, Y.; Denga, L. Fe3O4/PVDF-HFP photothermal membrane with in-situ heating for sustainable, stable and efficient pilot-scale solar-driven membrane distillation. Desalination 2020, 478, 114288. [Google Scholar] [CrossRef]
- Chen, Y.R.; Xin, R.; Huang, X.; Zuo, K.; Tung, K.-L.; Li, Q. Wetting-resistant photothermal nanocomposite membranes for direct solar membrane distillation. J. Membr. Sci. 2021, 620, 118913. [Google Scholar] [CrossRef]
- Guan, Y.F.; Huang, B.C.; Qian, C.; Wang, L.F.; Yu, H.Q. Improved PVDF membrane performance by doping extracellular polymeric substances of activated sludge. Water Res. 2017, 113, 89–96. [Google Scholar] [CrossRef]
- Yang, R.; Li, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Facile synthesis of hollow mesoporous nickel sulfide nanoparticles for highly efficient combinatorial photothermal–chemotherapy of cancer. J. Mater. Chem. B 2020, 8, 7766. [Google Scholar] [CrossRef]
- Zhanga, X.; Wua, J.; Williamsb, G.R.; Niua, S.; Qiana, Q.; Li-Min Zhu, L.M. Functionalized MoS2-nanosheets for targeted drug delivery and chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2019, 173, 101–108. [Google Scholar] [CrossRef]
- Wang, X.-M.; Huang, L.; Wang, Y.-J.; Xuan, L.; Li, W.W.; Tian, L.-J. Highly efficient near-infrared photothermal antibacterial membrane with incorporated biogenic CuSe nanoparticles. Chem. Eng. J. 2021, 405, 126711. [Google Scholar] [CrossRef]
- Abramovich, S.; Dutta, D.; Rizza, C.; Santoro, S.; Aquino, M.; Cupolillo, A.; Occhiuzzi, J.; La Russa, M.F.; Ghosh, B.; Farias, D.; et al. NiSe and CoSe Topological Nodal-Line Semimetals: A Sustainable Platform for Efficient Thermoplasmonics and Solar-Driven Photothermal Membrane Distillation. Small 2022, 18, 2201473. [Google Scholar] [CrossRef]
- Tavker, N.; Sharma, M. Designing of waste fruit peels extracted cellulose supported molybdenum sulfide nanostructures for photocatalytic degradation of RhB dye and industrial effluent. J. Environ. Manag. 2020, 255, 109906. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, P.A.; Oluwalana, A.E.; Andrew, F.P. Morphological Studies, Photocatalytic Activity, and Electrochemistry of Platinum Disulfide Nanoparticles from Bis (morpholinyl-4-carbodithioato)-platinum(II). ACS Omega 2020, 5, 27142–27153. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.C.; Gao, S.P.; Dong, P.; Baines, R.; Ajayan, P.M.; Ye, M.X.; Shen, J.F. Insight into the hydrogen evolution reaction of nickel dichalcogenide nanosheets: Activities related to non-metal ligands. Nanoscale 2017, 9, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- De Silva, U.; Masud, J.; Zhang, N.; Hong, Y.; Liyanage, W.P.R.; Zaeem, M.A.; Nath, M. Nickel telluride as a bifunctional electrocatalyst for efficient water splitting in alkaline medium. J. Mater. Chem. A 2018, 6, 7608–7622. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Y.F.; Jiang, J.; Yu, S.H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Reddy, P.L.; Deshmukh, K.; Kovářík, T.; Reiger, D.; Nambiraj, N.A.; Lakshmipathy, R.; Pasha, S.K. Enhanced dielectric properties of green synthesized Nickel Sulphide (NiS) nanoparticles integrated polyvinylalcohol nanocomposites. Mater. Res. Express 2020, 7, 064007. [Google Scholar] [CrossRef]
- Shen, L.; Chen, Z.X. Critical review of the impact of tortuosity on diffusion. Chem. Eng. Sci. 2007, 62, 3748–3755. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, 864. [Google Scholar]
- Draxel, C.A.; Sofo, J.O. Linear optical properties of solids within the full-potential linearized augmented plane wave method. Comput. Phys. Commun. 2006, 175, 1. [Google Scholar]
- Schwarz, K.; Blaha, P.; Trickey, S.B. Electronic structure of solids with WIEN2k. Mol. Phys. 2010, 108, 3147. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation. Phys. Rev. 1965, 140, A1133. [Google Scholar]
- Yu, P.; Cardona, M. Fundamentals of Semiconductors Physics and Materials Properties; Springer: Berlin, Germany, 2010. [Google Scholar]
- Lide, R. David, CRC Handbook of Chemistry and Physics, 85th ed.; CRS Press: Boca Raton, FL, USA, 2004; p. 6. [Google Scholar]
- Buonomenna, M.G.; Lopez, L.C.; Favia, P.; d’Agostino, R.; Gordano, A.; Drioli, E. New PVDF membranes: The effect of plasma surface modification on retention in nanofiltration of aqueous solution containing organic compounds. Water Res. 2007, 41, 4309–4316. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).