Inimitable Impacts of Ceramides on Lipid Rafts Formed in Artificial and Natural Cell Membranes
Abstract
1. Introduction
2. Characteristic Properties of Ceramides and Their Domain Formation in Phospholipid Membranes
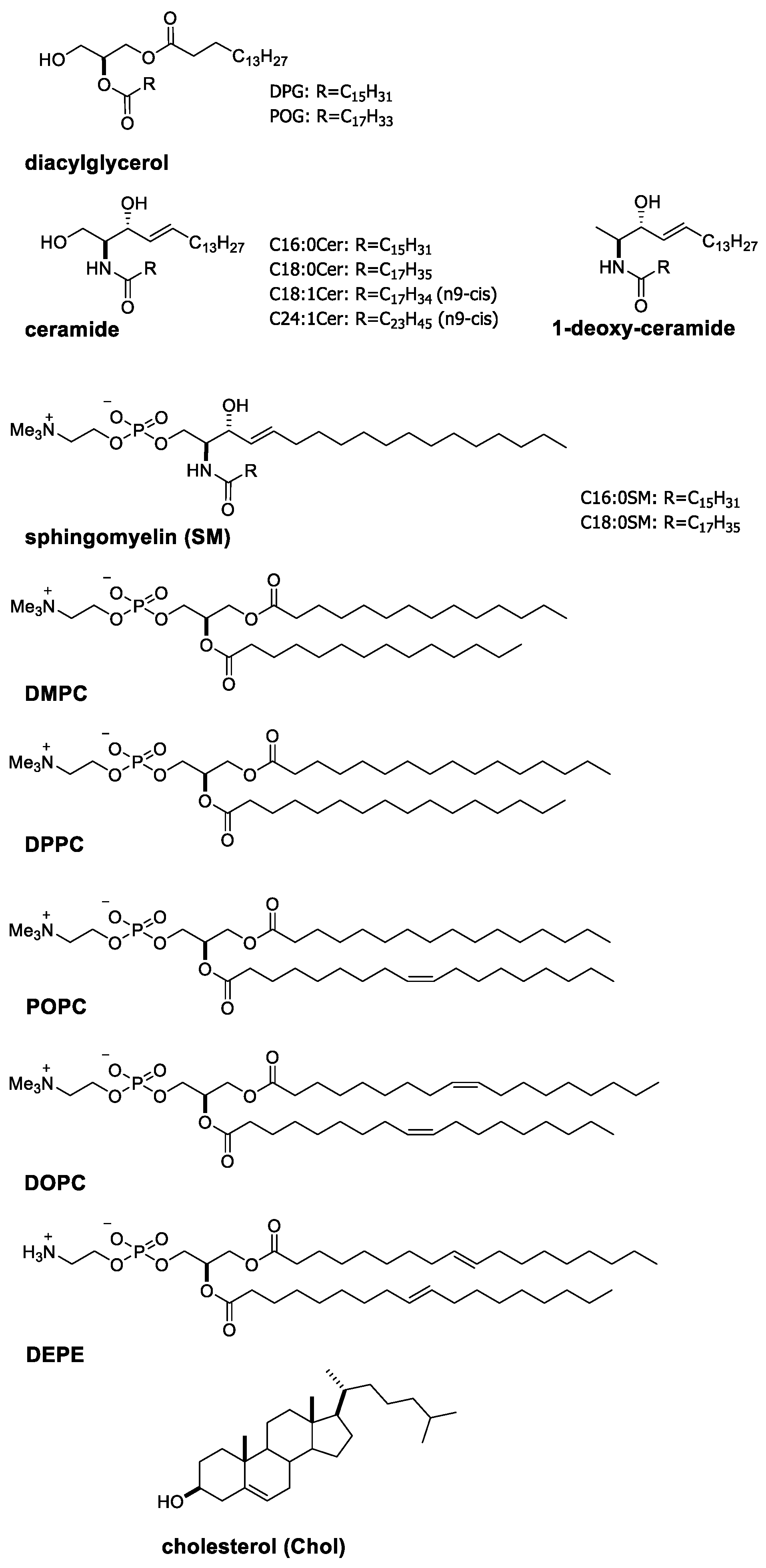
2.1. Characteristic Properties of Ceramide Molecules
2.2. Formation of Ceramide-Rich Gel Domains in Phospholipid Membranes
2.3. Ceramide-Induced Fusion of Raft-like Domains
2.4. Devised Methodologies for Visualising Ceramide Distribution
2.5. Ceramide-Induced Compositional Alteration of Raft-like Ordered Domains
3. Formation of Ceramide-Enriched Signal Platforms and Their Biological Functions
3.1. Ceramide-Induced Signal Platform Formation and Transmembrane Signalling
3.2. A Possible Structure of Signal Platforms
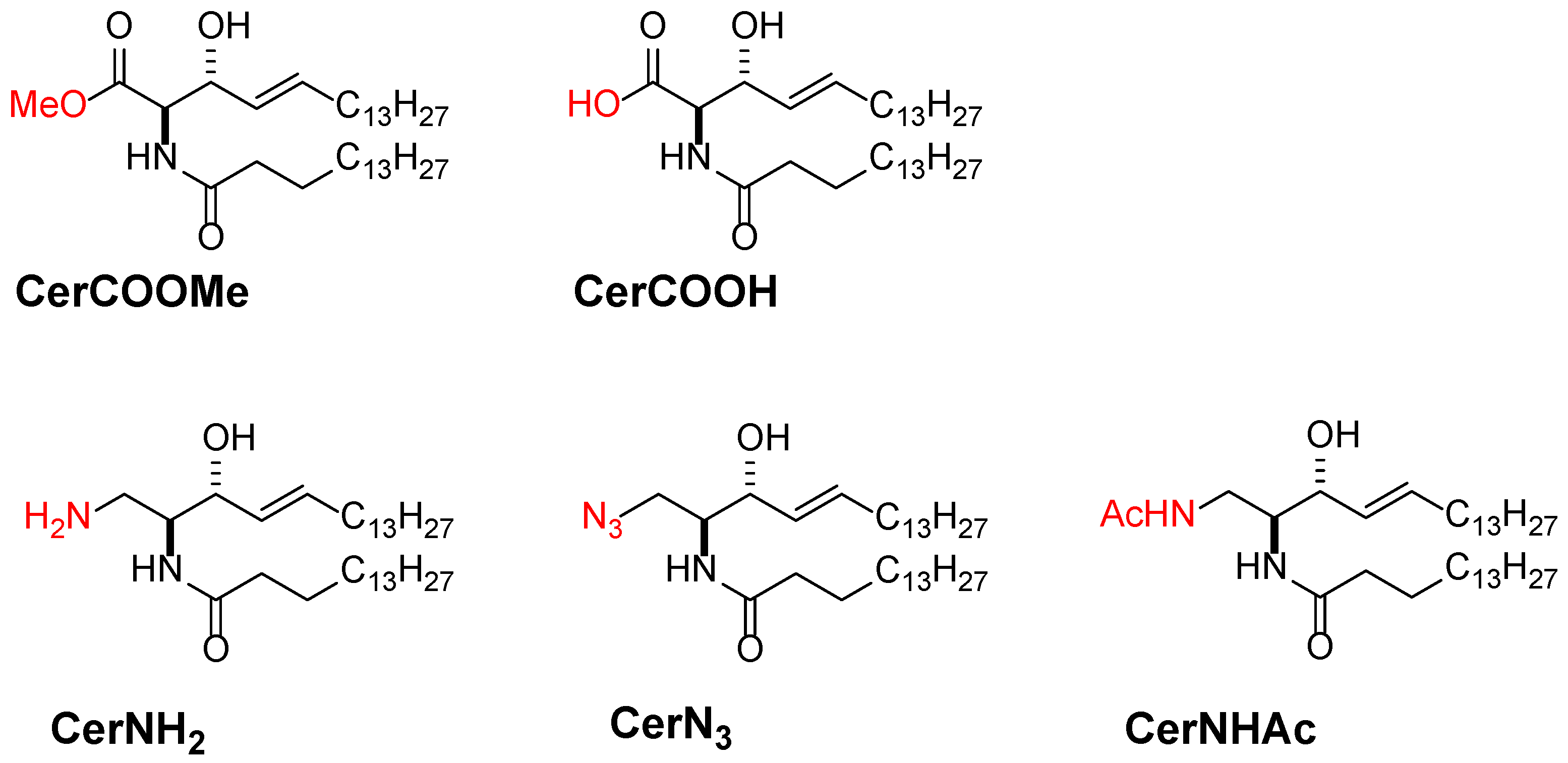
3.3. Promising Ceramide-Analogues for Identifying Intrinsic Ceramide Functions
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shabbir, M.A.; Mehak, F.; Khan, Z.M.; Ahmad, W.; Khan, M.R.; Zia, S.; Rahaman, A.; Aadil, R.M. Interplay between ceramides and phytonutrients: New insights in metabolic syndrome. Trends Food Sci. Technol. 2021, 111, 483–494. [Google Scholar] [CrossRef]
- Skácel, J.; Slusher, B.S.; Tsukamoto, T. Small molecule inhibitors targeting biosynthesis of ceramide, the central hub of the sphingolipid network. J. Med. Chem. 2021, 64, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.C.; Aguilera-Albesa, S.; Pujol, A. Ceramide signalling in inherited and multifactorial brain metabolic diseases. Neurobiol. Dis. 2020, 143, 105014. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Okazaki, T. Role of ceramide/sphingomyelin (SM) balance regulated through “SM cycle” in cancer. Cell. Signal. 2021, 87, 110119. [Google Scholar] [CrossRef] [PubMed]
- Stith, J.L.; Velazquez, F.N.; Obeid, L.M. Advances in determining signaling mechanisms of ceramide and role in disease. J. Lipid Res. 2019, 60, 913–918. [Google Scholar] [CrossRef]
- Walchuk, C.; Wang, Y.; Suh, M. The impact of EPA and DHA on ceramide lipotoxicity in the metabolic syndrome. Br. J. Nutr. 2021, 125, 863–875. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Rouser, G. The fatty acid composition of brain sphingolipids: Sphingomyelin, ceramide, cerebroside, and cerebroside sulfate. J. Lipid Res. 1964, 5, 339–3420. [Google Scholar] [CrossRef]
- Akino, T. Sphingosine base and fatty acid compositions of pig brain sphingolipids. Tohoku J. Exp. Med. 1969, 98, 87–97. [Google Scholar] [CrossRef][Green Version]
- Summers, S.A.; Garza, L.A.; Zhou, H.; Birnbaum, M.J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 1998, 18, 5457–5464. [Google Scholar] [CrossRef]
- Straczkowski, M.; Kowalska, I.; Baranowski, M.; Nikolajuk, A.; Otziomek, E.; Zabielski, P.; Adamska, A.; Blachnio, A.; Gorski, J.; Gorska, M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 2007, 50, 2366–2373. [Google Scholar] [CrossRef]
- Masamune, A.; Igarashi, Y.; Hakomori, S.I. Regulatory role of ceramide in interleukin (IL)-1Β-induced E-selectin expression in human umbilical vein endothelial cells: Ceramide enhances IL-1Β action, but is not sufficient for E-selectin expression. J. Biol. Chem. 1996, 271, 9368–9375. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Dixit, V.M. Ceramide in apoptosis-Does it really matter? Trends Biochem. Sci. 1998, 23, 374–377. [Google Scholar] [CrossRef]
- Jin, Z.H.; Li, W.; Pan, H.Z. Ceramide and apoptosis. Prog. Biochem. Biophys. 1999, 26, 540–541. [Google Scholar]
- Obeid, L.M.; Linardic, C.M.; Karolak, L.A.; Hannun, Y.A. Programmed cell death induced by ceramide. Science 1993, 259, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Bielawska, A.; Linardic, C.M.; Hannun, Y.A. Modulation of cell growth and differentiation by ceramide. FEBS Lett. 1992, 307, 211–214. [Google Scholar] [CrossRef]
- Jayadev, S.; Liu, B.; Bielawska, A.E.; Lee, J.Y.; Nazaire, F.; Pushkareva, M.Y.; Obeid, L.M.; Hannun, Y.A. Role for ceramide in cell cycle arrest. J. Biol. Chem. 1995, 270, 2047–2052. [Google Scholar] [CrossRef]
- Venable, M.E.; Lee, J.Y.; Smyth, M.J.; Bielawska, A.; Obeid, L.M. Role of ceramide in cellular senescence. J. Biol. Chem. 1995, 270, 30701–30708. [Google Scholar] [CrossRef] [PubMed]
- Slotte, P.J. Molecular properties of various structurally defined sphingomyelins-correlation of structure with function. Prog. Lipid Res. 2013, 52, 206–219. [Google Scholar] [CrossRef]
- Slotte, J.P. The Importance of hydrogen bonding in sphingomyelin’s membrane interactions with co-Lipids. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 304–310. [Google Scholar] [CrossRef]
- Cebecauer, M.; Amaro, M.; Jurkiewicz, P.; Sarmento, M.J.; Šachl, R.; Cwiklik, L.; Hof, M. Membrane lipid nanodomains. Chem. Rev. 2018, 118, 11259–11297. [Google Scholar] [CrossRef]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.L. Plasma membrane organization and function: Moving past lipid rafts. Mol. Biol. Cell 2013, 24, 2765–2768. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Codini, M.; Garcia-Gil, M.; Albi, E. Cholesterol and sphingolipid enriched lipid rafts as therapeutic targets in cancer. Int. J. Mol. Sci. 2021, 22, 726. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. Sphingolipid symmetry governs membrane lipid raft structure. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Atienza, J.M.; Duclos, R.I.; Rawlings, A.V.; Dong, Z.; Shipley, G.G. Structural and thermotropic properties of synthetic C16:0 (palmitoyl) ceramide: Effect of hydration. J. Lipid Res. 1995, 36, 1936–1944. [Google Scholar] [CrossRef]
- Souza, S.L.; Capitán, M.J.; Álvarez, J.; Funari, S.S.; Lameiro, M.H.; Melo, E. Phase behavior of aqueous dispersions of mixtures of N-palmitoyl ceramide and cholesterol: A lipid system with ceramide-cholesterol crystalline lamellar phases. J. Phys. Chem. B 2009, 113, 1367–1375. [Google Scholar] [CrossRef]
- López-García, F.; Villalaín, J.; Gómez-Fernández, J.C.; Quinn, P.J. The phase behavior of mixed aqueous dispersions of dipalmitoyl derivatives of phosphatidylcholine and diacylglycerol. Biophys. J. 1994, 66, 1991–2004. [Google Scholar] [CrossRef]
- Ekman, P.; Maula, T.; Yamaguchi, S.; Yamamoto, T.; Nyholm, T.K.M.; Katsumura, S.; Slotte, J.P. Formation of an ordered phase by ceramides and diacylglycerols in a fluid phosphatidylcholine bilayer-correlation with structure and hydrogen bonding capacity. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 2111–2117. [Google Scholar] [CrossRef]
- Jiménez-Monreal, A.M.; Villalaín, J.; Aranda, F.J.; Gómez-Fernández, J.C. The phase behavior of aqueous dispersions of unsaturated mixtures of diacylglycerols and phospholipids. Biochim. Biophys. Acta-Biomembr. 1998, 1373, 209–219. [Google Scholar] [CrossRef]
- Gulbins, E.; Kolesnick, R. Raft Ceramide in molecular medicine. Oncogene 2003, 22, 7070–7077. [Google Scholar] [CrossRef] [PubMed]
- Pascher, I. Molecular arrangements in sphingolipids conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim. Biophys. Acta-Biomembr. 1976, 455, 433–451. [Google Scholar] [CrossRef]
- Siskind, L.J.; Davoody, A.; Lewin, N.; Marshall, S.; Colombini, M. Enlargement and contracture of C2-ceramide channels. Biophys. J. 2003, 85, 1560–1575. [Google Scholar] [CrossRef]
- Kinnunen, P.K.J.; Holopainen, J.M. Sphingomyelinase activity of LDLA link between atherosclerosis, ceramide, and apoptosis? Trends Cardiovasc. Med. 2002, 12, 37–42. [Google Scholar] [CrossRef]
- Jiménez-Rojo, N.; Sot, J.; Busto, J.V.; Shaw, W.A.; Duan, J.; Merrill, A.H.; Alonso, A.; Goñi, F.M. Biophysical properties of novel 1-deoxy-(dihydro)ceramides occurring in mammalian cells. Biophys. J. 2014, 107, 2850–2859. [Google Scholar] [CrossRef]
- Garidel, P.; Fölting, B.; Schaller, I.; Kerth, A. The microstructure of the stratum corneum lipid barrier: Mid-infrared spectroscopic studies of hydrated ceramide:palmitic acid:cholesterol model systems. Biophys. Chem. 2010, 150, 144–156. [Google Scholar] [CrossRef]
- Moore, D.J.; Rerek, M.E.; Mendelsohn, R. FTIR spectroscopy studies of the conformational order and phase behavior of ceramides. J. Phys. Chem. B 1997, 101, 8933–8940. [Google Scholar] [CrossRef]
- Notman, R.; Den Otter, W.K.; Noro, M.G.; Briels, W.J.; Anwar, J. The permeability enhancing mechanism of DMSO in ceramide bilayers simulated by molecular dynamics. Biophys. J. 2007, 93, 2056–2068. [Google Scholar] [CrossRef]
- Wang, E.; Klauda, J.B. Molecular dynamics simulations of ceramide and ceramide-phosphatidylcholine Bilayers. J. Phys. Chem. B 2017, 121, 10091–10104. [Google Scholar] [CrossRef]
- Pandit, S.A.; Scott, H.L. Molecular-dynamics simulation of a ceramide bilayer. J. Chem. Phys. 2006, 124, 014708. [Google Scholar] [CrossRef]
- Matsufuji, T.; Kinoshita, M.; Matsumori, N. Preparation and membrane distribution of fluorescent derivatives of ceramide. Langmuir 2019, 35, 2392–2398. [Google Scholar] [CrossRef] [PubMed]
- Möuts, A.; Vattulainen, E.; Matsufuji, T.; Kinoshita, M.; Matsumori, N.; Slotte, J.P. On the importance of the C(1)-OH and C(3)-OH functional groups of the long-chain base of ceramide for interlipid interaction and lateral segregation into ceramide-rich domains. Langmuir 2018, 34, 15864–15870. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.N.; Silva, L.C.; Futerman, A.H.; Prieto, M. Effect of ceramide structure on membrane biophysical properties: The role of acyl chain length and unsaturation. Biochim. Biophys. Acta-Biomembr. 2011, 1808, 2753–2760. [Google Scholar] [CrossRef]
- Holopainen, J.M.; Lehtonen, J.Y.A.; Kinnunen, P.K.J. Lipid microdomains in dimyristoylphosphatidylcholine–ceramide liposomes. Chem. Phys. Lipids 1997, 88, 1–13. [Google Scholar] [CrossRef]
- Veiga, M.P.; Arrondo, J.L.R.; Goñi, F.M.; Alonso, A. Ceramides in phospholipid membranes: Effects on bilayer stability and transition to nonlamellar phases. Biophys. J. 1999, 76, 342–350. [Google Scholar] [CrossRef]
- Sot, J.; Aranda, F.J.; Collado, M.I.; Goñi, F.M.; Alonso, A. Different effects of long- and short-chain ceramides on the gel-fluid and lamellar-hexagonal transitions of phospholipids: A calorimetric, NMR, and x-ray diffraction study. Biophys. J. 2005, 88, 3368–3380. [Google Scholar] [CrossRef]
- López-Montero, I.; Monroy, F.; Vélez, M.; Devaux, P.F. Ceramide: From lateral segregation to mechanical stress. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 1348–1356. [Google Scholar] [CrossRef]
- Catapano, E.R.; Arriaga, L.R.; Espinosa, G.; Monroy, F.; Langevin, D.; López-Montero, I. Solid character of membrane ceramides: A surface rheology study of their mixtures with sphingomyelin. Biophys. J. 2011, 10, 2721–2730. [Google Scholar] [CrossRef]
- López-Montero, I.; Catapano, E.R.; Espinosa, G.; Arriaga, L.R.; Langevin, D.; Monroy, F. Shear and compression rheology of Langmuir monolayers of natural ceramides: Solid character and plasticity. Langmuir 2013, 29, 6634–6644. [Google Scholar] [CrossRef]
- Catapano, E.R.; Natale, P.; Monroy, F.; López-Montero, I. The enzymatic sphingomyelin to ceramide conversion increases the shear membrane viscosity at the air-water interface. Adv. Coll. Interf. Sci. 2017, 247, 555–560. [Google Scholar] [CrossRef]
- Ipsen, J.H.; Karlström, G.; Mourtisen, O.G.; Wennerström, H.; Zuckermann, M.J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta-Biomembr. 1987, 905, 162–172. [Google Scholar] [CrossRef]
- Quinn, P.J.; Wolf, C. The liquid-ordered phase in membranes. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Chachaty, C.; Rainteau, D.; Tessier, C.; Quinn, P.J.; Wolf, C. Building up of the liquid-ordered phase formed by sphingomyelin and cholesterol. Biophys. J. 2005, 88, 4032–4044. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Tanaka, K.; Matsumori, N. The influence of ceramide and its dihydro analog on the physico-chemical properties of sphingomyelin bilayers. Chem. Phys. Lipids 2020, 226, 104835. [Google Scholar] [CrossRef]
- Maulik, P.R.; Shipley, G.G. Interaction of N-steraroyl sphingomyelin with cholesterol and dipalmitoylphosphatidylcholine in bilayer membranes. Biophys. J. 1996, 760, 2256–2265. [Google Scholar] [CrossRef]
- Siavashi, R.; Phaterpekar, T.; Leung, S.S.W.; Alonso, A.; Goñi, F.M.; Thewalt, J.L. Lamellar phases composed of phospholipid, cholesterol, and ceramide, as studied by 2H NMR. Biophys. J. 2019, 117, 296–306. [Google Scholar] [CrossRef]
- Murthy, A.V.R.; Guyomarc’h, F.; Lopez, C. Palmitoyl ceramide promotes milk sphingomyelin gel phase domains formation and affects the mechanical properties of the fluid phase in milk-SM/DOPC supported membranes. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 635–644. [Google Scholar] [CrossRef]
- Ira; Johnston, L.J. Sphingomyelinase generation of ceramide promotes clustering of nanoscale domains in supported bilayer membranes. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 185–197. [Google Scholar]
- Silva, L.C.; De Almeida, R.F.M.; Castro, B.M.; Fedorov, A.; Prieto, M. Ceramide-domain formation and collapse in lipid rafts: Membrane reorganization by an apoptotic lipid. Biophys. J. 2007, 92, 502–516. [Google Scholar] [CrossRef]
- Sot, J.; Ibarguren, M.; Busto, J.V.; Montes, L.R.; Goñi, F.M.; Alonso, A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008, 582, 3230–3236. [Google Scholar] [CrossRef]
- Ira; Linda, J.J. Ceramide promotes restructuring of model raft membranes. Langmuir 2006, 22, 11284–11289. [Google Scholar] [CrossRef] [PubMed]
- Chiantia, S.; Kahya, N.; Ries, J.; Schwille, P. Effects of ceramide on liquid-ordered domains investigated by simultaneous AFM and FCS. Biophys. J. 2006, 90, 4500–4508. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.J. Nanoscale imaging of domains in supported lipid membranes. Langmuir 2007, 23, 5886–5895. [Google Scholar] [CrossRef] [PubMed]
- Canals, D.; Salamone, S.; Hannun, Y.A. Visualizing bioactive ceramides. Chem. Phys. Lipids 2018, 216, 142–151. [Google Scholar] [CrossRef]
- Popov, J.; Vobornik, D.; Coban, O.; Keating, E.; Miller, D.; Francis, J.; Petersen, N.O.; Johnston, L.J. Chemical mapping of ceramide distribution in sphingomyelin-rich domains in monolayers. Langmuir 2008, 24, 13502–13508. [Google Scholar] [CrossRef]
- Zheng, L.; McQuaw, C.M.; Ewing, A.G.; Winograd, N. Sphingomyelin/phosphatidylcholine and cholesterol interactions studied by imaging mass spectrometry. J. Am. Chem. Soc. 2007, 129, 15730–15731. [Google Scholar] [CrossRef]
- McQuaw, C.M.; Sostarecz, A.G.; Zheng, L.; Ewing, A.G.; Winograd, N. Lateral heterogeneity of dipalmitoylphosphatidylethanolamine-cholesterol Langmuir-Blodgett films investigated with imaging time-of-flight secondary ion mass spectrometry and atomic force microscopy. Langmuir 2005, 21, 807–813. [Google Scholar] [CrossRef]
- Kinoshita, M.; Suzuki, K.G.N.; Murata, M.; Matsumori, N. Evidence of lipid rafts based on the partition and dynamic behavior of sphingomyelins. Chem. Phys. Lipids 2018, 215, 84–95. [Google Scholar] [CrossRef]
- Passarelli, M.K.; Winograd, N. Lipid imaging with time-of-flight secondary ion mass spectrometry (ToF-SIMS). Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1811, 976–990. [Google Scholar] [CrossRef]
- Fletcher, J.S. Latest applications of 3D ToF-SIMS bio-imaging. Biointerphases 2015, 10, 018902. [Google Scholar] [CrossRef]
- Kinoshita, M.; Suzuki, K.G.N.; Matsumori, N.; Takada, M.; Ano, H.; Morigaki, K.; Abe, M.; Makino, A.; Kobayashi, T.; Hirosawa, K.M.; et al. Raft-based sphingomyelin interactions revealed by new fluorescent sphingomyelin analogs. J. Cell Biol. 2017, 216, 1183–1204. [Google Scholar] [CrossRef] [PubMed]
- Megha; London, E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): Implications for lipid raft structure and function. J. Biol. Chem. 2004, 279, 9997–10004. [Google Scholar] [CrossRef] [PubMed]
- Megha; Sawatzki, P.; Kolter, T.; Bittman, R.; London, E. Effect of Ceramide N-acyl chain and polar headgroup structure on the properties of ordered lipid domains (lipid rafts). Biochim. Biophys. Acta-Biomembr. 2007, 1768, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Maula, T.; Urzelai, B.; Peter Slotte, J. The effects of N-acyl chain methylations on ceramide molecular properties in bilayer membranes. Eur. Biophys. J. 2011, 40, 857–863. [Google Scholar] [CrossRef]
- Alanko, S.M.K.; Halling, K.K.; Maunula, S.; Slotte, J.P.; Ramstedt, B. Displacement of sterols from sterol/sphingomyelin domains in fluid bilayer membranes by competing molecules. Biochim. Biophys. Acta-Biomembr. 2005, 1715, 111–121. [Google Scholar] [CrossRef][Green Version]
- Taniguchi, Y.; Ohba, T.; Miyata, H.; Ohki, K. Rapid phase change of lipid microdomains in giant vesicles induced by conversion of sphingomyelin to ceramide. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 145–153. [Google Scholar] [CrossRef]
- González-Ramírez, E.J.; Artetxe, I.; García-Arribas, A.B.; Goni, F.M.; Alonso, A. Homogeneous and heterogeneous bilayers of ternary lipid compositions containing equimolar ceramide and cholesterol. Langmuir 2019, 35, 5305–5315. [Google Scholar] [CrossRef]
- Busto, J.V.; Sot, J.; Requejo-Isidro, J.; Goni, F.M.; Alonso, A. Cholesterol displaces palmitoylceramide from its tight packing with palmitoylsphingomyelin in the absence of a liquid-disordered phase. Biophys. J. 2010, 99, 1119–1128. [Google Scholar] [CrossRef]
- García-Arribas, A.B.; Axpe, E.; Mujika, J.I.; Mérida, D.; Busto, J.V.; Sot, J.; Alonso, A.; Lopez, X.; García, J.Á.; Ugalde, J.M.; et al. Cholesterol-ceramide interactions in phospholipid and sphingolipid bilayers as observed by positron annihilation lifetime spectroscopy and molecular dynamics simulations. Langmuir 2016, 32, 5434–5444. [Google Scholar] [CrossRef]
- Grassmé, H.; Jendrossek, V.; Riehle, A.; Von Kürthy, G.; Berger, J.; Schwarz, H.; Weller, M.; Kolesnick, R.; Gulbins, E. Host defense against pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 2003, 9, 322–330. [Google Scholar] [CrossRef]
- Cremesti, A.E.; Goni, F.M.; Kolesnick, R. Role of sphingomyelinase and ceramide in modulating rafts: Do biophysical properties determine biologic outcome? FEBS Lett. 2002, 531, 47–53. [Google Scholar] [CrossRef]
- Bollinger, C.R.; Teichgräber, V.; Gulbins, E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta-Mol. Cell Res. 2005, 1746, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, A.; Fujiwara, T.K.; Tsunoyama, T.A.; Kasai, R.S.; Liu, A.A.; Hirosawa, K.M.; Kinoshita, M.; Matsumori, N.; Komura, N.; Ando, H.; et al. Defining raft domains in the plasma membrane. Traffic 2020, 21, 106–137. [Google Scholar] [CrossRef]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid rafts: Controversies resolved, mysteries remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.F. Lipid rafts: Contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 2006, 7, 456–462. [Google Scholar] [CrossRef]
- Shaw, A.S. Lipid rafts: Now you see them, Now you don’t. Nat. Immunol. 2006, 7, 1139–1142. [Google Scholar] [CrossRef]
- Grassmé, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef]
- Grassmé, H.; Schwarz, H.; Gulbins, E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem. Biophys. Res. Commun. 2001, 284, 1016–1030. [Google Scholar] [CrossRef]
- Stancevic, B.; Kolesnick, R. Ceramide-rich platforms in transmembrane Signaling. FEBS Lett. 2010, 584, 1728–1740. [Google Scholar] [CrossRef]
- Suzuki, K.G.N.; Kasai, R.S.; Hirosawa, K.M.; Nemoto, Y.L.; Ishibashi, M.; Miwa, Y.; Fujiwara, T.K.; Kusumi, A. Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 2012, 8, 774–783. [Google Scholar] [CrossRef]
- Adam, D.; Heinrich, M.; Kabelitz, D.; Schütze, S. Ceramide: Does it matter for T cells? Trends Immunol. 2002, 23, 1–4. [Google Scholar] [CrossRef]
- Grassmé, H.; Jendrossek, V.; Bock, J.; Riehle, A.; Gulbins, E. Ceramide-rich membrane rafts mediate CD40 clustering. J. Immunol. 2002, 168, 298–307. [Google Scholar] [CrossRef]
- Janes, P.W.; Ley, S.C.; Magee, A.I.; Kabouridis, P.S. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 2000, 12, 23–34. [Google Scholar] [CrossRef]
- Gupta, N.; DeFranco, A.L. Lipid rafts and B cell signaling. Semin. Cell Dev. Biol. 2007, 18, 616–626. [Google Scholar] [CrossRef]
- Bionda, C.; Hadchity, E.; Alphonse, G.; Chapet, O.; Rousson, R.; Rodriguez-Lafrasse, C.; Ardail, D. Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radic. Biol. Med. 2007, 43, 681–694. [Google Scholar] [CrossRef]
- Yamaji, A.; Sekizawa, Y.; Emoto, K.; Sakuraba, H.; Inoue, K.; Kobayashi, H.; Umeda, M. Lysenin, a novel sphingomyelin-specific binding protein. J. Biol. Chem. 1998, 273, 5300–5306. [Google Scholar] [CrossRef]
- Kiyokawa, E.; Makino, A.; Ishii, K.; Otsuka, N.; Yamaji-Hasegawa, A.; Kobayashi, T. Recognition of sphingomyelin by lysenin and lysenin-related proteins. Biochemistry 2004, 43, 9766–9773. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Carpinteiro, A.; Trarbach, T.; Hengge, U.R.; Gulbins, E. Doxorubicin enhances TRAIL-induced cell death via ceramide-enriched membrane platforms. Apoptosis 2007, 12, 1533–1541. [Google Scholar] [CrossRef]
- Bock, J.; Szabó, I.; Gamper, N.; Adams, C.; Gulbins, E. Ceramide inhibits the potassium channel Kv1.3 by the formation of membrane platforms. Biochem. Biophys. Res. Commun. 2003, 305, 890–897. [Google Scholar] [CrossRef]
- Holmes, T.C.; Fadool, D.A.; Levitan, I.B. Tyrosine phosphorylation of the Kv1.3 potassium channel. J. Neurosci. 1996, 16, 1581–1590. [Google Scholar] [CrossRef]
- Filipp, D.; Zhang, J.; Leung, B.L.; Shaw, A.; Levin, S.D.; Veillette, A.; Julius, M. Regulation of Fyn through translocation of activated Lck into lipid rafts. J. Exp. Med. 2003, 197, 1221–1227. [Google Scholar] [CrossRef]
- Yasuda, H.; Torikai, K.; Kinoshita, M.; Sazzad, M.A.A.; Tsujimura, K.; Slotte, J.P.; Matsumori, N. Preparation of nitrogen analogues of ceramide and studies of their aggregation in sphingomyelin bilayers. Langmuir 2021, 37, 12438–12446. [Google Scholar] [CrossRef]
- Matsufuji, T.; Kinoshita, M.; Möuts, A.; Slotte, J.P.; Matsumori, N. Preparation and membrane properties of oxidized ceramide derivatives. Langmuir 2018, 34, 465–471. [Google Scholar] [CrossRef]
- Moro, K.; Nagahashi, M.; Gabriel, E.; Takabe, K.; Wakai, T. Clinical application of ceramide in cancer treatment. Breast Cancer 2018, 72, 2964–2979. [Google Scholar] [CrossRef]
- Boojar, M.M.A.; Boojar, M.M.A.; Golmohammad, S. Ceramide pathway: A novel approach to cancer chemotherapy. Egypt. J. Basic Appl. Sci. 2018, 5, 237–244. [Google Scholar]
- Brachtendorf, S.; El-Hindi, K.; Grösch, S. Ceramide synthases in cancer therapy and chemoresistance. Prog. Lipid Res. 2019, 74, 160–185. [Google Scholar] [CrossRef]
- Nagahashi, M.; Ramachandran, S.; Kim, E.Y.; Allegood, J.C.; Rashid, O.M.; Yamada, A.; Zhao, R.; Milstien, S.; Zhou, H.; Spiegel, S.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012, 72, 726–735. [Google Scholar] [CrossRef]
- Salas, A.; Ponnusamy, S.; Senkal, C.E.; Meyers-Needham, M.; Selvam, S.P.; Saddoughi, S.A.; Apohan, E.R.; Sentelle, D.; Smith, C.; Gault, C.R.; et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood 2011, 117, 5941–5952. [Google Scholar] [CrossRef]
- Young, M.M.; Kester, M.; Wang, H.G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, M.; Matsumori, N. Inimitable Impacts of Ceramides on Lipid Rafts Formed in Artificial and Natural Cell Membranes. Membranes 2022, 12, 727. https://doi.org/10.3390/membranes12080727
Kinoshita M, Matsumori N. Inimitable Impacts of Ceramides on Lipid Rafts Formed in Artificial and Natural Cell Membranes. Membranes. 2022; 12(8):727. https://doi.org/10.3390/membranes12080727
Chicago/Turabian StyleKinoshita, Masanao, and Nobuaki Matsumori. 2022. "Inimitable Impacts of Ceramides on Lipid Rafts Formed in Artificial and Natural Cell Membranes" Membranes 12, no. 8: 727. https://doi.org/10.3390/membranes12080727
APA StyleKinoshita, M., & Matsumori, N. (2022). Inimitable Impacts of Ceramides on Lipid Rafts Formed in Artificial and Natural Cell Membranes. Membranes, 12(8), 727. https://doi.org/10.3390/membranes12080727