Separation of Chromium (VI), Copper and Zinc: Chemistry of Transport of Metal Ions across Supported Liquid Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Methods
2.3.1. Procedure for Study on Extraction
2.3.2. Procedure for Study on Stripping
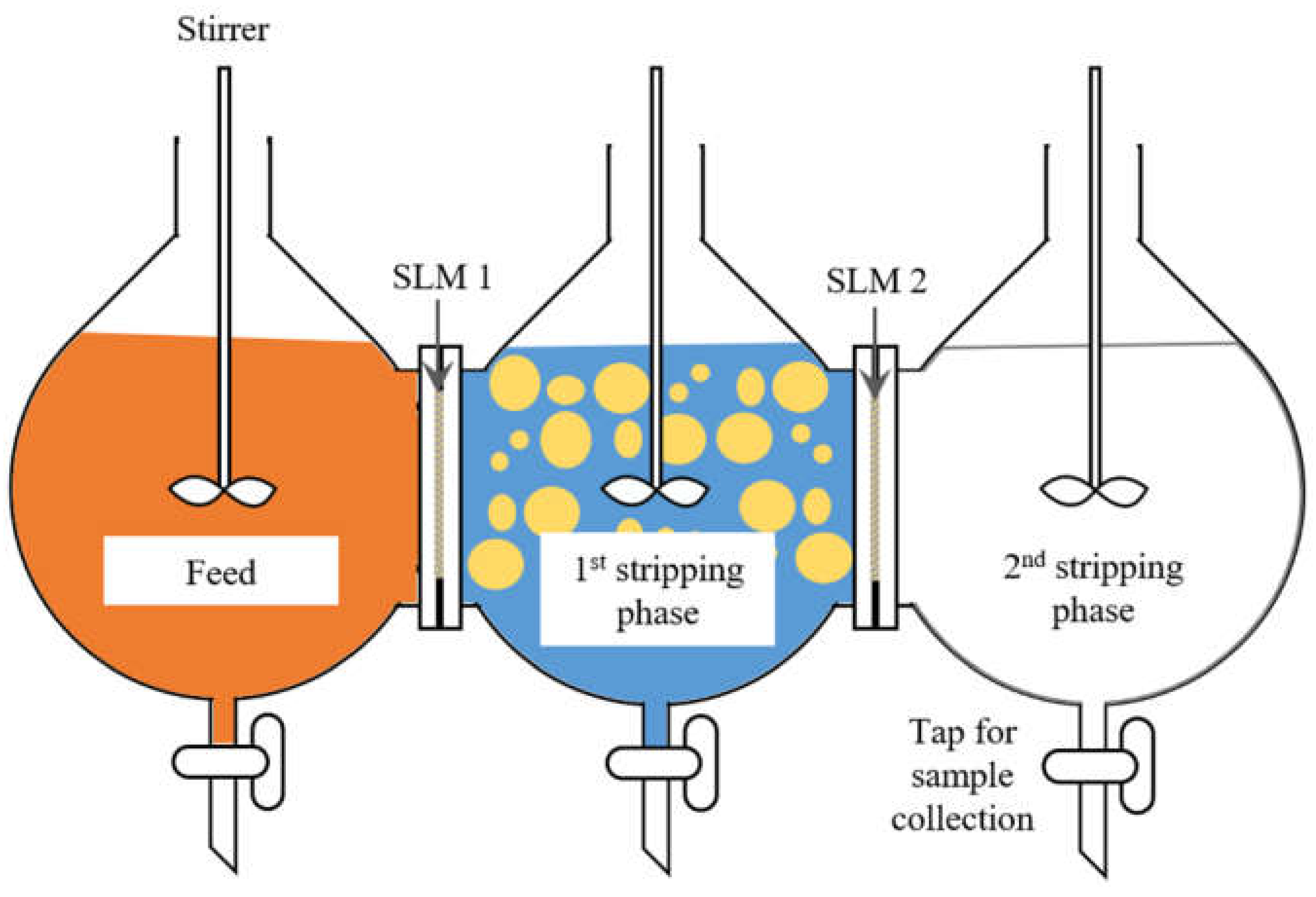
2.3.3. Metal Ions Transport and Separation Using SLM with Strip Dispersion
2.4. Theory and Associated Equations
3. Results and Discussion
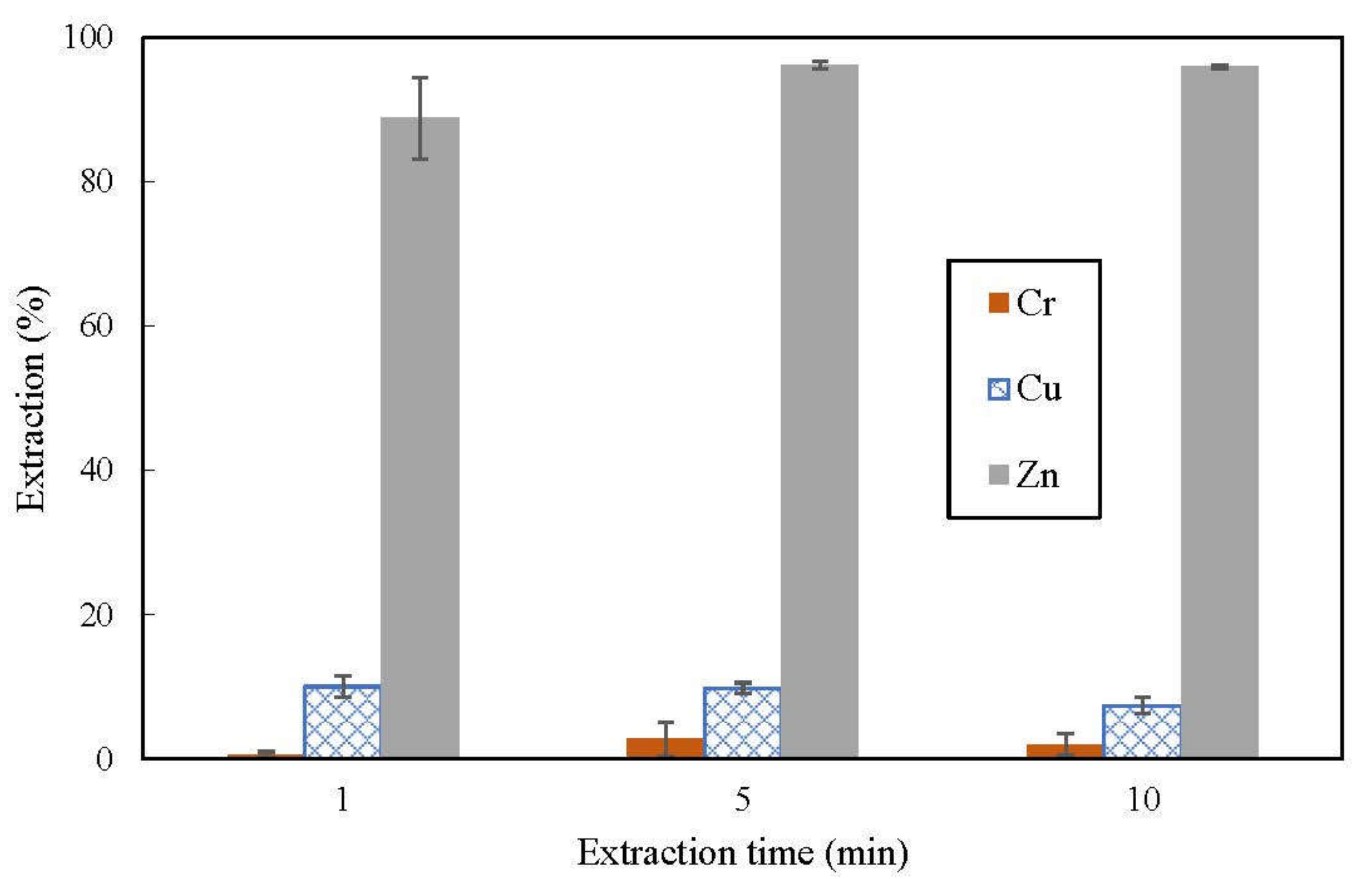
3.1. Effect of Shaking Time on Extraction
3.2. Effect of pHeq on Extraction
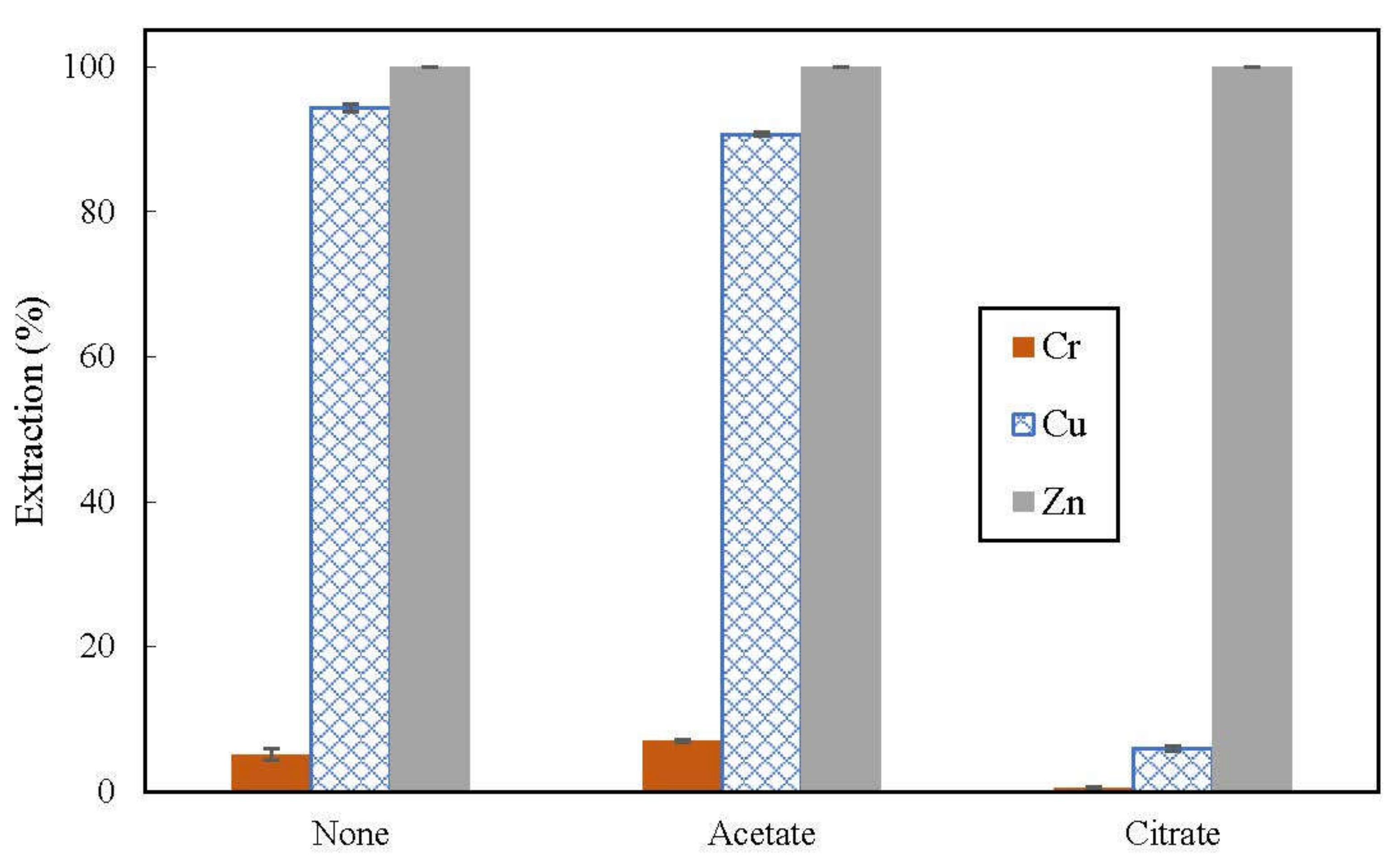
3.3. Effect of Buffer in Feed on Extraction
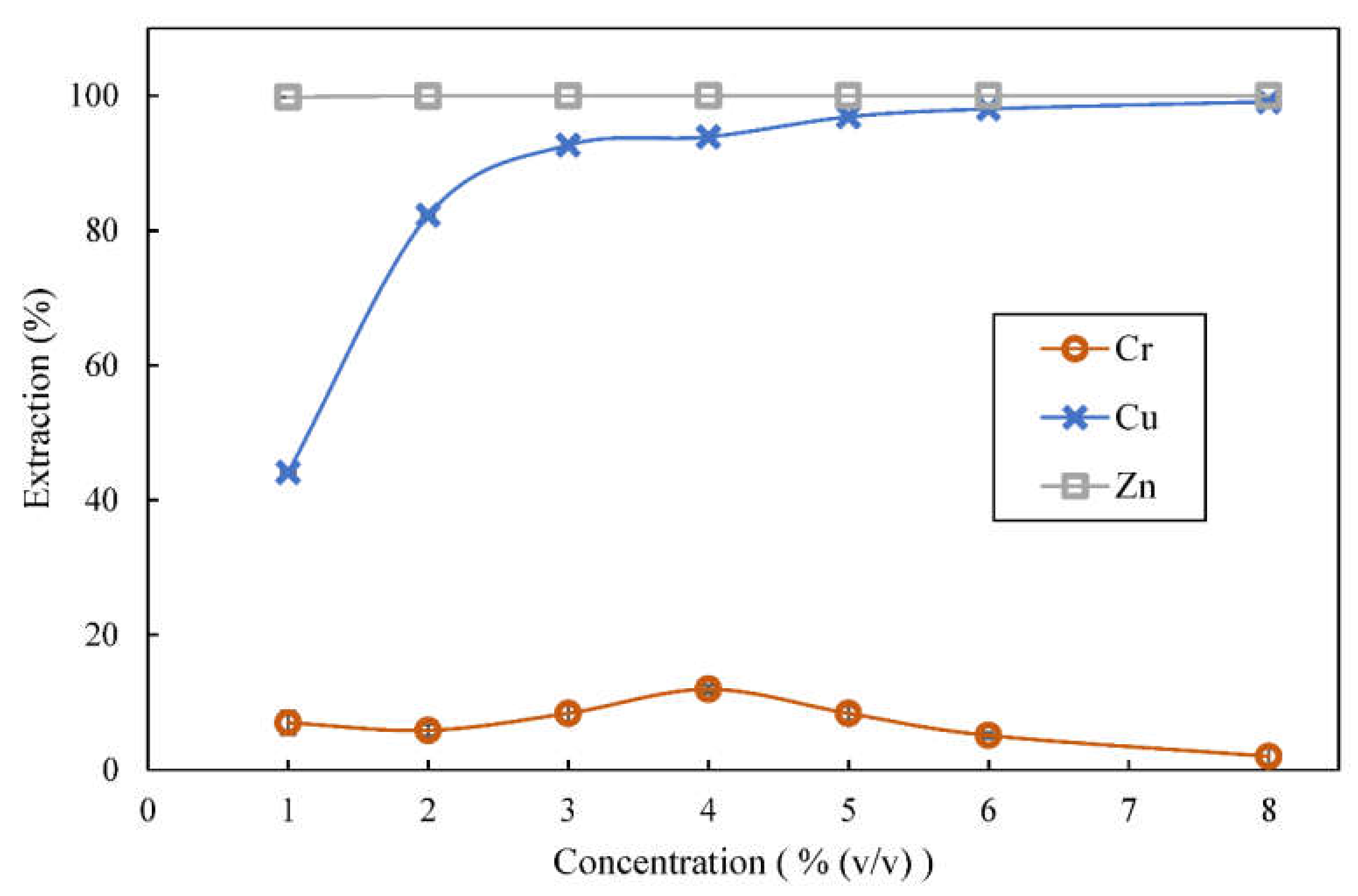
3.4. Effect of Extractant Concentration on Extraction
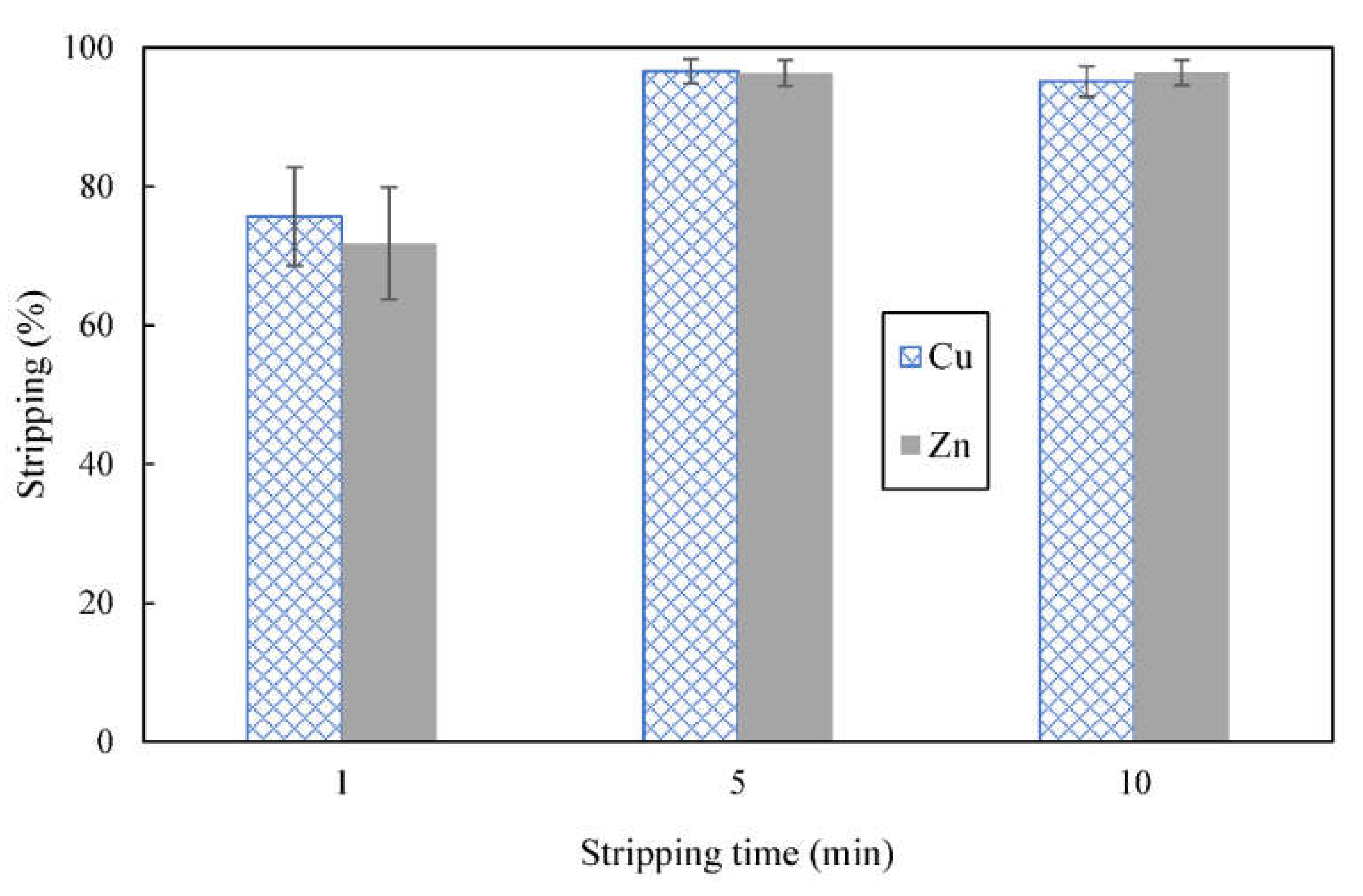
3.5. Effect of Shaking Time on Stripping
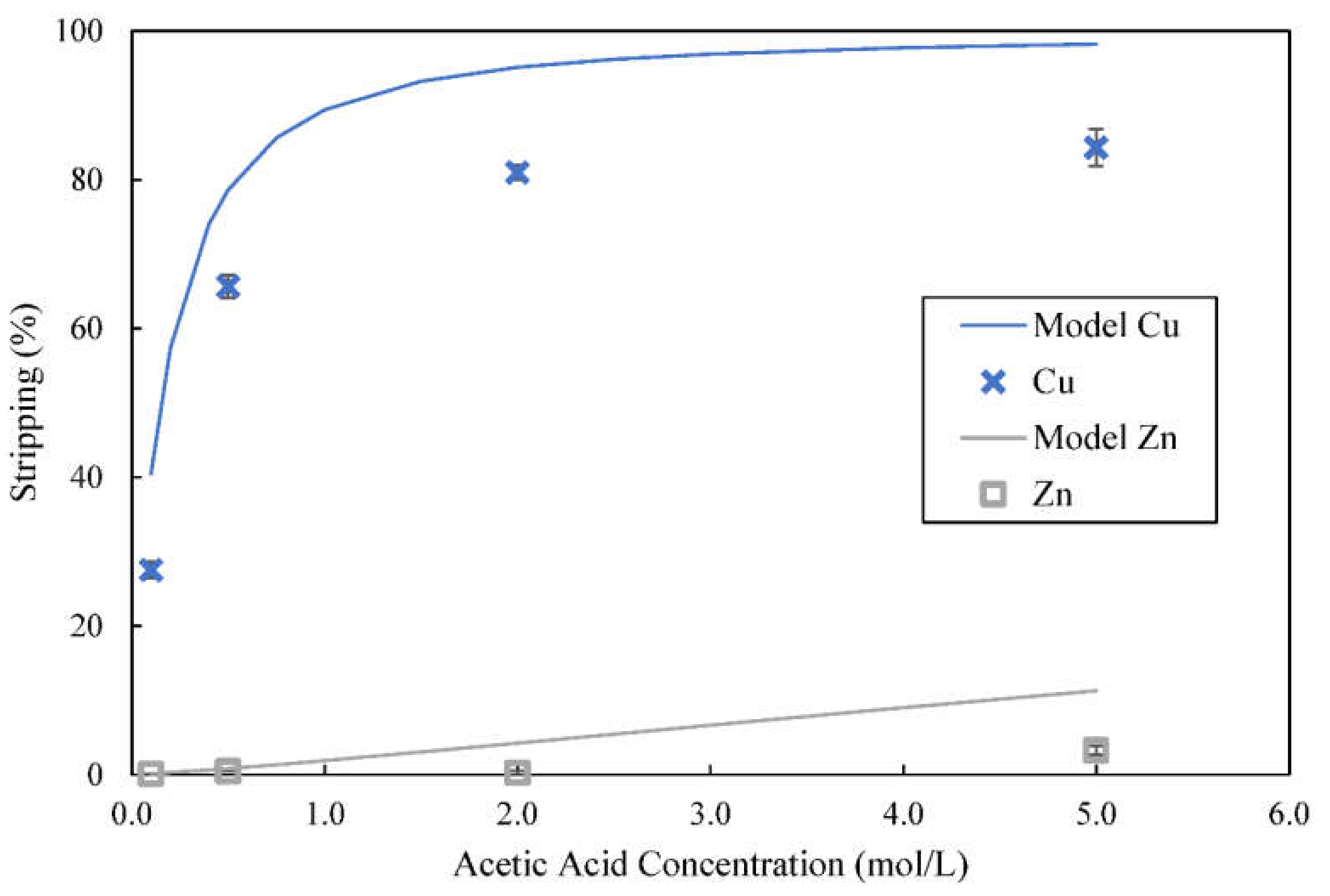
3.6. Use of Acetic Acid as a Stripping Reagent
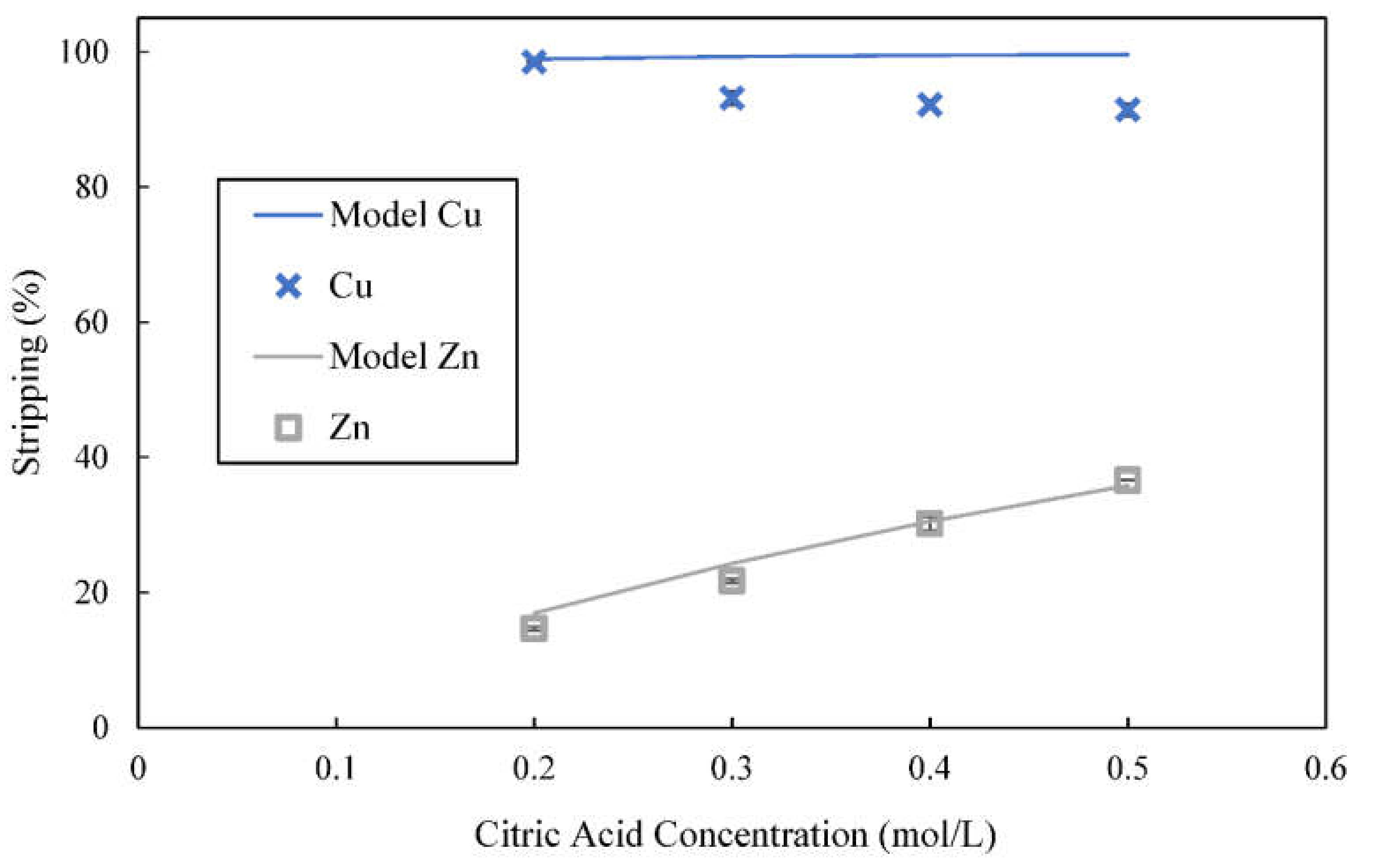
3.7. Use of Citric Acid and Citrate Buffer as Stripping Reagents
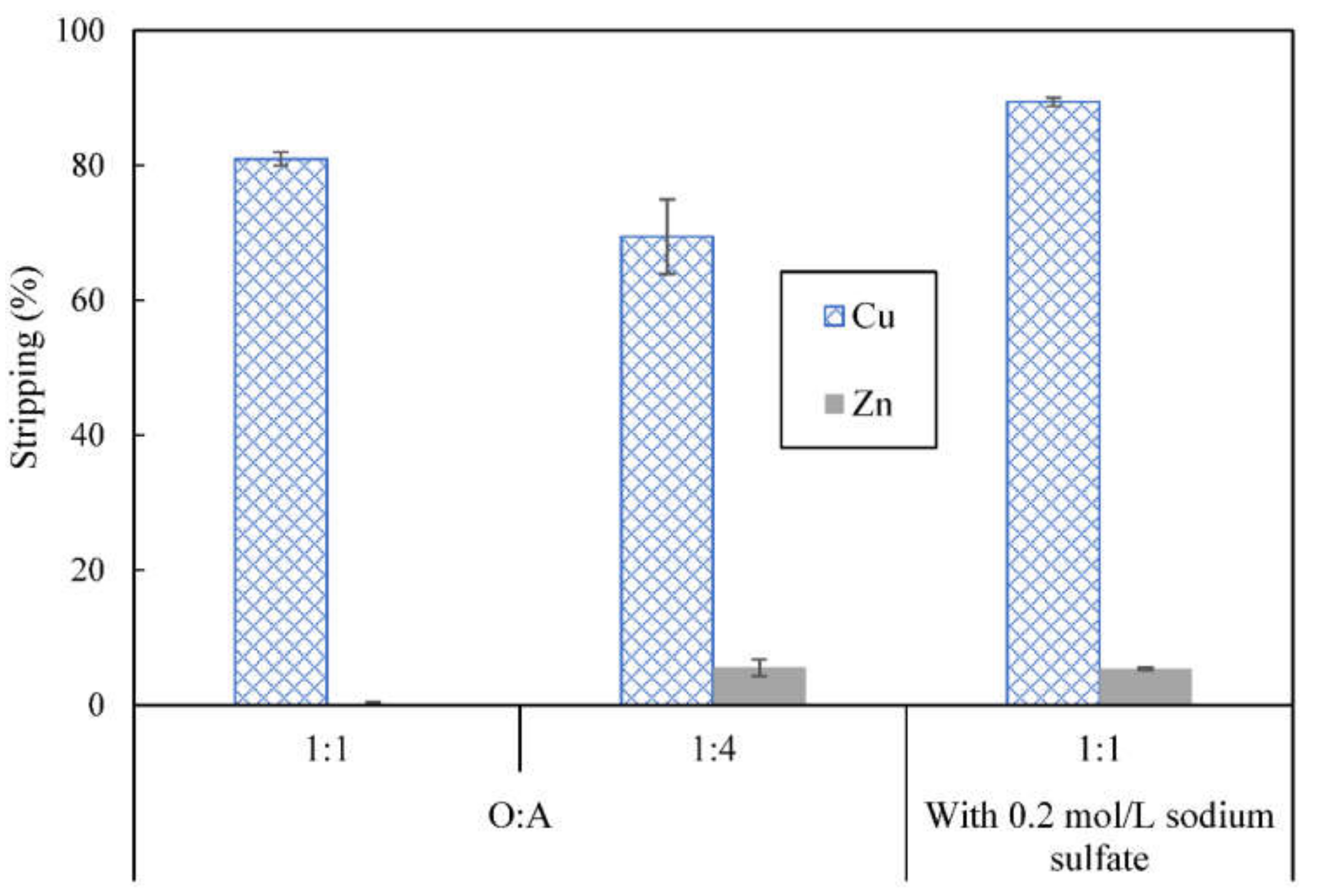
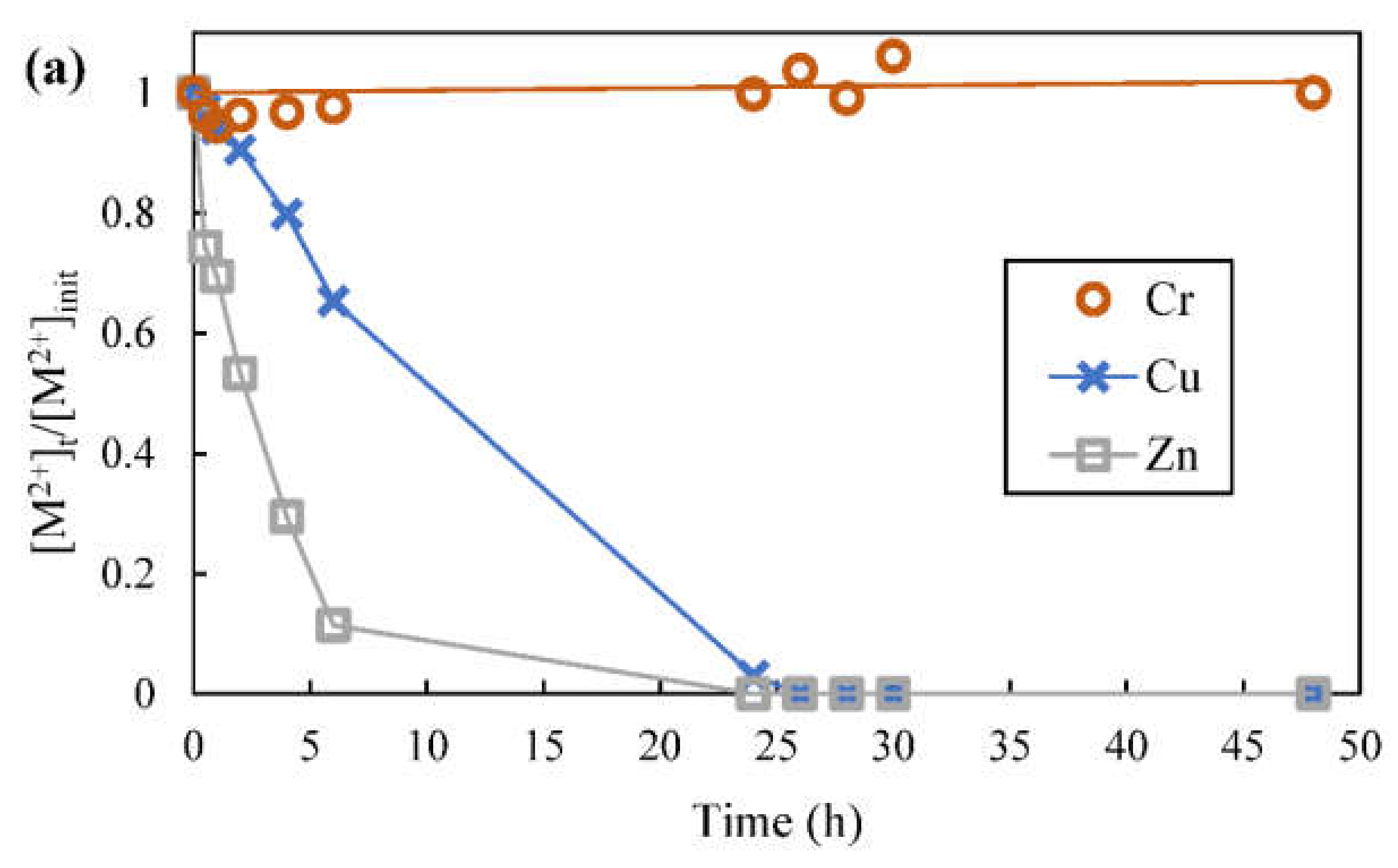
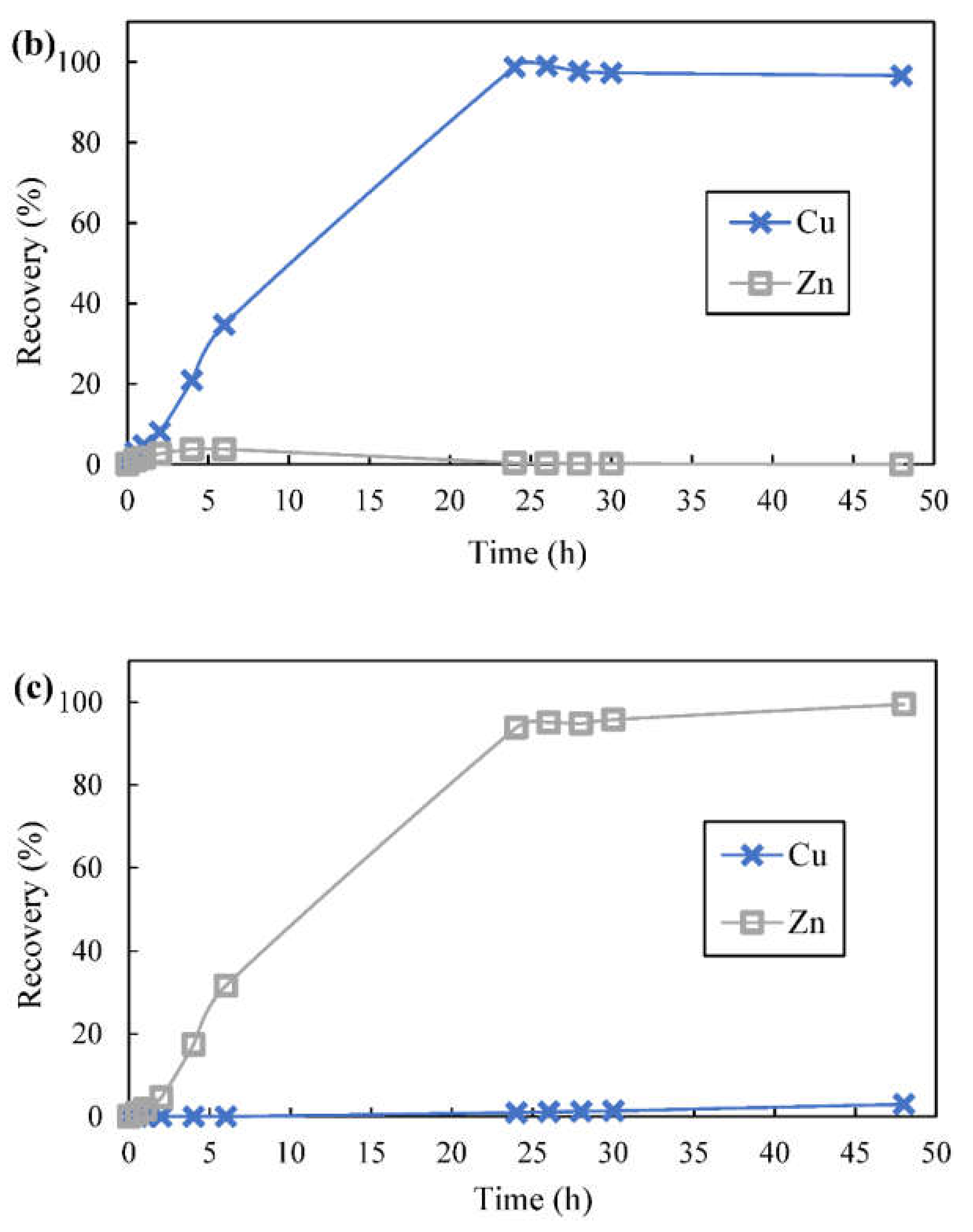
3.8. Metal Ions Transport and Separation Using SLM with Strip Dispersion
- First compartment contained 400 mL feed with mixture of 100 ppm each chromium (VI), copper and zinc in 0.1 mol/L acetate buffer at starting pH of 3.96 (pHeq of 3.60 could be attained when this starting feed pH was applied during solvent extraction)
- Organic phase contained 4% (v/v) D2EHPA in kerosene
- The effective membrane area was 28.3 cm2
- Second compartment contained 300 mL first stripping phase, 0.2 mol/L pH 3 citrate buffer, mixed with 50 mL organic phase to form a strip dispersion
- Third compartment contained 400 mL second stripping phase, which was 1 mol/L sulfuric acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng. Rev. 2017, 4, 37–59. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edsbl&AN=RN383348893&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 9 March 2018). [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. EXS 2012, 101, 133–164. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edsbas&AN=edsbas.6E3B6AFA&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 13 March 2018). [PubMed]
- Cox, M.; Reinhardt, H. The Use of Solvent Extraction in the Recovery of Waste. In Solvent Extraction Principles and Practice, Revised and Expanded, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker: New York, NY, USA, 2004; p. 38. [Google Scholar]
- Barakat, M.A. Review Article: New Trends in Removing Heavy Metals from Industrial Wastewater. Arab J. Chem. 2011, 4, 361–377. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edselp&AN=S1878535210001334&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 9 March 2018). [CrossRef]
- Jia, L.; Huang, J.; Ma, Z.; Liu, X.; Chen, X.; Li, J.; He, L.; Zhao, Z. Research and development trends of hydrometallurgy: An overview based on Hydrometallurgy literature from 1975 to 2019. Trans. Nonferr. Met. Soc. China 2020, 30, 3147–3160. Available online: https://www.sciencedirect.com/science/article/pii/S1003632620654504 (accessed on 30 June 2021). [CrossRef]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. Available online: https://www.sciencedirect.com/science/article/pii/S0959652619304834 (accessed on 30 June 2021). [CrossRef]
- El-Nadi, Y.A. Solvent extraction and its applications on ore processing and recovery of metals: Classical approach. Sep. Purif. Rev. 2017, 46, 195–215. [Google Scholar] [CrossRef]
- Tavlarides, L.L.; Bae, J.H.; Lee, C.K. Solvent extraction, membranes, and ion exchange in hydrometallurgical dilute metals separation. Sep. Sci. Technol. 1987, 22, 581–617. [Google Scholar] [CrossRef]
- Goh, S.S.; Morad, N.; Ismail, N.; Rafatullah, M. Developments in supported liquid membranes for treatment of metal-bearing wastewater. Sep. Purif. Rev. 2022, 51, 38–56. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, J.; Kim, B.; Kim, M.S.; Kobayashi, M. Separation of Copper and Zinc Ions by Hollow Fiber Supported Liquid Membrane Containing LIX84 and PC-88A. Mater. Trans. JIM 2004, 45, 1915–1919. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edsjst&AN=edsjst.DN.JALC.00240510949&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 6 March 2018). [CrossRef][Green Version]
- Prakorn, R.; Kwanta, N.; Ura, P. One-through Selective Separation of Copper, Chromium and Zinc Ions by Hollow Fiber Supported Liquid Membrane. Korean J. Chem. Eng. 2004, 21, 1212–1217. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edselc&AN=edselc.2-52.0-13444310574&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 17 December 2018). [CrossRef]
- Parhi, P.K.; Sarangi, K. Separation of Copper, Zinc, Cobalt and Nickel Ions by Supported Liquid Membrane Technique Using LIX 84I, TOPS-99 and Cyanex 272. Sep. Purif. Technol. 2008, 59, 169–174. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edselp&AN=S1383586607002894&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 9 January 2019). [CrossRef]
- Alguacil, F.J.; Lopez, F.A. Separation iron(III)-manganese(II) via supported liquid membrane technology in the treatment of spent alkaline batteries. Membranes 2021, 11, 991. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M. Chromium(VI) Removal Through Facilitated Transport Using CYANEX 923 as Carrier and Reducing Stripping with Hydrazine Sulfate. Environ. Sci. Technol. 2003, 37, 1043–1047. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edscal&AN=edscal.14611935&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 16 February 2019). [CrossRef] [PubMed]
- Zhou, H.; Ye, Y.; Tan, Y.; Zhu, K.; Liu, X.; Tian, H.; Guo, Q.; Wang, L.; Zhao, S.; Liu, Y. Supported Liquid Membranes Based on Bifunctional Ionic Liquids for Selective Recovery of Gallium. Membranes 2022, 12, 376. [Google Scholar] [CrossRef]
- Alguacil, F.J. Non-dispersive Extraction of Gold(III) with Ionic Liquid Cyphos IL101. Sep. Purif. Technol. 2017, 179, 72–76. Available online: http://www.sciencedirect.com/science/article/pii/S1383586616318032 (accessed on 29 April 2020). [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Lithium Extraction from Complex Aqueous Solutions Using Supported Ionic Liquid Membranes. J. Membr. Sci. 2019, 580, 62–76. Available online: http://www.sciencedirect.com/science/article/pii/S0376738819302157 (accessed on 29 April 2020). [CrossRef]
- Duan, H.; Wang, Z.; Yuan, X.; Wang, S.; Guo, H.; Yang, X. A Novel Sandwich Supported Liquid Membrane System for Simultaneous Separation of Copper, Nickel and Cobalt in Ammoniacal Solution. Sep. Purif. Technol. 2017, 173, 323–329. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edselp&AN=S1383586616305597&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 1 March 2019). [CrossRef]
- Luo, X.; He, D.; Ma, M. Simultaneous Transport and Separation of Cu(II) and Zn(II) in Cu-Zn-Co Sulfate Solution by Double Strip Dispersion Hybrid Liquid Membrane (SDHLM). Sep. Sci. Technol. 2010, 45, 2130–2140. [Google Scholar] [CrossRef]
- De los Ríos, A.P.; Hernández-Fernández, F.J.; Lozano, L.J.; Sánchez-Segado, S.; Ginestá-Anzola, A.; Godínez, C.; Tomás-Alonso, F.; Quesada-Medina, J. On the Selective Separation of Metal Ions from Hydrochloride Aqueous Solution by Pertraction through Supported Ionic Liquid Membranes. J. Membr. Sci. 2013, 444, 469–481. [Google Scholar] [CrossRef]
- Hong, H.; Ban, G.; Kim, H.S.; Jeong, H.S.; Park, M.S. Fabrication of cylindrical 3D cellulose nanofibril(CNF) aerogel for continuous removal of copper (Cu2+) from wastewater. Chemosphere 2021, 278, 130288. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Lo, S.L.; Li, C.M.; Kuan, W.H. Treating chemical mechanical polishing (CMP) wastewater by electro-coagulation-flotation process with surfactant. J. Hazard. Mater. 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.L.; Wong, Z.J.; Sarjadi, M.S.; Soloi, S.; Arshad, S.E.; Bidin, K.; Musta, B. Heavy Metals Removal from Electroplating Wastewater by Waste Fiber-Based Poly(amidoxime) Ligand. Water 2021, 13, 1260. [Google Scholar] [CrossRef]
- Wang, X.; Buer, G.; Fan, W.; Gao, L.; Huo, M. Copper removal from semiconductor CMP wastewater in the presence of nano-SiO2 through biosorption. J. Water Reuse Desalin. 2021, 11, 289–300. [Google Scholar] [CrossRef]
- Rahman, R.A.; Mohamad, A.B.; Surif, S.; Basri, H. Treatment of Metal Finishing Wastewater in Sequencing Batch Process. J. Kejuruter 1994, 6, 3–13. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edsbas&AN=edsbas.2996B6CB&site=eds-live&scope=site&authtype=shib&custid=70180632 (accessed on 29 March 2018).
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Shah, K.; Gupta, K.; Sengupta, B. Selective separation of copper and zinc from spent chloride brass pickle liquors using solvent extraction and metal recovery by precipitation-stripping. J. Environ. Chem. Eng. 2017, 5, 5260–5269. Available online: http://www.sciencedirect.com/science/article/pii/S2213343717304992 (accessed on 14 December 2020). [CrossRef]
- Chang, S.H.; Teng, T.T.; Ismail, N. Efficiency, stoichiometry and structural studies of Cu(II) removal from aqueous solutions using di-2-ethylhexylphosphoric acid and tributylphosphate diluted in soybean oil. Chem. Eng. J. 2011, 166, 249–255. Available online: https://www.sciencedirect.com/science/article/pii/S1385894710010429 (accessed on 4 January 2022). [CrossRef]
- Ghebghoub, F.; Barkat, D. The effect of diluents on extraction of copper(II) with di(2-ethylhexyl)phosphoric acid. J. Coord. Chem. 2009, 62, 1449–1456. [Google Scholar] [CrossRef]
- Mansur, M.B.; Slater, M.J.; Biscaia, E.C. Equilibrium analysis of the reactive liquid-liquid test system ZnSO4/D2EHPA/n-heptane. Hydrometallurgy 2002, 63, 117–126. [Google Scholar] [CrossRef]
- Halim, S.F.A.; Chang, S.H.; Morad, N. Extraction of Cu(II) Ions from Aqueous Solutions by Free Fatty Acid-Rich Oils as Green Extractants. J. Water Process. Eng. 2020, 33, 100997. Available online: http://www.sciencedirect.com/science/article/pii/S2214714419308037 (accessed on 9 February 2020). [CrossRef]
- Sulaiman, R.N.R.; Othman, N. Synergetic Facilitated Transport of Nickel via Supported Liquid Membrane Process by a Mixture of Di (2-ethylhexyl) Phosphoric Acid and N-octanol: Kinetic Permeation Study and Approach for a Green Process. Chem. Eng. Process.-Process. Intensif. 2018, 134, 9–19. Available online: http://www.sciencedirect.com/science/article/pii/S0255270118304288 (accessed on 19 December 2019). [CrossRef]
- Silberberg, M.S. Chemistry: The Molecular Nature of Matter and Change, 4th ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Martins, J.M.; Guimarães, A.S.; Dutra, A.J.B.; Mansur, M.B. Hydrometallurgical separation of zinc and copper from waste brass ashes using solvent extraction with D2EHPA. J. Mater. Res. Technol. 2020, 9, 2319–2330. Available online: https://www.sciencedirect.com/science/article/pii/S2238785419307367 (accessed on 15 June 2021). [CrossRef]
- Jafari, H.; Abdollahi, H.; Gharabaghi, M.; Balesini, A.A. Solvent extraction of zinc from synthetic Zn-Cd-Mn chloride solution using D2EHPA: Optimization and thermodynamic studies. Sep. Purif. Technol. 2018, 197, 210–219. Available online: https://www.sciencedirect.com/science/article/pii/S1383586617340650 (accessed on 30 June 2021). [CrossRef]
- Agrawal, A.; Pal, C.; Sahu, K.K. Extractive removal of chromium (VI) from industrial waste solution. J. Hazard. Mater. 2008, 159, 458–464. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=edscal&AN=edscal.20824413&site=eds-live&scope=site&authtype=ip,shib&custid=s5243931 (accessed on 15 February 2019). [CrossRef]
- Zhang, G.; Chen, D.; Zhao, W.; Zhao, H.; Wang, L.; Wang, W.; Qi, T. A novel D2EHPA-based synergistic extraction system for the recovery of chromium (III). Chem. Eng. J. 2016, 302, 233–238. Available online: https://www.sciencedirect.com/science/article/pii/S1385894716306866 (accessed on 30 June 2021). [CrossRef]
- Stoll, V.S.; Blanchard, J.S. Chapter 6 buffers: Principles and practice1. In Methods in Enzymology, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 43–56. Available online: https://www.sciencedirect.com/science/article/pii/S0076687909630068 (accessed on 27 April 2021).
- Belkhouche, N.E.; Didi, M.A.; Villemin, D. Separation of nickel and copper by solvent extraction using Di-2 ethylhexylphosphoric acid-based synergistic mixture. Solvent. Extr. Ion Exch. 2005, 23, 677–693. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López-Delgado, A.; Alonso, M.; Sastre, A.M. The Phosphine Oxides Cyanex 921 and Cyanex 923 as Carriers for Facilitated Transport of Chromium (VI)-chloride Aqueous Solutions. Chemosphere 2004, 57, 813–819. Available online: http://www.sciencedirect.com/science/article/pii/S004565350400606X (accessed on 20 February 2019). [CrossRef] [PubMed]
- Kahlert, H.; Scholz, F. Acid-base Diagrams, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2013; pp. 1–111. [Google Scholar]
- Ritcey, G.M. Development of industrial solvent extraction processes. In Solvent Extraction Principles and Practice, Revised and Expanded, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker: New York, NY, USA, 2004; p. 57. [Google Scholar]


| Concentration (mol/L) | HA(aq) | ⇌ | H+(aq) | + | A−(aq) |
|---|---|---|---|---|---|
| Initial | [HA] | 0 | 0 | ||
| During stripping | [HA] − (2[Cu2+] + 2[Zn2+]) | 2[Cu2+] + 2[Zn2+] (Used up for stripping) | 2[Cu2+] + 2[Zn2+] | ||
| Stripping equilibrium | [HA] − [H+] − 2[Cu2+] − 2[Zn2+] | [H+] | [H+] + 2[Cu2+] + 2[Zn2+] |
| Time (h) | Cu in 1st Stripping Phase | Zn in 2nd Stripping Phase | ||
|---|---|---|---|---|
| Recovery (%) | Purity (%) | Recovery (%) | Purity (%) | |
| 24 | 98.8 | 99.7 | 93.8 | 98.7 |
| 26 | 99.1 | 99.8 | 95.1 | 98.4 |
| 28 | 97.7 | 99.8 | 94.8 | 98.3 |
| 30 | 97.3 | 99.9 | 95.8 | 98.2 |
| 48 | 96.7 | ~100 | 99.5 | 96.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, S.S.; Rafatullah, M.; Ismail, N.; Alam, M.; Siddiqui, M.R.; Seow, E.-K. Separation of Chromium (VI), Copper and Zinc: Chemistry of Transport of Metal Ions across Supported Liquid Membrane. Membranes 2022, 12, 685. https://doi.org/10.3390/membranes12070685
Goh SS, Rafatullah M, Ismail N, Alam M, Siddiqui MR, Seow E-K. Separation of Chromium (VI), Copper and Zinc: Chemistry of Transport of Metal Ions across Supported Liquid Membrane. Membranes. 2022; 12(7):685. https://doi.org/10.3390/membranes12070685
Chicago/Turabian StyleGoh, Saik Su, Mohd Rafatullah, Norli Ismail, Mahboob Alam, Masoom Raza Siddiqui, and Eng-Keng Seow. 2022. "Separation of Chromium (VI), Copper and Zinc: Chemistry of Transport of Metal Ions across Supported Liquid Membrane" Membranes 12, no. 7: 685. https://doi.org/10.3390/membranes12070685
APA StyleGoh, S. S., Rafatullah, M., Ismail, N., Alam, M., Siddiqui, M. R., & Seow, E.-K. (2022). Separation of Chromium (VI), Copper and Zinc: Chemistry of Transport of Metal Ions across Supported Liquid Membrane. Membranes, 12(7), 685. https://doi.org/10.3390/membranes12070685










