Abstract
Mitochondria are capable of synchronized oscillations in many variables, but the underlying mechanisms are still unclear. In this study, we demonstrated that rat liver mitochondria, when exposed to a pulse of Sr2+ ions in the presence of valinomycin (a potassium ionophore) and cyclosporin A (a specific inhibitor of the permeability transition pore complex) under hypotonia, showed prolonged oscillations in K+ and Sr2+ fluxes, membrane potential, pH, matrix volume, rates of oxygen consumption and H2O2 formation. The dynamic changes in the rate of H2O2 production were in a reciprocal relationship with the respiration rate and in a direct relationship with the mitochondrial membrane potential and other indicators studied. The pre-incubation of mitochondria with Ca2+(Sr2+)-dependent phospholipase A2 inhibitors considerably suppressed the accumulation of free fatty acids, including palmitic and stearic acids, and all spontaneous Sr2+-induced cyclic changes. These data suggest that the mechanism of ion efflux from mitochondria is related to the opening of short-living pores, which can be caused by the formation of complexes between Sr2+(Ca2+) and endogenous long-chain saturated fatty acids (mainly, palmitic acid) that accumulate due to the activation of phospholipase A2 by the ions. A possible role for transient palmitate/Ca2+(Sr2+)-induced pores in the maintenance of ion homeostasis and the prevention of calcium overload in mitochondria under pathophysiological conditions is discussed.
1. Introduction
Currently, there are numerous data indicating the involvement of mitochondria in the regulation of the intracellular dynamics of free Ca2+, including generation, distribution, and synchronization of Ca2+ waves in the cell cytoplasm [1,2,3,4]. Accumulating evidence suggests that the formation and propagation of “calcium waves” in cells is mediated by oscillations in ion fluxes in mitochondria [4,5]. In isolated mitochondria, a pulse addition of Ca2+ or its chemical analog Sr2+ can trigger complex dynamic modes of ion transport accompanied by fluctuations in transmembrane potential, respiration rate, volume of the mitochondrial matrix, and ATP production [6,7,8,9,10,11,12].
As early as 50 years ago, it was shown that mitochondria could generate ion oscillations with a wide variety of periods and waveforms. The history of the study of oscillatory processes in mitochondria begins with the works of B. Chance, L. Packer, H. Lardy, B. Pressman, and others [13]. The main stimulus for the development of mitochondrial oscillations was found to be the transport of the divalent metal ions Ca2+ or Sr2+ across the inner mitochondrial membrane. The co-addition of the potassium ionophore valinomycin to mitochondria promoted long-term fluctuations in ion fluxes across the mitochondrial membrane. Studies by A. Gyulkhandanyan, Yu. Evtodienko, and others demonstrated sustained Sr2+/valinomicin-induced oscillations in the Sr2+, K+ and H+ fluxes, oxygen consumption, and light scattering of suspensions of isolated rat liver mitochondria under hypotonic conditions [5,6,11].
Mitochondria are now recognized to contain several systems involved in Ca2+ transport: the mitochondrial Ca2+ uniporter complex (MCUC), the rapid mode of Ca2+ uptake (RaM), the leucine zipper and EF-hand-containing transmembrane protein 1 (Letm1), mitochondrial ryanodine receptor type 1, uncoupling proteins (UCPs), Na+/Li+/Ca2+ exchanger (NCLX), the Ca2+/H+ exchanger, and the mitochondrial permeability transition pore (mPTP) [14,15,16]. Among them, MCUC is believed to be the predominant calcium import system, which plays a crucial role in controlling ion uptake by mitochondria under pathophysiological states [12,16,17]. It was found that mitochondrial influx of Ca2+(Sr2+) upon Ca2+(Sr2+)-induced ion oscillations occurred primarily via MCUC [6,7,8,9,10,11,12]. As for the mechanism of ion efflux from mitochondria in oscillatory modes, this issue is still not clear. Some studies have shown that the opening of the mPTP, a multiprotein mega-channel complex at outer and inner membrane contact sites, is not related to the release of ions from mitochondria in Ca2+(Sr2+)-induced ion oscillations, since the selective mPTP blocker cyclosporin A (CsA) did not affect the cycling of ions across mitochondrial membranes [12,18,19,20,21]. In addition, the loading of mitochondria with Sr2+ ions, which are unable to induce mPTP opening, not only did not prevent but even favored continuous ion oscillations in mitochondria [8,10,11]. In this regard, the participation of the mPTP in Ca2+(Sr2+)-induced self-oscillations in ion fluxes has been questioned.
Earlier, we found that one of the pathways of ion transport can be through the formation of lipid pores in mitochondrial and artificial (liposomes and black lipid) membranes by the mechanism of chemotropic phase transition [22,23,24]. The mechanism can be realized due to the capacity of long-chain saturated fatty acids to bind Ca2+ with high affinity [25]. Unlike the mPTP, the CsA-independent palmitate/Ca2+-induced permeability transition pore (PA-mPT pore) can be induced by lower concentrations of Ca2+ or Sr2+ ions in the mitochondrial matrix under conditions close to physiological ones [26,27,28,29]. Furthermore, one of the main features of the PA-mPT pore is its ability to spontaneously close, with a rapid restoration of membrane integrity [26,29,30]. As is already known, the ability of lipid pores to rapidly heal, if their diameter does not exceed a certain value, is an intrinsic property [31]. It has been observed that induction of the PA-mPT pore can lead to the transient permeabilization of the mitochondrial membrane, but the mitochondria remain functionally active [26,29,30]. We suggest that the intermittent opening/closure of the PA-mPT pore underlies the so-called “flickering” mode of permeability transition, which is reversible and appears to have a role in maintaining mitochondrial calcium homeostasis by providing the organelles with an emergency pathway for the rapid outflow of Ca2+ ions [30].
The aim of this work was to reveal whether PA-mPT pore formation is involved in the mechanism of ion efflux upon ion fluctuations triggered by Sr2+ in the presence of valinomycin in rat liver mitochondria. Using a number of inhibitors of Ca2+(Sr2+)-dependent phospholipase A2, we found that a decrease in the content of free fatty acids, including palmitic and stearic acids, suppressed continuous Sr2+-induced cyclic changes in ion fluxes and the main functions of mitochondria.
2. Materials and Methods
2.1. Materials
SrCl2 and tetraphenylphosphonium chloride were purchased from Merck KGaA (Darmstadt, Germany); arachidonyl trifluoromethyl ketone (AACOCF3), 4-(4-Octadecylphenyl)-4-oxobutenoic acid (OBAA), and palmitoyl trifluoromethyl ketone (PACOCF3) were obtained from Tocris BioScience (Bristol, UK); aristolochic acid I (AA), 6E-bromoenol lactone (BEL), trifluoperazine dihydrochloride (TFP), 4′-bromophenacyl bromide (BrB), valinomycin (Val), CsA, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). The stock solutions of AACOCF3, PACOCF3, and OBAA were prepared in dimethylsulfoxide (DMSO). The stock solutions of AA, BEL, TFP, BrB, Val, and CsA were prepared in 95% ethanol. In the control experiments, an equivalent volume of the solvent was used. The final concentrations of DMSO and ethanol in the incubation medium did not exceed 0.1% (volume).
2.2. Animals
Experiments were performed on Wistar male rats weighting 200–250 g. The animals were housed under standard conditions at a room temperature of 18–22 °C, relative humidity 60–70%, and regular 12 h light-dark cycles and received commercial pellets and water ad libitum. All assays were carried out in accordance with Protocol No. 19/2020 of 18.02.2020 of the Ethics Committees at the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences and the University of Helsinki.
2.3. Isolation of Rat Liver Mitochondria
Mitochondria were isolated from the liver of mature male Wistar rats (220–250 g) by a standard differential centrifugation technique using a Sigma 3-16K centrifuge (Sigma-Aldrich, St. Louis, MO, USA) [27]. The livers were cooled in saline, pressed through a plate, and homogenized with a Potter-type glass homogenizer. The medium contained 210 mM mannitol, 70 mM sucrose, 10 mM HEPES/KOH (pH 7.4), and 1 mM EDTA. The homogenate was centrifuged at 700× g (10 min), and mitochondria were sedimented for 15 min at 7000× g. The mitochondrial pellet was resuspended in a washing medium containing 210 mM mannitol, 70 mM sucrose, 10 mM HEPES/KOH (pH 7.4), and 0.1 mM EGTA and centrifuged for 15 min at 7000× g. The pellet was resuspended in the washing medium (0.1 mL/g of the liver tissue) and stored on ice. The concentration of mitochondrial protein was determined by the Lowry method [32]. The resulting suspension of rat liver mitochondria contained 70–80 mg of protein per mL.
2.4. Estimation of the Functional Parameters of Mitochondria
The mitochondrial membrane potential (ΔΨm) was estimated by the distribution of the lipophilic cation tetraphenylphosphonium (TPP+) (1.5 µM), which was measured with a TPP+-sensitive electrode (Nico-Analyt, Moscow, Russia). Changes in the concentration of TPP+ in the incubation medium were inversely proportional to changes in the membrane potential [33]. The concentrations of Sr2+ and K+ ions in the incubation medium were determined with Sr2+- and K+-selective electrodes (Nico-Analyt, Moscow, Russia). Changes in the medium pH were registered by a pH microelectrode InLab Micro (Metler Toledo, Switzerland). Changes in the concentrations of TPP+, K+, H+, and Sr2+ were recorded simultaneously in a 1 mL temperature-controlled cell with constant stirring at 26 °C using an original multichannel electrometrical system Record 4 (Pushchino, Russia), as described previously [12]. The ion-selective electrodes were calibrated at the beginning of each experiment.
The swelling of mitochondria (0.4 mg/mL) was measured as a decrease in absorbance at 540 nm (A540) in a stirred cuvette at room temperature (24 °C) using a USB-2000 spectroscopy fiber-optic system (Ocean Optics, Dunedin, FL, USA) [26].
The rate of oxygen consumption by mitochondria was measured polarographically using an Oxygraph-2k (O2k) high-resolution respirometer equipped with DatLab software (Oroboros Instruments, Innsbruck, Austria), as described previously [34]. Temperature was maintained at 25 °C under continuous stirring at 600 rpm. The experiments were carried out with oxygen concentrations in the range of 220–50 µM O2. In additional control experiments, the respiratory control ratio (RCR) was measured under conventional conditions [34]. The RCR of isolated mitochondria was in the region of 5–6 when using 5 mM potassium succinate as a respiration substrate.
The rate of H2O2 production in mitochondria was determined using the fluorescent dye Amplex red (AR), as described previously [34]. The fluorescence of resorufin, an oxidized product of AR (excitation/emission at 563/587 nm) was measured using a CARY fluorimeter (Varian Inc., Palo Alto, CA, USA) at 36 °C under continuous stirring. The medium was supplemented with 10 μM AR and 1 U/mL of horseradish peroxidase; then, 0.1 mg/mL of the mitochondrial protein was added. The level of H2O2 was determined from the calibration curve. The concentration of a standard H2O2 solution was calculated using the molar absorption coefficient E240 = 43.6 M−1·cm−1. The rate of H2O2 generation was calculated based on the first derivative function of the H2O2 concentration vs. time and expressed as pmol H2O2/s·mL.
The incubation medium contained 20 mM sucrose, 1 mM KCl, 1 μM tetraphenylphosphonium chloride (TPP+), 1 μM cyclosporin A (CsA), 1 μM rotenone, 5 mM succinic acid, and 12.5 mM Tris (pH 7.3). SrCl2 and valinomycin were added to mitochondria 1 min after the start of incubation. The inhibitors of phospholipase A2 were added to the mitochondria 1 min before the addition of Sr2+. Stock solutions of the inhibitors were prepared in ethanol. The final concentration of ethanol in the incubation medium was <0.1 volume percent.
2.5. Determination of Free Fatty Acids upon Mitochondrial Oscillations by Gas Chromatography
The levels of free fatty acids (FFAs) in rat liver mitochondria were determined by gas chromatography using a Pye-Unicam 304 gas chromatograph (Pye Unicam Ltd., Cambridge, UK) equipped with a (1 m × 2 mm ID) glass column packed with Porapak Q, 80–100 mesh (Fluka Chemie AG, Buchs, Switzerland), in accordance with the conventional technique [35]. The content of main FFAs was analyzed before and 12 min after the addition of Sr2+ and valinomycin to the mitochondria in the absence (0.1% DMSO) or presence of 25 μM aristolochic acid (ArA), a phospholipase A2 inhibitor. The inhibitor of PLA2 ArA or 0.1% DMSO were added to mitochondrial suspensions 1 min before the addition of Sr2+ and valinomycin. The oscillatory mode of mitochondrial functioning was confirmed spectrophotometrically by reversible changes in optical density at 540 nm. The mitochondrial suspensions were fixed with a mixture of chloroform–methanol in the volume ratio 2:1. All samples were supplemented with 300 µg heptadecanoic acid (C17:0), which was used as an external standard. Chloroform and water–methanol layers were separated by centrifugation at 10,000× g for 10 min at 4 °C. The lower layer of the extract was collected, and a specific amount of anhydrous sodium sulfate was added for dehydration. The dehydrated extract liquor was dried under a gentle stream of nitrogen gas at room temperature. The obtained fractions were methylated, as described previously [35]. Methyl ethers were extracted by four volumes of hexane, and an aliquot was injected into the chromatograph. The injector and detector were kept at 200 and 250 °C, respectively. The carrier gas was nitrogen at a flow rate of 10 mL/min. Values were expressed as µg FFA per mg mitochondrial protein and represent the means of four samples.
2.6. Detection of the Group IV Cytosolic Phospholipase A2 in Isolated Rat Liver Mitochondria by Immunoelectron Microscopy
Immunoelectron microscopy of isolated rat liver mitochondria was performed to determine the distribution pattern of Ca2+(Sr2+)-dependent cytosolic phospholipase A2 (cPLA2) in the organelles. The immunochemical analysis was performed using the commercial group IV cPLA2 antibody (cat. no. sc-438, Santa Cruz Biotechnology, Dallas, TX, USA) and 10 nm colloidal gold-labeled antibody (cat. no. G7402, Sigma-Aldrich, St. Louis, MO, USA) as primary and secondary antibodies, correspondingly. The suspensions of rat liver mitochondria (1 mg/mL) were fixed at room temperature for 4 h in 4% paraformaldehyde and 0.5% glutaraldehyde solution in 0.1 M PBS buffer (pH 7.4). After washing with the buffer, the mitochondrial suspensions were postfixed for 2 h with a 1% solution of osmic acid in PBS, dehydrated in alcohols of increasing concentrations, and enclosed in Epon-812. The treatment of mitochondria with the antibodies was carried out in a dampening chamber on the grids with ultrathin sections, which were prepared on an ultramicrotome Leica EM UC6 (Wetzlar, Germany). To get rid of resin and osmium, the slices were incubated in solutions of periodic acid and sodium periodate for 3 min, with further washing with 0.1 M PBS buffer. The nonspecific labeling of proteins was blocked by the superblock reagent (Pierse, Rockford, IL, USA). All further procedures were performed using PSB buffer supplemented with 0.1% glycine and 0.1% Triton X-100. The cPLA2 antibody was used in a 1:50 dilution. The procedure was performed at 4 °C during the night. After thorough washing, the ultrathin sections were treated with the secondary antibody conjugated with 10 nm colloidal gold microparticles (dilution 1:20) for 1 h at room temperature, washed and stained with lead citrate and uranyl acetate. In control experiments, 0.1 M PBS buffer was used in place of the primary antibody (in the presence of the secondary antibody) as a negative control. The slices were viewed using a Tesla BS-500 electron microscope (Brno, Czech Republic) and scanned using an Epson V700 scanner (Epson, Long Beach, CA, USA). About 30 electron microscopic preparations were examined.
2.7. Data Processing
The data are expressed as the means ± standard derivation (m ± SD). Characteristic curves typical for each of the independent experiments (n = 7–10) are presented. Statistical analysis of the data was carried out using GraphPad Prism version 6.0 software for Windows (San Diego, CA, USA). Normality of the sample distributions was verified using the Shapiro–Wilk test before using parametric analyses. Repeated-measures analysis of variance (ANOVA) was used with post hoc Bonferroni tests to compare FFA levels. The differences were considered statistically significant at p < 0.05.
3. Results
3.1. Generation of Spontaneous Oscillations in Ion Fluxes and Respiration Rate of Rat Liver Mitochondria
Figure 1 shows that a single addition of Sr2+ (45 nmol/mg protein) to rat liver mitochondria energized by succinate and incubated in hypotonic medium triggered short-term synchronous oscillations (two oscillating waves) of fluxes of Sr2+, K+, and TPP+ ions across the mitochondrial membrane, as well as reversible changes in the rate of O2 consumption. As one can see, the mitochondrial membrane potential (ΔΨm) estimated by the distribution of the molecular sensor TPP+ and the mitochondrial respiration rate recovered rapidly, and the added Sr2+ was eventually accumulated by the mitochondria. At the same time, the influx of K+ ions into the mitochondria was significantly slowed down. Under the resting-state conditions, the rate of transport of K+ across the mitochondrial membrane is known to be much lower than that of Ca2+ or Sr2+ [36,37]. One can suppose that the low rate of potassium transport limits the propagation of oscillating waves and is the main reason for the rapid damping of the Sr2+-induced spontaneous oscillations in ion fluxes and ΔΨm.
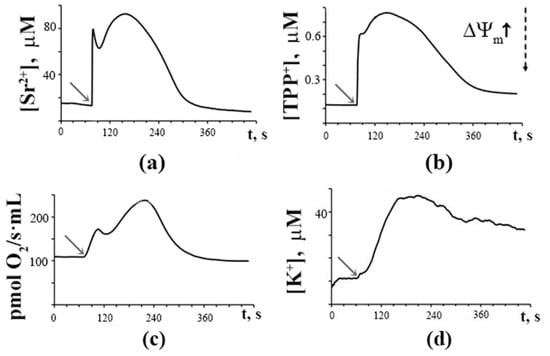
Figure 1.
Single pulse addition of Sr2+ (shown by the arrow) induces rapid reversible changes in Sr2+ fluxes (a), membrane potential (b), respiratory rate (c), and slow changes in K+ fluxes (d) in the suspension of rat liver mitochondria. The incubation medium contained 20 mM sucrose, 1 mM KCl, 1 μM TPP+, 1 μM CsA, 1 μM rotenone, 5 mM succinic acid, and 12.5 mM Tris (pH 7.3). Addition: 45 nmol SrCl2/mg of mitochondrial protein. The dotted arrow indicates a change in the membrane potential. The typical traces are presented (n = 7).
As seen from Figure 2, the co-addition of the potassium ionophore valinomycin to the mitochondrial suspension increased the number of spontaneous Sr2+-induced oscillation waves of the fluxes of Sr2+ and K+ ions, as well as ΔΨm. The long-term (within 30 min) reversible oscillations in ion fluxes and ΔΨm were induced by the addition of Sr2+ ions at concentrations of 35–50 nmol/mg protein and small amounts of valinomycin (2 ng/mg protein); in this case, three to five “cycles” of spontaneous ion oscillations in mitochondria could be observed (Figure 2).
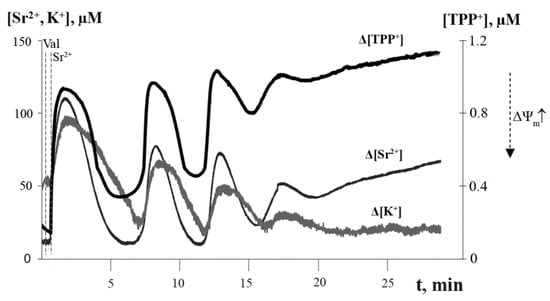
Figure 2.
Simultaneous recording of Sr2+/valinomycin-induced oscillations in Sr2+ and K+ fluxes and membrane potential of rat liver mitochondria. The medium contained 20 mM sucrose, 1 mM KCl, 1.5 μM TPP+, 1 μM CsA, 1 μM rotenone, 5 mM succinic acid, and 12.5 mM Tris (pH 7.3). Additions: 2 ng valinomycin and 45 nmol SrCl2/mg of protein. The typical traces are presented (n = 5).
Along with a temporary dissipation of ΔΨm, the influx of Sr2+ and K+ ions into mitochondria was also accompanied by a synchronous decline in the pHout of the medium (alkalization of the mitochondrial matrix) (Figure S1). The decrease in the electrochemical transmembrane potential was enhanced with each new wave of the oscillations, which ultimately impaired the ability of mitochondria to accumulate Sr2+, K+, and H+ ions. Our experiments suggest that the number of oscillation waves depends on the degree of coupling of isolated mitochondria and that the higher this is, the greater number of waves is registered.
Figure 3 shows that the cyclic transport of K+ ions across the mitochondrial membrane was accompanied by periodic changes in the volume of the mitochondrial matrix, as determined by the light scattering technique. One can see that the cycles of the low-amplitude swelling of mitochondria and their subsequent spontaneous contraction correspond to the influx and efflux of K+ ions, respectively. Following the last oscillation wave, irreversible mitochondrial swelling was observed.
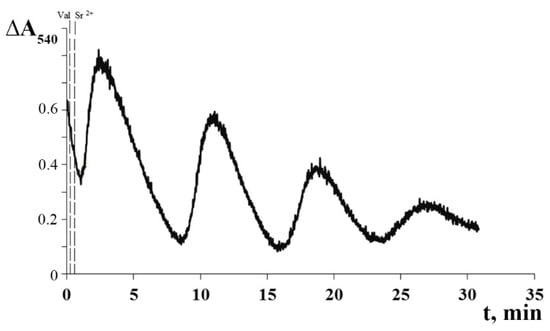
Figure 3.
Sr2+/valinomycin-induced cyclic changes in matrix volume of rat liver mitochondria. The experimental conditions were the same as in Figure 2. Additions: 2 ng valinomycin and 45 nmol SrCl2/mg of protein. The typical traces are presented (n = 5).
Parallel studies of the dynamic changes in the rates of mitochondrial oxygen consumption and H2O2 generation under the experimental conditions demonstrated that these indicators also changed in an oscillatory mode (Figure 4A). One can see that when mitochondrial respiration decreased, that is, the degree of reduction of the electron transport chain complexes was higher, the rate of H2O2 production was maximal. Thus, there was an inverse relationship between the dynamic changes in mitochondrial respiration and H2O2 production. It should also be noted that the temporary decrease in ΔΨm corresponded to a synchronous drop in the rate of H2O2 production (Figure 4B). On the contrary, when the mitochondrial membrane potential returned to a high level, the rate of H2O2 formation also became maximal, which indicates a direct relationship between the dynamic changes in ΔΨm and H2O2 production. These synchronous periodic changes in mitochondrial function could persist for 20–30 min.
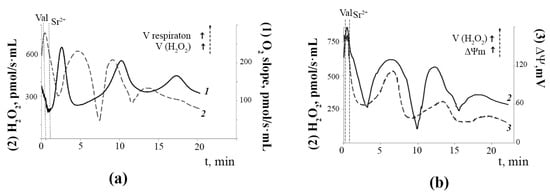
Figure 4.
Sr2+/valinomycin-induced dynamic changes in the respiration rate (trace 1), H2O2 production rate (trace 2), and the membrane potential (trace 3) of rat liver mitochondria: (a) Reciprocal changes in the rates of oxygen consumption and H2O2 production by rat liver mitochondria after the addition of Sr2+ and valinomycin. The conditions were the same as in Figure 2. The rate of mitochondrial respiration (O2 slope, (pmol/s·ml)) was measured polarographically using an Oxygraph-2k respirometer and DatLab software (Oroboros Instruments, Innsbruck, Austria). The rate of H2O2 production (H2O2, (pmol/s·ml)) by the mitochondria was determined by the Amplex Red/peroxidase assay. The typical traces are presented (n = 5); (b) Synchronous changes in the rate of H2O2 production and the membrane potential of rat liver mitochondria after the addition of Sr2+ ions and valinomycin. The conditions were the same as in Figure 2. The mitochondrial membrane potential was estimated with the use of tetraphenylphosphonium (TPP+) and an electrode selective for TPP+ as described in the Materials and Methods section. The amount of TPP+ accumulated in mitochondria was determined by measuring the difference between its initial concentration and the concentration after the addition of mitochondria. The typical traces are presented (n = 5).
The specific blocker of MCUC ruthenium red (1 µM) suppresses the formation of the next oscillation wave of Sr2+ flux (Figure S2) and the other functional parameters of mitochondria. In contrast, the inhibitor of cyclophilin D, a key regulator of mPTP opening, cyclosporin A (CsA), did not affect mitochondrial oscillations, and it was added to the incubation medium in all the above experiments to exclude mPTP-related mitochondrial damage.
3.2. Accumulation of Free Fatty Acids and Effects of Inhibitors of PLA2 on the Long-Term Oscillatory Mode of Mitochondrial Functioning
It is known that, under hypotonic conditions, the activity of phospholipases A2 (PLA2) in mitochondria is enhanced [38,39]. The influx of Sr2+ in the mitochondrial matrix can also contribute to the activation of PLA2 and the accumulation of free fatty acids (FFAs) [39].
Our data showed that after the addition of Sr2+ ions and valinomycin, the level of total FFAs in rat liver mitochondria increased 1.7 times (Figure 5 and Figure S3). Among saturated long-chain fatty acids, palmitic (C16:0) and stearic (C18:0) acids accounted for the largest proportion; their total contents more than doubled. The addition of the inhibitor of Ca2+(Sr2+)-dependent PLA2 aristolochic acid (25 µM) before the start of long-term ion oscillations in mitochondria significantly blocked the accumulation of FFAs, including palmitic and stearic acids (Figure 5).
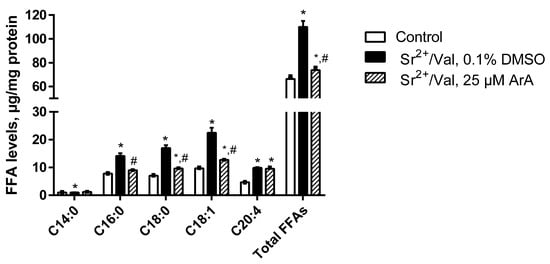
Figure 5.
A comparative analysis of the content of the main free fatty acids in rat liver mitochondria before (control) and after the onset of Sr2+/valinomycin (Val)-induced ion oscillations in the absence (0.1% DMSO) or presence of 25 μM aristolochic acid (ArA), a phospholipase A2 (PLA2) inhibitor. The medium and conditions were the same as in Figure 2. The inhibitor of PLA2 ArA or 0.1% DMSO was added to a mitochondrial suspension 1 min before the addition of Sr2+. Data represent the means ± SEM of at least four independent experiments. * p < 0.05 vs. the control group (without additions); # p < 0.05 vs. the experimental group without PLA2 inhibitor (0.1% DMSO).
As has been found earlier, free palmitic and stearic acids in complexes with Sr2+(Ca2+) ions can induce the opening of the PA-mPT short-lived pores that are insensitive to CsA and other blockers of the classical mPTP [26,29,30]. To elucidate whether the PA-mPT pore is involved in the ion efflux pathway under long-term Sr2+-induced ion oscillations, we investigated the effect of several inhibitors of Ca2+(Sr2+)-dependent and Ca2+(Sr2+)-independent PLA2 on oscillation propagation.
Figure 6 shows the synchronous changes in the ion fluxes of mitochondria pre-incubated with the Ca2+(Sr2+)-dependent PLA2 inhibitor aristolochic acid (ArA). One can see that the incubation of mitochondria with ArA at a concentration of 25 µM eliminated Sr2+-induced cyclic changes in Sr2+ and K+ ion transport, ΔΨm, mitochondrial matrix volume, as well as the rates of mitochondrial respiration and H2O2 production.
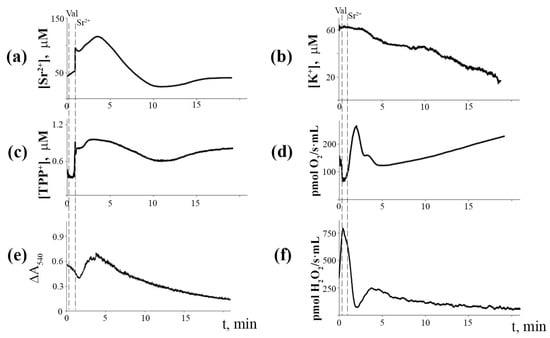
Figure 6.
Blocking effect of aristolochic acid (25 μM), a phospholipase A2 inhibitor, on Sr2+/valinomycin-induced cyclic changes in the fluxes of Sr2+ (a), K+ (b), TPP+ (c), respiration rate (d), matrix volume (e), and H2O2 production rate (f) in rat liver mitochondria. The medium and conditions were the same as in Figure 2. Additions: 2 ng valinomycin and 45 nmol SrCl2/mg of protein. The typical traces are presented (n = 5).
Similar effects were observed with other inhibitors of Ca2+(Sr2+)-dependent PLA2, namely, 15 µM arachidonyl trifluoromethyl ketone (AACOCF3), 1 µM 4-(4-Octadecylphenyl)-4-oxobutenoic acid (OBAA), 10 µM trifluoperazine dihydrochloride (TFP), 40 µM 4′-bromophenacyl bromide (BrB), and 15 µM bromoenol lactone (BEL) (Table 1, Figure S4). At the same time, the inhibitor of Ca2+-independent PLA2 palmityl trifluoromethyl ketone (PACOCF3, 20 µM) was ineffective (Figure S5).

Table 1.
Phospholipase A2 inhibitors capable of preventing the Sr2+/valinomycin-induced oscillatory state of rat liver mitochondria.
3.3. Ultrastructural Localization of the Group IV Ca2+(Sr2+)-Dependent Cytosolic Phospholipase A2 in Isolated Rat Liver Mitochondria
Recent data suggest that only Ca2+(Sr2+)-dependent cytosolic phospholipase A2β3 (group IVB PLA2; encoded by PLA2G4B) is an endogenous protein and constitutively associated with mitochondria in liver tissue [40]. However, the exact localization and distribution of this type of PLA2 in the organelles has not yet been determined.
Using specific anti-cPLA2 and 10 nm colloidal gold-labeled secondary antibodies, we performed an immunoelectron microscopic study to determine the localization and distribution pattern of the group IV Ca2+(Sr2+)-dependent cPLA2 in isolated rat liver mitochondria (Figure 7).
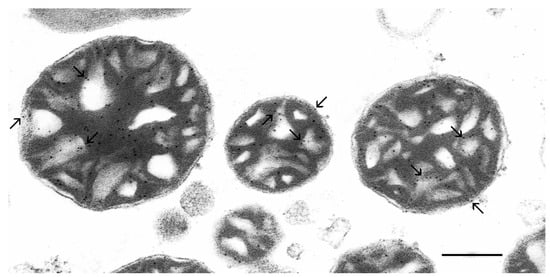
Figure 7.
Ultrastructural localization of the group IV Ca2+(Sr2+)-dependent cytosolic phospholipase A2 (cPLA2) in isolated rat liver mitochondria. Mitochondria were incubated with specific antibodies against cPLA2 and secondary antibodies labeled with 10 nm colloidal gold nanoparticles. Black granules (shown by the arrows) are the binding sites for the antibodies to the target proteins. The scale bar is 0.25 μm.
As can be seen, electron-dense (black) gold labels were predominantly located on the outer and crista membranes of mitochondria. It should be noted that no colloidal gold labels were observed in the control experiments, when the primary antibody was replaced with 0.1 M PBS buffer (Figure S6).
4. Discussion
In the current study, we provide evidence relating to the oscillatory mode of mitochondrial function when a single pulse addition of Sr2+ (35–50 nmol/mg protein) to rat liver mitochondria in a hypotonic medium initiates the cycling of ions across the inner mitochondrial membrane. The triggering of long-term oscillations in ion fluxes required an increase in the permeability of the mitochondrial membrane to potassium ions. This can be explained by the fact that, under the resting-state conditions, the rate of the transport of K+ ions across the mitochondrial membrane is one order of magnitude lower than that of Ca2+ or Sr2+ [36,37,41]. Therefore, potassium influx can limit the next oscillation wave. The addition of small amounts of the highly specific potassium ionophore valinomycin resulted in prolonged oscillations in the following parameters of mitochondria: (1) membrane potential; (2) fluxes of Sr2+, K+, and H+ ions across the membrane; (3) the volume of the mitochondrial matrix; (4) the rate of mitochondrial respiration; and (5) the rate of H2O2 production. Additional experiments showed that the specific inhibitor of the mPTP opening CsA did not affect ion cycling in the mitochondria, which indicates that the efflux of ions in this mode of mitochondrial function could not be related to the opening and closure of the mPTP pore. Furthermore, strontium ions were used instead of Ca2+, as they are not able to induce mPTP pore opening [42,43]. Another feature of the Sr2+/valinomycin-induced mitochondrial oscillations is their strong dependence on the state of strontium transport. It is known that strontium ions enter mitochondria via the same pathway as Ca2+, i.e., MCUC [2,37]. The specific MCUC inhibitor RR rapidly stopped all the Sr2+/valinomycin-induced cyclic changes in ion fluxes in the mitochondria.
The fact that valinomycin can promote periodic changes in the movement of K+ and H+ ions across the mitochondrial membrane and in mitochondrial matrix volume has been found by many authors, starting with the classical works of B. Chance, R. Packer, and others [6,13]. However, the molecular mechanism of K+ release in exchange for H+ in mitochondria is as yet unclear [41]. With regard to the release pathway of Sr2+(Ca2+), K+, and other ions from mitochondria in the oscillatory mode, we suggest that it is mediated by the opening of mitochondrial palmitate/Ca2+-induced short-lived lipid pores. Our study showed that the accumulation of free Sr2+ in the mitochondrial matrix was accompanied by the hydrolysis of membrane phospholipids and the accumulation of endogenous FFAs, while the level of palmitic and stearic acids increased more than two times. It is known that mitochondrial PLA2s (the Ca2+-dependent cPLA2β and Ca2+-independent iPLA2γ, iPLA2β) can hydrolyze both sn-1 and sn-2 fatty acyl groups of phospholipids [40,44,45,46], with saturated fatty acids being predominantly at the sn-1 position [47]. Current data indicate that the group IV Ca2+-dependent cPLA2β3 (group IVB PLA2, encoded by PLA2G4B) displays PLA1, PLA2, and more powerful lysophospholipase activities and is constitutively associated with mitochondria [40]. Our results suggest that the Ca2+-dependent cPLA2 is localized mainly on the crista membranes of rat liver mitochondria. The accumulation of saturated long-chain FFAs in liver mitochondria incubated with divalent cations was confirmed previously [38,46], and their participation in Ca2+/H+ and K+/H+ exchange in the organelles was proposed [48]. Recent data also suggest that fatty acids in the presence of calcium ions can induce self-sustaining fluctuations in transmembrane voltage and current in biomimetic membranes without proteins [49,50].
As has been found earlier, palmitic and stearic acids can bind with Ca2+ ions with high affinity [25], and the formation of complexes of these fatty acids with Ca2+ in the membrane leads to the opening of short-lived Pal-mPT pores by the mechanism of the chemotropic phase transition in the lipid bilayer, as described previously for artificial membranes (liposomes and black lipid membranes) [22,23,24,25], erythrocytes [51], and mitochondria [21,22]. The formation of the non-selective lipid pore ensures the transport of ions along the gradient of their concentrations: the release of Sr2+ and K+ from mitochondria and the entry of H+ into the mitochondrial matrix. This promotes transient mitochondrial depolarization, and shrinking mitochondria become more condensed and their optical density is increased. Due to the ability of lipid pores to close spontaneously, the integrity and low permeability of the mitochondrial membrane to ions can be restored [21,22,26,29]. Pore closure contributes to the recovery of ΔΨm, the main driving force of ion transport in mitochondria, K+ re-uptake by mitochondria (in complex with valinomycin), and swelling of the organelles (optical density is decreased). Under the conditions of recovered ΔΨm, the mitochondria are able to re-uptake the released Sr2+ ions via MCUC, and the cycle can be repeated.
The experiments on pre-incubation of mitochondria with Ca2+(Sr2+)-dependent PLA2 inhibitors showed that the suppression of the enzyme activity led to a decline in the content of FFAs in the mitochondria in response to a Sr2+ pulse and the prevention of the second and subsequent spontaneous oscillation waves of the membrane potential, along with declines in the cycling of Sr2+ and K+ ions across the membrane, matrix volume, as well as spontaneous periodic changes in the rates of mitochondrial respiration and H2O2 generation. These results are consistent with our previous data showing that palmitic acid can re-start the CsA-insensitive Sr2+-induced release of Sr2+ after it has been abolished by the Ca2+-dependent PLA2 inhibitor AACOCF3 [12]. Therefore, it becomes clear that, to produce Sr2+-induced spontaneous oscillations in mitochondria, several factors are needed. One of the factors is a sharp increase in free Sr2+(Ca2+) in the mitochondrial matrix. It should be noted that, with slow continuous infusion of the same amount of strontium into a suspension of mitochondria, ion oscillations do not occur [10]. It may be that this is due to the binding of Sr2+ in the mitochondrial matrix and a decrease in the concentration of free ions required for the induction of Pal-mPT pores in the mitochondrial membrane. Another factor for triggering spontaneous oscillations in ion fluxes is an increase in the activity of mitochondrial PLA2 and accumulation of endogenous FFAs. The next factor for the occurrence of prolonged spontaneous oscillations in mitochondrial parameters is the maintenance of respiratory chain activity and membrane potential. As can be seen from Figure 2, a decrease in ΔΨm in each subsequent cycle attenuates ion oscillations; Sr2 + is accumulated by mitochondria at a lower rate, and the intramitochondrial concentration of the free ion may be insufficient to induce the opening of Pal-mPT pores. Most probably, under physiological conditions, mitochondrial oscillations exist for a longer time.
Accumulating evidence shows that the pulse mode of changes in the concentrations of ions in the cytoplasm and mitochondria has physiological importance [4,5,52,53]. This way of transmitting the signal within the cell is faster than simple diffusion and provides the spatiotemporal regulation of Ca2+-dependent cell functions, since the encoding of a calcium signal can be realized via the modulation of the amplitude and frequency of oscillations in ion concentration. Synchronous Ca2+ oscillations in mitochondria are involved in the propagation of intracellular calcium waves that regulate multiple signaling cascades in the cytoplasm of both excitable and non-excitable cells [52,53,54].
A transient change in membrane permeability to calcium ions due to the formation of short-lived pores in the mitochondrial membrane may regulate intracellular calcium homeostasis and the volume of mitochondria, thereby protecting them from calcium overload and osmotic shock. As is known, mitochondrial calcium overload is the major cause of the formation of the mPTP, the collapse of mitochondrial membrane potential, and, ultimately, the induction of cell death [55,56]. The Pal-mPT pore may be one of the systems for a rapid release of calcium ions and the maintenance of mitochondrial function under conditions accompanied by a sharp increase in [Ca2+] in the matrix. The futile cycles of the ion mediated by MCUC and Pal-mPT pore functioning in mitochondria may lead to a mild uncoupling accompanied by a significant decrease in ROS production. As shown in this work, there is a direct relationship between the dynamic changes in the rate of H2O2 production by mitochondria and the value of the mitochondrial membrane potential (Figure 4). Earlier, a similar correlation has been shown only in static models using a low concentration of the uncouplers of oxidative phosphorylation and blockers of mitochondrial respiration [57,58]. The data confirm the idea that cells may be protected from oxidative stress and over-reduction of respiratory chain complexes by a mild but persistent decrease in the membrane potential of mitochondria [58,59]. It is known that membrane phospholipids are the primary targets of ROS. Periodic influx and efflux of Ca2+ during Ca2+-induced oscillations in mitochondria may provide hydrolysis of oxidized phospholipids by Ca2+-dependent PLA2 in the phase of Ca2+ accumulation in the matrix, followed by a decrease in the activity of phospholipase A2 after the ion release. This suggestion is supported by the data on periodic changes in the content of lysophospholipids in mitochondria during Sr2+-dependent oscillations [60]. Therefore, the oscillatory mode of mitochondrial function may contribute to the protection of cells and mitochondria from oxidative stress and calcium overload, which is associated with the development of many pathologies, such as cardiovascular diseases, neurological disorders, diabetes, obesity, cancer, and others [4,5,16,61].
5. Conclusions
In this work, it was found that, during prolonged ion oscillations in response to a Sr2+ pulse, the efflux of ions from mitochondria down their concentration gradients can be mediated by the opening of the short-living pore induced by fatty acids (mainly, palmitic and stearic acids) and Sr2+. The data obtained allow us to suggest that the transient palmitate/Sr2+(Ca2+)-induced pore is a tool for the rapid release of calcium ions from mitochondria, which may be involved in preventing mitochondrial calcium overload and regulating cell ion homeostasis. Studies of the Pal/Ca2+(Sr2+)-induced lipid pore hold promise for understanding the mechanism of the oscillatory mode of mitochondrial function under physiological and pathological conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/membranes12070667/s1, Figure S1: Simultaneous recording of Sr2+/valinomycin-induced oscillations in K+ fluxes and external pH in the suspension of rat liver mitochondria; Figure S2: Ruthenium red (RR, 1 μM) prevents the extension of the Sr2+/valinomycin-induced cyclic changes in strontium ion fluxes in rat liver mitochondria; Figure S3: Gas–liquid chromatography profile of FFA methyl esters obtained from rat liver mitochondria before (control) and after the onset of Sr2+/valinomycin-induced ion oscillations in the absence (0.1% DMSO) or presence of 25 μM aristolochic acid (ArA), a phospholipase A2 inhibitor; Figure S4: Blocking effect of AACOCF3 (15 μM), a Ca2+-dependent phospholipase A2 inhibitor, on Sr2+/valinomycin-induced cyclic changes in the fluxes of Sr2+, K+, and TPP+ in rat liver mitochondria; Figure S5: The Ca2+-independent phospholipase A2 inhibitor PACOCF3 (20 μM) has no effect on Sr2+/valinomycin-induced cyclic changes in the fluxes of Sr2+, K+, TPP+, and the respiration rate of rat liver mitochondria; Figure S6: Typical immunoelectron micrographs of isolated rat liver mitochondria treated with antibodies against the group IV cytosolic phospholipase A2 or 0.1 M PBS buffer in the presence of 10 nm colloidal gold-labeled antibodies.
Author Contributions
Conceptualization, N.V.B. and G.D.M.; investigation, N.V.B., L.L.P., K.N.B. and M.I.S.; writing—original draft preparation, N.V.B.; writing—review and editing, N.V.B., G.D.M. and N.-E.L.S.; project administration, G.D.M.; funding acquisition, G.D.M. and N.-E.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
Institutional Review Board Statement
All manipulations with rats were performed in accordance with the European Convention for the Protection of Vertebrates used for experimental and other purposes (Strasbourg, 1986) and the principles of the Helsinki Declaration (2000). All the protocols were approved by the Ethics Committees of the University of Helsinki and the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences (Protocol No. 19/2020 of 18.02.2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wacquier, B.; Combettes, L.; Dupont, G. Cytoplasmic and mitochondrial calcium signaling: A two-way relationship. Cold Spring Harb. Perspect. Biol. 2020, 12, a038802. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.-E.L.; Carafoli, E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry 2005, 70, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell. Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Mitochondrial oscillations in physiology and pathophysiology. Adv. Exp. Med. Biol. 2008, 641, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Wacquier, B.; Combettes, L.; Van Nhieu, G.T.; Dupont, G. Interplay between intracellular Ca2+ oscillations and Ca2+-stimulated mitochondrial metabolism. Sci. Rep. 2016, 6, 19316. [Google Scholar] [CrossRef]
- Gylkhandanyan, A.V.; Evtodienko, Y.V.; Zhabotinsky, A.M.; Kondrashova, M.N. Continuous Sr2+-induced oscillations of the ionic fluxes in mitochondria. FEBS Lett. 1976, 66, 644–647. [Google Scholar] [CrossRef]
- Evtodienko, Y.V.; Zinchenko, V.P.; Holmuhamedov, E.L.; Gylkhandanyan, A.V.; Zhabotinsky, A.M. The stoichiometry of ion fluxes during Sr2+-induced oscillations in mitochondria. Biochim. Biophys. Acta 1980, 589, 157–161. [Google Scholar] [CrossRef]
- Holmuhamedov, E.L.; Teplova, V.V.; Chukhlova, E.A. Excitability of inner mitochondrial membrane. II. Reversible Sr2+-induced Sr2+ release from mitochondria. Biol. Membr. 1991, 8, 612–620. [Google Scholar]
- Teplova, V.V.; Sidash, S.S.; Makarov, P.R.; Evdotienko, Y.V. Characteristics of reversible and irreversible Ca2+-induced Ca2+ release from mitochondria in permeabilized Ehrlich ascites carcinoma cells. Biokhimiia 1995, 60, 944–950. [Google Scholar]
- Holmuhamedov, E.L.; Teplova, V.V.; Chukhlova, E.A.; Evtodienko, Y.V.; Ulrich, R.G. Strontium excitability of the inner mitochondrial membrane: Regenerative strontium-induced strontium release. Biochem. Mol. Biol. Int. 1995, 36, 39–49. [Google Scholar]
- Sidash, S.S.; Evtodienko, I.V.; Kholmokhamedov, E.L.; Teplova, V.V. Characteristics of Sr2+-induced permeability increase in the inner mitochondrial membrane. Membr. Cell Biol. 1995, 8, 447–454. [Google Scholar]
- Mironova, G.D.; Saris, N.E.; Belosludtseva, N.V.; Agafonov, A.V.; Elantsev, A.B.; Belosludtsev, K.N. Involvement of palmitate/Ca2+(Sr2+)-induced pore in the cycling of ions across the mitochondrial membrane. Biochim. Biophys. Acta 2015, 1848, 488–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chance, B.; Ghosh, A.; Pye, E. Biological and Biochemical Oscillators, 1st ed.; Academic Press: New York, NY, USA; London, UK, 1973; p. 534. [Google Scholar]
- Garbincius, J.F.; Elrod, J.W. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022, 102, 893–992. [Google Scholar] [CrossRef] [PubMed]
- Elustondo, P.A.; Nichols, M.; Robertson, G.S.; Pavlov, E.V. Mitochondrial Ca2+ uptake pathways. J. Bioenerg. Biomembr. 2017, 49, 113–119. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Dubinin, M.V.; Belosludtseva, N.V.; Mironova, G.D. Mitochondrial Ca2+ transport: Mechanisms, molecular structures, and role in cells. Biochemistry 2019, 84, 593–607. [Google Scholar] [CrossRef]
- Grant, C.W.; Usachev, Y.M. MCU (mitochondrial Ca2+ uniporter) makes the calcium go round. J. Biol. Chem. 2022, 298, 101604. [Google Scholar] [CrossRef]
- Buckman, J.F.; Reynolds, I.J. Spontaneous changes in mitochondrial membrane potential in cultured neurons. J. Neurosci. 2001, 21, 5054–5065. [Google Scholar] [CrossRef]
- Vergun, O.; Votyakova, T.V.; Reynolds, I.J. Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria. Biophys. J. 2003, 85, 3358–3366. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; Marbán, E.; O’Rourke, B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003, 278, 44735–44744. [Google Scholar] [CrossRef]
- Mironova, G.D.; Belosludtsev, K.N.; Belosludtseva, N.V.; Gritsenko, E.N.; Khodorov, B.I.; Saris, N.-E.L. Mitochondrial Ca2+cycle mediated by the palmitate-activated cyclosporin A-insensitive pore. J. Bioenerg. Biomembr. 2007, 39, 167–174. [Google Scholar] [CrossRef]
- Mironova, G.D.; Gritsenko, E.; Gateau-Roesch, O.; Levrat, C.; Agafonov, A.; Belosludtsev, K.; Prigent, A.F.; Muntean, D.; Dubois, M.; Ovize, M. Formation of palmitic acid/Ca2+complexes in the mitochondrial membrane: A possible role in the cyclosporin-insensitive permeability transition. J. Bioenerg. Biomembr. 2004, 36, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Agafonov, A.; Gritsenko, E.; Belosludtsev, K.; Kovalev, A.; Gateau-Roesch, O.; Saris, N.-E.L.; Mironova, G.D. A permeability transition in liposomes induced by the formation of Ca2+/palmitic acid complexes. Biochim. Biophys. Acta 2003, 1609, 153–160. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Gritsenko, E.N.; Shlyapnikova, E.A.; Kharakoz, D.P.; Belosludtseva, N.V.; Lezhnev, E.I.; Saris, N.-E.L.; Mironova, G.D. Ca2+-induced phase separation in the membrane of palmitate-containing liposomes and its possible relation to membrane permeabilization. J. Membr. Biol. 2007, 215, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Mironova, G.D.; Gateau-Roesch, O.; Levrat, C.; Gritsenko, E.; Pavlov, E.; Lazareva, A.V.; Limarenko, E.; Rey, C.; Louisot, P.; Saris, N.-E.L. Palmitic and stearic acids bind Ca2+ with high affinity and form nonspecific channels in black-lipid membranes. Possible relation to Ca2+-activated mitochondrial pores. J. Bioenerg. Biomembr. 2001, 33, 319–331. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Mironova, G.D. Possible mechanism for formation and regulation of the palmitate-induced cyclosporin A-insensitive mitochondrial pore. Biochemistry 2005, 70, 815–821. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Saris, N.E.; Belosludtseva, N.V.; Trudovishnikov, A.S.; Lukyanova, L.D.; Mironova, G.D. Physiological aspects of the mitochondrial cyclosporin A-insensitive palmitate/Ca2+-induced pore: Tissue specificity, age profile and dependence on the animal’s adaptation to hypoxia. J. Bioenerg. Biomembr. 2009, 41, 395–401. [Google Scholar] [CrossRef]
- Sultan, A.; Sokolove, P.M. Free fatty acid effects on mitochondrial permeability: An overview. Arch. Biochem. Biophys. 2001, 386, 52–61. [Google Scholar] [CrossRef]
- Sultan, A.; Sokolove, P.M. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 2001, 386, 37–51. [Google Scholar] [CrossRef]
- Mironova, G.D.; Pavlov, E.V. Mitochondrial cyclosporine A-independent palmitate/Ca2+-induced permeability transition pore (PA-mPT Pore) and its role in mitochondrial function and protection against calcium overload and glutamate toxicity. Cells 2021, 10, 125. [Google Scholar] [CrossRef]
- Antonov, V.F.; Shevchenko, E.V. Lipid pores and stability of cell membranes. Prog. Biophys. Mol. Biol. 1996, 65, 319–325. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Aiuchi, T.; Matsunaga, M.; Nakaya, K.; Nakamura, Y. Calculation of membrane potential in synaptosomes with use of a lipophilic cation (tetraphenylphosphonium). Chem. Pharm. Bull. 1989, 37, 3333–3337. [Google Scholar] [CrossRef] [PubMed]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Talanov, E.Y.; Tenkov, K.S.; Starinets, V.S.; Agafonov, A.A.; Pavlik, L.L.; Dubinin, M.V. Effect of bedaquiline on the functions of rat liver mitochondria. Biochim. Biophys. Acta Biomembr. 2019, 1861, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Garlid, K.D.; Paucek, P. The mitochondrial potassium cycle. IUBMB Life 2001, 52, 153–158. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Kargapolov, A.V. Changes in the phospholipid content of intact mitochondria under mitochondrial swelling in hypotonic sucrose solutions. Biokhimiia 1979, 44, 293–296. [Google Scholar]
- Saris, N.-E.L. Stimulation of phospholipase A2 activity in mitochondria by magnesium and polyamines. Magnes. Res. 1994, 7, 5–10. [Google Scholar]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating phospholipase A2 biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef]
- Kravenska, Y.; Checchetto, V.; Szabo, I. Routes for potassium ions across mitochondrial membranes: A biophysical point of view with special focus on the ATP-sensitive K+ channel. Biomolecules 2021, 11, 1172. [Google Scholar] [CrossRef]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Mironova, G.D.; Belosludtsev, K.N.; Surin, A.M.; Trudovishnikov, A.S.; Belosludtseva, N.V.; Pinelis, V.G.; Krasilynikova, I.A.; Khodorov, B.I. Mitochondrial lipid pore in the mechanism of glutamate-induced calcium disturbance of neurons. Biochem. Suppl. Ser. A Membr. Cell Biol. 2012, 6, 45–55. [Google Scholar] [CrossRef]
- Ghosh, M.; Loper, R.; Gelb, M.H.; Leslie, C.C. Identification of the expressed form of human cytosolic phospholipase A2β(cPLA2β). cPLA2β3 is a novel variant localized to mitochondria and early endosomes. J. Biol. Chem. 2006, 281, 16615–16624. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Taketomi, Y.; Miki, Y.; Sato, H.; Hirabayashi, T.; Yamamoto, K. Recent progress in phospholipase A2 research: From cells to animals to humans. Prog. Lipid Res. 2011, 50, 152–192. [Google Scholar] [CrossRef]
- Moon, S.H.; Jenkins, C.M.; Liu, X.; Guan, S.; Mancuso, D.J.; Gross, R.W. Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J. Biol. Chem. 2012, 287, 14880–14895. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, P.; Wieckowski, M.R.; Wojtczak, L. Long-chain fatty acid-promoted swelling of mitochondria: Further evidence for the protonophoric effect of fatty acids in the inner mitochondrial membrane. FEBS Lett. 2000, 471, 108–112. [Google Scholar] [CrossRef]
- Kocherginsky, N. Biomimetic membranes with aqueous nanochannels. Phase transitions and oscillations. Membr. Membr. Technol. 2021, 3, 442–447. [Google Scholar] [CrossRef]
- Kocherginsky, N. Biomimetic membranes without proteins but with aqueous nanochannels and facilitated transport. Membr. Membr. Technol. 2021, 3, 434–441. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Trudovishnikov, A.S.; Belosludtseva, N.V.; Agafonov, A.V.; Mironova, G.D. Palmitic acid induces the opening of a Ca2+-dependent pore in the plasma membrane of red blood cells: The possible role of the pore in erythrocyte lysis. J. Membr. Biol. 2010, 237, 13–19. [Google Scholar] [CrossRef]
- Wang, F.; Li, A.; Meng, T.G.; Wang, L.-Y.; Wang, L.-J.; Hou, Y.; Schatten, H.; Sun, Q.-Y.; Ou, X.-H. Regulation of [Ca2+]i oscillations and mitochondrial activity by various calcium transporters in mouse oocytes. Reprod. Biol. Endocrinol. 2020, 18, 87. [Google Scholar] [CrossRef]
- Cortassa, S.; Aon, M.A.; Winslow, R.L.; O’Rourke, B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys. J. 2004, 87, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Briston, T.; Selwood, D.L.; Szabadkai, G.; Duchen, M.R. Mitochondrial permeability transition: A molecular lesion with multiple drug targets. Trends Pharmacol. Sci. 2019, 40, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Morciano, G.; Naumova, N.; Koprowski, P.; Valente, S.; Sardão, V.A.; Potes, Y.; Rimessi, A.; Wieckowski, M.R.; Oliveira, P.J. The mitochondrial permeability transition pore: An evolving concept critical for cell life and death. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2489–2521. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef]
- Kotova, E.A.; Antonenko, Y.N. Fifty years of research on protonophores: Mitochondrial uncoupling as a basis for therapeutic action. Acta Nat. 2022, 14, 4–13. [Google Scholar] [CrossRef]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial uncoupling: A key controller of biological processes in physiology and diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef]
- Wiswedel, I.; Barnstorf, U.; Augustin, W.; Holmuhamedov, E.; Medvedev, B.; Evtodienko, Y. Involvement of periodic deacylation-acylation cycles of mitochondrial phospholipids during Sr2+-induced oscillatory ion transport in rat liver mitochondria. Biochim. Biophys. Acta 1982, 688, 597–604. [Google Scholar] [CrossRef]
- Samanta, K.; Mirams, G.R.; Parekh, A.B. Sequential forward and reverse transport of the Na+/Ca2+ exchanger generates Ca2+ oscillations within mitochondria. Nat. Commun. 2018, 9, 156. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).