Alcohol Diffusion in Alkali-Metal-Doped Polymeric Membranes for Using in Alkaline Direct Alcohol Fuel Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
2.2.1. Membrane Doping
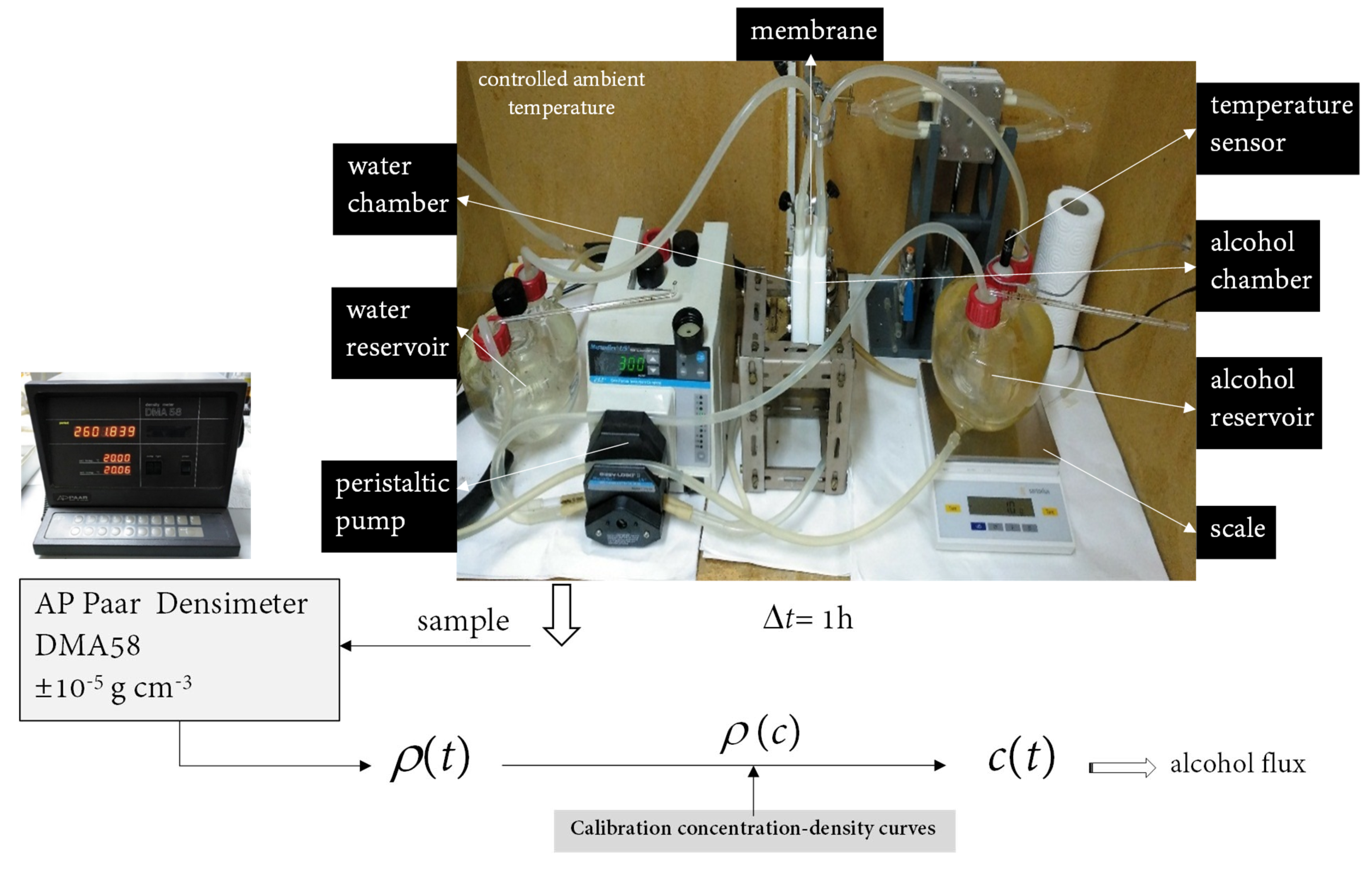
2.2.2. Alcohol Permeability
3. Results and Discussion
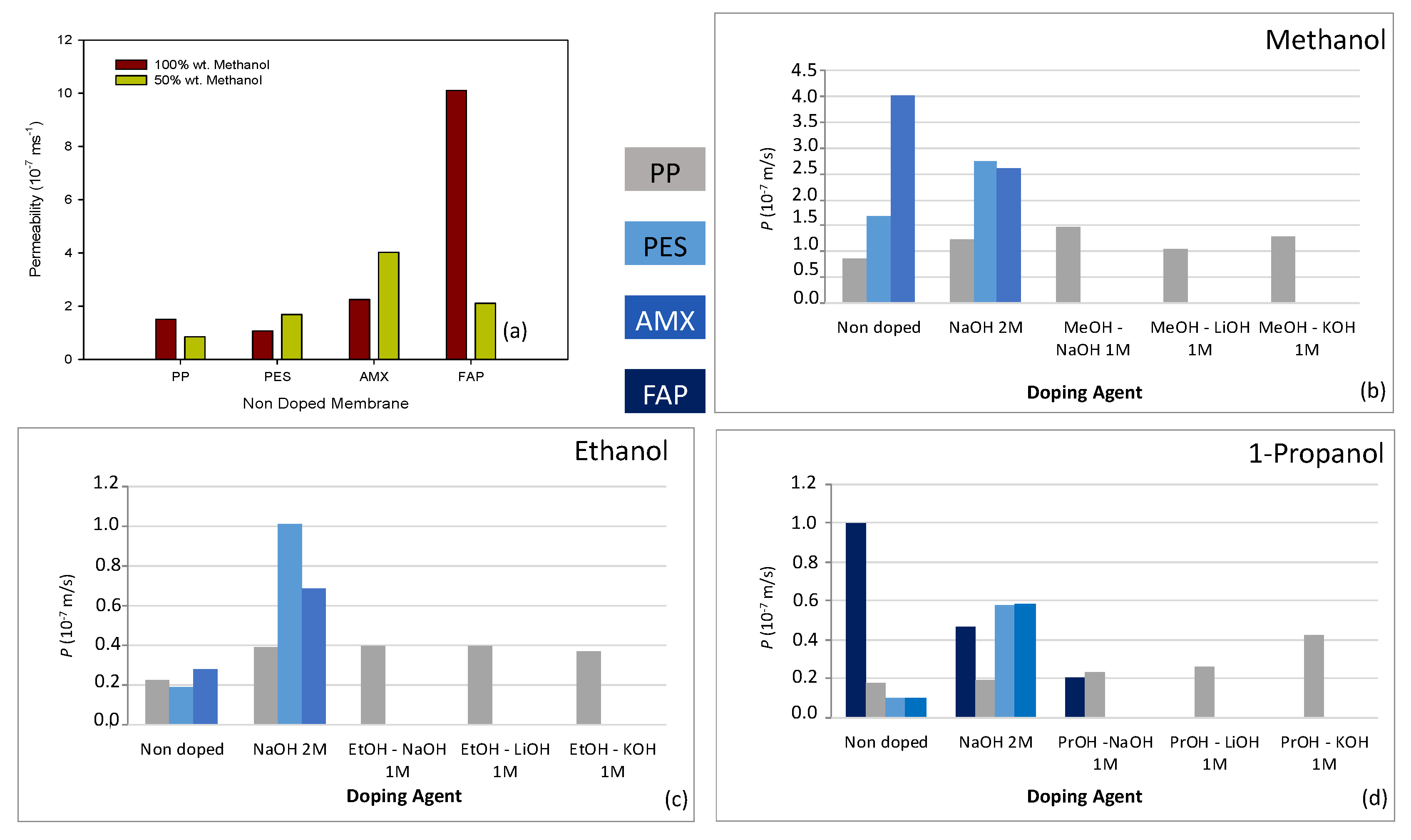
3.1. Alcohol Permeability for Nondoped Membranes
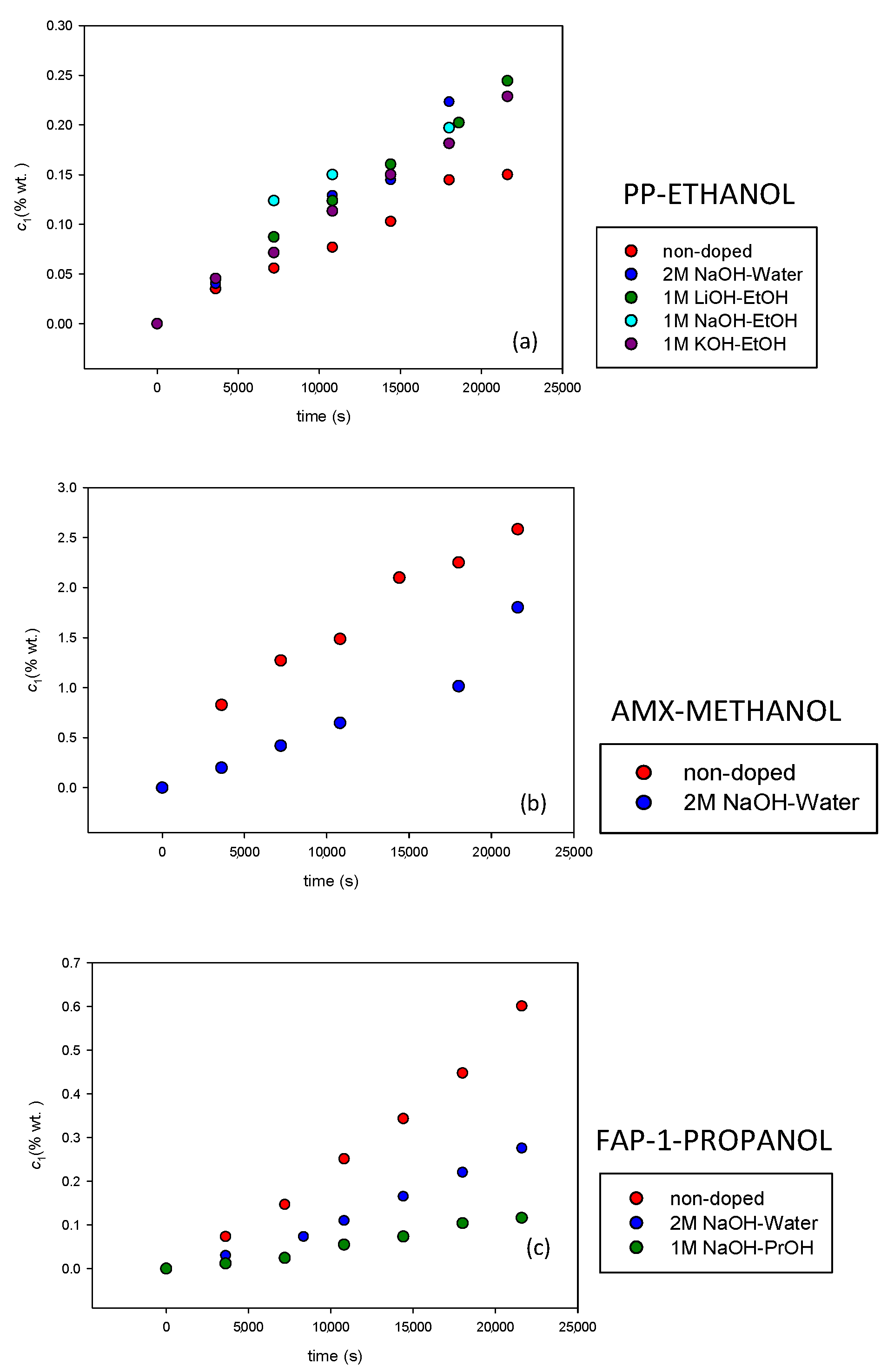
3.2. Alcohol Permeability for Doped Membranes
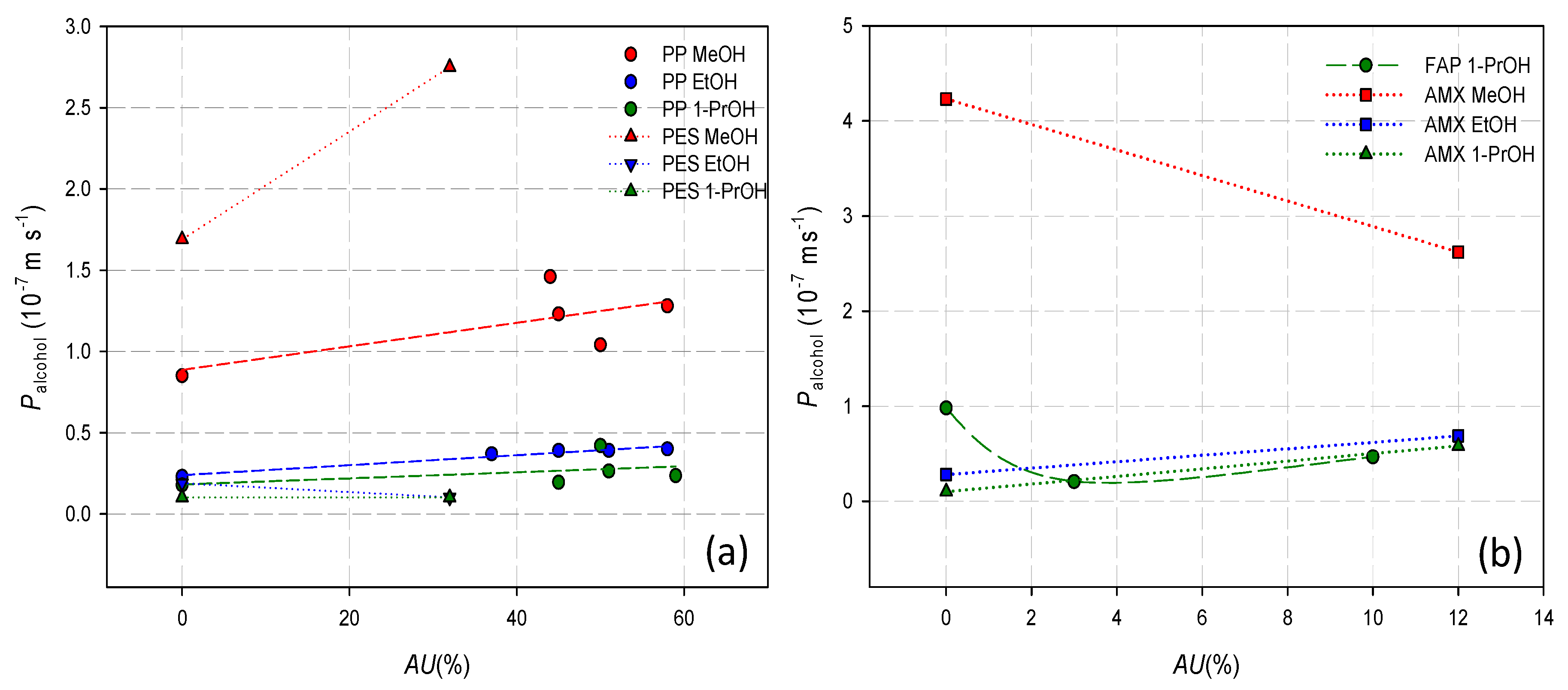
3.3. Alcohol Permeability and Doping Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board
Data Availability Statement
Conflicts of Interest
References
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. RSC Energy Environ. Ser. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Teik, J.; Goh, E.; Rasyidah, A.; Rahim, A.; Masdar, M.S.; Shyuan, L.K. Enhanced Performance of Polymer Electrolyte Membranes via Modification with Ionic Liquids for Fuel Cell Applications. Membranes 2021, 11, 395. [Google Scholar]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Paidar, M.; Fateev, V.; Bouzek, K. Membrane electrolysis—History, current status and perspective. Electrochim. Acta 2016, 209, 737–756. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Choi, J.-H.; Khanh, P.; Nguyen, T.; Kim, D.-J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef]
- López-Fernández, E.; Gómez-Sacedón, G.; Gi-Rostra, J.; Yubero, F.; González-Elipe, A.R.; de Lucas-Consuegra, A. Recent advances in alkaline exchange membrane water electrolysis and electrode manufacturing. Molecules 2021, 26, 6326. [Google Scholar] [CrossRef]
- Xu, F.; Su, Y.; Lin, B. Progress of Alkaline Anion Exchange Membranes for Fuel Cells: The Effects of Micro-Phase Separation. Front. Mater. 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.A.; Lister, T.E.; Rae, C.; Wood, N.D. Anion Exchange Membrane Electrolyzers as Alternative for Upgrading of Biomass-Derived Molecules. ACS Sustain. Chem. Eng. 2018, 6, 8458−8467. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sust. Energ. Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Hren, M.; Bozic, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline membrane fuel cells: Anion exchange membranes and fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- Halim, E.M.; Chemchoub, S.; El Attar, A.; Salih, F.E.; Oularbi, L.; El Rhazi, M. Recent Advances in Anode Metallic Catalysts Supported on Conducting Polymer-Based Materials for Direct Alcohol Fuel Cells. Front. Energy 2022, 10, 843736. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Effect of alkali doping on alkaline stability and cell performance of quaternization polyvinyl alcohol/graphene oxide membranes for passive DEFCs. Mater. Lett. 2021, 292, 129651. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wa, L.-Y.; Han, Y.; Hong, Y.; Huang, L.; Yang, X.; Wang, Y.; Zaghib, K.; Zhou, Z. KOH-doped polybenzimidazole membrane for direct hydrazine fuel cell. J. Colloid Int. Sci. 2020, 563, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Fadzillah, D.M.; Kamarudin, S.K.; Zainoodin, M.A.; Masdar, M.S. Critical challenges in the system development of direct alcohol fuel cells as portable power supplies: An overview. Int. J. Hydrogen Energy 2019, 44, 3031–3054. [Google Scholar] [CrossRef]
- Penchev, H.; Borisov, G.; Petkucheva, E.; Ublekov, F.; Sinigersky, V.; Radev, I.; Slavcheva, E. Highly KOH Doped para-Polybenzimidazole Anion Exchange Membrane and its Performance in Pt/TinO2n–1 Catalyzed Water Electrolysis Cell. Mater. Lett. 2018, 221, 128–130. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Tsampas, M.N.; Fredriksson, H.O.; Gracia, J.M.; Niemantsverdriet, J. Hydrogen from electrochemical reforming of C1–C3 alcohols using proton conducting membranes. Int. J. hydrogen Energy 2017, 42, 10762–10774. [Google Scholar] [CrossRef] [Green Version]
- Ulusoy, I.; Uzunoglu, A.; Ata, A.; Ozturk, O.; Ider, M. Hydrogen generation from alkaline solutions of methanol and ethanol by electrolysis. ECS Trans. 2009, 19, 77–94. [Google Scholar] [CrossRef]
- Shen, P.-K.; Wang, S.-L.; Hu, Z.-Y.; Li, Y.-L.; Zeng, R.; Huang, Y.-Q. Hydrogen production by alcohol electrolysis. Acta Phys. Chim. Sin. 2007, 23, 107–110. [Google Scholar]
- Sasikimar, G.; Muthumeenal, A.; Pethaiah, S.S.; Nachiappan, N.; Balaji, R. Aqueous methanol electrolysis using proton conducting membrane for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 5905–5910. [Google Scholar] [CrossRef]
- Yu, E.H.; Krever, U.; Scott, K. Principles and materials aspect of direct alkaline alcohol fuel cells. Energies 2010, 3, 1499–1528. [Google Scholar] [CrossRef]
- Chu, Y.J.; Shul, Y.G. Alcohol crossover behaviour in direct alcohol fuel cells (DDAFCs) System. Fuel Cells 2012, 1, 109–115. [Google Scholar] [CrossRef]
- An, L.; Zhao, T. Transport phenomena in alkaline direct ethanol fuel cells for sustainable energy production. J. Power Sources 2006, 160, 181–186. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y.J. Recent advancements in applications of alkaline anion exchange membranes for polymer electrolyte fuel cells. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Laín, L.; Barragán, V.M. Swelling properties of alkali-metal doped polymeric anion-exchange membranes in alcohol media for application in fuel cells. Int. J. Hydrogen Energy 2016, 41, 14160–14170. [Google Scholar] [CrossRef]
- González, B.; Calvar, N.; Gómez, E.; Dominguez, A. Density, dynamic viscosity, and derived properties of binary mixtures of methanol or ethanol with water, ethyl acetate, and methyl acetate at T = 293.15, 298.15, and 303.15 K. J. Chem. Thermodyn. 2007, 39, 1578–1588. [Google Scholar] [CrossRef]
- Pang, F.M.; Seng, C.E.; Teng, T.T.; Ibrahim, M.H. Densities and viscosities of aqueous solutions of 1-propanol and 2-propanol at temperatures from 293.15 K to 333.15 K. J. Mol. Liq. 2007, 136, 71–78. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; An, L.; Zhao, G.; Yan, X.H. Physicochemical properties of alkaline doped polybenzimidazole membranes for anion exchange membrane fuel cells. J. Membr. Sci. 2015, 493, 340–348. [Google Scholar] [CrossRef]
- Godino, M.P.; Barragán, V.M.; Villaluenga, J.P.G.; Izquierdo-Gil, M.A. Influence of the cationic form of an ion-exchange membrane in the permeability and solubility of methanol/water mixtures. Sep. Purif. Technol. 2015, 148, 10–14. [Google Scholar] [CrossRef]
- Pan, W.-H.; Lue, S.J.; Chang, C.-M.; Liu, Y.-L. Alkali doped polyvinyl alcohol/multi-walled carbon nanotube electrolyte for direct methanol alkaline fuel cell. J. Membr. Sci. 2011, 376, 225–232. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhao, T.S.; Yang, W.W. Measurements of water uptake and transport properties in anion-exchange membranes. Int. J. Hydrogen Energy 2010, 35, 5656–5665. [Google Scholar] [CrossRef]
- Mukoma, P.; Jooste, B.R.; Vosloo, H.C.M. A comparison of methanol permeability in Chitosan and Nafion 117 membranes at high to medium methanol concentrations. J. Membr. Sci. 2004, 243, 293–299. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Zakaria, Z. Enhanced alkaline stability and performance of alkali-doped quaternized poly(vinyl alcohol) membranes for passive direct ethanol fuel cell. Int. J. Energy Res. 2019, 43, 5252–5265. [Google Scholar] [CrossRef]
- Khattab, S.; Bandarkar, F.; Fakhree, M.A.A.; Jonyban, A. Density, viscosity, and surface tension of water+ethanol mixtures from 293 to 323 K. Korean J. Chem. Eng. 2012, 29, 812–817. [Google Scholar] [CrossRef]
- Calculators. Available online: http://www.handymath.com/ (accessed on 26 May 2022).
- Herráez, J.V.; Belda, R. Refractive indices, densities and excess molar volumes and monoalcohols + water. J. Solut. Chem. 2006, 35, 1315–1328. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Khinnavar, R.S. Diffusion and sorption of organic liquids through polymer membranes: 10- Polyurethane, nitrile-butadiene rubber and epichlorohydrin versus aliphatic alcohols (C1–C6). Polymer 1993, 34, 5. [Google Scholar] [CrossRef]
- Bernardo, G. Diffusivity of alcohols in amorphous polystyrene. J. Appl. Polym. Sci. 2013, 127, 1803–1811. [Google Scholar] [CrossRef]
- Xue, S.; Yin, G. Methanol permeability in sulfonated poly(etheretherketone) membranes: A comparison with Nafion membranes. Eur. Polym. J. 2006, 42, 776–785. [Google Scholar] [CrossRef]
- Godino, M.P.; Barragán, V.M.; Villaluenga, J.P.G.; Izquierdo-Gil, M.A.; Ruiz-Bauzá, C.; Seoane, B. Liquid transport through sulfonated cation-exchange membranes for different water-alcohol solutions. Chem. Eng. J. 2010, 162, 643–648. [Google Scholar] [CrossRef]
- Zhao, T.S.; Li, Y.S.; Shen, S.Y. Anion-exchange membrane direct ethanol fuel cells: Status and perspective. Front. Energy Power Eng. China 2010, 4, 443–458. [Google Scholar] [CrossRef]

| Membrane | d (μm) * | IEC (meq g−1) * | Selectivity * | R (Ω cm2) * | ρ (kg m−3) ** |
|---|---|---|---|---|---|
| PES | 450 | 1.8 | >0.95 | <7.5 | 945 |
| PP | 440 | 1.8 | >0.95 | <8.0 | 917 |
| AMX | 140 | 1.5 | ≥0.98 | <3.5 | 1090 |
| FAP | 50 | 1.2 | >0.92 | <1.5 | 1132 |
| Pure Liquid | ρ (kg m−3) * | ν* (mPa s) | M (10−3 kg mol−1) | |
|---|---|---|---|---|
| Water | H2O | 995.7 | 0.797 | 18.0 |
| MeOH | CH4O | 782.0 | 0.508 | 32.04 |
| EtOH | C2H6O | 781.3 | 0.987 | 46.07 |
| 1-PrOH | C3H8O | 796.4 | 1.726 | 60.09 |
| Water–Alcohol Mixture | ρ(kg m−3) * | ν*(mPa s) | ||
| 1M | MeOH | 999.2 | 0.756 | |
| EtOH | 976.4 | 0.709 | ||
| 1-PrOH | 990.2 | 0.920 | ||
| P (10−8 ms−1) | ||||
|---|---|---|---|---|
| PP | PES | AMX | FAP | |
| MeOH 100% | 15.0 ± 0.1 | 10.6 ± 0.1 | 22 ± 4 | 101 ± 8 |
| MeOH 50% | 8.3 ± 0.1 | 16.7 ± 0.1 | 42.1 ± 0.1 | 20.9 ± 0.1 |
| EtOH 50% | 2.27 ± 0.14 | 1.88 ± 0.14 | 2.82 ± 0.15 | -- |
| 1-PrOH 50 % | 1.82 ± 0.14 | 1.01 ± 0.18 | 1.02 ± 0.18 | 9.7 ± 0.4 |
| P (10−8 ms−1) | |||
|---|---|---|---|
| PP | MeOH | EtOH | 1-PrOH |
| Nondoped | 8.3 ± 0.1 | 2.27 ± 0.15 | 1.82 ± 0.14 |
| NaOH 2M Water | 12.1 ± 0.1 | 3.9 ± 0.3 | 1.93 ± 0.07 |
| LiOH 1M | 10.3 ± 0.2 | 3.92 ± 0.07 | 2.62 ± 0.25 |
| NaOH 1M | 14.4 ± 0.1 | 4.00 ± 0.07 | 2.31 ± 0.25 |
| KOH 1M | 12.6 ± 0.2 | 3.68 ± 0.07 | 4.17 ± 0.20 |
| P (10−8 ms−1) | |||
|---|---|---|---|
| MeOH | EtOH | 1-PrOH | |
| PES | |||
| Nondoped | 16.7 ± 0.1 | 1.88 ± 0.14 | 1.01 ± 0.18 |
| NaOH 2M Water | 27.3 ± 0.3 | 10.1 ± 0.3 | 5.8 ± 0.3 |
| AMX | |||
| Nondoped | 42.1 ± 0.3 | 2.82 ± 0.15 | 1.02 ± 0.18 |
| NaOH 2M Water | 26.0 ± 0.3 | 6.83 ± 0.14 | 5.78 ± 0.18 |
| FAP | |||
| Nondoped | 20.9 ± 0.1 | -- | 9.7 ± 0.4 |
| NaOH 2M Water | -- | -- | 4.65 ± 0.25 |
| NaOH 1M | -- | -- | 2.05 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Nieto, A.; Muñoz, S.; Barragán, V.M. Alcohol Diffusion in Alkali-Metal-Doped Polymeric Membranes for Using in Alkaline Direct Alcohol Fuel Cells. Membranes 2022, 12, 666. https://doi.org/10.3390/membranes12070666
Fernández-Nieto A, Muñoz S, Barragán VM. Alcohol Diffusion in Alkali-Metal-Doped Polymeric Membranes for Using in Alkaline Direct Alcohol Fuel Cells. Membranes. 2022; 12(7):666. https://doi.org/10.3390/membranes12070666
Chicago/Turabian StyleFernández-Nieto, Andrea, Sagrario Muñoz, and Vicenta María Barragán. 2022. "Alcohol Diffusion in Alkali-Metal-Doped Polymeric Membranes for Using in Alkaline Direct Alcohol Fuel Cells" Membranes 12, no. 7: 666. https://doi.org/10.3390/membranes12070666
APA StyleFernández-Nieto, A., Muñoz, S., & Barragán, V. M. (2022). Alcohol Diffusion in Alkali-Metal-Doped Polymeric Membranes for Using in Alkaline Direct Alcohol Fuel Cells. Membranes, 12(7), 666. https://doi.org/10.3390/membranes12070666







