Separation Mechanisms and Anti-Fouling Properties of a Microporous Polyvinylidene Fluoride–Polyacrylic Acid–Graphene Oxide (PVDF-PAA-GO) Composite Membrane with Salt and Protein Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Testing of the Composite Membrane
2.2.1. Preparation of GO
2.2.2. Fabrication of the Non-Woven/PVDF-PAA Membrane
2.2.3. Fabrication of the Non-Woven/PVDF-PAA-GO Membrane
2.3. Material and Membrane Characterizations
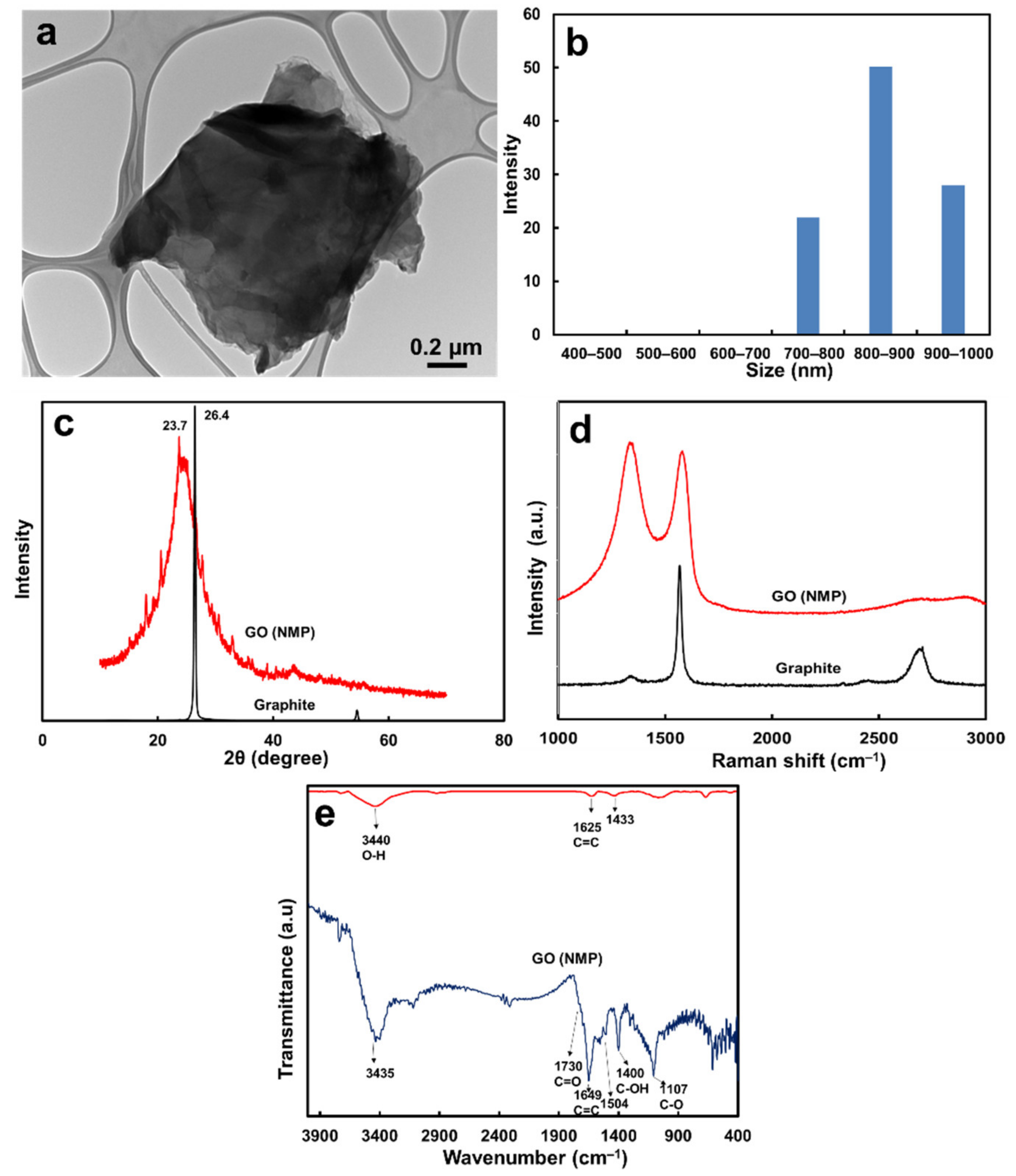
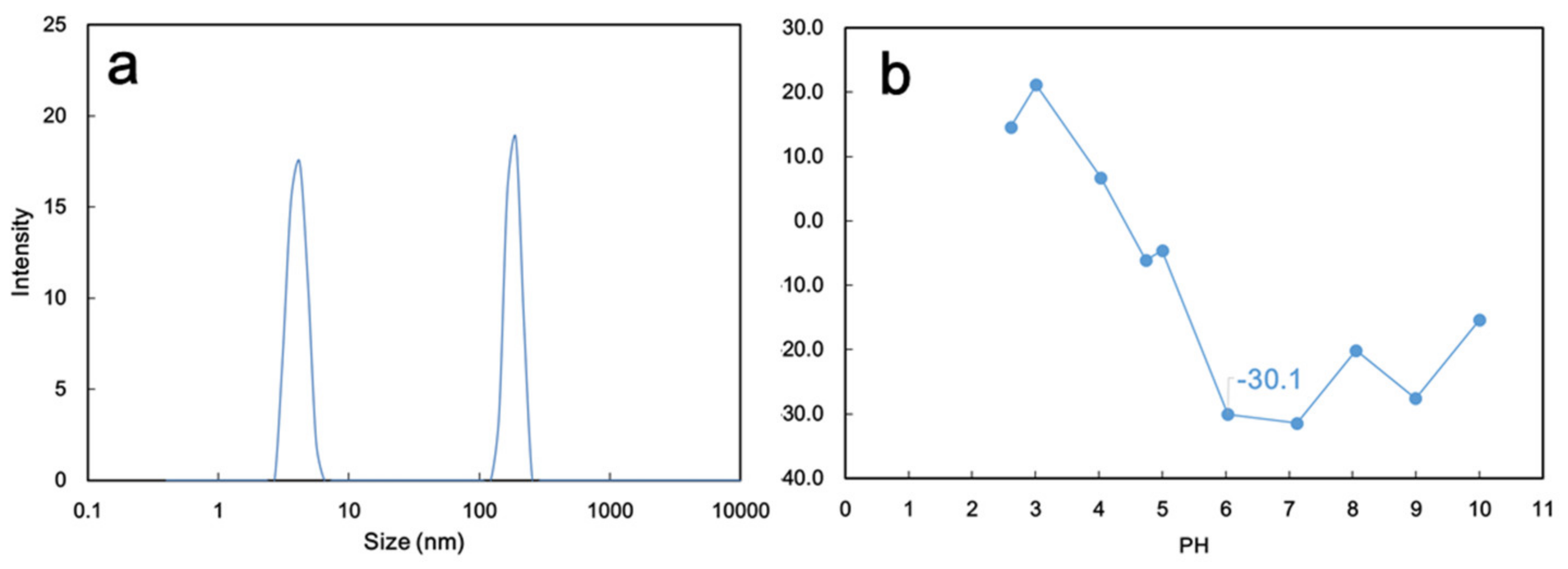
2.3.1. Physicochemical Properties of GO
2.3.2. Physicochemical Analysis of the Composite Membrane
2.4. Membrane Filtration Performances
2.4.1. Preparation of Feed Solutions and Calibration Curves
2.4.2. Membrane Filtration Performance Test
3. Results and Discussion
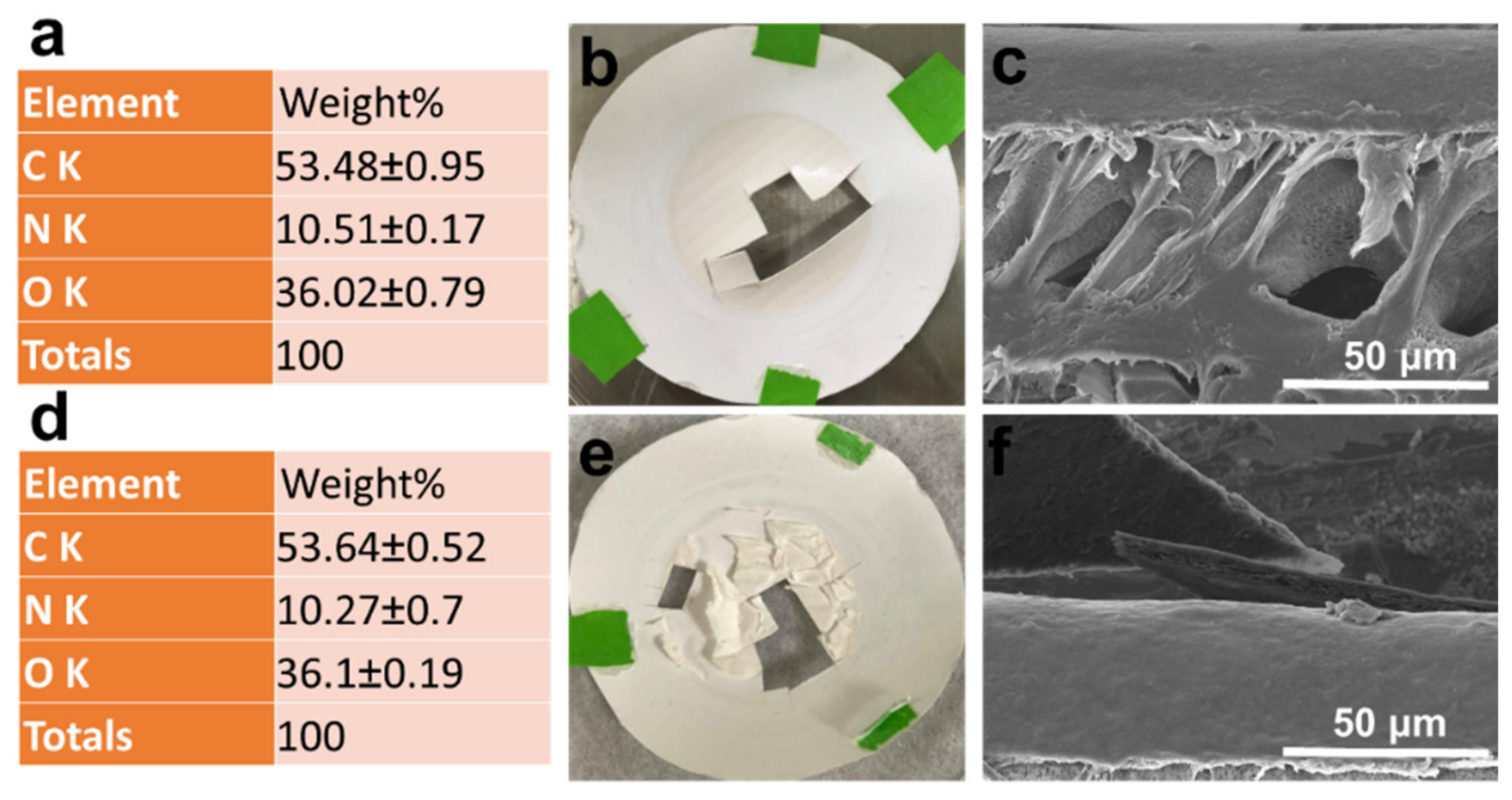
3.1. Characterization of Polymeric Composite Membranes
3.2. Performance of Non-Woven/PVDF-PAA Membrane
3.2.1. Permeance and Separation Performance on Salt Solutions
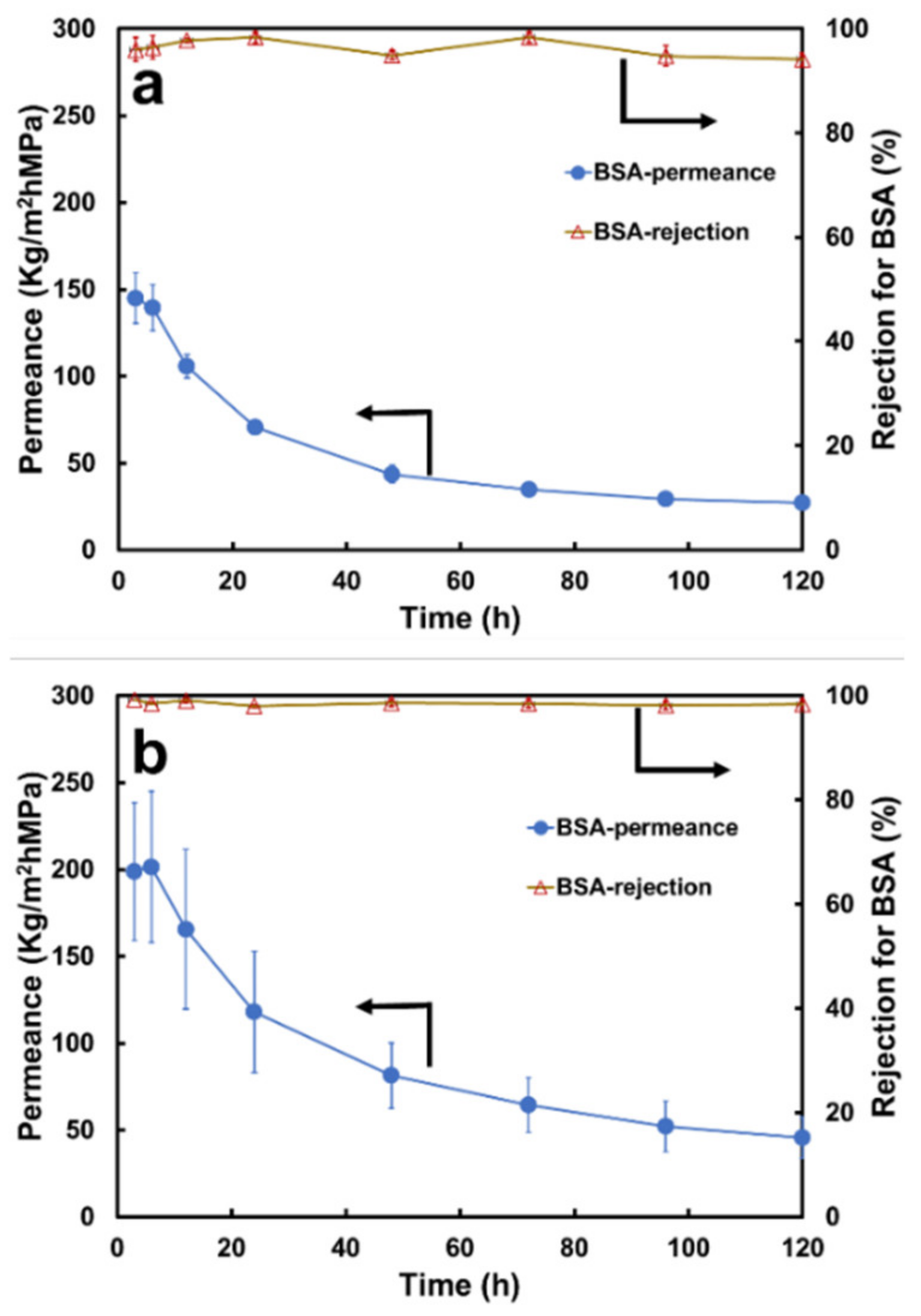
3.2.2. Separation Performance on BSA Solution
3.3. Performance of Non-Woven/PVDF-PAA-GO Composite Membrane
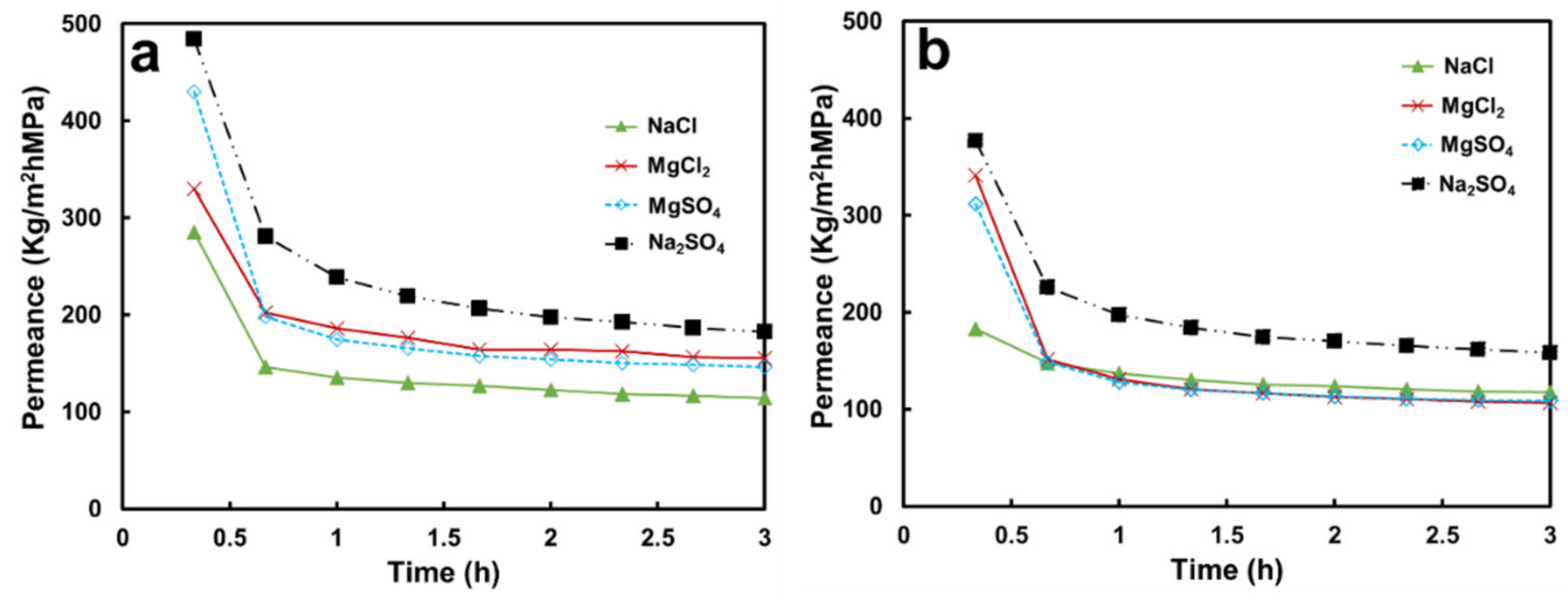
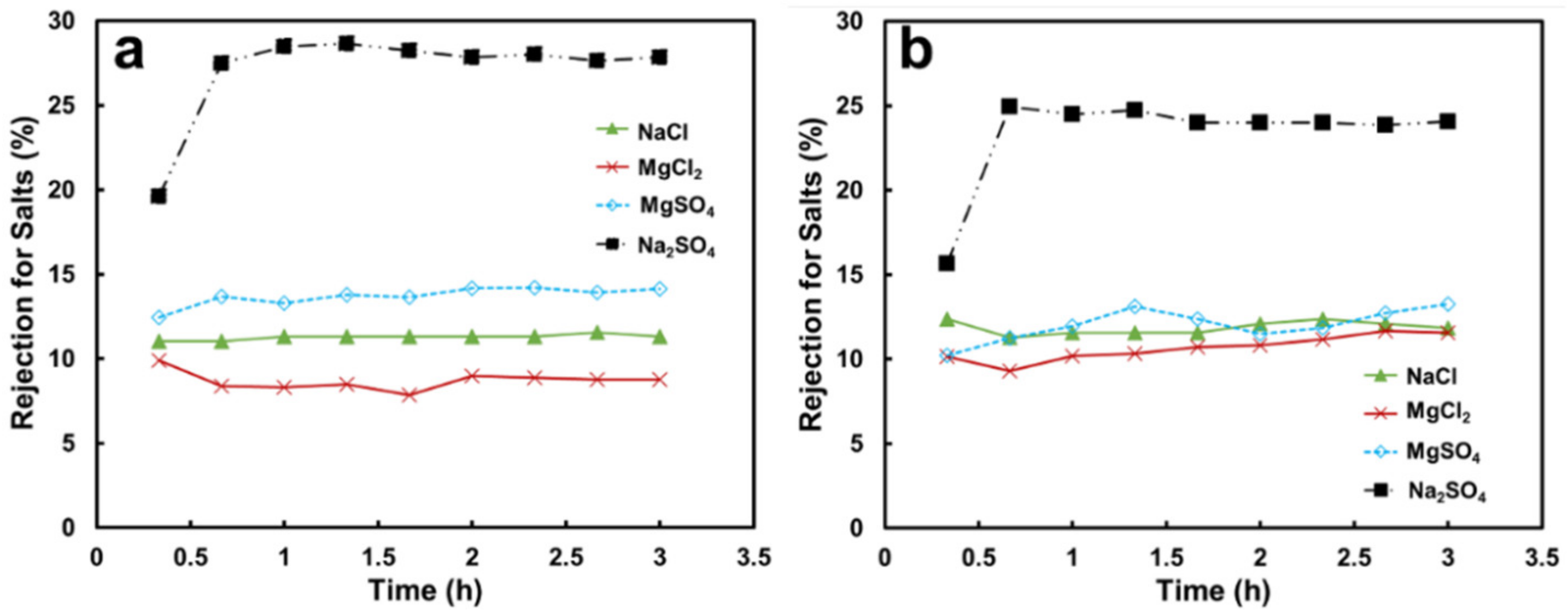
3.3.1. Permeance and Separation Performance with Salt Solutions
3.3.2. Separation Performance on the BSA Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Issaoui, M.; Jellali, S.; Zorpas, A.A.; Dutournie, P. Membrane technology for sustainable water resources management: Challenges and future projections. Sustain. Chem. Pharm. 2022, 25, 100590. [Google Scholar] [CrossRef]
- Zhou, Y.; Tol, R.S.J. Evaluating the costs of desalination and water transport. Water Resour. Res. 2005, 41. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Bopape, M.F.; Mahlangu, O.T.; Mamba, B.B.; Van der Bruggen, B.; Quist-Jensen, C.A.; Richards, H. Fouling, performance and cost analysis of membrane-based water desalination technologies: A critical review. J. Environ. Manag. 2022, 301, 113922. [Google Scholar] [CrossRef] [PubMed]
- Vigneswaran, S.; Ngo, H.H.; Chaudhary, D.S.; Hung, Y.-T. Physicochemical Treatment Processes for Water Reuse. Physicochem. Treat. Process. 2005, 3, 635–676. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Majumdar, R.; Mishra, U.; Bhunia, B. Advanced Functional Membranes for Microfiltration and Ultrafiltration. Adv. Funct. Membr. Mater. Appl. 2022, 120, 43–71. [Google Scholar]
- Dong, X.; Li, S.; Zhang, Q.; Zhang, S. Preparation and characterization of novel positively charged copolymer composite membranes for nanofiltration. RSC Adv. 2014, 4, 22625–22631. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Amaral, M.C.S. Enhancing industries exploitation: Integrated and hybrid membrane separation processes applied to industrial effluents beyond the treatment for disposal. Chem. Eng. J. 2022, 430, 133006. [Google Scholar] [CrossRef]
- Said, N.; Lau, W.J.; Ho, Y.-C.; Lim, S.K.; Zainol Abidin, M.N.; Ismail, A.F. A review of commercial developments and recent laboratory research of dialyzers and membranes for hemodialysis application. Membranes 2021, 11, 767. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-Food Industry Waste as Resource of Chemicals: The Role of Membrane Technology in Their Sustainable Recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chao, C.-M.; Wang, D.K.; Liu, K.-M.; Tseng, H.-H. Enhancing the antifouling properties of a PVDF membrane for protein separation by grafting branch-like zwitterions via a novel amphiphilic SMA-HEA linker. J. Membr. Sci. 2021, 624, 119126. [Google Scholar] [CrossRef]
- Carter, B.; DiMarzo, L.; Pranata, J.; Barbano, D.M.; Drake, M. Efficiency of removal of whey protein from sweet whey using polymeric microfiltration membranes. J. Dairy Sci. 2021, 104, 8630–8643. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, J.; Liu, F.; Xu, B.-M.; Pan, Y. Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes 2022, 12, 519. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Kumar, S.R.; Lue, S.J. Separation mechanisms of binary dye mixtures using a PVDF ultrafiltration membrane: Donnan effect and intermolecular interaction. J. Membr. Sci. 2019, 575, 38–49. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly (vinylidene fluoride)(PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Preparation of a Novel Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane Modified with Reduced Graphene Oxide/Titanium Dioxide (TiO2) Nanocomposite with Enhanced Hydrophilicity and Antifouling Properties. Ind. Eng. Chem. Res. 2014, 53, 140819070522009. [Google Scholar] [CrossRef]
- An, Z.; Xu, R.; Dai, F.; Xue, G.; He, X.; Zhao, Y.; Chen, L. PVDF/PVDF-g-PACMO blend hollow fiber membranes for hemodialysis: Preparation, characterization, and performance. RSC Adv. 2017, 7, 26593–26600. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Kumar, S.R.; Nguyen, C.H.; Lee, J.W.; Tsai, H.-A.; Hsieh, C.-H.; Lue, S.J. High-permeability graphene oxide and poly (vinyl pyrrolidone) blended poly (vinylidene fluoride) membranes: Roles of additives and their cumulative effects. J. Membr. Sci. 2021, 619, 118773. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, C.-B.; Kumar, S.R.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. Graphene oxide-cation interaction: Inter-layer spacing and zeta potential changes in response to various salt solutions. J. Membr. Sci. 2018, 554, 253–263. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Pan, F.; He, G.; Fang, C.; Cao, K.; Xing, R.; Jiang, Z. Fabricating graphene oxide-based ultrathin hybrid membrane for pervaporation dehydration via layer-by-layer self-assembly driven by multiple interactions. J. Membr. Sci. 2015, 487, 162–172. [Google Scholar] [CrossRef]
- Geng, C.; Zhao, F.; Wang, Q.; Zheng, S.; Liu, Y.; Niu, H.; Zhang, J.; Dong, H. Anti-biofouling property and anti-leaching investigation of modifier for PVDF ultrafiltration membrane by incorporating antibacterial graphene oxide derivatives. J. Environ. Chem. Eng. 2022, 10, 108558. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of Graphene Oxide Formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Wang, L.-T.; Chen, Y.-H.; Kumar, S.R.; Lue, S.J. Morphological, electrical, and compositional changes of graphene oxide nanosheets from aqueous to organic solution. in review.
- Chang, W.-T.; Chao, Y.-H.; Li, C.-W.; Lin, K.-L.; Wang, J.-J.; Kumar, S.R.; Lue, S.J. Graphene oxide synthesis using microwave-assisted vs. modified Hummer’s methods: Efficient fillers for improved ionic conductivity and suppressed methanol permeability in alkaline methanol fuel cell electrolytes. J. Power Sources 2019, 414, 86–95. [Google Scholar] [CrossRef]
- Krishna, R.; Titus, E.; Okhay, O.; Campos Gil, J.; Ventura, J.; Ramana, E.; Gracio, J.J.A. Rapid Electrochemical Synthesis of Hydrogenated Graphene Oxide Using Ni Nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 4054–4069. [Google Scholar]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- King, A.A.K.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene oxide and its Derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef]
- Yan, S.; Qi, T.-T.; Chen, D.-W.; Li, Z.; Li, X.-J.; Pan, S.-Y. Magnetic solid phase extraction based on magnetite/reduced graphene oxide nanoparticles for determination of trace isocarbophos residues in different matrices. J. Chromatogr. A 2014, 1347, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Giarola, J.D.F.; Borges, K.B.; Tarley, C.R.T.; de Oliveira, F.M.; Ribeiro, E.S.; Pereira, A.C. Development and application of graphite-SiO2/Al2O3/Nb2O5-methylene blue (GRP-SiAlNb-MB) composite for electrochemical determination of dopamine. Arab. J. Chem. 2017, 10, 430–438. [Google Scholar] [CrossRef]
- Monteserín, C.; Blanco, M.; Aranzabe, E.; Aranzabe, A.; Laza, J.M.; Larrañaga-Varga, A.; Vilas, J.L. Effects of Graphene Oxide and Chemically-Reduced Graphene Oxide on the Dynamic Mechanical Properties of Epoxy Amine Composites. Polymers 2017, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 465. [Google Scholar] [CrossRef]
- Baig, N.; Kawde, A.-N. A cost-effective disposable graphene-modified electrode decorated with alternating layers of Au NPs for the simultaneous detection of dopamine and uric acid in human urine. RSC Adv. 2016, 6, 80756–80765. [Google Scholar] [CrossRef]
- Hu, K.; Dickson, J.M. Development and characterization of poly(vinylidene fluoride)–poly(acrylic acid) pore-filled pH-sensitive membranes. J. Membr. Sci. 2007, 301, 19–28. [Google Scholar] [CrossRef]
- Zhi, S.-H.; Wan, L.-S.; Xu, Z.-K. Poly(vinylidene fluoride)/poly(acrylic acid)/calcium carbonate composite membranes via mineralization. J. Membr. Sci. 2014, 454, 144–154. [Google Scholar] [CrossRef]
- Mbareck, C.; Nguyen, Q.T.; Alaoui, O.T.; Barillier, D. Elaboration, characterization and application of polysulfone and polyacrylic acid blends as ultrafiltration membranes for removal of some heavy metals from water. J. Hazard. Mater. 2009, 171, 93–101. [Google Scholar] [CrossRef]
- Ravishankar, H.; Christy, J.; Jegatheesan, V. Graphene Oxide (GO)-Blended Polysulfone (PSf) Ultrafiltration Membranes for Lead Ion Rejection. Membranes 2018, 8, 77. [Google Scholar] [CrossRef]
- Ekambaram, K.; Doraisamy, M. Surface modification of PVDF nanofiltration membrane using Carboxymethylchitosan-Zinc oxide bionanocomposite for the removal of inorganic salts and humic acid. Colloids Surf. A Physicochem. Eng. Asp. 2017, 525, 49–63. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kumar, S.R.; Shih, C.M.; Hung, W.S.; An, Q.F.; Hsu, H.C.; Huang, S.H.; Lue, S.J. High permeance nanofiltration thin film composites with a polyelectrolyte complex top layer containing graphene oxide nanosheets. J. Membr. Sci. 2017, 540, 391–400. [Google Scholar] [CrossRef]
- Peeters, J.M.M.; Boom, J.P.; Mulder, M.H.V.; Strathmann, H. Retention measurements of nanofiltration membranes with electrolyte solutions. J. Membr. Sci. 1998, 145, 199–209. [Google Scholar] [CrossRef]
- Flecha, F.L.G.; Levi, V. Determination of the molecular size of BSA by fluorescence anisotropy. Biochem. Mol. Biol. Educ. 2003, 31, 319–322. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed]
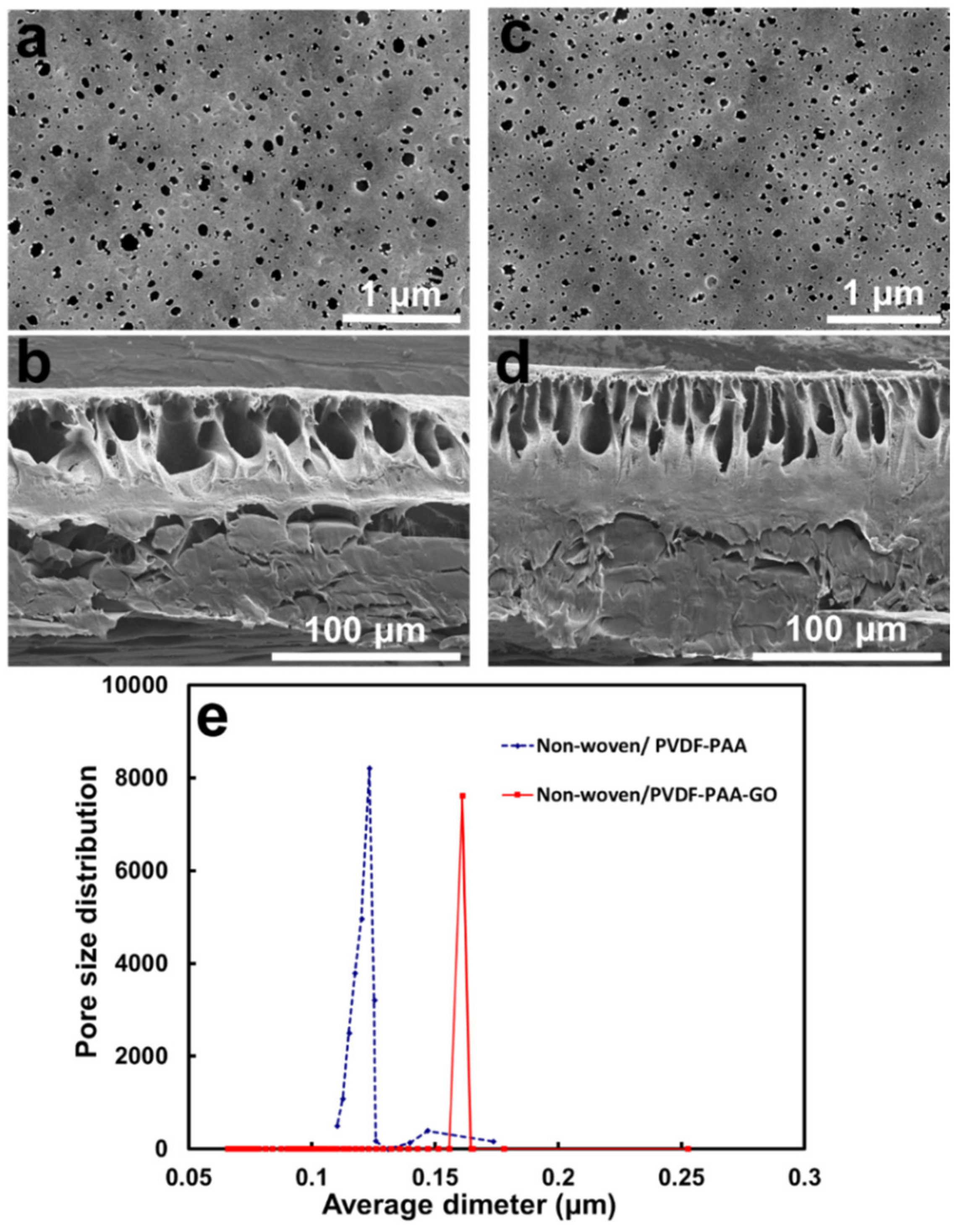


| Membrane | Thickness (μm) | Mean Pore Size b (μm) | Volume Porosity d (%) | Surface Zeta Potential (mV) | Contact Angle (°) |
|---|---|---|---|---|---|
| Non-woven | 97 | 7.13 ± 3.33 | - | −3.91 ± 0.58 | 77.2 |
| Non-woven/PVDF-PAA | 97/56 a | 0.12 ± 0.02 c | 15.6 d | −19.36 ± 0.57 | 74.9 |
| Non-woven/PVDF-PAA-GO | 97/54 a | 0.16 ± 0.07 c | 28.7 d | −30.88 ± 1.14 | 59.3 |
| Membrane | Permeance (kg/m2hMPa) | Ref. |
|---|---|---|
| PVDF-PAA | 260 | [37] |
| PVDF/CMC-ZnO (0.05 wt%) | 128.9 | [40] |
| Non-woven/PVDF-PAA | 203.1 | [20] |
| Non-woven/PVDF-PAA | 246 | This work |
| Non-woven/PVDF-PAA-GO | 276 | This work |
| Salt | Mole Con. (M) | Ionic Strength (M) | Activity Coefficient | Salt Activity |
|---|---|---|---|---|
| MgCl2 | 0.01 | 0.03 | 0.709 | 0.021 |
| NaCl | 0.034 | 0.034 | 0.832 | 0.028 |
| MgSO4 | 0.017 | 0.066 | 0.382 | 0.025 |
| Na2SO4 | 0.006 | 0.019 | 0.754 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-T.; Chen, Y.-H.; Chang, W.-T.; Kumar, S.R.; Chen, C.-C.; Lue, S.J. Separation Mechanisms and Anti-Fouling Properties of a Microporous Polyvinylidene Fluoride–Polyacrylic Acid–Graphene Oxide (PVDF-PAA-GO) Composite Membrane with Salt and Protein Solutions. Membranes 2023, 13, 40. https://doi.org/10.3390/membranes13010040
Wang L-T, Chen Y-H, Chang W-T, Kumar SR, Chen C-C, Lue SJ. Separation Mechanisms and Anti-Fouling Properties of a Microporous Polyvinylidene Fluoride–Polyacrylic Acid–Graphene Oxide (PVDF-PAA-GO) Composite Membrane with Salt and Protein Solutions. Membranes. 2023; 13(1):40. https://doi.org/10.3390/membranes13010040
Chicago/Turabian StyleWang, Li-Ting, Yu-Han Chen, Wei-Ting Chang, Selvaraj Rajesh Kumar, Chien-Chang Chen, and Shingjiang Jessie Lue. 2023. "Separation Mechanisms and Anti-Fouling Properties of a Microporous Polyvinylidene Fluoride–Polyacrylic Acid–Graphene Oxide (PVDF-PAA-GO) Composite Membrane with Salt and Protein Solutions" Membranes 13, no. 1: 40. https://doi.org/10.3390/membranes13010040
APA StyleWang, L.-T., Chen, Y.-H., Chang, W.-T., Kumar, S. R., Chen, C.-C., & Lue, S. J. (2023). Separation Mechanisms and Anti-Fouling Properties of a Microporous Polyvinylidene Fluoride–Polyacrylic Acid–Graphene Oxide (PVDF-PAA-GO) Composite Membrane with Salt and Protein Solutions. Membranes, 13(1), 40. https://doi.org/10.3390/membranes13010040








