Membrane Distillation Crystallizer Applied for Separation of NaCl Solutions Contaminated with Oil
Abstract
:1. Introduction
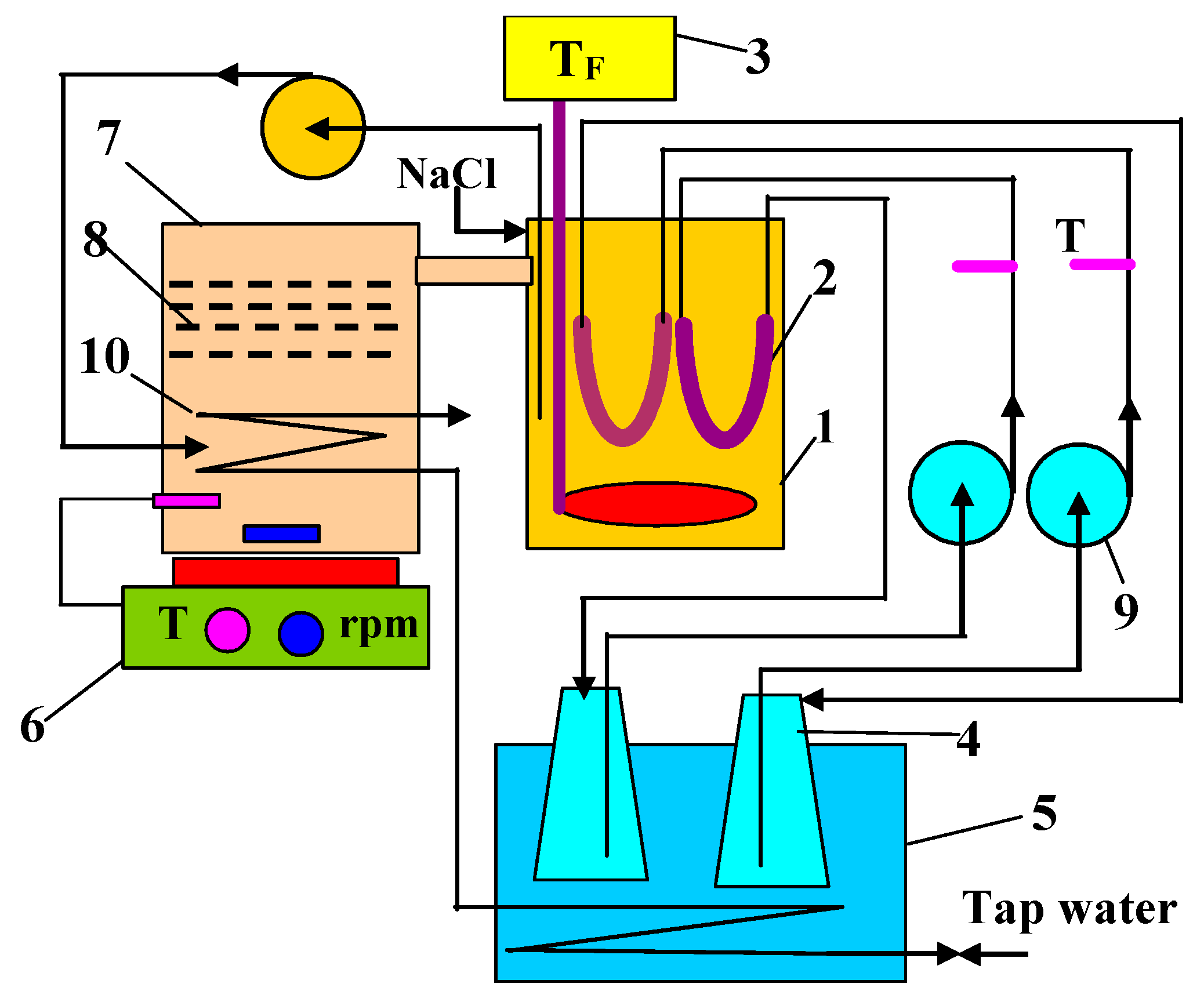
2. Materials and Methods
3. Results and Discussion
3.1. MD of NaCl Solutions Contaminated by Oil
3.2. Two-Chamber MDC Installation
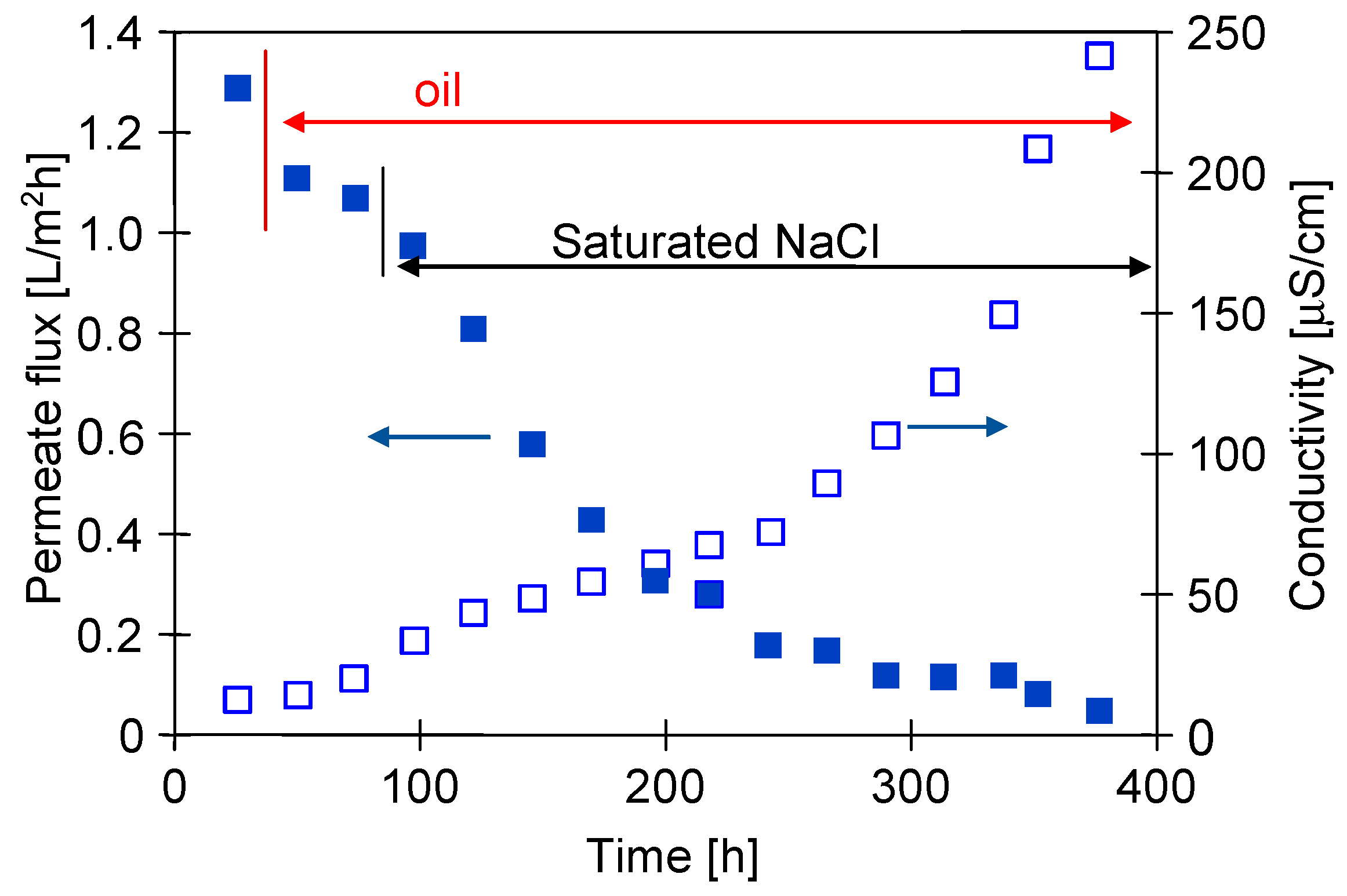
3.3. Long-Term MDC Studies
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lou, J.; Johnston, J.; Cath, T.Y.; Martinand, D.; Tilton, N. Computational fluid dynamics simulations of unsteady mixing in spacer-filled direct contact membrane distillation channels. J. Membr. Sci. 2021, 622, 118931. [Google Scholar] [CrossRef]
- Edwie, F.; Chung, T.-S. Development of hollow fiber membranes for water and salt recovery from highly concentrated brine via direct contact membrane distillation and crystallization. J. Membr. Sci. 2012, 421, 111–123. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C.; El Bakouri, H.; Riaza, A. Desalination and concentration of saline aqueous solutions up to supersaturation by air gap membrane distillation and crystallization fouling. Desalination 2016, 393, 39–51. [Google Scholar] [CrossRef]
- Gryta, M. The Application of Submerged Modules for Membrane Distillation. Membranes 2020, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Bush, J.A.; Vanneste, J.; Cath, T.Y. Membrane distillation for concentration of hypersaline brines from the Great Salt Lake: Effects of scaling and fouling on performance, efficiency, and salt rejection. Sep. Purif. Technol. 2016, 170, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Nakoa, K.; Rahaoui, K.; Date, A.; Akbarzadeh, A. Sustainable zero liquid discharge desalination (SZLDD). Sol. Energy 2016, 135, 337–347. [Google Scholar] [CrossRef]
- Hou, D.; Yuan, Z.; Tang, M.; Wang, K.; Wang, J. Effect and mechanism of an anionic surfactant on membrane performance during direct contact membrane distillation. J. Membr. Sci. 2020, 595, 117495. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, V.J.H.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Gryta, M. Application of polypropylene membranes hydrophilized by plasma for water desalination by membrane distillation. Desalination 2021, 515, 115187. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, L.; Gu, J.-Q.; Yang, M.-Y.; Xu, X.; Chen, C.-S.; Wang, H.-T.; Agathopoulos, S. Highly stable hydrophobic SiNCO nanoparticle-modified silicon nitride membrane for zero-discharge water desalination. AIChE J. 2017, 63, 1272–1277. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Membrane distillation of saline water contaminated with oil and surfactants. Membranes 2021, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Guan, K.; Zhang, P.; Shen, Q.; Li, Z.; Istirokhatun, T.; Matsuyama, H. Asymmetric superwetting Janus structure for fouling- and scaling-resistant membrane distillation. J. Membr. Sci. 2022, 657, 120697. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.-W.; Tijing, L.D.; Shon, H.K.; Hong, S. Elucidation of physicochemical scaling mechanisms in membrane distillation (MD): Implication to the control of inorganic fouling. Desalination 2022, 527, 115573. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Jeong, S.; Vigneswaran, S.; Lee, S.; Wang, R.; Fane, A.G. Experimental comparison of submerged membrane distillation configurations for concentrated brine treatment. Desalination 2017, 420, 54–62. [Google Scholar] [CrossRef]
- Julian, H.; Ye, Y.; Li, H.; Chen, V. Scaling mitigation in submerged vacuum membrane distillation and crystallization (VMDC) with periodic air-backwash. J. Membr. Sci. 2018, 547, 19–33. [Google Scholar] [CrossRef]
- Chen, G.; Lu, Y.; Krantz, W.B.; Wang, R.; Fane, A.G. Optimization of operating conditions for a continuous membrane distillation crystallization process with zero salty water discharge. J. Membr. Sci. 2014, 450, 1–11. [Google Scholar] [CrossRef]
- Semblante, G.U.; Lee, J.Z.; Lee, L.Y.; Ong, S.L.; Ng, H.Y. Brine pre-treatment technologies for zero liquid discharge systems. Desalination 2018, 441, 96–111. [Google Scholar] [CrossRef]
- Janson, A.; Benyahia, F.; Adhama, S.; Minier-Matar, J.; Hussain, A. Field evaluation of membrane distillation technologies for desalination of highly saline brines. Desalination 2014, 351, 101–108. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, H.; Lee, S.; Lee, S.; Hong, S. Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 2017, 403, 172–178. [Google Scholar] [CrossRef]
- Nariyoshi, Y.N.; Pantoja, C.E.; Seckler, M.M. Evaluation of sodium chloride crystallization in membrane distillation crystallization applied to water desalination. Braz. J. Chem. Eng. 2016, 33, 675–690. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Jeong, S.; Lee, S.; Vigneswaran, S. Fractional-submerged membrane distillation crystallizer (F-SMDC) for treatment of high salinity solution. Desalination 2018, 440, 59–67. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, D.; Xiao, W.; Ruan, X.; Fang, J.; He, G. Membrane assisted cooling crystallization: Process model, nucleation, metastable zone, and crystal size distribution. AIChE J. 2016, 62, 829–841. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Lee, S.; Vigneswaran, S. Effect of inorganic and organic compounds on the performance of fractional submerged membrane distillation-crystallizer. J. Membr. Sci. 2019, 582, 9–19. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, D.; Xiao, W.; Li, G.; Zhao, R.; Li, X.; He, G.; Ruan, X. Interface-Based Crystal Particle Autoselection via Membrane Crystallization: From Scaling to Process Control. AIChE J. 2019, 65, 723–733. [Google Scholar] [CrossRef]
- Pantoja, C.E.; Nariyoshi, Y.N.; Seckler, M.M. Membrane Distillation Crystallization Applied to Brine Desalination: Additional Design Criteria. Ind. Eng. Chem. Res. 2016, 55, 1004–1012. [Google Scholar] [CrossRef]
- Schwantesa, R.; Bauera, L.; Chavana, K.; Dückerb, D.; Felsmannc, C.; Pfafferottd, J. Air gap membrane distillation for hypersaline brine concentration: Operational analysis of a full-scale module—New strategies for wetting mitigation. Desalination 2018, 444, 13–25. [Google Scholar] [CrossRef]
- Zhang, P.; Knötig, P.; Gray, S.; Duke, M. Scale reduction and cleaning techniques during direct contact membrane distillation of seawater reverse osmosis brine. Desalination 2015, 374, 20–30. [Google Scholar] [CrossRef]
- Gryta, M. Bilge water separation by membrane distillation. Sep. Purif. Technol. 2020, 237, 116332. [Google Scholar] [CrossRef]
- Gryta, M. Concentration of NaCl solution by membrane distillation integrated with crystallization. Sep. Sci. Technol. 2002, 37, 3535–3558. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Shao, Y.; Zhang, D.; Ruan, X.; Xiao, W.; Li, X.; Wu, X.; Jiang, X. Enhanced performance of superhydrophobic polypropylene membrane with modified antifouling surface for high salinity water treatment. Sep. Purif. Technol. 2019, 214, 11–20. [Google Scholar] [CrossRef]
- Boukhriss, M.; Khemili, S.; Hamida, M.B.H.; Bacha, H.B. Simulation and experimental study of an AGMD membrane distillation pilot for the desalination of seawater or brackish water with zero liquid discharged. Heat Mass Transfer. 2018, 54, 3521–3531. [Google Scholar] [CrossRef]
- Gryta, M. Resistance of Polypropylene Membrane to Oil Fouling during Membrane Distillation. Membranes 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Gryta, M. Scaling diminution by heterogeneous crystallization in a filtration element integrated with membrane distillation module. Pol. J. Chem. Technol. 2009, 11, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Edwie, F.; Chung, T.-S. Development of simultaneous membrane distillation–crystallization (SMDC) technology for treatment of saturated brine. Chem. Eng. Sci. 2013, 98, 160–172. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryta, M. Membrane Distillation Crystallizer Applied for Separation of NaCl Solutions Contaminated with Oil. Membranes 2023, 13, 35. https://doi.org/10.3390/membranes13010035
Gryta M. Membrane Distillation Crystallizer Applied for Separation of NaCl Solutions Contaminated with Oil. Membranes. 2023; 13(1):35. https://doi.org/10.3390/membranes13010035
Chicago/Turabian StyleGryta, Marek. 2023. "Membrane Distillation Crystallizer Applied for Separation of NaCl Solutions Contaminated with Oil" Membranes 13, no. 1: 35. https://doi.org/10.3390/membranes13010035
APA StyleGryta, M. (2023). Membrane Distillation Crystallizer Applied for Separation of NaCl Solutions Contaminated with Oil. Membranes, 13(1), 35. https://doi.org/10.3390/membranes13010035





