Membrane Tubulation with a Biomembrane Force Probe
Abstract
1. Introduction
2. Materials and Methods
2.1. Erythrocyte Biotinylation
2.2. Streptavidin Beads
2.3. Giant Unilamellar Vesicle Preparation
2.4. Micropipette Formation
- HEAT = 350, FIL = 4, VEL = 55, DEL = 255, PUL = 255.
- HEAT = 350, FIL = 5, VEL = 40, DEL = 50, PUL = 40
- HEAT = 350, FIL = 5, VEL = 40, DEL = “blank”, PUL = 255
2.5. Lipids
3. Results
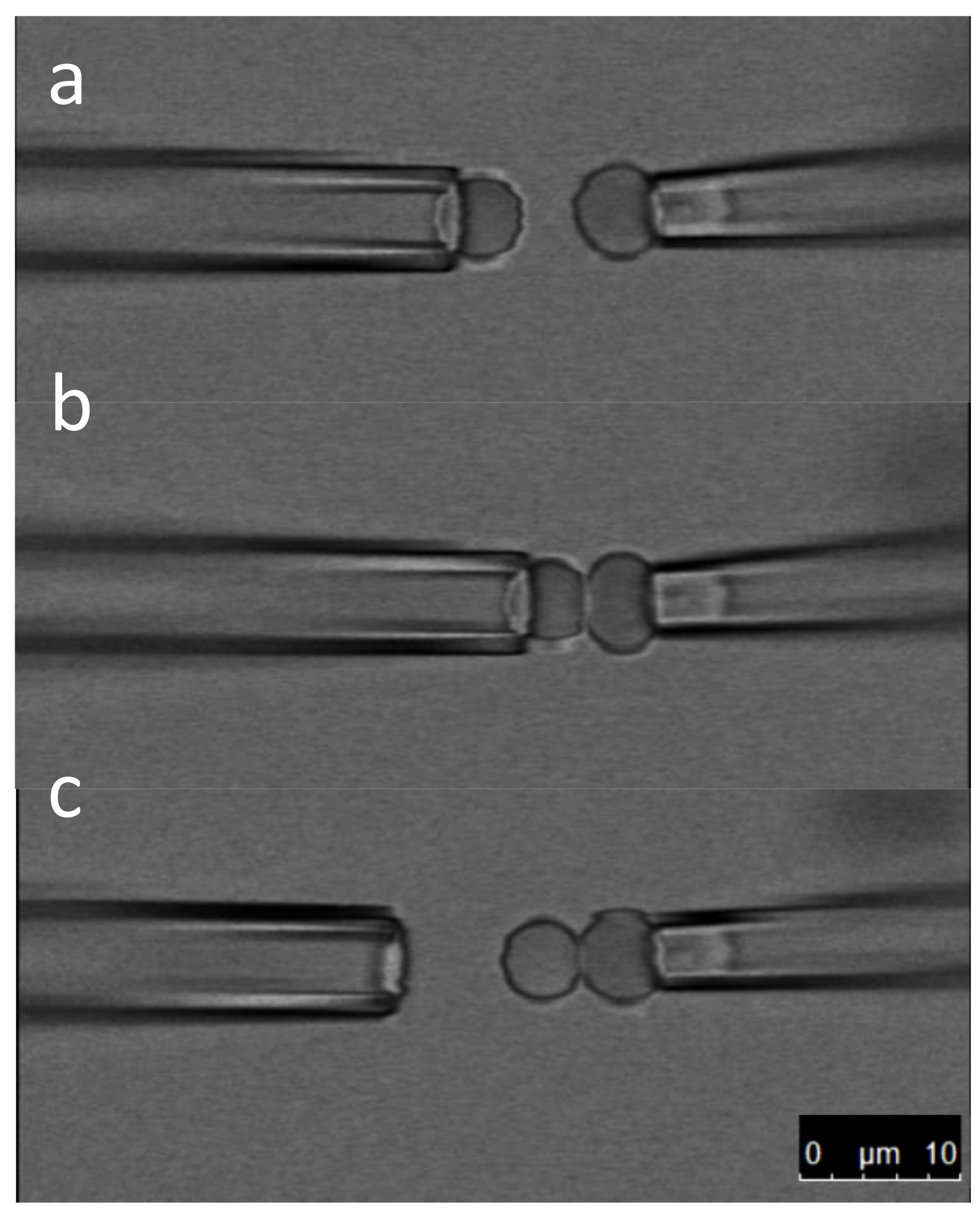
3.1. How to Form a Membrane Tube with the Biomembrane Force Probe
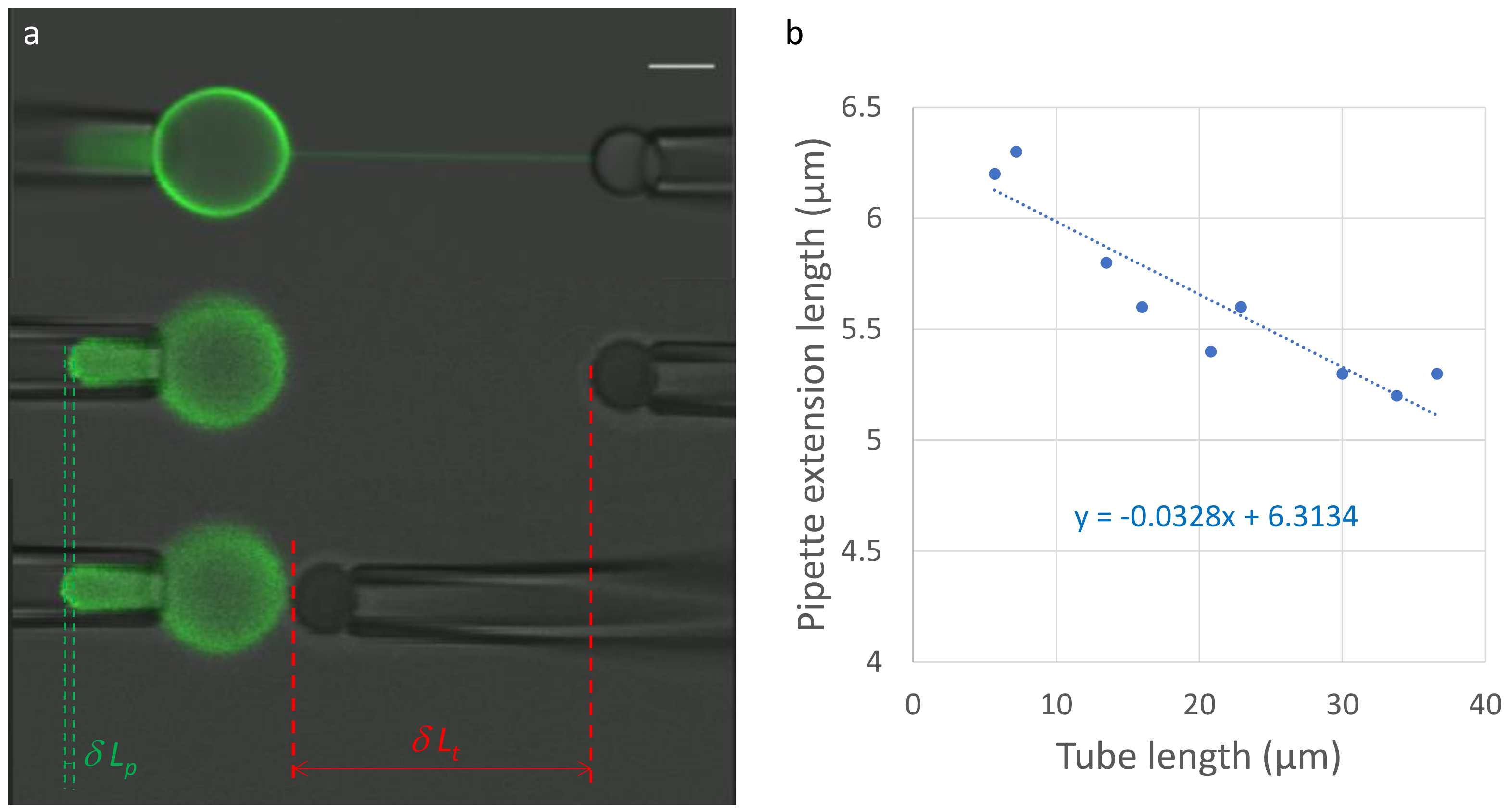
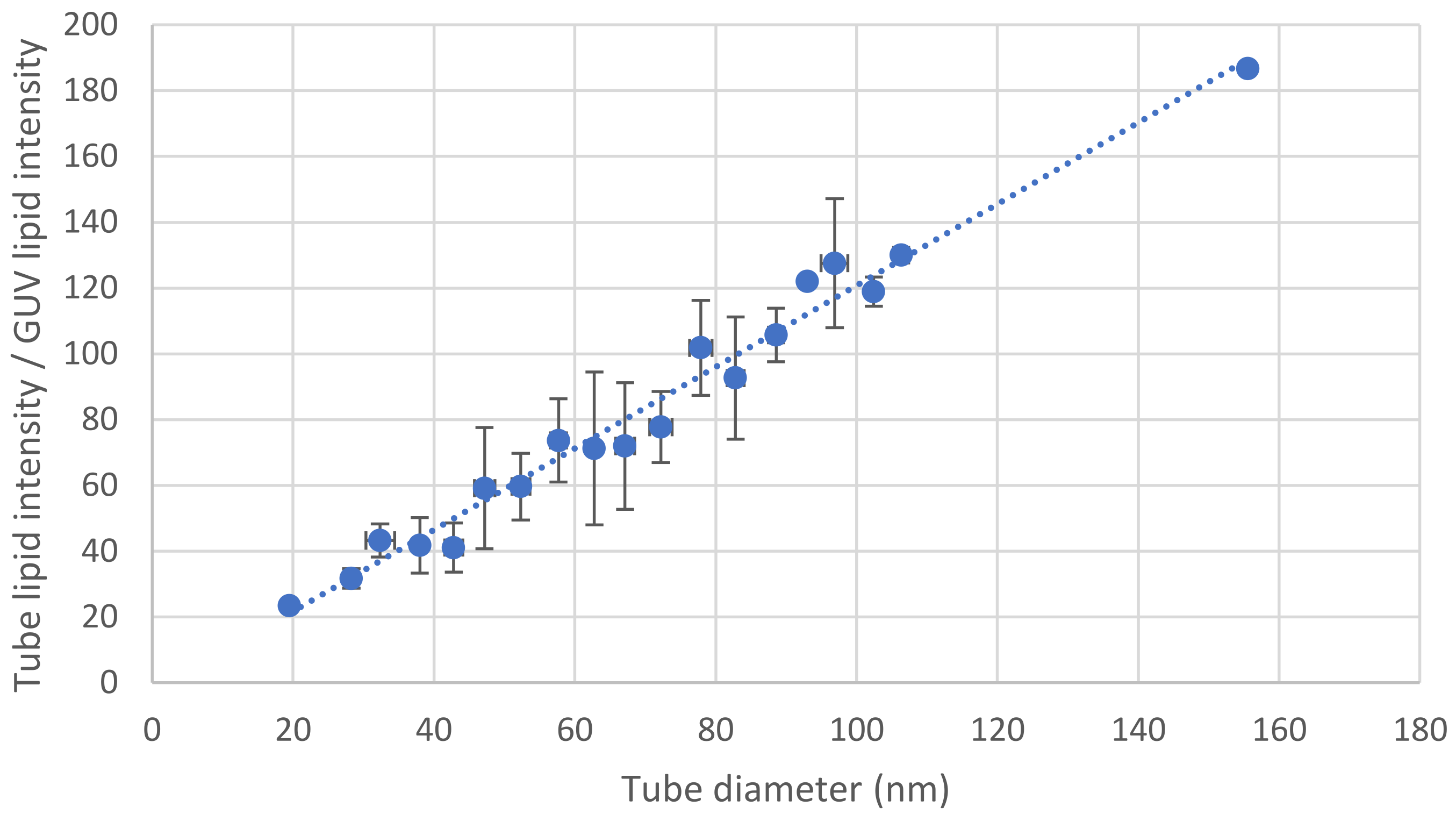
3.2. How to Measure the Tube Diameter
3.3. Tube Diameter, Surface Tension and Bending Modulus
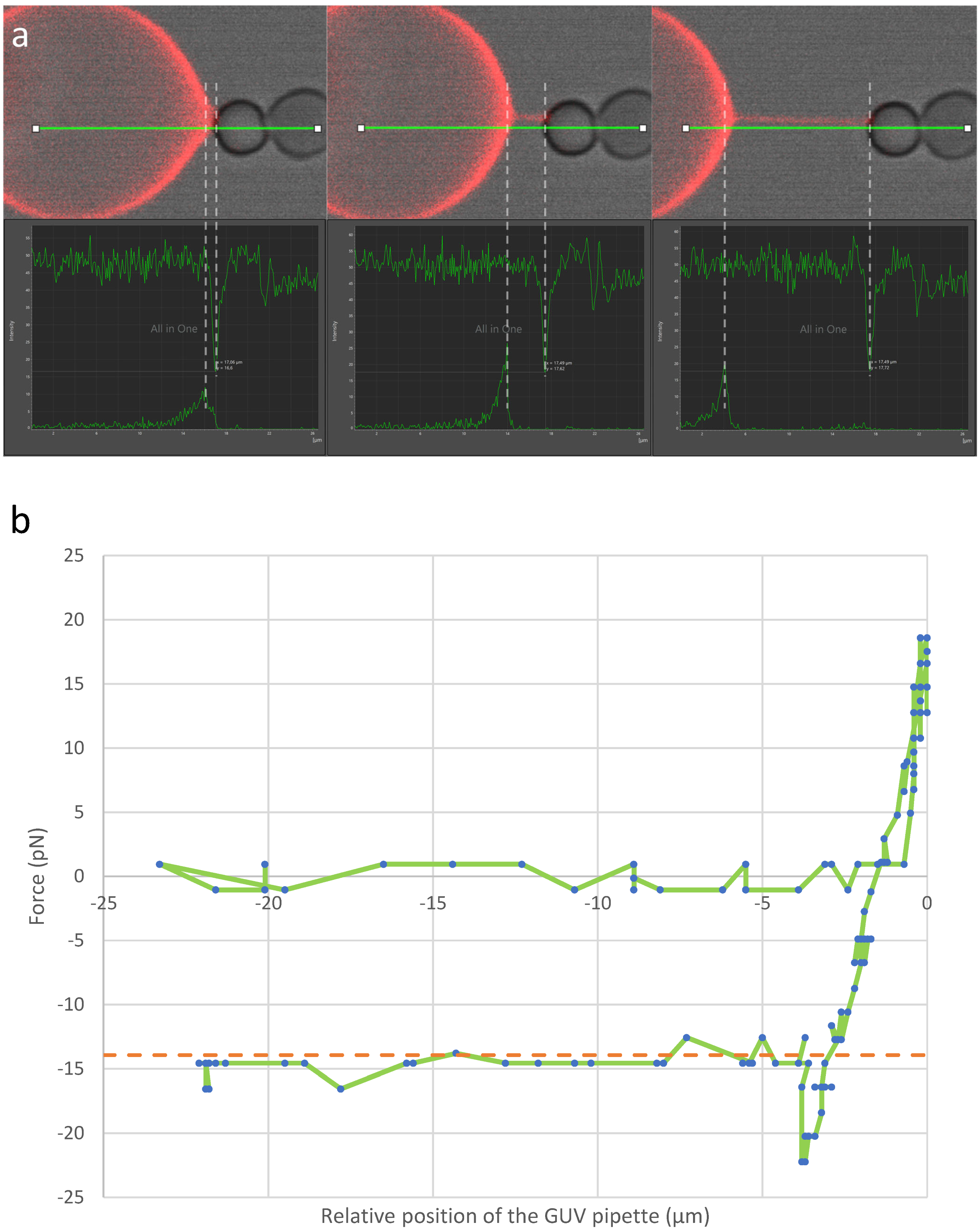
3.4. Force Required for Tubulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Z.; Yu, C.H. PI(3,4)P(2)-mediated membrane tubulation promotes integrin trafficking and invasive cell migration. Proc. Natl. Acad. Sci. USA 2021, 118, e2017645118. [Google Scholar] [CrossRef]
- Rahajeng, J.; Kuna, R.S.; Makowski, S.L.; Tran, T.T.T.; Buschman, M.D.; Li, S.; Cheng, N.; Ng, M.M.; Field, S.J. Efficient Golgi Forward Trafficking Requires GOLPH3-Driven, PI4P-Dependent Membrane Curvature. Dev. Cell. 2019, 50, 573–585.e5. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Zurzolo, C. Tunneling nanotubes: Reshaping connectivity. Curr. Opin. Cell Biol. 2021, 71, 139–147. [Google Scholar] [CrossRef]
- Abounit, S.; Zurzolo, C. Wiring through tunneling nanotubes—From electrical signals to organelle transfer. J. Cell Sci. 2012, 125, 1089–1098. [Google Scholar] [CrossRef]
- Ariazi, J.; Benowitz, A.; De Biasi, V.; Den Boer, M.L.; Cherqui, S.; Cui, H.; Douillet, N.; Eugenin, E.A.; Favre, D.; Goodman, S.; et al. Tunneling Nanotubes and Gap Junctions-Their Role in Long-Range Intercellular Communication during Development, Health, and Disease Conditions. Front. Mol. Neurosci. 2017, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Bowman, H.; Leung, A.; Needham, D.; Tirrell, D. Biomembrane templates for nanoscale conduits and networks. Science 1996, 273, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Baumgart, T. Sorting of lipids and proteins in membrane curvature gradients. Biophys. J. 2009, 96, 2676–2688. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, D.; Derenyi, I.; Bassereau, P.; Nassoy, P. Coalescence of membrane tethers: Experiments, theory, and applications. Biophys. J. 2005, 88, 2714–2726. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Cuvelier, D.; Nassoy, P.; Prost, J.; Bassereau, P.; Goud, B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 2005, 24, 1537–1545. [Google Scholar] [CrossRef]
- Roux, A.; Cappello, G.; Cartaud, J.; Prost, J.; Goud, B.; Bassereau, P. A minimal system allowing tubulation with molecular motors pulling on giant liposomes. Proc. Natl. Acad. Sci. USA 2002, 99, 5394–5399. [Google Scholar] [CrossRef]
- Heinrich, V.; Waugh, R.E. A piconewton force transducer and its application to measurement of the bending stiffness of phospholipid membranes. Ann. Biomed. Eng. 1996, 24, 595–605. [Google Scholar] [CrossRef]
- Prevost, C.; Tsai, F.C.; Bassereau, P.; Simunovic, M. Pulling Membrane Nanotubes from Giant Unilamellar Vesicles. J. Vis. Exp. 2017, 130, e56086. [Google Scholar] [CrossRef]
- Derenyi, I.; Julicher, F.; Prost, J. Formation and interaction of membrane tubes. Phys. Rev. Lett. 2002, 88, 238101. [Google Scholar] [CrossRef]
- Brochard-Wyart, F.; Borghi, N.; Cuvelier, D.; Nassoy, P. Hydrodynamic narrowing of tubes extruded from cells. Proc. Natl. Acad. Sci. USA 2006, 103, 7660–7663. [Google Scholar] [CrossRef] [PubMed]
- Rossier, O.; Cuvelier, D.; Borghi, N.; Puech, P.H.; Derényi, I.; Buguin, A.; Nassoy, P.; Brochard-Wyart, F. Giant vesicles under flows:: Extrusion and retraction of tubes. Langmuir 2003, 19, 575–584. [Google Scholar] [CrossRef]
- Evans, E.; Ritchie, K.; Merkel, R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys. J. 1995, 68, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Ritchie, K. Dynamic strength of molecular adhesion bonds. Biophys. J. 1997, 72, 1541–1555. [Google Scholar] [CrossRef]
- Merkel, R.; Nassoy, P.; Leung, A.; Ritchie, K.; Evans, E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 1999, 397, 50–53. [Google Scholar] [CrossRef]
- Evans, E. Energy landscapes of biomolecular adhesion and receptor anchoring at interfaces explored with dynamic force spectroscopy. Faraday Discuss. 1998, 111, 1–16. [Google Scholar] [CrossRef]
- Pincet, F.; Husson, J. The solution to the streptavidin-biotin paradox: The influence of history on the strength of single molecular bonds. Biophys. J. 2005, 89, 4374–4381. [Google Scholar] [CrossRef]
- Husson, J.; Pincet, F. Analyzing single-bond experiments: Influence of the shape of the energy landscape and universal law between the width, depth, and force spectrum of the bond. Phys. Rev. E 2008, 77, 026108. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Heinrich, V.; Leung, A.; Kinoshita, K. Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. I. Membrane separation from the cytoskeleton. Biophys. J. 2005, 88, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, V.; Leung, A.; Evans, E. Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. II. Tether flow terminated by P-selectin dissociation from PSGL-1. Biophys. J. 2005, 88, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- King, M.R.; Heinrich, V.; Evans, E.; Hammer, D.A. Nano-to-micro scale dynamics of P-selectin detachment from leukocyte interfaces. III. Numerical simulation of tethering under flow. Biophys. J. 2005, 88, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Jegou, A.; Pincet, F.; Perez, E.; Wolf, J.P.; Ziyyat, A.; Gourier, C. Mapping mouse gamete interaction forces reveal several oocyte membrane regions with different mechanical and adhesive properties. Langmuir 2008, 24, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Gourier, C.; Jegou, A.; Husson, J.; Pincet, F. A Nanospring Named Erythrocyte. The Biomembrane Force Probe. Cell. Mol. Bioeng. 2008, 1, 263–275. [Google Scholar] [CrossRef]
- Xu, X.H.; Spring, C.M.; Ju, L.N.; Wang, Y.M.; Reheman, A.; Yang, H.; Jin, J.W.; Lei, X.; Yang, Y.; Reddy, E.C.; et al. Apolipoprotein A-IV Is a Novel Ligand of Platelet αIIbβ3 Integrin and an Endogenous Thrombosis Inhibitor: Measurement of Single-Molecular Interactions By Biomembrane Force Probe. Blood 2014, 124, 92. [Google Scholar] [CrossRef]
- Ju, L.N.; Chen, Y.F.; Li, K.T.; Yuan, Z.; Liu, B.Y.; Jackson, S.P.; Zhu, C. Dual Biomembrane Force Probe enables single-cell mechanical analysis of signal crosstalk between multiple molecular species. Sci. Rep. 2017, 7, 14185. [Google Scholar] [CrossRef]
- Smit, D.; Fouquet, C.; Doulazmi, M.; Pincet, F.; Trembleau, A.; Zapotocky, M. BFPTool: A software tool for analysis of Biomembrane Force Probe experiments. BMC Biophys. 2017, 10, 2. [Google Scholar] [CrossRef]
- Smit, D.; Fouquet, C.; Pincet, F.; Zapotocky, M.; Trembleau, A. Axon tension regulates fasciculation/defasciculation through the control of axon shaft zippering. Elife 2017, 6, e19907. [Google Scholar] [CrossRef]
- Moldovan, L.; Song, C.H.; Chen, Y.C.; Wang, H.J.; Ju, L.A. Biomembrane force probe (BFP): Design, advancements, and recent applications to live-cell mechanobiology. Exploration 2023, 3, 20230004. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Ju, L.; Hong, J.; Ji, Q.; Chen, W.; Zhu, C. Fluorescence Biomembrane Force Probe: Concurrent Quantitation of Receptor-ligand Kinetics and Binding-induced Intracellular Signaling on a Single Cell. J. Vis. Exp. 2015, 102, e52975. [Google Scholar] [CrossRef]
- Mathivet, L.; Cribier, S.; Devaux, P.F. Shape change and physical properties of giant phospholipid vesicles prepared in the presence of an AC electric field. Biophys. J. 1996, 70, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Simson, D.A.; Ziemann, F.; Strigl, M.; Merkel, R. Micropipet-based pico force transducer: In depth analysis and experimental verification. Biophys. J. 1998, 74, 2080–2088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rawicz, W.; Olbrich, K.C.; McIntosh, T.; Needham, D.; Evans, E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pincet, L.; Pincet, F. Membrane Tubulation with a Biomembrane Force Probe. Membranes 2023, 13, 910. https://doi.org/10.3390/membranes13120910
Pincet L, Pincet F. Membrane Tubulation with a Biomembrane Force Probe. Membranes. 2023; 13(12):910. https://doi.org/10.3390/membranes13120910
Chicago/Turabian StylePincet, Lancelot, and Frédéric Pincet. 2023. "Membrane Tubulation with a Biomembrane Force Probe" Membranes 13, no. 12: 910. https://doi.org/10.3390/membranes13120910
APA StylePincet, L., & Pincet, F. (2023). Membrane Tubulation with a Biomembrane Force Probe. Membranes, 13(12), 910. https://doi.org/10.3390/membranes13120910





