Innovative Approaches to Poultry Processing Wastewater Treatment: The Stainless Steel Ultrafiltration Membrane as a Viable Option
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
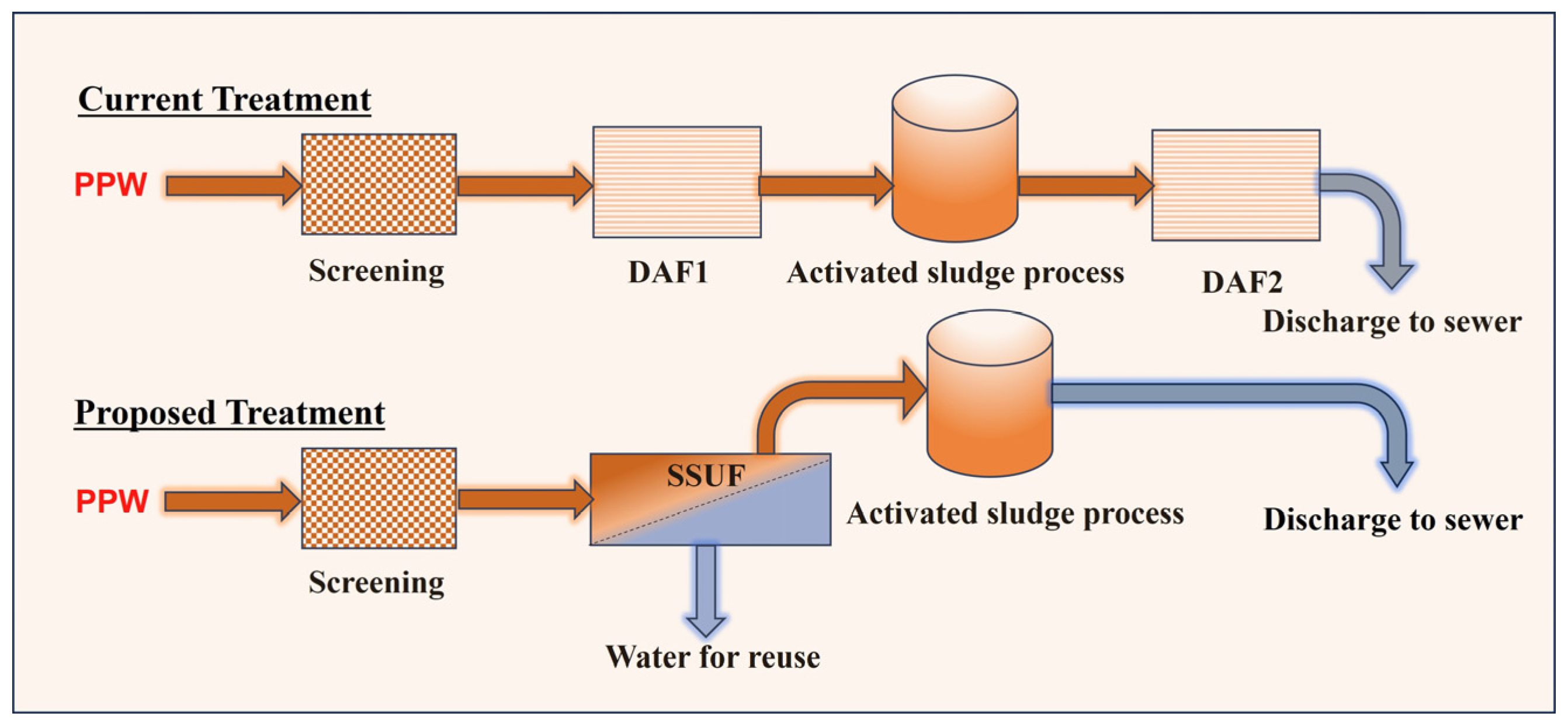
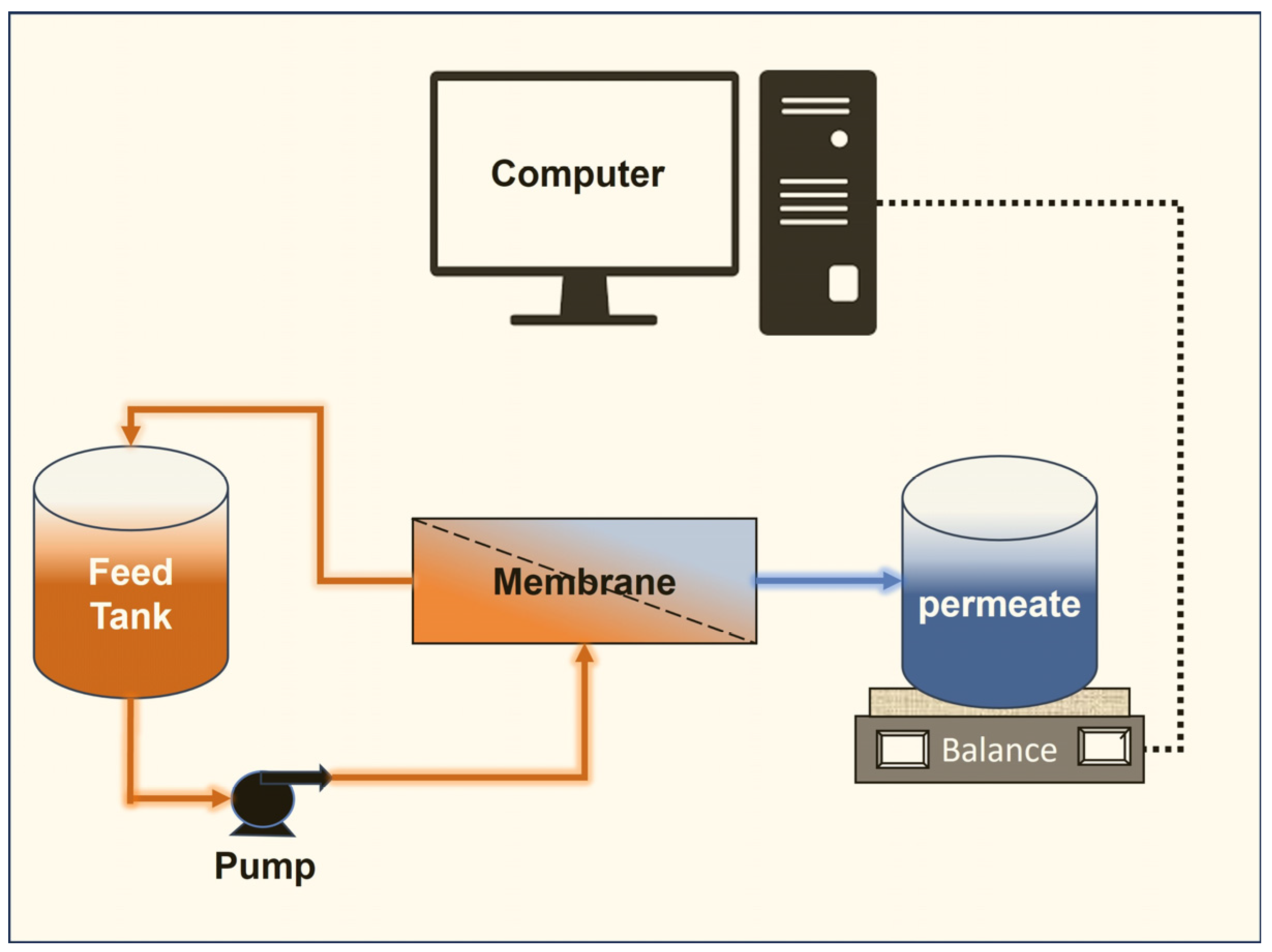
2.2. Experimental Methods
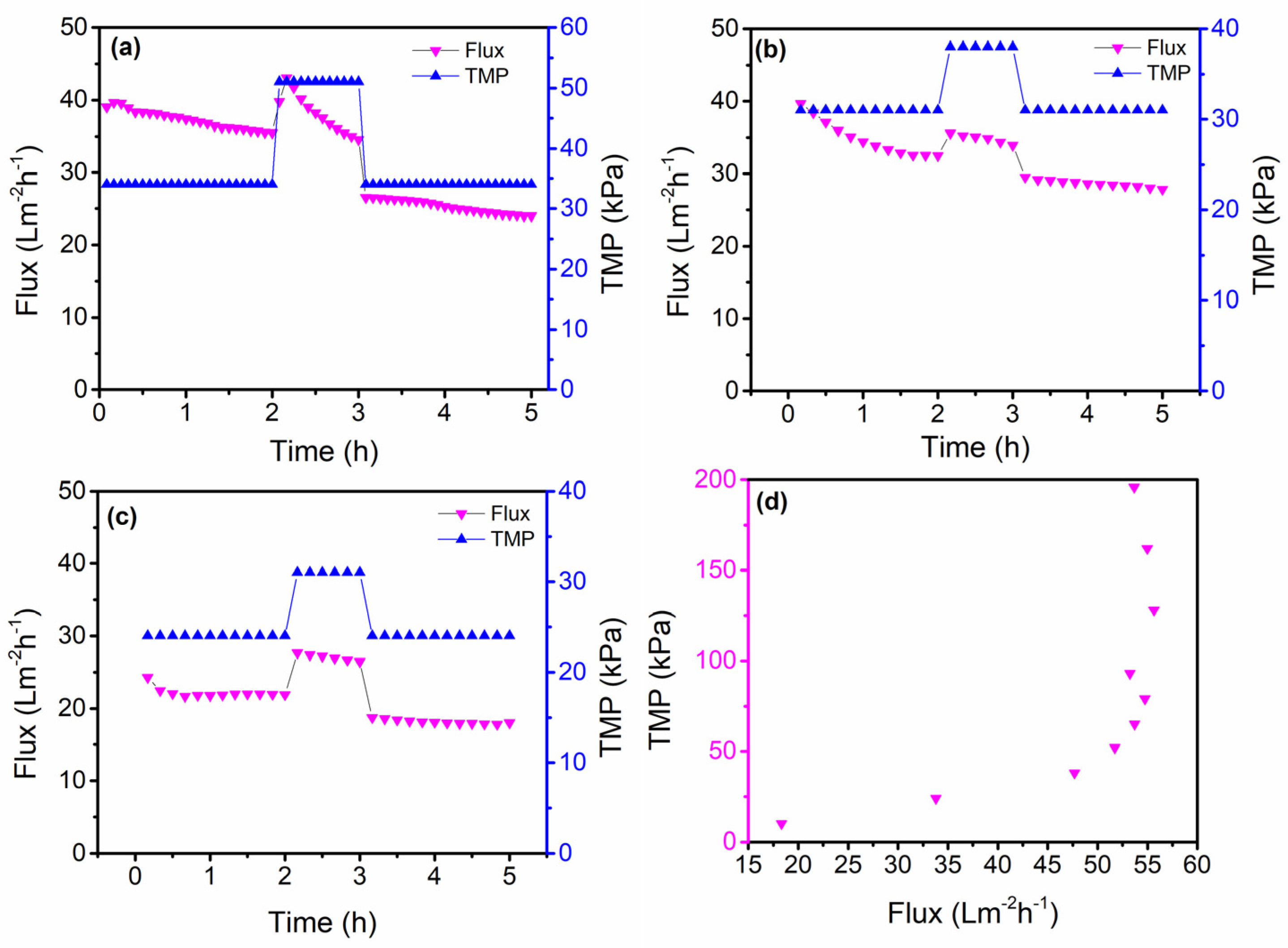
2.3. Critical Flux Experiments
2.4. Analytical Methods
2.5. Membrane Regeneration
3. Results
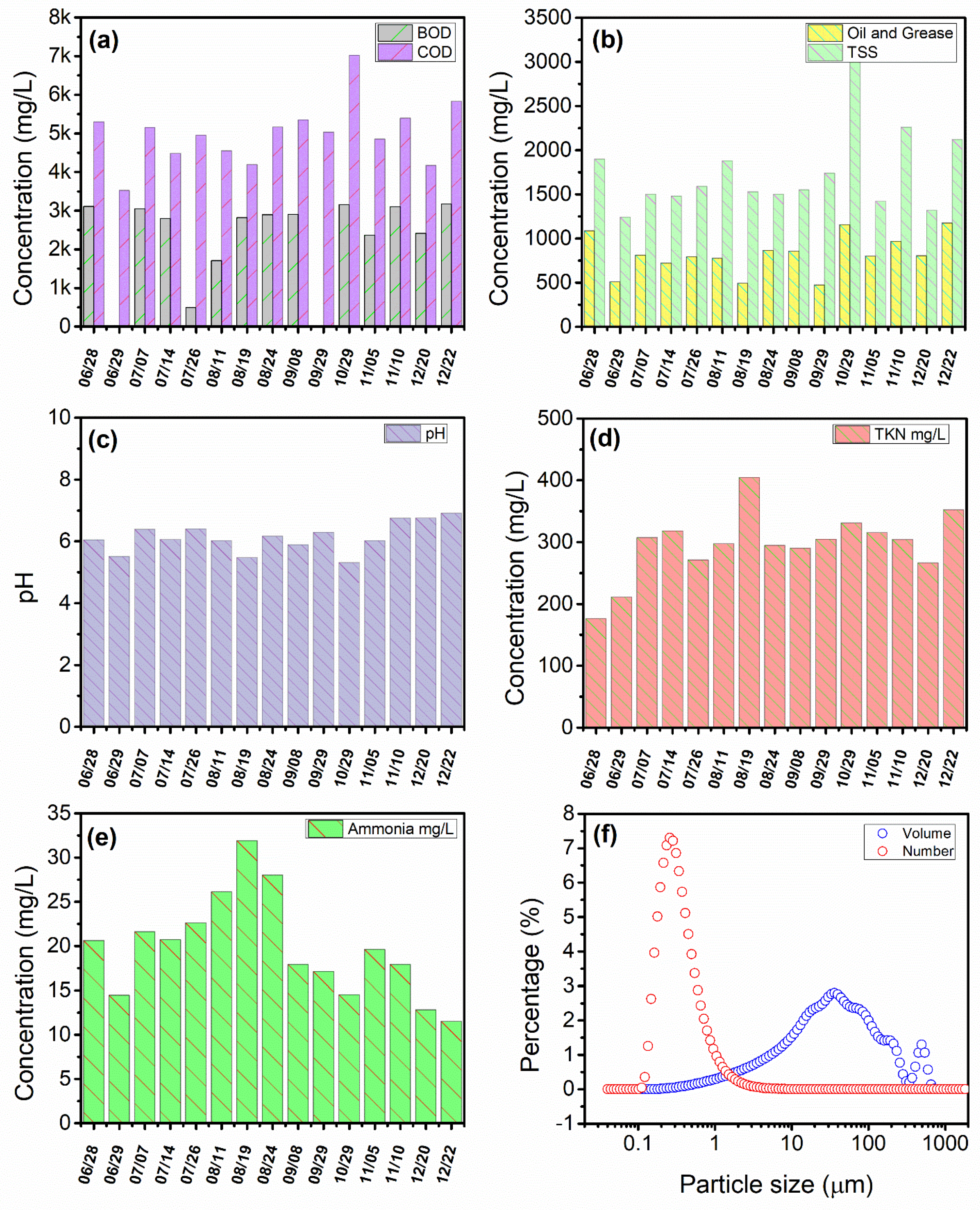
3.1. PPW Characterization
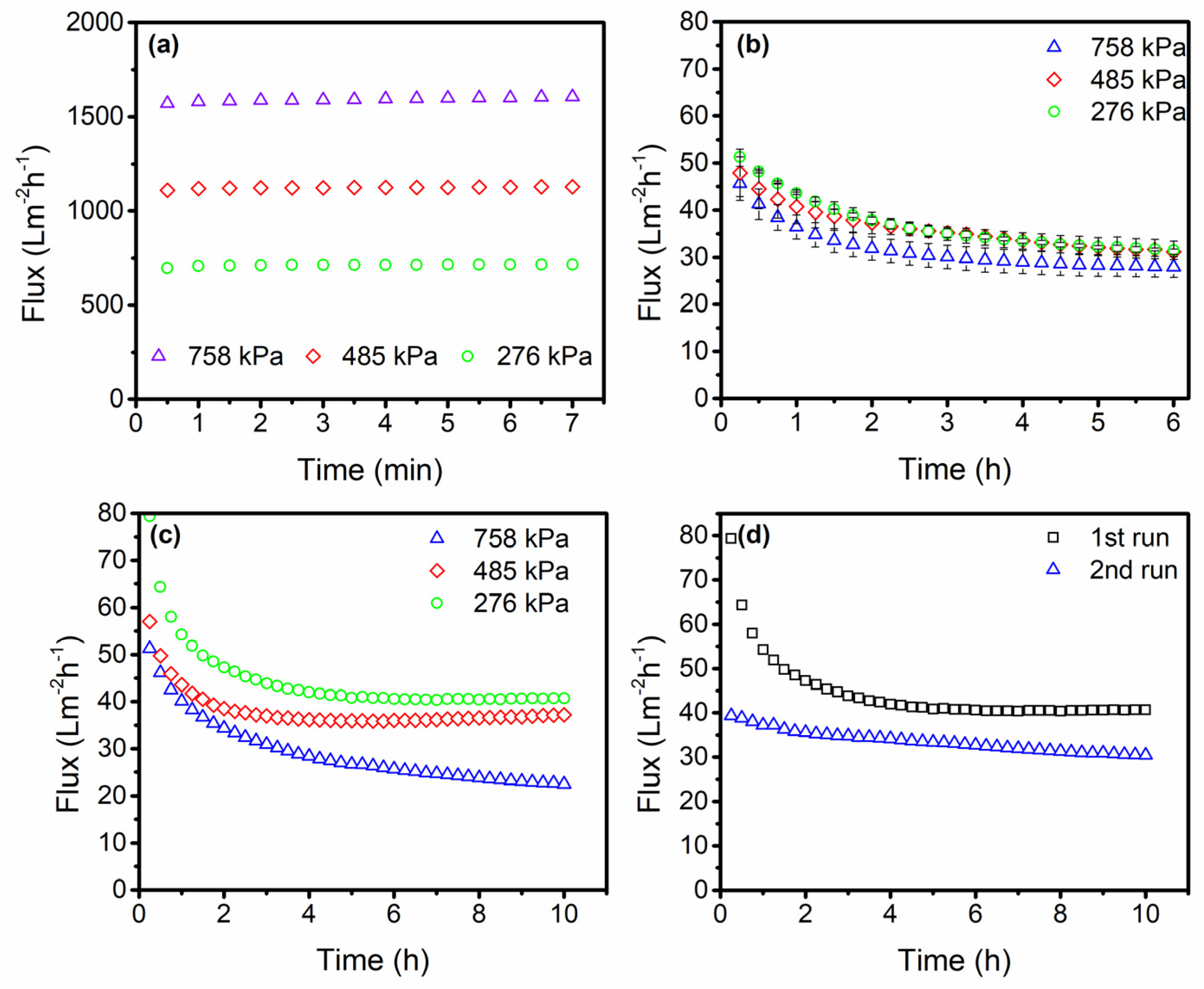
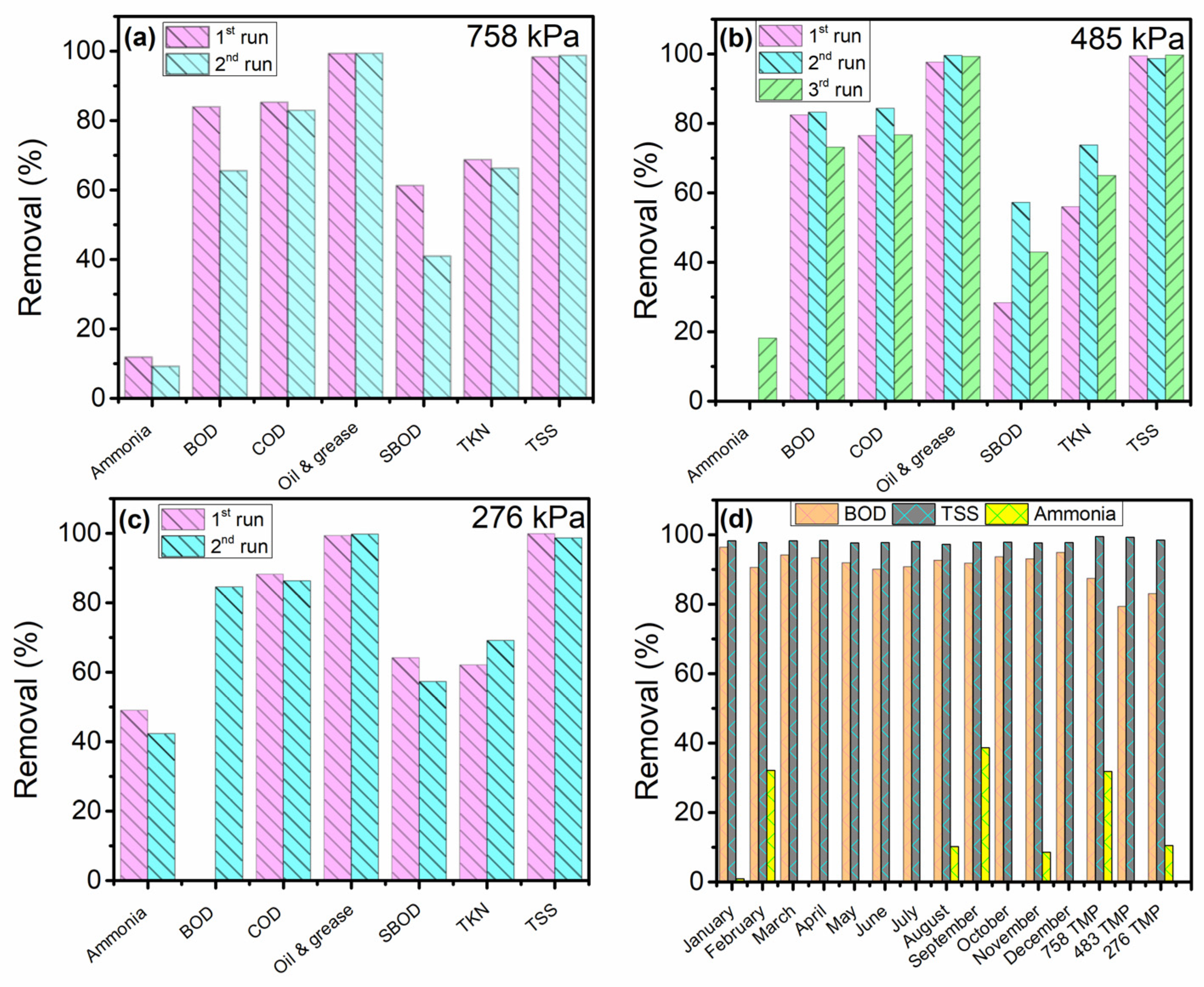
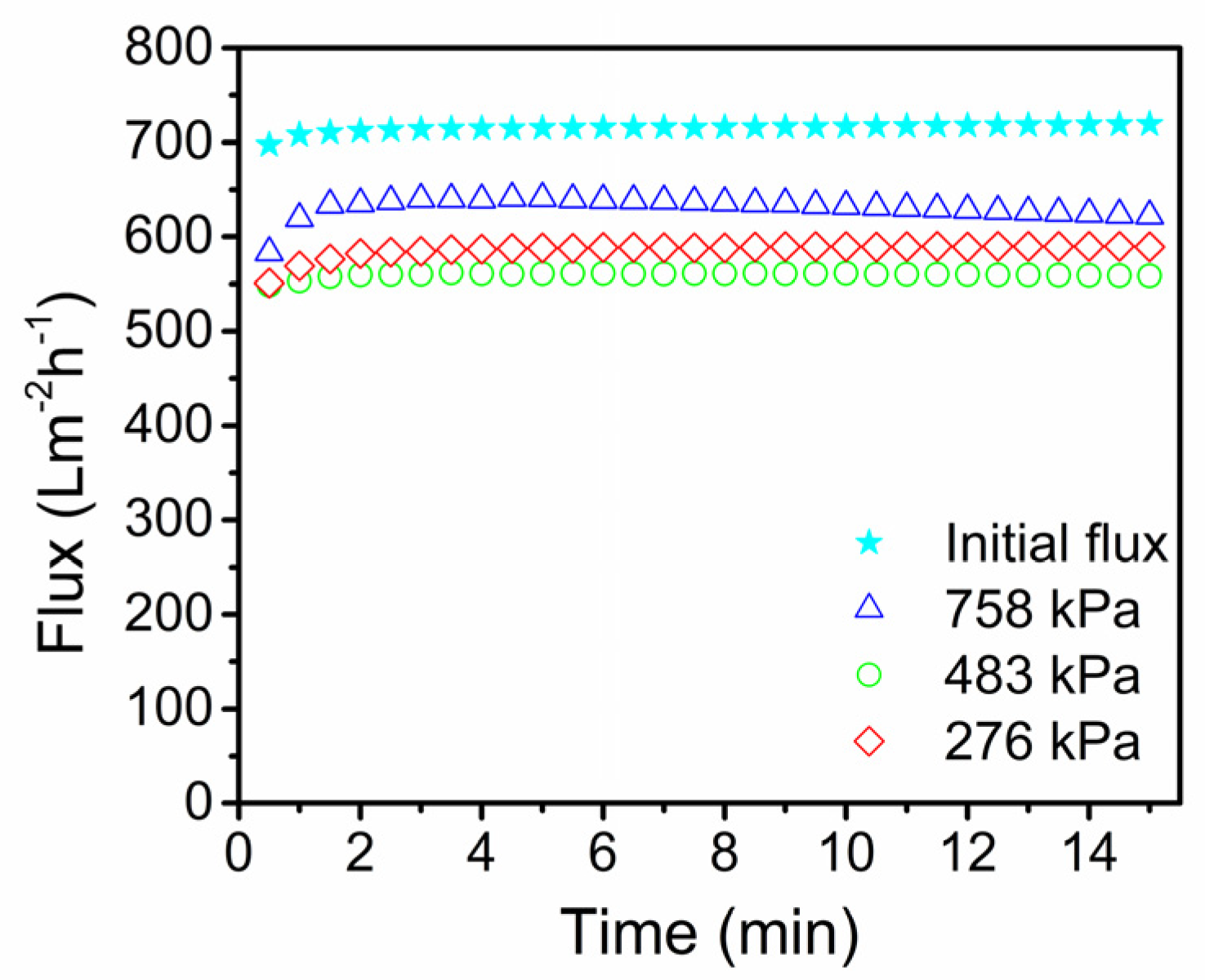
3.2. Stainless Steel Membrane Performance
3.2.1. Treatment of PPW before the First DAF
3.2.2. Pathogen Removal Validation
3.2.3. Particles Removal
3.3. Critical Flux Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, C.R.; Cross, A.J.; Koebnick, C.; Sinha, R. Trends in meat consumption in the USA. Public Health Nutr. 2011, 14, 575–583. [Google Scholar] [CrossRef]
- Parlasca, M.C.; Qaim, M. Meat Consumption and Sustainability. Annu. Rev. Resour. Econ. 2022, 14, 17–41. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Poultry Slaughter 2021 Summary 02/25/2022; United States Department of Agriculture: Washington, DC, USA, 2021.
- United States Department of Agriculture. Poultry Slaughter 2022 Summary 02/28/2023; United States Department of Agriculture: Washington, DC, USA, 2022.
- Malmali, M.; Askegaard, J.; Sardari, K.; Eswaranandam, S.; Sengupta, A.; Wickramasinghe, S.R. Evaluation of ultrafiltration membranes for treating poultry processing wastewater. J. Water Process Eng. 2018, 22, 218–226. [Google Scholar] [CrossRef]
- Kiepper, B.H.; Merka, W.C.; Fletcher, D.L. Proximate Composition of Poultry Processing Wastewater Particulate Matter from Broiler Slaughter Plants. Poult. Sci. 2008, 87, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Plumber, H.S.; Kiepper, B.H. Impact of Poultry Processing By-Products on Wastewater Generation, Treatment, and Discharges. In Proceedings of the 2011 Georgia Water Resources Conference, Athens, GA, USA, 11–13 April 2011. [Google Scholar]
- Fatima, F.; Du, H.; Kommalapati, R.R. Treatment of Poultry Slaughterhouse Wastewater with Membrane Technologies: A Review. Water 2021, 13, 1905. [Google Scholar] [CrossRef]
- Northcutt, J.K.; Jones, D.R. A Survey of Water Use and Common Industry Practices in Commercial Broiler Processing Facilities. J. Appl. Poult. Res. 2004, 13, 48–54. [Google Scholar] [CrossRef]
- Avula, R.Y.; Nelson, H.M.; Singh, R.K. Recycling of poultry process wastewater by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2009, 10, 1–8. [Google Scholar] [CrossRef]
- Abboah-Afari, E.; Kiepper, B.H. Membrane Filtration of Poultry Processing Wastewater: I. Pre-DAF (Dissolved Air Flotation). Appl. Eng. Agric. 2012, 28, 231–236. [Google Scholar] [CrossRef]
- Vidal, G.; Carvalho, A.; Méndez, R.; Lema, J.M. Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Bioresour. Technol. 2000, 74, 231–239. [Google Scholar] [CrossRef]
- Sardari, K.; Askegaard, J.; Chiao, Y.-H.; Darvishmanesh, S.; Kamaz, M.; Wickramasinghe, S.R. Electrocoagulation followed by ultrafiltration for treating poultry processing wastewater. J. Environ. Chem. Eng. 2018, 6, 4937–4944. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Zhumagulov, M.; Saspugayeva, G.; Jakupova, Z.; Mussimkhan, Ð. Treatment of poultry slaughterhouse wastewater with combined system. Potravin. Slovak J. Food Sci. 2019, 13, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Terán Hilares, R.; Garcia Bustos, K.A.; Sanchez Vera, F.P.; Colina Andrade, G.J.; Pacheco Tanaka, D.A. Acid precipitation followed by microalgae (Chlorella vulgaris) cultivation as a new approach for poultry slaughterhouse wastewater treatment. Bioresour. Technol. 2021, 335, 125284. [Google Scholar] [CrossRef] [PubMed]
- Del Nery, V.; de Nardi, I.R.; Damianovic, M.H.R.Z.; Pozzi, E.; Amorim, A.K.B.; Zaiat, M. Long-term operating performance of a poultry slaughterhouse wastewater treatment plant. Resour. Conserv. Recycl. 2007, 50, 102–114. [Google Scholar] [CrossRef]
- Lo, Y.M.; Cao, D.; Argin-Soysal, S.; Wang, J.; Hahm, T.-S. Recovery of protein from poultry processing wastewater using membrane ultrafiltration. Bioresour. Technol. 2005, 96, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Bingo, M.N.; Njoya, M.; Basitere, M.; Ntwampe, S.K.O.; Kaskote, E. Performance evaluation of an integrated multi-stage poultry slaughterhouse wastewater treatment system. J. Water Process Eng. 2021, 43, 102309. [Google Scholar] [CrossRef]
- Rinquest, Z.; Basitere, M.; Ntwampe, S.K.O.; Njoya, M. Poultry slaughterhouse wastewater treatment using a static granular bed reactor coupled with single stage nitrification-denitrification and ultrafiltration systems. J. Water Process Eng. 2019, 29, 100778. [Google Scholar] [CrossRef]
- Basitere, M.; Rinquest, Z.; Njoya, M.; Sheldon, M.S.; Ntwampe, S.K.O. Treatment of poultry slaughterhouse wastewater using a static granular bed reactor (SGBR) coupled with ultrafiltration (UF) membrane system. Water Sci. Technol. 2017, 76, 106–114. [Google Scholar] [CrossRef]
- Shih, J.C.H.; Kozink, M.B. Ultrafiltration Treatment of Poultry Processing Wastewater and Recovery of a Nutritional By-Product1. Poult. Sci. 1980, 59, 247–252. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Cai, C.; Sun, W.; He, S.; Zhang, Y.; Wang, X. Ceramic membrane fouling mechanisms and control for water treatment. Front. Environ. Sci. Eng. 2023, 17, 126. [Google Scholar] [CrossRef]
- Kotobuki, M.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules 2021, 26, 3331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qu, Y.; Liu, Y.; Yang, F.; Zhang, X.; Furukawa, K.; Yamada, Y. Experimental study of domestic sewage treatment with a metal membrane bioreactor. Desalination 2005, 177, 83–93. [Google Scholar] [CrossRef]
- Du, N.; Pan, L.; Liu, J.; Wang, L.; Li, H.; Li, K.; Xie, C.; Hang, F.; Lu, H.; Li, W. Clarification of Limed Sugarcane Juice by Stainless Steel Membranes and Membrane Fouling Analysis. Membranes 2022, 12, 910. [Google Scholar] [CrossRef] [PubMed]
- Le Clech, P.; Jefferson, B.; Chang, I.S.; Judd, S.J. Critical flux determination by the flux-step method in a submerged membrane bioreactor. J. Membr. Sci. 2003, 227, 81–93. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Fane, A.G.; Wiley, D.E. Factors influencing critical flux in membrane filtration of activated sludge. J. Chem. Technol. Biotechnol. 1999, 74, 539–543. [Google Scholar] [CrossRef]
- Youravong, W.; Lewis, M.J.; Grandison, A.S. Critical Flux in Ultrafiltration of Skimmed Milk. Food Bioprod. Process. 2003, 81, 303–308. [Google Scholar] [CrossRef]
- Maruf, S.H.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Critical flux of surface-patterned ultrafiltration membranes during cross-flow filtration of colloidal particles. J. Membr. Sci. 2014, 471, 65–71. [Google Scholar] [CrossRef]
- Espinasse, B.; Bacchin, P.; Aimar, P. On an experimental method to measure critical flux in ultrafiltration. Desalination 2002, 146, 91–96. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F.; Othman, M.H.D.; Shirazi, M.M.A. Critical Flux and Fouling Analysis of PVDF-Mixed Matrix Membranes for Reclamation of Refinery-Produced Wastewater: Effect of Mixed Liquor Suspended Solids Concentration and Aeration. Membranes 2022, 12, 161. [Google Scholar] [CrossRef]
- Allie, Z.; Jacobs, E.P.; Maartens, A.; Swart, P. Enzymatic cleaning of ultrafiltration membranes fouled by abattoir effluent. J. Membr. Sci. 2003, 218, 107–116. [Google Scholar] [CrossRef]
- Marchesi, C.M.; Paliga, M.; Oro, C.E.D.; Dallago, R.M.; Zin, G.; Di Luccio, M.; Oliveira, J.V.; Tres, M.V. Use of membranes for the treatment and reuse of water from the pre-cooling system of chicken carcasses. Environ. Technol. 2021, 42, 126–133. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Values |
|---|---|
| Surface area m2 | 0.0058 |
| Length m | 0.30 |
| Pore Size (µm) | 0.02 |
| Diameter (mm) | 6 |
| Material | Stainless steel |
| Type | Tubular |
| Flow type | Tangential |
| Sample Name | (A) E. coli (MPN/100 mL) A | (A) Total Coliform (MPN/100 mL) A | (B) E. coli (MPN/100 mL) B | (B) Total Coliform (MPN/100 mL) B | (C) E. coli (MPN/100 mL) C | (C) Total Coliform (MPN/100 mL) C |
|---|---|---|---|---|---|---|
| PPW | >24,196,000 | >24,196,000 | >24,196,000 | >24,196,000 | >24,196,000 | >24,196,000 |
| Permeate | 345 | 68,670 | 308 | 92,080 | 115 | 29,090 |
| Removal % | 99.999 | 99.7 | 99.999 | 99.6 | 99.999 | 99.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dada, S.O.; Thamariselvan, C.; Jebur, M.; Wickramasinghe, S.R. Innovative Approaches to Poultry Processing Wastewater Treatment: The Stainless Steel Ultrafiltration Membrane as a Viable Option. Membranes 2023, 13, 880. https://doi.org/10.3390/membranes13110880
Dada SO, Thamariselvan C, Jebur M, Wickramasinghe SR. Innovative Approaches to Poultry Processing Wastewater Treatment: The Stainless Steel Ultrafiltration Membrane as a Viable Option. Membranes. 2023; 13(11):880. https://doi.org/10.3390/membranes13110880
Chicago/Turabian StyleDada, Saubana Olorunsola, Chidambaram Thamariselvan, Mahmood Jebur, and Sumith Ranil Wickramasinghe. 2023. "Innovative Approaches to Poultry Processing Wastewater Treatment: The Stainless Steel Ultrafiltration Membrane as a Viable Option" Membranes 13, no. 11: 880. https://doi.org/10.3390/membranes13110880
APA StyleDada, S. O., Thamariselvan, C., Jebur, M., & Wickramasinghe, S. R. (2023). Innovative Approaches to Poultry Processing Wastewater Treatment: The Stainless Steel Ultrafiltration Membrane as a Viable Option. Membranes, 13(11), 880. https://doi.org/10.3390/membranes13110880







