Surface Optimization of Commercial Porous Ti Substrates by EPD of Titanium Nitride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Preparation of TiN Suspensions
2.3. TiN Powders Infiltration by EPD
Single Steps Tests
2.4. Multi-Step Tests
2.5. Characterization
3. Results and Discussion
3.1. Characterization of TiN Powders
3.2. Characterization of As-Received and Polished Ti Substrates
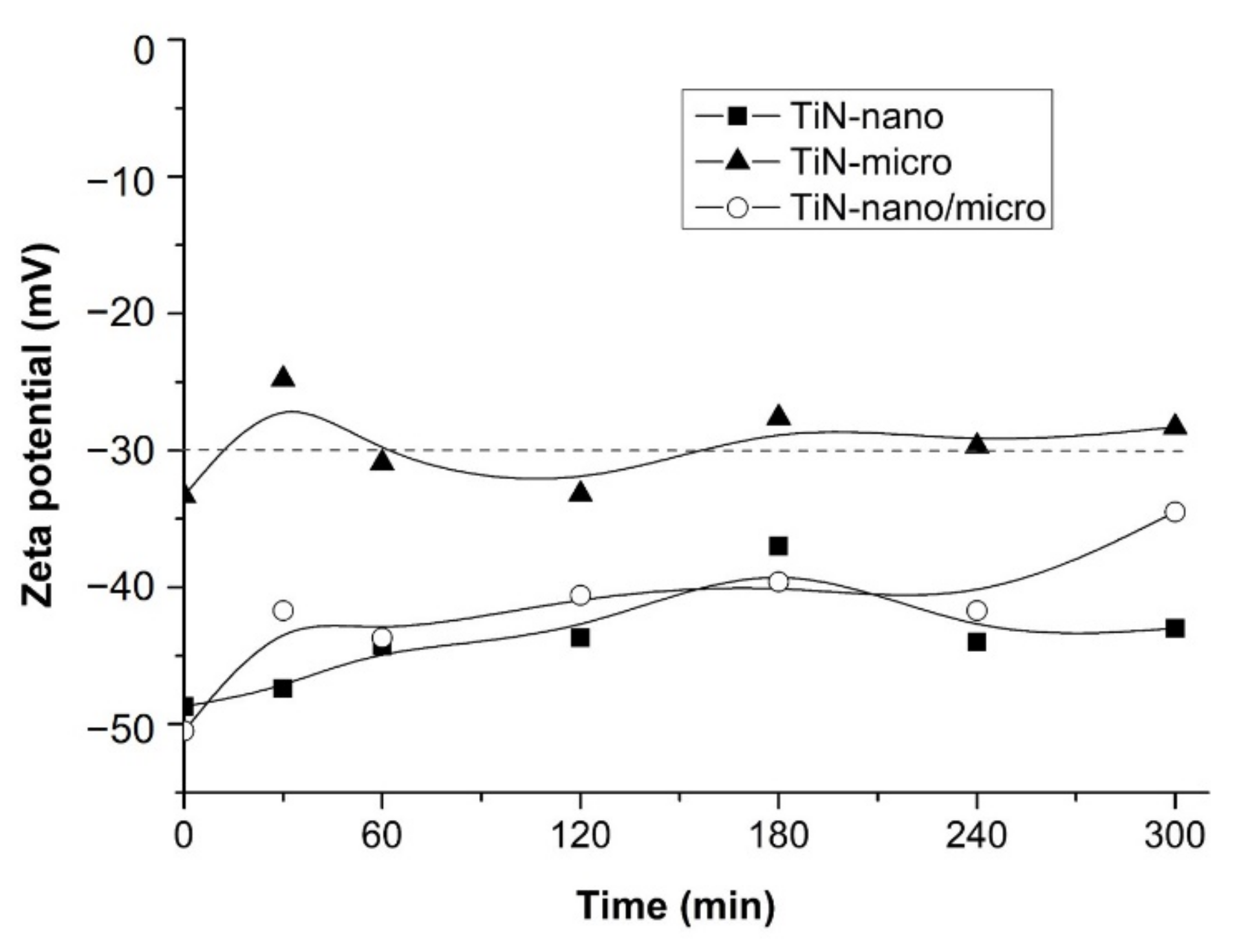
3.3. Characterization of TiN Suspensions
3.4. TiN Powders Infiltration by EPD
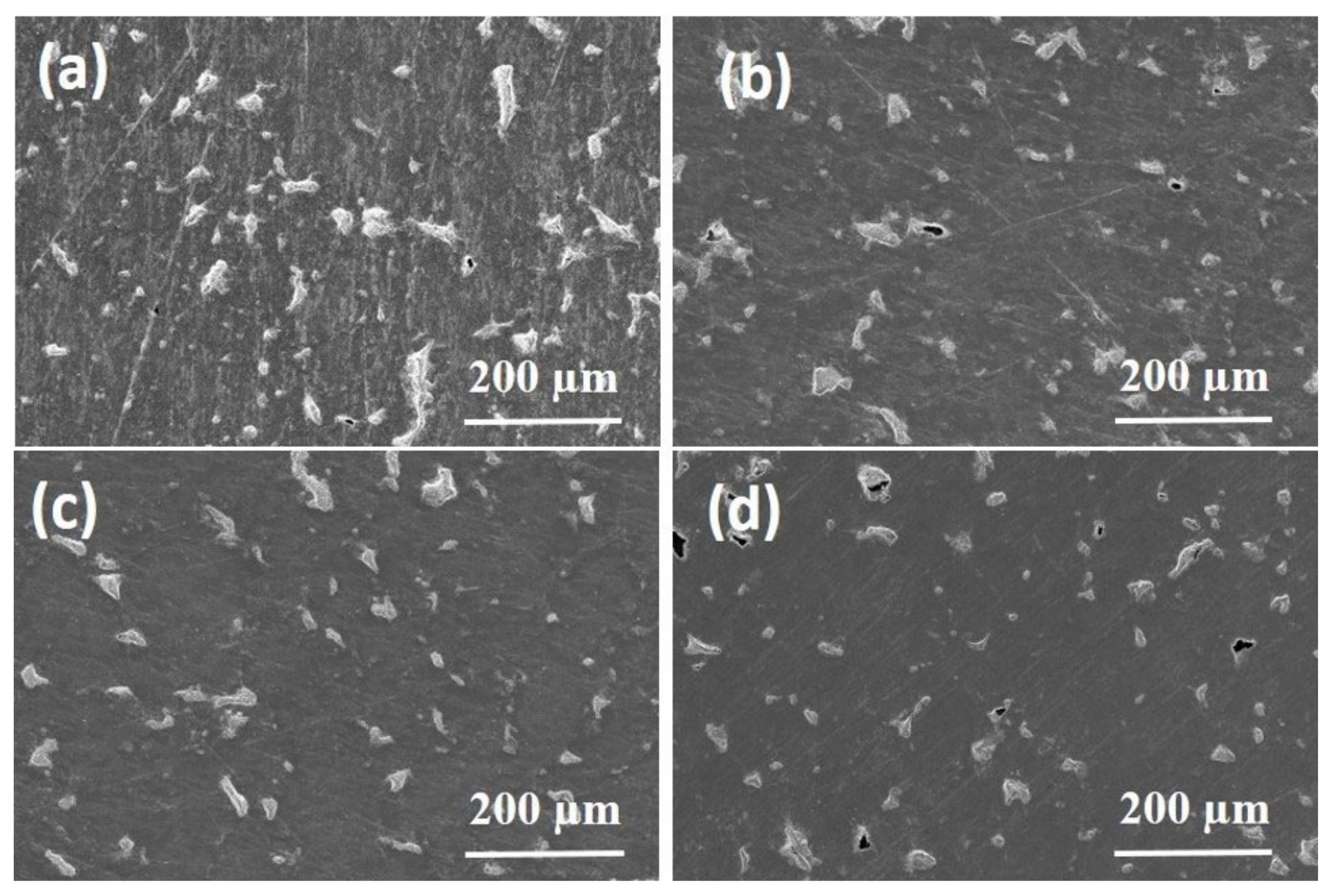
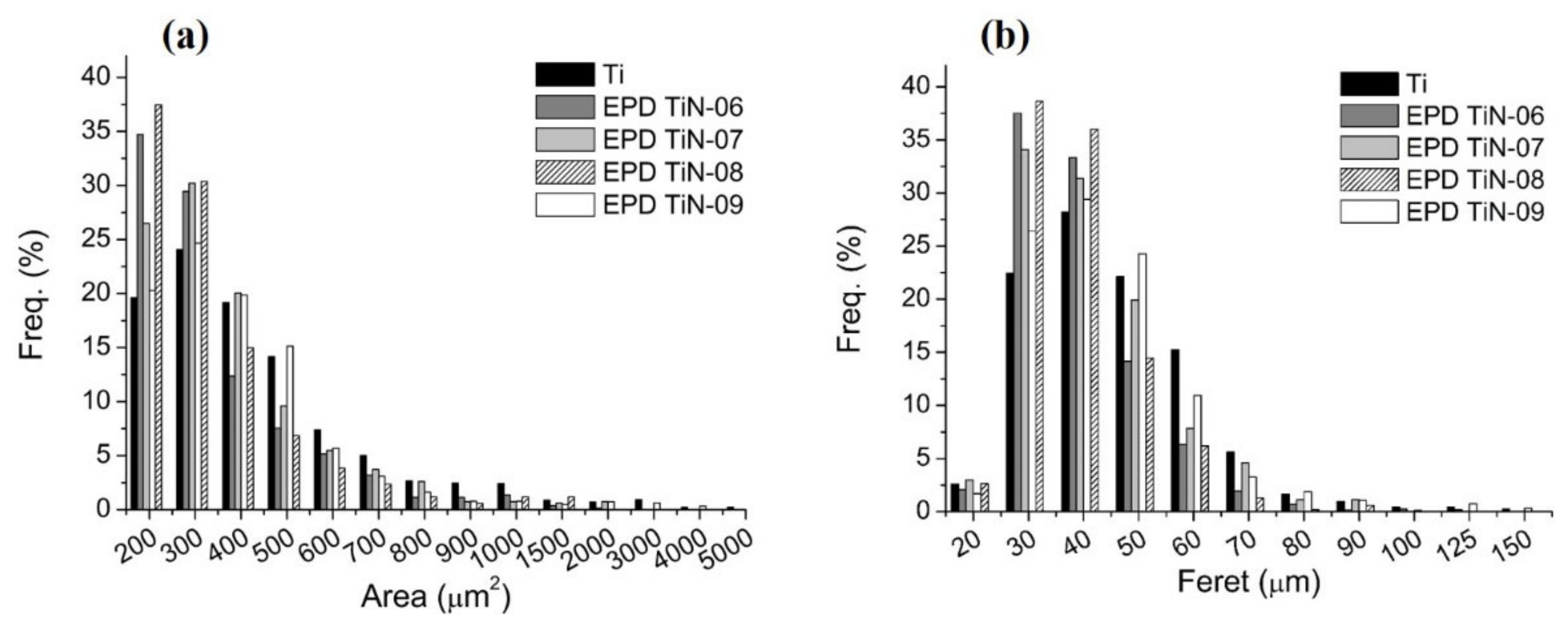
3.5. Surface Topology Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva Veras, T.; Mozer, T.S.; dos Santos, M.; Da Costa, R.; Da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New perspectives on fuel cell technology: A brief review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, F.; Medrano, J.A.; Fernandez, E.; Melendez, J.; van Sint Annaland, M.; Pacheco-Tanaka, D.A. Advances on high temperature Pd-based membranes and membrane reactors for hydrogen purifcation and production. J. Membr. Sci. Res. 2017, 3, 142–156. [Google Scholar] [CrossRef]
- Bredesen, R.; Peters, T.A.; Stange, M.; Vicinanza, N.; Venvik, J.H. Palladium-based membranes in hydrogen production. In Membrane Engineering for the Treatment of Gases: Volume 2: Gas-Separation Problems Combined with Membrane Reactors, 1st ed.; Drioli, E., Barbieri, G., Eds.; RSC Publishing: London, UK, 2011; pp. 40–86. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-based membranes for hydrogen separation: Review of alloying elements and their influence on membrane properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Nooijer, N.D.; Arratibel Plazaola, A.; Meléndez Rey, J.; Fernandez, E.; Pacheco Tanaka, D.A.; Sint Annaland, M.V.; Gallucci, F. Long-term stability of thin-film Pd-based supported membranes. Processes 2019, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.kitco.com/charts/livepalladium.html (accessed on 25 March 2022).
- Mortalò, C.; Barison, S.; Rebollo, E.; Fabrizio, M. Membrane Engineering for the Treatment of Gases: Gas-Separation Issues Combined with Membrane Reactors; Drioli, E., Barbieri, G., Brunetti, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; Volume 2, pp. 273–302. [Google Scholar] [CrossRef]
- Meulenberg, W.A.; Schulze-Küppers, F.; Deibert, W.; Van Gestel, T.; Baumann, S. Ceramic membranes: Materials–components–potential applications. Chem. Biol. Eng. Rev. 2019, 6, 198–208. [Google Scholar] [CrossRef]
- Hashim, S.S.; Somalu, M.R.; Loh, K.S.; Liu, S.; Zhou, W.; Sunarso, J. Perovskite-based proton conducting membranes for hydrogen separation: A review. Int. J. Hydrog. Energy 2018, 43, 15281–15305. [Google Scholar] [CrossRef]
- Montaleone, D.; Mercadelli, E.; Escolástico, S.; Gondolini, A.; Serra, J.M.; Sanson, A. All-ceramic asymmetric membranes with superior hydrogen permeation. J. Mater. Chem. A 2018, 6, 15718–15727. [Google Scholar] [CrossRef]
- Mortalò, C.; Rebollo, E.; Escolástico, S.; Deambrosis, S.; Haas-Santo, K.; Rancan, M.; Dittmeyer, R.; Armelao, L.; Fabrizio, M. Enhanced sulfur tolerance of BaCe0.65Zr0.20Y0.15O3-δ-Ce0.85Gd0.15O2-δ composite for hydrogen separation membranes. J. Membr. Sci. 2018, 564, 123–132. [Google Scholar] [CrossRef]
- Mortalò, C.; Santoru, A.; Pistidda, C.; Rebollo, E.; Boaro, M.; Leonelli, C.; Fabrizio, M. Structural evolution BaCe0.65Zr0.20Y0.15O3-δ-Ce0.85Gd0.15O2-δ composite MPEC membrane by in-situ synchrotron XRD analyses. Mater. Today Energy 2019, 13, 331–341. [Google Scholar] [CrossRef]
- Mortalò, C.; Boaro, M.; Rebollo, E.; Zin, V.; Aneggi, E.; Fabrizio, M.; Trovarelli, A. Insights on the interfacial processes involved in the mechanical and redox stability of the BaCe0.65Zr0.20Y0.15O3−δ−Ce0.85Gd0.15O2−δ composite. ACS Appl. Energy Mater. 2020, 3, 9877–9888. [Google Scholar] [CrossRef]
- Kim, K.-I.; Hong, T.-W. Evaluations of hydrogen permeation on TiN-5 wt% Ni membrane by spark plasma sintering. Int. J. Hydrog. Energy 2010, 35, 12981–12985. [Google Scholar] [CrossRef]
- Kim, K.-I.; Hong, T.-W. Hydrogen permeation of TiN-graphene membrane by hot press sintering (HPS) process. Solid State Ion. 2012, 225, 699–702. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kim, S.-H.; Kim, W.-G.; Hur, S.-C.; Honh, T.-W. Evaluations of hydrogen permeation on TiN-20wt% Co membrane by hot press sintering. Defect Diffus. Forum 2010, 297–301, 549–554. [Google Scholar] [CrossRef]
- Nozaki, T.; Hatano, Y.; Yamakawa, E.; Hachikawa, A.; Ichinose, K. Improvement of high temperature stability of Pd coating on Ta by HfN intermediate layer. Int. J. Hydrog. Energy 2010, 35, 12454–12460. [Google Scholar] [CrossRef]
- Kura, C.; Fujimoto, S.; Kunisada, Y.; Kiwalski, D.; Tsuji, E.; Zhu, C.; Hazabaki, H.; Aoki, Y. Enhanced hydrogen permeability of hafnium nitride nanocrystalline membranes by interfacial hydride conduction. J. Mater. Chem. A 2018, 6, 2730–2741. [Google Scholar] [CrossRef]
- Kura, C.; Kunisada, Y.; Tsuji, E.; Zhu, C.; Habazaki, H.; Nagata, S.; Müller, M.P.; De Souza, R.A.; Aoki, Y. Hydrogen separation by nanocrystalline titanium nitride membranes with high hydride ion conductivity. Nat. Energy 2017, 2, 786–794. [Google Scholar] [CrossRef]
- Saito, M.; Kura, C.; Toriumi, H.; Hinokuma, S.; Ina, T.; Habazaki, H.; Aoki, Y. Formation of mobile hydridic defects in zirconium nitride films with n-type semiconductor properties. ACS Appl. Electron. Mater. 2021, 3, 3980–3989. [Google Scholar] [CrossRef]
- Sucheewa, N.; Wongwiriyapan, W.; Klamchuen, A.; Obata, M.; Fujishige, M.; Takeuchi, K.; Lertvanithphol, T.; Wutikhun, T.; Kullyakool, S.; Auttasiri, W.; et al. Tailoring properties of hafnium nitride thin film via reactive gas-timing RF magnetron sputtering for surface enhanced-raman scattering substrates. Crystals 2022, 12, 78. [Google Scholar] [CrossRef]
- Vahl, A.; Veziroglu, S.; Henkel, B.; Strunskus, T.; Polonskyi, O.; Aktas, O.C.; Faupel, F. Pathways to tailor photocatalytic performance of TiO2 thin films deposited by reactive magnetron sputtering. Materials 2019, 12, 2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarakinos, K. CAP 3 Exploring the potential of high power impulse magnetron sputtering for tailoring the chemical composition and the properties in metal oxynitride films. In Metallic Oxynitride Thin Films by Reactive Sputtering and Related Deposition Methods: Processes, Properties and Applications; Vaz, F., Martin, N., Fenker, M., Eds.; Bentham Books: London, UK, 2013; ISBN 978-1-60805-157-1. [Google Scholar]
- Mishra, S.K. Toughening of nanocomposite hard coatings. Rev. Adv. Mater. Sci. 2020, 59, 553–585. [Google Scholar] [CrossRef]
- Riekkinen, T.; Molarius, J.; Laurila, T.; Nurmela, A.; Suni, I.; Kivilahti, J.K. Reactive sputter deposition and properties of TaxN thin films. Microelectron. Eng. 2002, 64, 289–297. [Google Scholar] [CrossRef]
- Mortalò, C.; Deambrosis, S.M.; Montagner, F.; Zin, V.; Fabrizio, M.; Pasquali, L.; Capelli, R.; Montecchi, M.; Miorin, E. Production strategies of TiNx coatings via reactive high power impulse magnetron sputtering for selective H2 separation. Membranes 2021, 11, 360. [Google Scholar] [CrossRef]
- Machunze, R.; Ehiasarian, A.P.; Tichelaar, F.D.; Janssen, G.C.A.M. Stress and texture in HIPIMS TiN thin films. Thin Solid Films 2009, 518, 1561–1565. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Thirumurugesan, R.; Mohandas, E.; Sastikumar, D.; Kuppusami, P.; Song, J.I. Phase transition and thermal expansion studies of alumina thin films prepared by reactive pulsed laser deposition. J. Nanosci. Nanotechnol. 2014, 14, 7728–7733. [Google Scholar] [CrossRef]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A. Review of supported pd-based membranes preparation by electroless plating for ultra-pure hydrogen production. Membranes 2018, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Duke, M.; Dumée, L.F.; Merenda, A.; Des Ligneris, E.; Kong, L.; Hodgson, P.D.; Gray, S. Short review on porous metal membranes—Fabrication, commercial products, and applications. Membranes 2018, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhao, J.; Yang, P.; Chen, Y.; Fan, Y. Preparation of high stability Pd/ceramic/Ti-Al alloy composite membranes by electroless plating. Front. Chem. 2020, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Pałka, K.; Pokrowiecki, R.; Krzywicka, M. Chapter 3-Porous titanium materials and applications. In Titanium for Consumer Applications; Froes, F., Qian, M., Mitsuo, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–75. ISBN 9780128158203. [Google Scholar] [CrossRef]
- Tang, H.P.; Wang, J.; Qian, M. 28-Porous titanium structures and applications. In Titanium Powder Metallurgy; Qian, M., Francis, H., Froes, S., Eds.; Butterworth-Heinemann: London, UK, 2015; pp. 533–554. ISBN 9780128000540. [Google Scholar]
- Hackemüller, F.Z.; Borgardt, E.; Pachenko, O.; Müller, M.; Bram, M. Manufacturing of large-scale titanium-based porous transport layers for polymer membrane electrolysis by tape casting. Adv. Eng. Mater. 2019, 21, 1801201. [Google Scholar] [CrossRef]
- Hidnert, P. Thermal Expansion of Titanium; National Bureau of Standards: Gaithersburg, MA, USA, 1943; Volume 30, p. RP1520. [Google Scholar]
- Zhang, L.; Chen, L.; Wang, L. Surface modification of titanium and titanium alloys: Technologies, developments, and future interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Gaviria, J.; Alcudia, A.; Begines, B.; Beltrán, A.M.; Rodríguez-Ortiz, J.A.; Trueba, P.; Villarraga, J.; Torres, Y. Biofunctionalization of porous Ti substrates coated with Ag nanoparticles for potential antibacterial behavior. Metals 2021, 11, 692. [Google Scholar] [CrossRef]
- Rodríguez, Á.; Trueba, P.; Amado, J.M.; Tobar, M.J.; Giner, M.; Amigó, V.; Torres, Y. Surface modification of porous titanium discs using femtosecond laser structuring. Metals 2020, 10, 748. [Google Scholar] [CrossRef]
- Kaur, S.; Ghadirinejad, K.; Oskouei, H.R. An overview on the tribological performance of titanium alloys with surface modifications for biomedical applications. Lubricants 2019, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Singare, A.A.; Kale, B.S.; Bhole, K.S. Experimental characterization of meso-micro fractals from nanoparticle seeded resin in lifting plate hele-shaw cell. Mater. Today Proc. 2018, 5, 24213. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of Electrophoretic Deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Cannio, M.; Boccaccini, D.N.; Ponzoni, C.; Leonelli, C. Electrophoretic deposition: An effective technique to obtain functionalized nanocoatings. In Handbook of Modern Coating Technologies; Aliofkhazraei, M., Ali, N., Chipara, M., Laidani, N.B., de Hosson, J.T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 209–230. [Google Scholar] [CrossRef]
- Cannio, M.; Novak, S.; Besra, L.; Boccaccini, A.R. Electrophoretic Deposition. In Ceramic and Composites Processing Methods, 1st ed.; Bansal, N.P., Boccaccini, A.R., Eds.; The American Ceramic Society Published©, John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ab Aziz, S.A.B.; Amirnordin, S.H.; Hamimah, A.R.; Abdullah, H.Z.; Taib, H. Short review: Electrophoretic Deposition (EPD) on non-conductive substrate. Adv. Mater. Res. 2012, 488–489, 1358–1362. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Finklea, H.; Liu, X. A review of electrophoretic deposition of metal oxides and its application in solid oxide fuel cells. Adv. Colloid Interface Sci. 2020, 276, 102102. [Google Scholar] [CrossRef]
- Kreethawate, L.; Larpkiattaworn, S.; Jiemsirilers, S.; Besra, L.; Uchikoshi, T. Applications of electrophoretic deposition for inner surface coating of porous ceramic tubes. Surf. Coat. Technol. 2010, 205, 1922–1928. [Google Scholar] [CrossRef]
- Smeacetto, F.; De Miranda, A.; Cabanas Polo, S.; Molin, S.; Boccaccini, D.; Salvo, M.; Boccaccini, A.R. Electrophoretic deposition of Mn1.5Co1.5O4 on metallic interconnect and interaction with glass-ceramic sealant for solid oxide fuel cells application. J. Power Sources 2015, 280, 379–386. [Google Scholar] [CrossRef]
- Amrollahi, P.; Krasinski, J.S.; Vaidyanathan, R.; Tayebi, L.; Vashaee, D. Electrophoretic Deposition (EPD): Fundamentals and applications from nano to microscale structures. In Handbook of Nanoelectrochemistry; Aliofkhazraei, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Geneva, Switzerland, 2016; pp. 561–591. [Google Scholar] [CrossRef]
- Shikha Awasthi, S.; Pandey, S.K.; Pandey, C.P.; Balani, K. Progress in electrochemical and electrophoretic deposition of nickel with carbonaceous allotropes: A review. Adv. Mater. Interfaces 2019, 7, 1901096. [Google Scholar] [CrossRef]
- Myung, S.-T.; Kumagai, M.; Asaishi, R.; Sun, Y.-K.; Yashiro, H. Nanoparticle TiN-coated type 310S stainless steel as bipolar plates for polymer electrolyte membrane fuel cell. Electrochem. Comun. 2008, 10, 480. [Google Scholar] [CrossRef]
- Lee, D.; Lee, E.; Yoon, J.; Keith, B.; Oh, T.-S.; Woo, S.-P.; Yoon, Y.S.; Kim, D.-J. Electrophoretic deposition of titanium nitride onto 316 stainless steel as a bipolar plate for fuel cell application. ECS Trans. 2017, 80, 851–857. [Google Scholar] [CrossRef]
- Golchin, S.S.; Aghajani, H. Corrosion resistance and adhesion properties of electrophoretic deposited nano TiN powder. J. Nano Res. 2021, 70, 15–25. [Google Scholar] [CrossRef]
- Kavanlouei, M.; Akbari, A. Electrophoretic deposition of titanium nitride coating. J. Am. Cer. Soc. 2018, 10, 3288–3298. [Google Scholar] [CrossRef]
- Mendoza, C.; Gonzalez, Z.; Castro, Y.; Gordo, E.; Ferrari, B. Improvement of TiN nanoparticles EPD inducing steric stabilization in non-aqueous suspensions. J. Eur. Ceram. Soc. 2016, 36, 307–317. [Google Scholar] [CrossRef]
- Cui, X.; You, Z.; Ma, M.; Chu, P.K. Nanocrystalline titanium nitride films prepared by electrophoretic deposition. Surf. Coat. Technol. 2009, 204, 418. [Google Scholar] [CrossRef]
- Urena, J.; Mendoza, C.; Ferrari, B.; Castro, Y.; Tsipas, S.A.; Jimenez-Morales, A.; Gordo, E. Surface modification of powder metallurgy titanium by colloidal techniques and diffusion processes for biomedical applications. Adv. Eng. Mater. 2017, 19, 1600207. [Google Scholar] [CrossRef]
- Mendoza, C.; Gonzalez, Z.; Gordo, E.; Ferrari, B.; Castro, Y. Protective nature of nano-TiN coatings shaped by EPD on Ti substrates. J. Eur. Ceram. Soc. 2018, 38, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, Z.; Yus, J.; Moratalla, R.; Ferrari, B. Electrophoretic deposition of binder-free TiN nanoparticles to design 3D microstructures. The role of sintering in the microstructural robustness of supercapacitors electrodes. Electrochim. Acta 2021, 369, 137654. [Google Scholar] [CrossRef]
- Shih, C.-J.; Lung, B.-H.; Hon, M.-H. Colloidal processing of titanium nitride with poly-(methacrylic acid) polyelectrolyte. Mater. Chem. Phys. 1999, 60, 150–157. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, L.; Jiang, D.; Lin, Q.; Iwasa, M. Dispersion of TiN in aqueous media. J. Colloids Interface Sci. 2005, 286, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xiong, J.; Yang, M.; Xiong, S.; Chen, J.; Wu, Y.; Fan, H.; Sun, L.; Wang, J.; Wang, H. Dispersion of nano-TiN powder in aqueous media. J. Alloys Compd. 2010, 493, 362–367. [Google Scholar] [CrossRef]
- Tabelliion, J.; Clasen, R. Electrophoretic deposition from aqueous suspensions for near-shape manufacturing of advanced ceramics and glasses-applications. J. Mater. Sci. 2004, 39, 803–811. [Google Scholar] [CrossRef]
- Walton, W.H. Feret‘s statistical diameter as a measure of particle size. Nature 1948, 162, 329–330. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Shabanlou, E.; Jaleh, B.; Mohazzab, B.F.; Kakuee, O.; Golbedaghi, R.; Orooji, Y. TiN formation on Ti target by laser ablation method under different N2 gas pressure and laser 3canning cycles: A wettability study. Surf. Interfaces 2021, 27, 101509. [Google Scholar] [CrossRef]
- Oktay, S.; Kahraman, Z.; Urgen, M.; Kazmanli, K. XPS investigations of tribolayers formed on TiN and (Ti, Re)N coatings . Appl. Surf. Sci. 2015, 328, 255–261. [Google Scholar] [CrossRef]
- Moore, j.; Cerasoli, E. Particle light scattering methods and applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; John, C., Lindon, G.E., Tranter, D., Koppenaal, W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 543–553. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting zeta potential, the key feature of interfacial phenomena, with applications and recent advancements. Chem. Select 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Nakatuka, Y.; Yoshida, H.; Fukui, K.; Matuzawa, M. The effect of particle size distribution on effective zeta-potential by use of the sedimentation method. Adv. Powder Technol. 2015, 26, 650–656. [Google Scholar] [CrossRef]
- Carson, J.W.; Pittenger, B.H. Powder metal technologies and applications. In ASM Handbook; ASM International: Russell, OH, USA, 1998; Volume 7. [Google Scholar]
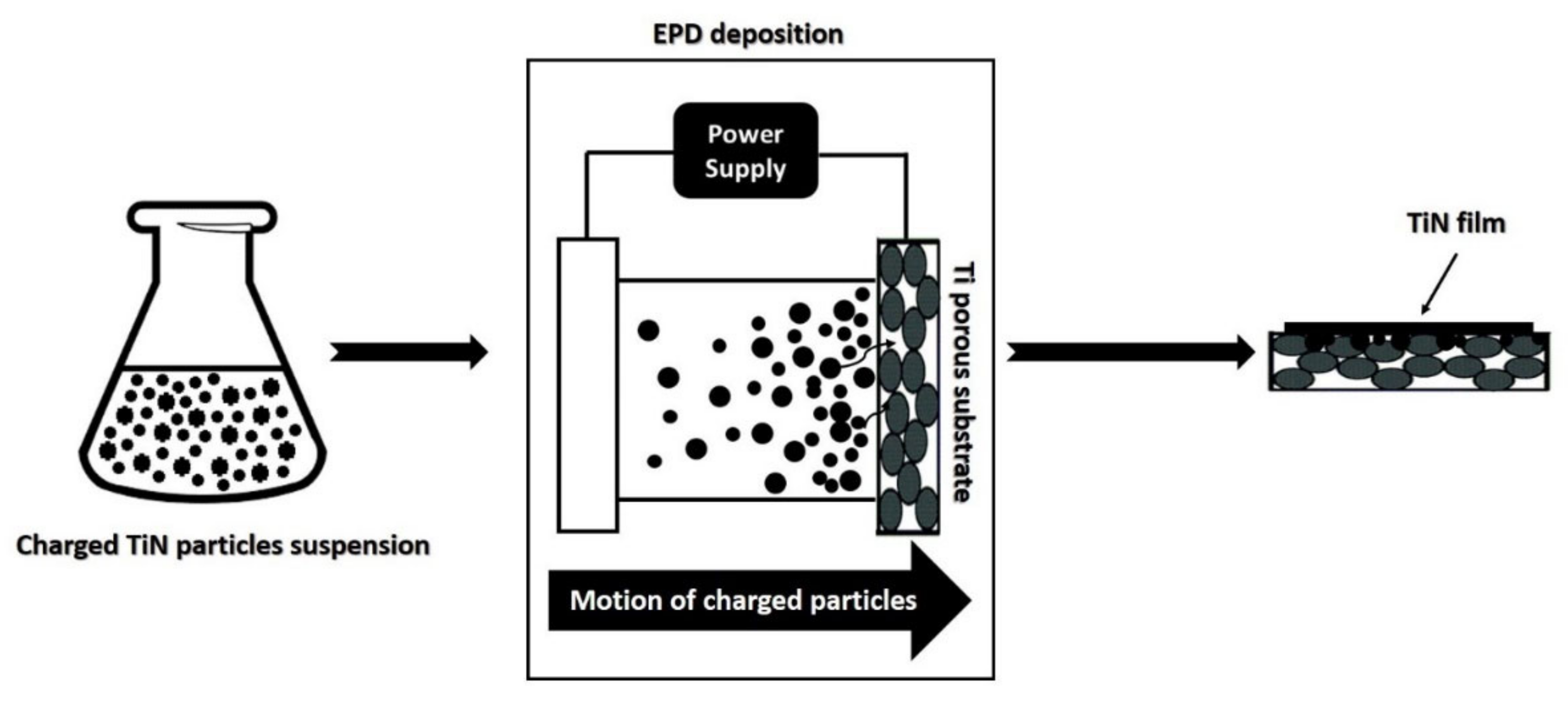

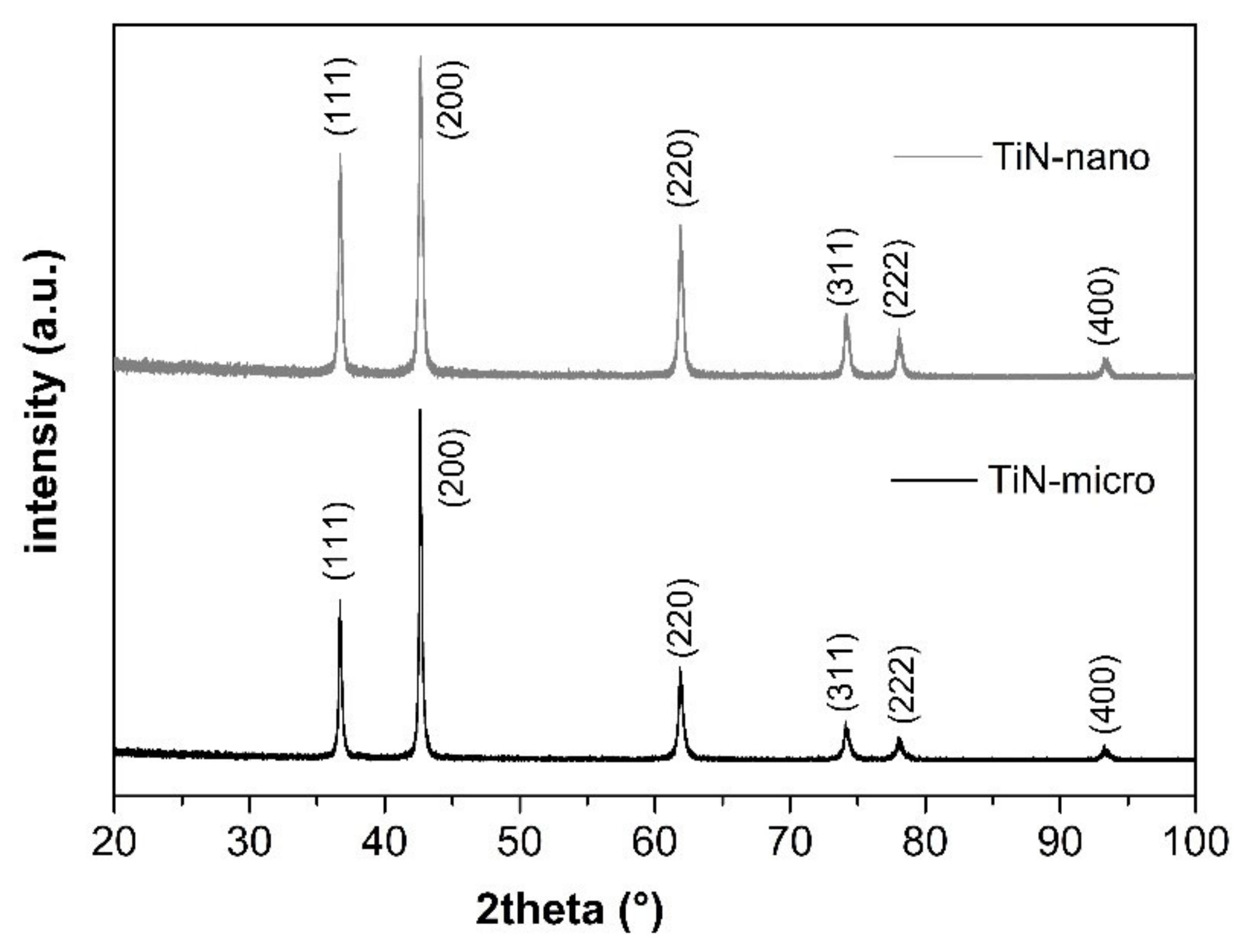
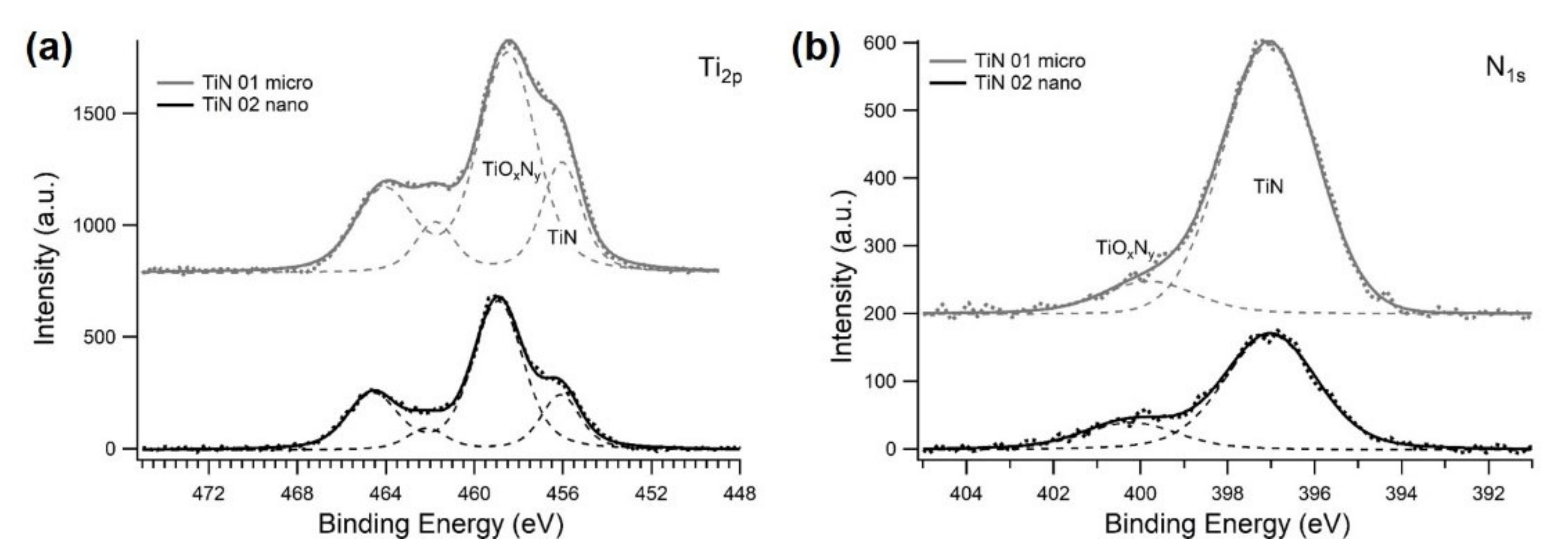



| Sample | TiN Suspension (1 wt%/) | Potential (V) | Number of Steps | Step Duration (s) | Duration (s) |
|---|---|---|---|---|---|
| Single-step | |||||
| EPD TiN-01 | TiN-micro | 10 | 1 | \ | 1200 |
| EPD TiN-02 | TiN-nano | 5 | 1 | \ | 1200 |
| EPD TiN-03 | TiN-nano/micro | 10 | 1 | \ | 1200 |
| EPD TiN-04 | TiN-nano/micro | 10 | 1 | \ | 3600 |
| EPD TiN-05 | TiN-nano | 5 | 1 | \ | 3600 |
| Multi-step | |||||
| EPD TiN-06 | TiN-nano/micro TiN-nano | 10 5 | 20 4 | 60 300 | 1200 1200 |
| EPD TiN-07 | TiN-micro TiN-nano | 10 5 | 30 4 | 60 300 | 1800 1200 |
| EPD TiN-08 | TiN-micro TiN-nano | 10 5 | 20 4 | 60 300 | 1200 1200 |
| EPD TiN-09 | TiN-nano | 5 | 8 | 300 | 2400 |
| Sample | ζ Potential (mV) |
|---|---|
| TiN-nano | −50.5 ± 3.7 |
| TiN-micro | −33.3 ± 3.1 |
| TiN-nano/micro | −46.7 ± 4.8 |
| Sample | Ra | Rq | Rsk | Rku |
|---|---|---|---|---|
| EPD TiN-06 | 0.309 ± 0.059 | 0.573 ± 0.170 | −2.916 ± 0.782 | 17.443 ± 5.07 |
| EPD TiN-07 | 0.366 ± 0.081 | 0.669 ± 0.189 | −3.514 ± 0.323 | 19.900 ± 2.729 |
| EPD TiN-08 | 0.179 ± 0.356 | 0.389 ± 0.110 | −3.079 ± 0.451 | 19.317 ± 2.052 |
| EPD TiN-09 | 0.399 ± 0.035 | 0.865 ± 0.080 | −4.829 ± 0.461 | 31.272 ± 6.086 |
| Ti | 0.376 ± 0.053 | 0.803 ± 0.137 | −4.888 ± 0.741 | 34.193 ± 7.943 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortalò, C.; Cannio, M.; Zin, V.; Miorin, E.; Montagner, F.; Pasquali, L.; Montecchi, M.; Boccaccini, D.N.; Fabrizio, M.; Deambrosis, S.M. Surface Optimization of Commercial Porous Ti Substrates by EPD of Titanium Nitride. Membranes 2022, 12, 531. https://doi.org/10.3390/membranes12050531
Mortalò C, Cannio M, Zin V, Miorin E, Montagner F, Pasquali L, Montecchi M, Boccaccini DN, Fabrizio M, Deambrosis SM. Surface Optimization of Commercial Porous Ti Substrates by EPD of Titanium Nitride. Membranes. 2022; 12(5):531. https://doi.org/10.3390/membranes12050531
Chicago/Turabian StyleMortalò, Cecilia, Maria Cannio, Valentina Zin, Enrico Miorin, Francesco Montagner, Luca Pasquali, Monica Montecchi, Dino Norberto Boccaccini, Monica Fabrizio, and Silvia Maria Deambrosis. 2022. "Surface Optimization of Commercial Porous Ti Substrates by EPD of Titanium Nitride" Membranes 12, no. 5: 531. https://doi.org/10.3390/membranes12050531
APA StyleMortalò, C., Cannio, M., Zin, V., Miorin, E., Montagner, F., Pasquali, L., Montecchi, M., Boccaccini, D. N., Fabrizio, M., & Deambrosis, S. M. (2022). Surface Optimization of Commercial Porous Ti Substrates by EPD of Titanium Nitride. Membranes, 12(5), 531. https://doi.org/10.3390/membranes12050531













