Abstract
The following paper offers a modern REE 1.0 computer application designed to model the behavior of REE ions in adsorptive materials and membranes. The current version of the application is based on several models, such as the Lagergren pseudo-first order, pseudo-second-order and Elovich kinetic models, and the intraparticle diffusion model, the diffusion-chemisorption model, and the Boyd model. The application has been verified on a sample of four different types of adsorptive materials and membranes. The proposed application allowed the analysis of kinetics, but also the mechanisms of the adsorption process, especially those responsible for the rate-determining steps. It was found that Lagergren pseudo-second-order kinetic model was the best-fit model to describe the adsorption behavior of REE ions onto the novel materials and membranes. Other models determined the process of chemisorption was in force for the analyzed cases, and the mechanisms controlling the adsorption processes are diffusion-chemisorption and adsorption is mostly controlled by film diffusion. Additionally, characteristic parameters, such as qe designated from two different models, showed very similar values, which indicates the correctness of the analysis.
1. Introduction
Nowadays, rare earth elements (REEs) become more and more important, especially because of their exceptional chemical, catalytic, physical, magnetic, and luminescent features and of course application in many modern technologies [1,2]. Due to China monopolizing in the production of REEs and the continuous increase in demand for these metals, there was a need to find new alternative sources and create new technologies for their recovery [2,3,4]. Unfortunately, the conventional REE mining techniques, which include ore deposits, are energy intensive and generate significant volumes of toxic wastes [5]. It turned out that coal fly ashes, generated annually worldwide in the amount of over 750 million tons, from which only 30% are utilized, can be their ideal source [1]. For REE recovery from coal fly ashes, physical, biological, and chemical methods (acid-base leaching) can be used [6,7,8,9,10,11,12,13,14,15,16,17,18]. However, they have many limitations. Therefore, alternative methods were introduced, such as membrane techniques and the application of appropriate materials, such as ion-imprinted polymers (IIP), which have recognition sites in a macromolecular matrix made by using a template molecule [19,20].
Currently, the most commonly used methods in water, municipal and industrial sewage purification, processing of extracts from chemical recovery methods, as well as the removal and recovery of them heavy metal ions are chemical precipitation, ion exchange, electrodialysis, ultrafiltration, nanofiltration, reverse osmosis, coagulation, flocculation, flotation, etc. [18,19,20,21,22,23,24]. However, they have several restrictions, such as a high consumption of reagents, generating toxic waste, and unpredictable removal of metal ions [25]. In turn, adsorption processes are by definition simple, economic, effective, and versatile. Of course, despite many advantages, they also have some disadvantages, such as low capacity, high costs, low selectivity, problems with their regeneration, and difficulties with scaling-up [26]. Therefore, further research is conducted towards the combination of advantages of usually used techniques, especially membrane techniques and adsorption processes, which was reflected in the introduction of a new type of adsorption membrane. As the name suggests, among the most important processes is adsorption, which is a mass transfer, during which the substance is transferred from the liquid phase to the surface of the solid and is associated with it as a result of physical or/and chemical interactions. As a rule, chemical adsorption is more suitable for removing or recovering metal ions, due to the stronger type of interaction and higher adsorption capacity relative to heavy metals. Special functional groups on the adsorbent surface that affect metal ions are responsible for this. That results in the adsorption separation of metals from the solution [27].
These special types of adsorption materials and membranes are a combination of functional groups (e.g., amino, carboxylic, and sulfone) with the surface and walls of pores of polymers [28,29,30,31,32,33,34,35,36]. They can also be hybrid materials and membranes that are a combination of polymer membranes with inorganic additives in the form of an adsorbent addition [37,38,39,40,41,42]. During the flow of processed sewage or extract through this type of membrane, functional active sites interact with separated ions, resulting in the separation of pollution or analytes at high speed and adsorption capacity. This is most likely caused by a very short intraparticle diffusion between the target substances and the active binding site in the adsorption membrane [28]. In recent years, the so-called ion-imprinted polymers (IIPs) are becoming more and more popular, due to their extremely high selectivity. IIPs are crosslinked polymers with pores and binding sites for the targeted ions [43,44,45]. They can be synthesized in a few steps during the reaction of the functional monomer, a crosslinker, an initiator and a template. In the first stage, complexes are formed based on monomers with functional groups and template ions (REE ions). In the second stage, polymerization of monomers is carried out by adding crosslinking agents and carrying out photo- or thermopolymerization. In the third step, template ions are removed from the polymers, thus creating specific binding sites that can later capture specific ions [46]. Ion-imprinted polymers are characterized by excellent ionic selectivity due to the presence of these specific sites that bind ions of the appropriate size and charge. Of course, their adsorption capacity depends on many factors, such as the ability of IIPs ligands to bind metal ions, the size of the ions, their charge, the electron configuration of the metals, and the degree of oxidation [47,48]. These types of materials are characterized by appropriate thermal and pH stability [49]. IIPs are developed to mimic the key and lock mechanisms for recognizing and removing target ions. Thus, IIPs are characterized by excellent selectivity and show a specific affinity for a given ion. Even pollutants or analytes present in low concentrations can be selectively removed by IIPs, which has not been successfully achieved by other methods [50]. A very important issue, detailed in the case of analyzing the received data from the course of experience using adsorption materials and membranes, is their proper interpretation and the answer to the question with what kind of adsorption we are dealing with and what types of mechanisms are responsible for separating processes. Therefore, in order to analyze process kinetics, Lagergren (Lagergren Pseudo-First-order and Pseudo-Second-order Kinetic Models) and Elovich models are used. However, to analyze the adsorption mechanisms, intraparticle diffusion, diffusion-chemisorption, and Boyd models were used [41]. Usually, researchers dealing with the analysis of metal ion adsorption processes use popular models for the interpretation of experimental data, without delving into the mechanisms responsible for these processes. As a result, there is no suitable comprehensive tool that would fulfill both functions. In addition, it is known that only such information on the mechanisms of the considered processes will enable setting the direction for further research and introducing appropriate modifications of the analyzed adsorbents, which will allow obtaining materials with improved adsorption properties.
The aim of this work is the creation of a novel computer application, REE 1.0, to model REE ion behavior in adsorptive materials and membranes to characterize the obtained experimental results and eventually to select the most optimal materials. The proposed application will be appropriate both for the analysis of kinetics and the mechanisms responsible for separation processes in adsorption materials. It will also enable determining characteristic parameters for adsorption and diffusion processes. The REE 1.0 application will be an ideal tool for researchers studying the adsorption processes of metal ions, not only REE but on various types of adsorbents. They will be able to find a full list of popular models designed to characterize them, as well as less frequently used models that provide valuable data on both the kinetics of processes and the mechanisms governing them. The proposed application is an initial version, which will be appropriately modified over time to a more advanced form.
2. Materials and Methods
The created application is based on various models, which are given below.
2.1. Lagergren Kinetic Models
Lagergren pseudo-first-order and pseudo-second-order kinetic models can be linearly expressed as Equations (1) and (2), respectively [51,52,53]:
where
ln (𝑞𝑒 − 𝑞𝑡) = ln 𝑞𝑒 − 𝑘1𝑡,
𝑡/𝑞𝑡 = 1/𝑘2𝑞2𝑒 + 𝑡/𝑞𝑒,
𝑘1 (min−1)—the rate constant of the pseudo-first-order kinetic model,
𝑘2 (gmg−1 min−1)—the rate constant of the pseudo-second-order kinetic model,
𝑞𝑡—the adsorption capacity of metal ions at time 𝑡 (min), and
𝑞𝑒 (mg/g)—the adsorption capacity of metal ions at equilibrium state.
Lagergren kinetic equations could be a helpful tool to assess adsorbent adsorption performance.
2.2. The Elovich Model
Usually, the Elovich model was used in the analysis of chemisorption kinetics of gases on solid surfaces. However, according to Wang et al. [54], it could be also used in the investigation of liquid state sorption of an adsorbent and could be expressed using the following equation [54,55]:
where 𝑎 (mg/g) and 𝑏 are the Elovich parameters. These parameters could be obtained from the intercept and slope of the created straight line.
𝑞𝑡 = 𝑎 + 𝑏ln (𝑡),
2.3. Mechanism Insights
To explain the adsorption behaviors of metal ions, it is important to gain insight into the adsorption mechanism, using the intraparticle diffusion model, the diffusion chemisorption model and the Boyd equation [56,57,58,59].
2.3.1. Intraparticle Diffusion
The intraparticle diffusion model [56,57] can be expressed using the following equation:
where
𝑞𝑡 = 𝑥𝑖 + 𝑘𝑝𝑡 0.5
qt [mg/g]—the adsorbed amount at time t [min],
kp [mg g−1min−1/2]—the intraparticle diffusion rate constant, and
xi [mg/g]—the intercept of straight-line qt (t 0.5), related to the boundary layer thickness.
It turns out that if the plot qt (t 0.5) is a straight line, the adsorption is controlled by the intraparticle diffusion. However, if we have to use multilinear curves, then two or more mechanisms influence the adsorption process.
2.3.2. The Diffusion-Chemisorption Model
This model can be expressed as the linear relationship [58]:
where KDC is the diffusion-chemisorption constant.
𝑡 0.5/𝑞𝑡 = 1/𝐾DC + 1/𝑞𝑒 𝑡 0.5
2.3.3. The Boyd Equation
This type of equation could be used to determine the rate controlling step during the adsorption [58,59,60,61]:
where
𝐹 = 1 − (6/𝜋2) exp (−𝐵𝑡)
Bt—a function of F, which is the fraction of solute adsorbed at different times. F values could be obtained using equation:
where
F = qt/qe
qt [mg/g]—the adsorbed amount at time t [min], and
𝑞𝑒 [mg/g]—the adsorbed amount of metal ions at equilibrium state.
The Bt values at different contact times could be calculated using the following formula (if F > 0.85) [3]:
Bt= −0.4977 − ln (1 − F)
The plot of the Boyd model can be obtained using the relationship between Bt versus time t.
Based on this model, the effective diffusion coefficient can be calculated by usage of the following equation:
ln [1/(1 − F2)] = π2/r2 Det
Creating the plot of ln [1/(1 − F2(t))] versus time t, the diffusion coefficient De can be calculated from the slope π2De/r2.
It is stated that if the chart Bt(t) is a straight line and passes through the beginning of the coordinate system, then the process controlling the rate of the mass transport will be pore diffusion (or particle diffusion mechanism). Otherwise, if the Bt(t) dependence chart is non-linear or linear, but it does not pass through the origin, the film-diffusion or external mass transport will be the main dominant factors.
2.4. Analysed Materials
The operation of the proposed computer application was verified on the basis of sample experimental data of the adsorption process of selected REE ions on various types of adsorption materials. For this purpose, experimental data presented in 4 different publications were used [62,63,64,65]. The first type was Ce (III) ion imprinted materials based on 2-hydroxyethyl methacrylate (HEMA) and N-methacryloylamido antipyrine (MAAP) as functional monomers [62]. The second analyzed material was synthesized as a Lu (III)-ion-imprinted polymer, based on Lu (III)-4-vinylpyridine-acetylacetone complex as a functional monomer and ethylene glycol dimethacrylate as a crosslinker [63]. The third type of adsorption material has been synthesized by Liu et al. [64] in the form of diglycolamide polymer-grafted silica. The last type of adsorbents [65] were the imprinted mesoporous cellulose nanocrystals films (IMCFs).
3. Results and Discussion
A Novel Computer Application, REE 1.0, for Modeling of REE Ion Behavior in Adsorptive Materials
In the initial stage of research, the authors created a computer program REE 1.0 in the Java programming language. In this program, the user will be able to choose the appropriate kinetic model and adsorption mechanism model for the adsorptive materials and membranes, such as the Lagergren pseudo-first order, pseudo-second-order and Elovich kinetic models and the intraparticle diffusion model, the diffusion-chemisorption model, and the Boyd model to study the various mechanisms. In this work, the experimental results found throughout the literature [62,63,64,65] were compared with the theoretical data predicted by means of the mentioned models. The user could enter the parameters, such as the adsorption capacity (qt) of REE ions at contact time (t). The program calculates the values of characteristic parameters, such as the adsorption capacity of metal ions at equilibrium state qe, rate constant k1 and k2, the intraparticle rate constant kp and boundary layer thickness xi, the diffusion-chemisorption constant KDC, effective coefficient De and determination coefficient R2. There is also the possibility to compare the experimental results with theoretical one. It will be an excellent tool for research scientists to study the adsorption kinetics and various mechanisms responsible for the adsorption of REE ions (and other metal ions) on the adsorptive membranes and other adsorptive materials.
The program consists of the following windows:
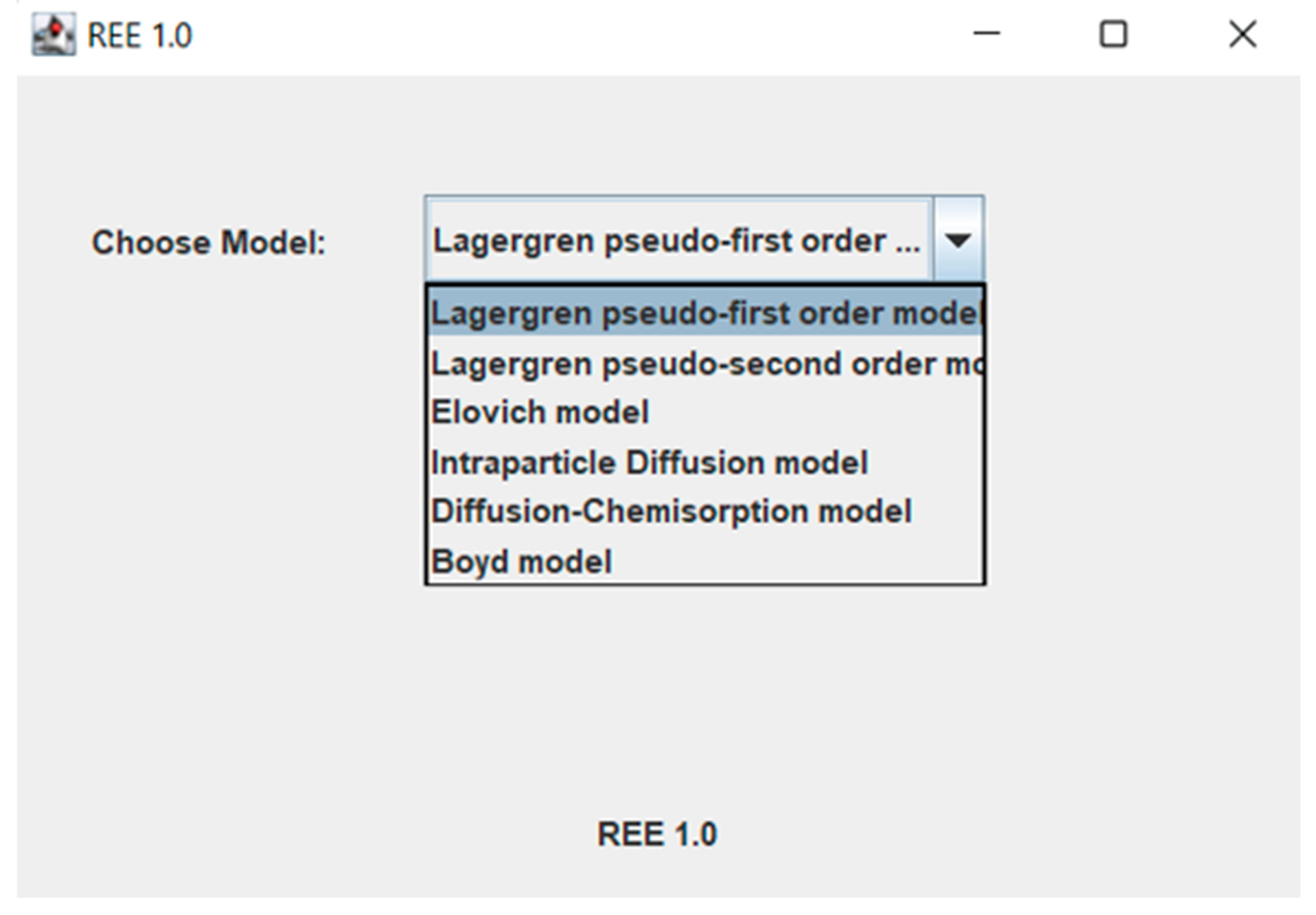
- Main window for model selection (Figure 1). Individual models are selected from the combo box.
 Figure 1. Main window for kinetic model or adsorption mechanism model selection.
Figure 1. Main window for kinetic model or adsorption mechanism model selection.
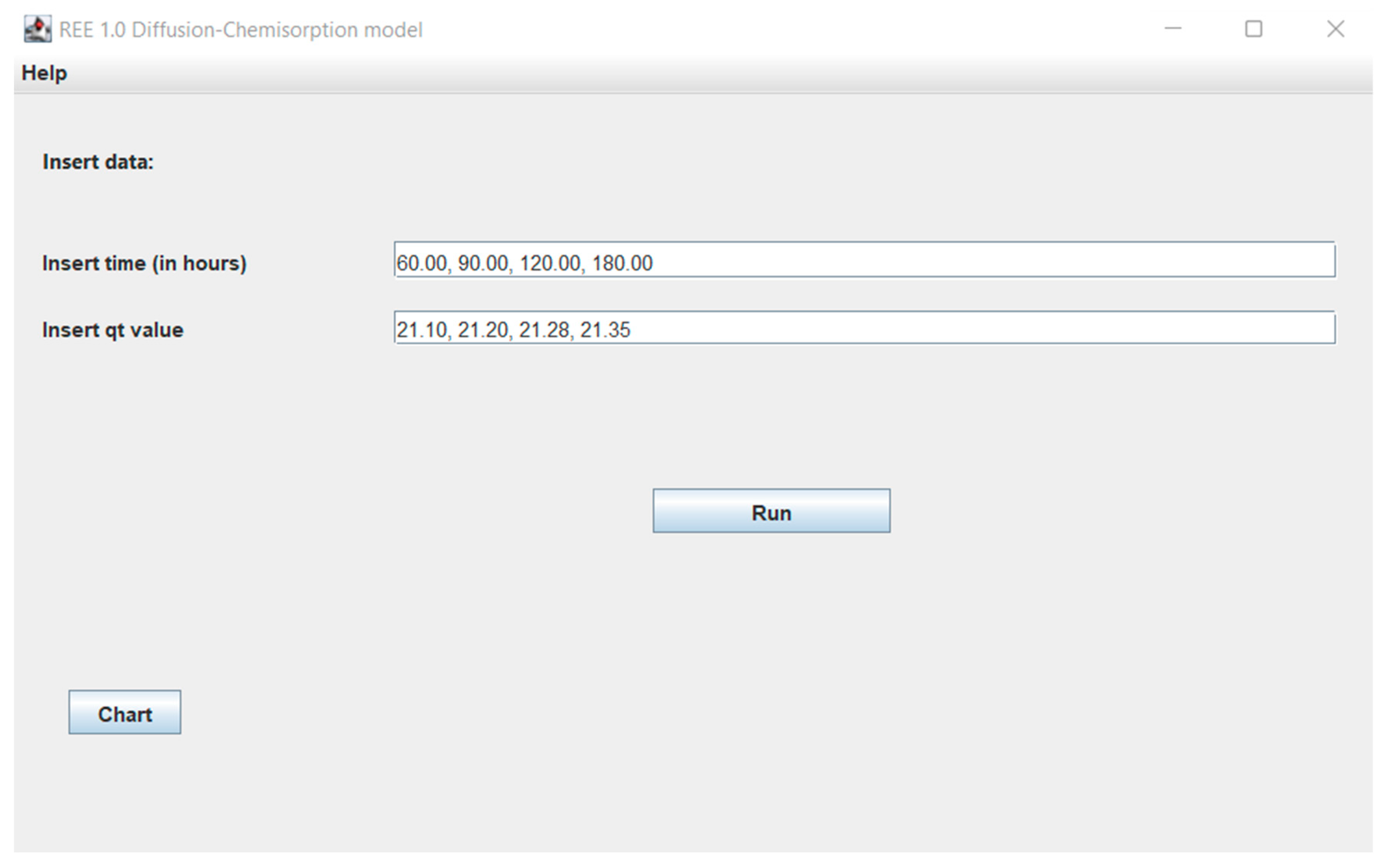
- In the next windows (Figure 2), additional tools such as the ability to add user’s experimental data, theoretical results from various models (Figure 3), the possibility to present the theoretical and experimental data at the graphs (Figure 4) are available.
 Figure 2. Window with diffusion-chemisorption model experimental data results for Eu ions.
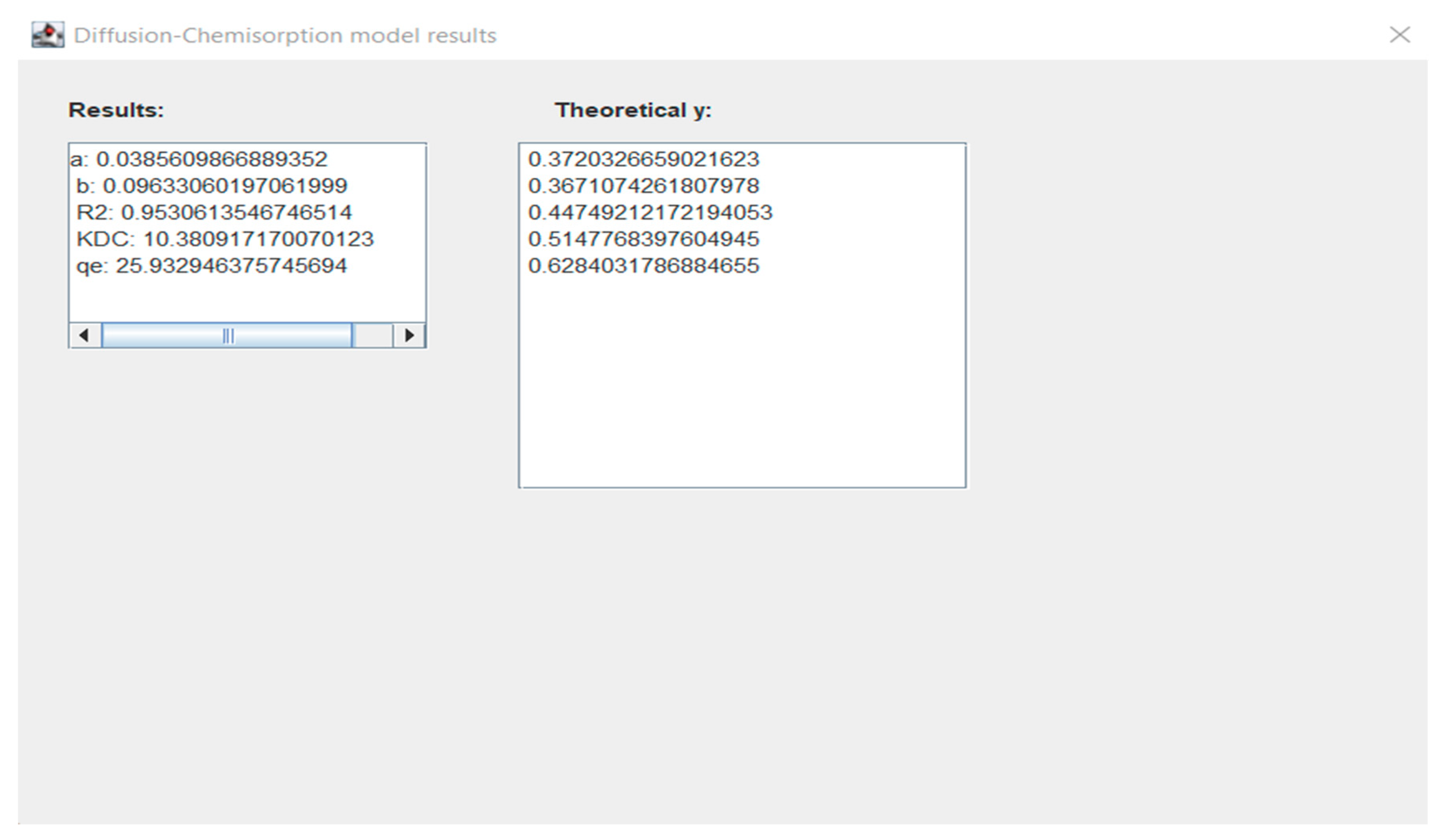
Figure 2. Window with diffusion-chemisorption model experimental data results for Eu ions.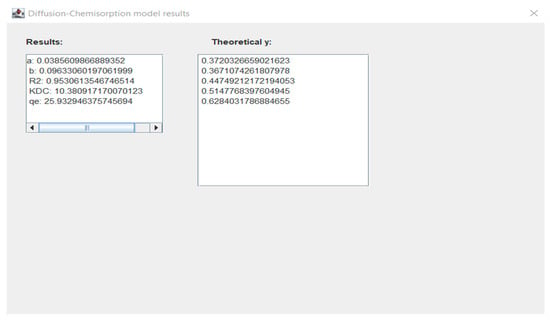 Figure 3. Window with diffusion-chemisorption model results for Eu ions.
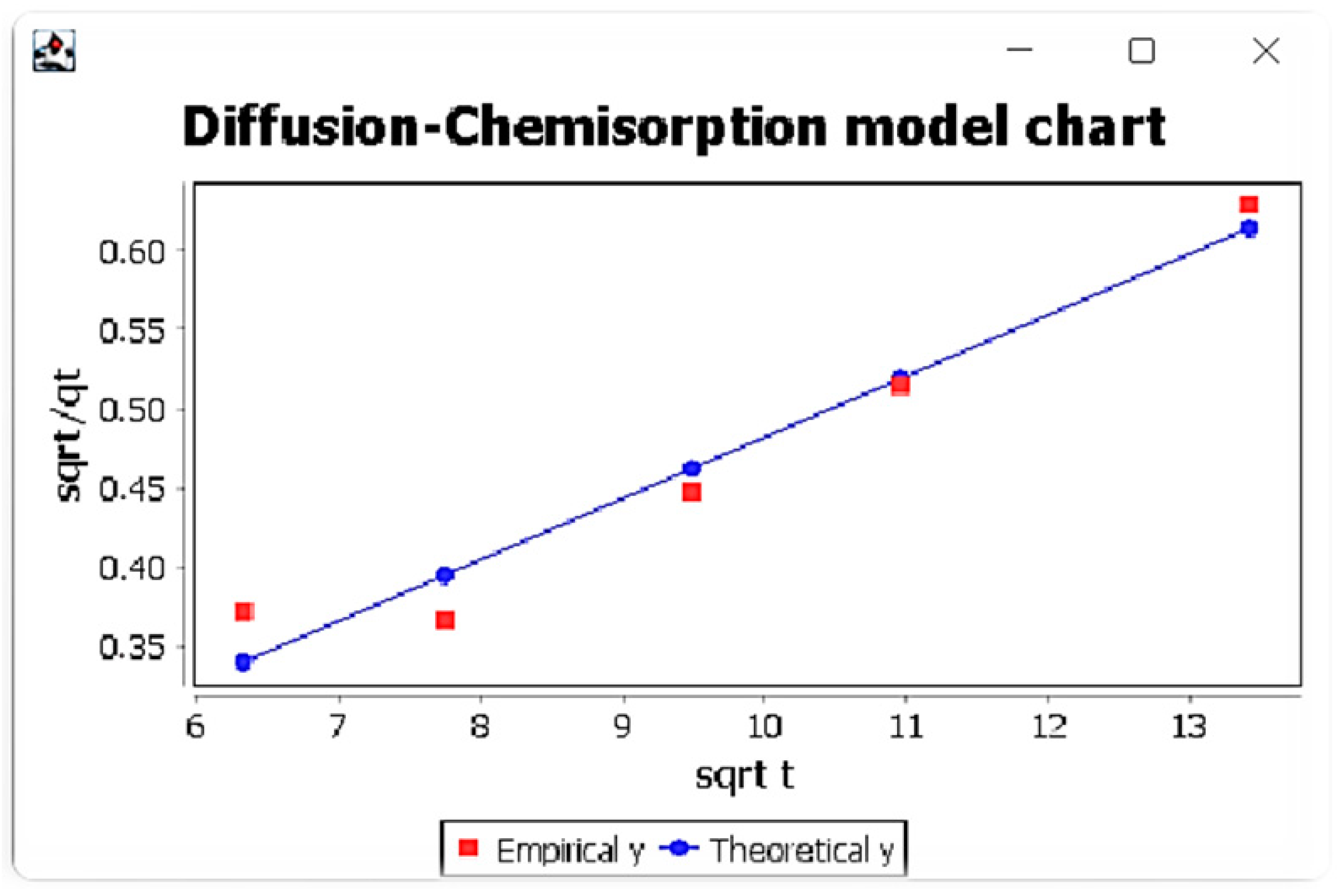
Figure 3. Window with diffusion-chemisorption model results for Eu ions.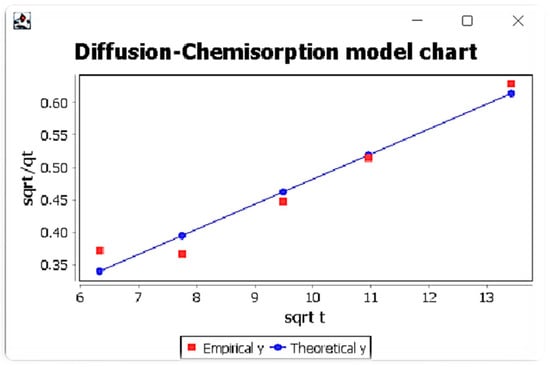 Figure 4. Window with diffusion-chemisorption model results and comparison of experimental and theoretical data graph for Eu ions.
Figure 4. Window with diffusion-chemisorption model results and comparison of experimental and theoretical data graph for Eu ions.
To compare the theoretical results obtained from the REE 1.0 application based on various models with the experimental results obtained from the literature, the correlation coefficient R2 was calculated. The data obtained during the simulation using REE 1.0 application for various models were presented in Table 1.

Table 1.
Comparison of simulation results for various REE ions obtained in REE 1.0 application.
As can be seen from Table 1, adsorptive materials and membranes are successfully used in the separation of rare earth metal ions from various types of aquatic solutions.
Kecili et al. [62] have examined the novel Ce (III) ion imprinted materials based on 2-hydroxyethyl methacrylate (HEMA) and N-methacryloylamido antipyrine (MAAP) as functional monomers. These materials were used for separation of Ce (III) ions from aquatic solutions and mixtures with other lanthanide ions, such as La (III) and Nd (III). However, the authors were only interested in examination of equilibrium parameters of adsorption process, using the Langmuir and Freundlich models. They have found that binding of Ce (III) ions to the ion imprinted poly (HEMA-co-(MAAP)2-Ce(H2O)2) is well fitted to the Langmuir isotherm model. It means that the binding of Ce (III) ions to the ion imprinted poly (HEMA-co-(MAAP)2Ce(H2O)2) is monolayer. They have also found that obtained IIPs exhibit high selectivity and sensitivity towards Ce (III) ions. In turn, Lai et al. [63] have synthesized a Lu (III)-ion-imprinted polymer, based on Lu (III)-4-vinylpyridine-acetylacetone complex as a functional monomer and ethylene glycol dimethacrylate as a crosslinker. Additionally, again, the authors have only used the Langmuir and Freundlich models and found that the Lu (III) ion adsorption was fitted to the Langmuir model, indicating that they have to use a monolayer adsorption of Lu (III) ions. The synthesized Lu (III) IIP had the properties of a large surface area, high adsorption capacity, a small cavity, a fast adsorption rate, and favorable heat stability. The results showed that the maximum adsorption of Lu (III) IIP was 64.2 mg g−1 with an adsorption equilibrium time of 30 min and the optimum pH was 5.5. The synthesized Lu (III) IIP had a good selective recognition ability for Lu (III) ion, compared with other ions. Liu et al. [64] have synthesized the new materials for REE ion adsorption, based on diglycolamide polymer-grafted silica. The prepared materials were used for separation of Eu (III) ions from solutions with other metal ions, such as K(I), Cr (II), Cu (II) or Fe (III). It was found that their occurrence did not decrease the adsorption of REE ion, so these materials had a good selective recognition ability for Eu (III) ion. In order to determine the equilibrium relationship between the adsorbent and the adsorbate, the authors have used the Freundlich and Langmuir isotherms. With their help, the ratio of the amount of adsorbed substance to its amount remaining in the solution (at a fixed temperature and in a state of equilibrium) and the sorption capacity of the adsorbent were determine. They found that the Langmuir adsorption isotherm was suited better to experimental data, which means the occurrence of monolayer adsorption, the energy equivalence of adsorption places and the lack of interactions between molecules adsorbed on adjacent active sites. In addition, all active places on the sorbent are free sites, ready to accept sorbate from the solution. In turn, using Lagergren models, the authors analyzed the kinetics of adsorption, and in particular the possible rate-determining step of the adsorption process. The experimental results were found to better fit the pseudo-second-order kinetic model. Further, the experimental qe,exp values agree well with the theoretical qe,theor for the pseudo-second-order kinetic model. This indicates that surface chemical sorption may be the step determining the rate of Eu (III) ion adsorption on analyzed IIP. Zheng et al. [65] have synthesized the imprinted mesoporous cellulose nanocrystals films (IMCFs) and used them for Nd (III) ions separation from aqueous solutions. For the analysis of experimental data, the Lagergren, Langmuir and Freundlich models were used. The authors have found that the Lagergren pseudo-second-order model fitted well the experimental data and the analyzed adsorption process belongs to chemical adsorption. In terms of the adsorption performance of IMCFs on Nd (III), again, the authors found a better fit of experimental results to the Langmuir isotherm, which proves monolayer sorption.
Analyzing the experimental results published in articles [62,63,64,65] on the adsorption of various rare earth metal ions from aqueous solutions, most often with accompanying ions (other REE and matrix ions) using the developed REE 1.0 application, the following conclusions were drawn. Of course, the results partly coincided with the conclusions of the authors using the Lagergren model, but they were supplemented with important information from the rest of the models used in the application.
It was found that in all cases, the experimental data from the adsorption analysis showed a better fit to the pseudo-second-order Lagergren model, which indicates the presence of chemical sorption. This was found based on higher R2 values compared to the pseudo-first-order model. In addition, the differences between the values of qe determined during the simulation and qe obtained experimentally were significantly smaller for the pseudo-second-order Lagergren model. In addition, it was also found that the experimental results fit the Elovich model, which proves the occurrence of chemisorption. It is also very important to determine what type of mechanism is responsible for the sorption and separation processes, and in particular which of them is responsible for the rate of these processes. The following models are used for this: intraparticle diffusion, diffusion-chemisorption, and Boyd. In all analyzed cases, a characteristic course of the relationship qt (t0.5) was found. Namely, they were in the form of multilinear curves (the value of R2 was sufficiently low), which proves the influence of two or more mechanisms on the adsorption process, and not only intraparticle diffusion. Using this model, it is possible to determine characteristic parameters, such as the intraparticle diffusion rate constant and the boundary layer thickness. It was found that the highest parameter values were obtained for Lu (III)IIP, and the lowest for Ce (III)IIP and Nd (III)IIPs. This is directly related to the form of adsorption materials.
It was also found that the diffusion-chemisorption mechanism was the dominant mechanism, taking into account its highest values of correlation coefficients among the considered models and the linear course of the relationship t0.5/qt (t0.5). This model also allowed to determine two parameters, namely the adsorbed amount of metal ions at equilibrium state qe and diffusion-chemisorption constant KDC. The highest values of these parameters were obtained for Lu (III)IIPs. In addition, it was found that the values of adsorbed amount of metal ions at equilibrium state qe are very close to their values determined from the pseudo-second-order Lagergren model.
Then, moving on to the model based on the Boyd equation, it was found that for Ce (III) and Eu (III), the relationship 𝐵𝑡(t) is non-linear, while for Lu (III) and Nd (III) despite the linear relationship, the lines do not pass through the origin, which proves that film-diffusion or external mass transport will be the main dominant factors. The calculated values of De fall well within the values characteristic for chemisorption systems.
Thus, we can see that the use of a computer application with an extensive range of models, concerning not only the analysis of kinetics, but also the mechanisms governing the adsorption processes of REE ions, both in modern materials and adsorption membranes, gives a number of possibilities, along with determining the characteristic parameters of the described processes.
4. Conclusions
In this paper, the authors proposed a modern computer application, REE 1.0, to model REE ion behavior in adsorptive membranes and materials. The current version of application was based on a few models, such as the Lagergren pseudo-first-order and pseudo-second-order and Elovich kinetic models and the intraparticle diffusion model, the diffusion-chemisorption model, and the Boyd model to study the various adsorption mechanisms. The operation of this application has been verified based on experimental results from the literature, regarding REE ion adsorption on four different types of adsorptive materials and membranes. It was found that the pseudo-second-order kinetic model was the best-fit model to describe the adsorption behavior of REE ions onto the novel materials and membranes and that the adsorption mechanism was a chemical coordination process. The good fit of experimental results to the Elovich model (R2 > 0.83) also indicates the existence of chemisorption. In the case of the intraparticle model, the linear fit exhibited multilinear curves (R2 < 0.84), so two or more steps will influence the adsorption process. In turn, the good fit to the diffusion-chemisorption model (R2 > 0.95) and the similarity of 𝑞𝑒 values with those obtained via the Lagergren pseudo-second-order kinetic model indicate that the adsorption of REE ions on the examined materials can be described using this model. The Boyd equation provided additional data, namely it was found that none of the obtained graphs passed through the origin. This indicated that the adsorption is mostly controlled by film diffusion. Additionally, the calculated values of De fall well within the values characteristic for chemisorption systems.
The created application, REE 1.0, could be an excellent tool for scientists studying adsorptive materials and membranes. REE 1.0 enables not only analyzing the kinetic mechanisms, but above all to studying the mechanisms responsible for the adsorption and behavior of REE ions in these types of materials and membranes. The proposed application is a preliminary version of the program and, during further research, it will be appropriately modified, especially as regards the number of available adsorption models and supplemented with elements regarding the type of adsorbents and their characteristics.
Author Contributions
Conceptualization, A.R. (Aleksandra Rybak), A.R. (Aurelia Rybak) and S.D.K.; methodology, A.R. (Aleksandra Rybak) and A.R. (Aurelia Rybak); software, A.R. (Aleksandra Rybak) and A.R. (Aurelia Rybak); writing—original draft preparation, A.R. (Aleksandra Rybak), A.R. (Aurelia Rybak) and S.D.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the Norway Grants 2014–2021 via the National Centre for Research and Development. The authors would like to thank them for providing financial support under the project no. NOR/SGS/MOHMARER/0284/2020-00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the extremely large size.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taggart, R.K.; Hower, J.C.; Hsu-Kim, H. Effects of Roasting Additives and Leaching Parameters on the Extraction of Rare Earth Elements from Coal Fly Ash. Int. J. Coal Geol. 2018, 196, 106–114. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Kononova, V.A.; Orlova, M.P.; Woolley, A.R. Alkaline Rocks of the World, Part 2: Former Soviet Union; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Mihalasky, M.J.; Tucker, R.D.; Renaud, K.; Verstraeten, I.M. Rare Earth Element and Rare Metal Inventory of Central Asia; USGS: Reston, VA, USA, 2018; Fact Sheet 2017–3089, p. 4. [Google Scholar]
- Xiong, X.; Liu, X.; Yu, I.K.M.; Wang, L.; Zhou, J.; Sun, X.; Rinklebe, J.; Shaheen, S.M.; Sik Ok, Y.; Lin, Z.; et al. Potentially toxic elements in solid waste streams: Fate and management approaches. Environ. Pollut. 2019, 253, 680–707. [Google Scholar] [CrossRef]
- Honaker, R.Q.; Groppo, J.; Yoon, R.-H.; Luttrell, G.H.; Noble, A.; Herbst, J. Process evaluation and flowsheet development for the recovery of rare earth elements from coal and associated byproducts. Miner. Metall. Proc. 2017, 34, 107–115. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A. Characteristics of Some Selected Methods of Rare Earth Elements Recovery from Coal Fly Ashes. Metals 2021, 11, 142. [Google Scholar] [CrossRef]
- Lin, R.; Howard, B.H.; Roth, E.A.; Bank, T.L.; Granite, E.J.; Soong, Y. Enrichment of rare earth elements from coal and coal by-products by physical separations. Fuel 2017, 200, 506–520. [Google Scholar] [CrossRef]
- Kashiwakura, S.; Kumagai, Y.; Kubo, H.; Wagatsuma, K. Dissolution of Rare Earth Elements from Coal Fly Ash Particles in a Dilute H2SO4 Solvent. Open J. Phys. Chem. 2013, 3, 69–75. [Google Scholar] [CrossRef]
- Peiravi, M.; Ackah, A.; Guru, R.; Liu, J.; Mohanty, M. Chemical extraction of rare earth elements from coal ash. Miner. Metall. Process. 2017, 34, 170–177. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, C.; Pan, J.; Liu, C.; Tang, M.; Ji, W.; Hu, T.; Zhang, N. Study on Influence Factors of Leaching of Rare Earth Elements from Coal Fly Ash. Energy Fuels 2018, 327, 8000–8005. [Google Scholar] [CrossRef]
- Bradło, D.; Żukowski, W.; Czupryński, P.; Witkowski, K. Potential of Metal Recovery from Coal Combustion Products. Part II. Leaching of Selected Elements. J. Pol. Miner. Eng. Soc. 2015, 16, 163–170. [Google Scholar]
- Lin, R.; Stuckman, M.; Howard, B.H.; Bank, T.L.; Roth, E.A.; Macala, M.K.; Lopano, C.; Soong, Y.; Granite, E.J. Application of sequential extraction and hydrothermal treatment for characterization and enrichment of rare earth elements from coal fly ash. Fuel 2018, 232, 124–133. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.A.; Equeenuddin, S.M. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. Int. J. Coal Sci. Technol. 2016, 3, 267–283. [Google Scholar] [CrossRef]
- Shin, D.; Kim, J.; Kim, B.-S.; Jeong, J.; Lee, J.-C. Use of phosphate solubilizing Bacteria to leach rare earth elements from monazite-bearing ore. Minerals 2015, 5, 189–202. [Google Scholar] [CrossRef]
- Ibrahim, H.; El-Sheikh, E. Bioleaching treatment of Abu Zeneima uraniferous gibbsite ore material for recovering U, REEs, Al and Zn. Res. J. Chem. Sci. 2011, 1, 55–66. [Google Scholar]
- Hassanien, W.A.G.; Desouky, O.A.N.; Hussien, S.S.E. Bioleaching of some rare earth elements from Egyptian monazite using Aspergillus ficuum and Pseudomonas aeruginosa. Walailak J. Sci. Technol. 2013, 11, 809–823. [Google Scholar]
- Muravyov, M.I.; Bulaev, A.G.; Melamud, V.S.; Kondrat’eva, T.F. Leaching of Rare Earth Elements from Coal Ashes Using Acidophilic Chemolithotrophic Microbial Communities. Microbiology 2015, 84, 194–201. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, F.; Liu, E.; Xu, X.; Yan, Y. Efficient recovery of neodymium in acidic system by free-standing dual-template docking oriented ionic imprinted mesoporous films. ACS Appl. Mater. Inter. 2017, 9, 730–739. [Google Scholar] [CrossRef]
- Liu, E.; Xu, X.; Zheng, X.; Zhang, F.; Liu, E.; Li, C. An ion imprinted macroporous chitosan membrane for efficiently selective adsorption of dysprosium. Sep. Purif. Technol. 2017, 189, 288–295. [Google Scholar] [CrossRef]
- Kononova, O.N.; Bryuzgina, G.L.; Apchitaeva, O.V.; Kononov, Y.S. Ion Exchange Recovery of Chromium (VI) and Manganese (II) from Aqueous Solutions. Arab. J. Chem. 2019, 12, 2713–2720. [Google Scholar] [CrossRef]
- Mollah, A.; Begum, A.; Rahman, M. Removal of Radionuclides from Low Level Radioactive Liquid Waste by Precipitation. J. Radioanal. Nucl. Chem. 1998, 229, 187–189. [Google Scholar] [CrossRef]
- Montaña, M.; Camacho, A.; Serrano, I.; Devesa, R.; Matia, L.; Vallés, I. Removal of Radionuclides in DrinkingWater by Membrane Treatment Using Ultrafiltration, Reverse Osmosis and Electrodialysis Reversal. J. Environ. Radioact. 2013, 125, 86–92. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of Various Pollutants from Water and Wastewater by Modified Chitosan Adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Lakherwa, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. 2014, 4, 2249–3131. [Google Scholar]
- Malik, D.S.; Jain, C.K.; Yadav, A. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl Water Sci. 2016, 7, 2113–2136. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Wat. Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Zheng, G.; Ye, H.; Zhang, Y.; Li, H.; Lin, L.; Ding, X. Removal of heavy metal in drinking water resource with cation-exchange resins (Type 110-H) mixed PES membrane adsorbents. J. Hazard Toxic Radioact Waste 2014, 19, 1–6. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Lamsayah, M.; Butylina, S.; Geng, S.; Esmaeili, M.; Giove, A.; Mouele, E.S.M.; Virolainen, S.; El Barkany, S.; Ouammou, A.; et al. Sustainable composite material based on glutenin biopolymeric-clay for efficient separation of rare earth elements. Chem. Eng. J. 2022, 440, 135959. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Chai, Y.; Sun, X. Recovery of rare earth by electro-sorption with sodium diphenylamine sulfonate modified activated carbon electrode. Sep. Purif. Technol. 2022, 292, 121005. [Google Scholar] [CrossRef]
- Qin, W.; Yu, A.; Han, X.; Wang, J.; Sun, J.; Zhang, J.; Weng, Y. Postsynthetic of MIL-101-NH2 MOFs supported on PVDF membrane for REEs recovery from waste phosphor. RSC Adv. 2022, 12, 24670–24680. [Google Scholar] [CrossRef]
- Bashiri, A.; Nikzad, A.; Malek, R.; Asadnia, M.; Razmjou, A. Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection. Membranes 2022, 12, 80. [Google Scholar] [CrossRef]
- Li, L.; Yu, B.; Davis, K.; King, A.; Dal-Cin, M.; Nicalek, A.; Du, N. Separation of Neodymium (III) and Lanthanum (III) via a Flat Sheet-Supported Liquid Membrane with Different Extractant-Acid Systems. Membranes 2022, 12, 1197. [Google Scholar] [CrossRef]
- Molina-Calderón, L.; Basualto-Flores, C.; Paredes, V.; Venegas-Yazigi, D. Advances of magnetic nanohydrometallurgy using superparamagnetic nanomaterials as rare earth ions adsorbents: A grand opportunity for sustainable rare earth recovery. Sep. Purif. Technol. 2022, 299, 121708. [Google Scholar] [CrossRef]
- Yadav, A.G.; Gujar, R.B.; Valsala, T.P.; Mohapatra, P.K. Comparative study on the uptake of lanthanides from acidic feeds using extraction chromatography resins containing N,N,N’,N’-tetra-n-alkyl diglycolamides with varying alkyl chain length in an ionic liquid. J. Chromatogr. A 2022, 1687, 463683. [Google Scholar] [CrossRef]
- Xiao, J.; Li, B.; Qiang, R.; Qiu, H.; Chen, J. Highly selective adsorption of rare earth elements by honeycomb-shaped covalent organic frameworks synthesized in deep eutectic solvents. Environ. Res. 2022, 214, 113977. [Google Scholar] [CrossRef]
- Liu, J.S.; Wang, X. Novel silica-based hybrid adsorbents: Lead (II) adsorption isotherms. Sci. World J. 2013, 6, 897159. [Google Scholar] [CrossRef]
- Liu, J.; Song, L.; Shao, G. Novel zwitterionic inorganic–organic hybrids: Kinetic and equilibrium model studies on Pb2+ removal from aqueous solution. J. Chem. Eng. Data 2011, 56, 2119–2127. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, J.S.; Song, L.; Shao, G.Q. Novel zwitterionic inorganic-organic hybrids: Synthesis of hybrid adsorbents and their applications for Cu2+ removal. J. Hazard Mater 2011, 186, 1335–1342. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wang, X.; Liu, J.S.; Wu, L.L. Removal of copper (Cu2+) from water using novel hybrid adsorbents: Kinetics and isotherms. J. Chem. Eng. Data 2013, 58, 1141–1150. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Li, M. Fundamental studies of novel zwitterionic hybrid membranes: Kinetic model and mechanism insights into strontium removal. Sci. World J. 2014, 7, 485820. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Wang, J. Removal of Co2+ from radioactive wastewater by polyvinyl alcohol PVA/chitosan magnetic composite. Prog. Nucl. Energy 2014, 71, 172–178. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A.; Sysel, P. Modeling of Gas Permeation through Mixed-Matrix Membranes Using Novel Computer Application MOT. Appl. Sci. 2018, 8, 1166. [Google Scholar] [CrossRef]
- Kusumkar, V.; Galamboš, M.; Viglašová, E.; Da, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Corazza, M.Z.; Somera, B.F.; Segatelli, M.G. Preparation of New Ion-Selective Cross-Linked Poly (Vinylimidazole-Co-Ethylene Glycol Dimethacrylate) Using a Double-Imprinting Process for the Preconcentration of Pb2+ Ions. J. Colloid Interface Sci. 2015, 450, 254–263. [Google Scholar] [CrossRef]
- Mahony, J.O.; Nolan, K.; Smyth, M.R.; Mizaikoff, B. Molecularly Imprinted Polymers—Potential and Challenges in Analytical Chemistry. Anal. Chim. Acta 2005, 534, 31–39. [Google Scholar] [CrossRef]
- Say, R.; Birlik, E.; Ersöz, A.; Yılmaz, F.; Gedikbey, T.; Denizli, A. Preconcentration of Copper on Ion-Selective Imprinted Polymer Microbeads. Anal. Chim. Acta 2003, 480, 251–258. [Google Scholar] [CrossRef]
- Nicholls, I.A.; Adbo, K.; Andersson, H.S.; Andersson, P.O.; Ankarloo, J.; Hedin-Dahlström, J.; Jokela, P.; Karlsson, J.G.; Olofsson, L.; Rosengren, J.; et al. Can We Rationally Design Molecularly Imprinted Polymers? Anal. Chim. Acta 2001, 435, 9–18. [Google Scholar] [CrossRef]
- Laatikainen, K.; Udomsap, D.; Siren, H.; Brisset, H.; Sainio, T.; Branger, C. Effect of Template Ion–Ligand Complex Stoichiometry on Selectivity of Ion-Imprinted Polymers. Talanta 2015, 134, 538–545. [Google Scholar] [CrossRef]
- Özkara, S.; Andaç, M.; Karakoç, V.; Say, R.; Denizli, A. Ion-imprinted PHEMA Based Monolith for the Removal of Fe3+ Ions from Aqueous Solutions. J. Appl. Polym. Sci. 2011, 120, 1829–1836. [Google Scholar] [CrossRef]
- Rao, T.P.; Daniel, S.; Gladis, J.M. Tailored Materials for Preconcentration or Separation of Metals by Ion-Imprinted Polymers for Solid-Phase Extraction (IIP-SPE). TrAC Trends Anal. Chem. 2004, 23, 28–35. [Google Scholar]
- Alfaro-Cuevas-Villanueva, R.; Hidalgo-V, A.; de Jes, C.; Cort, R. Thermodynamic, kinetic, and equilibrium parameters for the removal of lead and cadmium from aqueous solutions with calcium alginate beads. Sci. World J. 2014, 2014, 647512. [Google Scholar] [CrossRef]
- Krishna, R.H.; Swamy, A. Studies on removal of Cr (VI) from aqueous solutions using powder of mosambi fruit peelings (PMFP) as a low cost sorbent. E-J. Chem. 2012, 9, 1389–1399. [Google Scholar] [CrossRef]
- Osasona, I.; Ajayi, O.; Adebayo, A. Equilibrium, kinetics, and thermodynamics of the biosorption of Zn (II) from aqueous solution using powdered cow hooves. ISRN Phys. Chem. 2013, 2013, 865219. [Google Scholar] [CrossRef]
- Wang, X.S.; Lu, Z.; Miao, H.; He, W.; Shen, H. Kinetics of Pb (II) adsorption on black carbon derived from wheat residue. Chem. Eng. J. 2011, 166, 986–993. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Atia, A.A.; Donia, A.; Yousif, A. Removal of some hazardous heavy metals from aqueous solution using magnetic chelating resin with iminodiacetate functionality. Sep. Purif. Technol. 2008, 61, 348–357. [Google Scholar] [CrossRef]
- Guibal, E.; Milot, C.; Tobin, J.M. Metal–anion sorption by chitosan beats: Equilibrium and kinetic studies. Ind. Eng. Chem. Res. 1998, 37, 1454–1463. [Google Scholar] [CrossRef]
- Tavlieva, M.P.; Genieva, S.; Georgieva, V.; Vlaev, L.T. Kinetic study of brilliant green adsorption fromaqueous solution onto white rice husk ash. J. Colloid Interface Sci. 2013, 409, 112–122. [Google Scholar] [CrossRef]
- BHameed, H.; El-Khaiary, M.I. Equilibrium, kinetics and mechanism of malachite green adsorption on activated carbon prepared from bamboo by K2CO3 activation and subsequent gasification with CO2. J. Hazard. Mater. 2008, 157, 344–351. [Google Scholar] [CrossRef]
- C´Aceres-Jensen, L.; Rodr, J.; Parra-Rivero, J.; Escudey, M.; Barrientos, L.; Castro-Castillo, V. Sorption kinetics of diuron on volcanic ash derived soils. J. Hazard. Mater. 2013, 261, 602–613. [Google Scholar] [CrossRef]
- Keçili, R.; Dolak, I.; Gulları, B.; Ersoz, A.; Say, R. Ion imprinted cryogel-based supermacroporous traps for selective separation of cerium (III) in real samples. J. Rare Earths 2018, 36, 857–862. [Google Scholar] [CrossRef]
- Lai, X.; Hu, Y.; Fu, Y.; Wang, L.; Xiong, J. Synthesis and Characterization of Lu (III) Ion Imprinted Polymer. J. Inorg. Organomet. Polym. 2012, 22, 112–118. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Gong, A. Preparation of diglycolamide polymer modified silica and its application as adsorbent for rare earth ions. Desig. Monom. Polym. 2019, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Y.; Bian, T.; Wang, D.; Li, Z. One-step fabrication of imprinted mesoporous cellulose nanocrystals films for selective separation and recovery of Nd(III). Cellulose 2019, 26, 5571–5582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).