Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review
Abstract
:1. Introduction
2. Classifications of Adsorptive Ultrafiltration Membrane
2.1. Inorganic Filler-Based MMMs
2.2. Organic Filler-Based MMMs
2.3. Biomaterial-Based MMMs
2.4. Hybrid Filler-Based MMMs
| Type of MMMs | Membrane | Adsorbent | Pollutants | Rejection | Ref. |
|---|---|---|---|---|---|
| Inorganic filler-based MMMs | PES | Al2O3 | Cu2+ | 60.0% | [20] |
| PVDF | ZnO | Cu2+ | 83.3% | [21] | |
| PSf | MWNTs | Cr5+ | 94.2% | [22] | |
| PVC | CNT | Fe2+ | 95.1% | [23] | |
| PES | GO | Pb2+ | 98.0% | [24] | |
| PSF | NaX | Pb2+ | 91.0% | [25] | |
| PES | Carbonaceous materials | Cu2+ | 79.1% | [26] | |
| Organic filler-based MMMs | PAN | PVT | Cu2+ Pb2+ | 98.5% 51.0% | [31] |
| PVDF | PANI | Congo red | 74.0% | [32] | |
| PVC | Hyperbranched polyester | Sunset yellow | 96.4% | [33] | |
| PVDF | 2-aminobenzothiazole | Cr5+ | 82.1% | [34] | |
| Biomaterial-based MMMs | PMOXA15-PDMS110- PMOXA15 | Aquaporin Z | Urea, glucose, glycerol | 100% | [37] |
| PES | Banana peel, tea waste, and shaddock peel | Methylene blue Methyl violet 2B | 95.0% 96.0% | [38] | |
| Hybrid filler-based MMMs | PES | Iron (II, III) oxide and polyaniline | Cu2+ | 85.0% | [39] |
| PES | Citric acid–amylose-decorated multiwall carbon nanotubes | Humic acid | 97.4% | [40] | |
| PVDF | MIL | MB | 75.0% | [41] | |
| PVDF | PAA/ZIF-8 | Ni2+ | 99.0% | [42] |
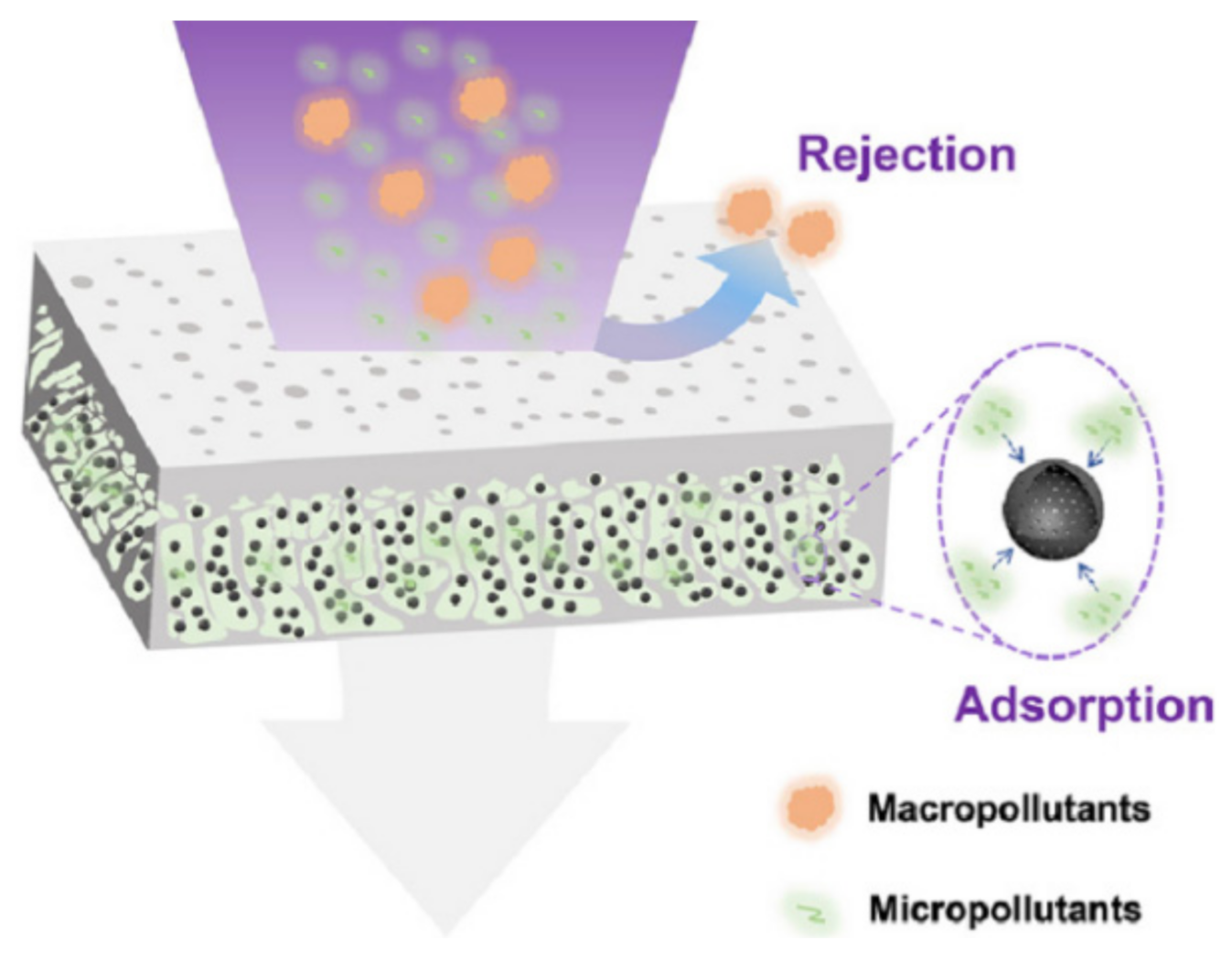
3. Mechanisms of Adsorptive Ultrafiltration MMMs
4. Preparation Techniques for Adsorptive Ultrafiltration Membrane
4.1. Blending
4.2. Surface Coating
4.3. Reverse Filtration

5. Future Trends and Challenges for Adsorptive Ultrafiltration Membranes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef] [Green Version]
- Song, M.L.; Wang, R.; Zeng, X.Q. Water resources utilization efficiency and influence factors under environmental restrictions. J. Clean. Prod. 2018, 184, 611–621. [Google Scholar] [CrossRef]
- Gao, X.R.; Zhao, Y.; Lu, S.B. Impact of coal power production on sustainable water resources management in the coal-fired power energy bases of Northern China. Appl. Energ. 2019, 250, 821–833. [Google Scholar] [CrossRef]
- Jagdish, G.P.; Sanjay, S.; Shi, W. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2021, 262, 128350. [Google Scholar]
- Wu, H.Y.; Xu, B.; Guan, Y.S. A metabolomic study on the association of exposure to heavy metals in the first trimester with primary tooth eruption. Sci. Total. Environ. 2020, 723, 138107. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Hullebusch, E.D.; Rodrigo, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Wu, C.R.; Wang, Z.Y.; Liu, S.H. Simultaneous permeability, selectivity and antibacterial property improvement of ultrafiltration membranes via in-situ quaternization. J. Membr. Sci. 2018, 548, 50–58. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Cao, C.Y.; Qu, J.; Yan, W.S.; Zhu, J.-F.; Wu, Z.-Y.; Song, W.-G. Low-cost synthesis of flowerlike α-Fe2O3 nanostructures for heavy metal ion removal: Adsorption property and mechanism. Langmuir 2012, 28, 4573–4579. [Google Scholar] [CrossRef]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, preparation, and applications (with emphasis on aqueous media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.; Wang, J. Adsorptive Separation of Furfural/5-Hydroxymethylfurfural in MAF-5 with Ellipsoidal Pores. Ind. Eng. Chem. Res. 2020, 59, 11734–11742. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, J.; Du, T.T. Zn (II)-imidazole derived metal azolate framework as an effective adsorbent for double coated solid-phase micro-extraction of sixteen polycyclic aromatic hydrocarbons. Talanta 2020, 214, 120866. [Google Scholar] [CrossRef] [PubMed]
- Ana, M.C.; Jose, G.S.; David, D. Using Aliphatic Alcohols to Tune Benzene Adsorption in MAF-6. Adv. Theor. Simul. 2019, 11, 1900112. [Google Scholar]
- Ehsan, B.E.; Sina, M.; Nazanin, M. Study on the performance of Cd2+ sorption using dimethylethylenediamine-modified zinc-based MOF (ZIF-8-mmen): Optimization of the process by RSM technique. Sep. Purif. Technol. 2020, 15, 2713–2728. [Google Scholar]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Acc. Chem. Res. 2012, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Su, Y.; Li, Q.; Gao, S.; Shang, J.K. Superparamagnetic magnesium ferrite nanoadsorbent for effective arsenic (III, V) removal and easy magnetic separation. Water Res. 2013, 47, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Wilke, C.M.; Wu, J.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.-F.; Gray, K.A. Combined toxicity of Nano-ZnO and Nano-TiO2: From single-to multinanomaterial systems. Environ. Sci. Technol. 2015, 49, 8113–8123. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular toxicity of carbonbased nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Du, Q.; Hua, M.; Jiao, T.F.; Gao, F.M.; Pan, B.C. Sorption Enhancement of Lead Ions from Water by Surface Charged Polystyrene-Supported Nano-Zirconium Oxide Composites. Environ. Sci. Technol. 2013, 47, 6536–6544. [Google Scholar] [CrossRef]
- Ghaemi, N. A new approach to copper ion removal from water by polymeric nanocomposite membrane embedded with γ-alumina nanoparticles. Appl. Surf. Sci. 2016, 364, 221–228. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, performances of PVDF/ZnO hybrid membranes and their applications in the removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–342. [Google Scholar] [CrossRef]
- Shah, P.; Murthy, C.N. Studies on the porosity control of MWCNT/polysulfone composite membrane and its effect on metal removal. J. Membr. Sci. 2013, 437, 90–98. [Google Scholar] [CrossRef]
- Farid, M.U.; Luan, H.Y.; Wang, Y.; Huang, H. Increased adsorption of aqueous zinc species by Ar/O2 plasma-treated carbon nanotubes immobilized in hollow-fiber ultrafiltration membrane. Chem. Eng. J. 2017, 325, 239–248. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard. Mater. 2016, 309, 53–64. [Google Scholar] [CrossRef]
- Hofman, M.; Pietrzak, R. Copper ions removal from liquid phase by polyethersulfone (PES) membranes functionalized by introduction of carbonaceous materials. Chem. Eng. J. 2013, 215, 216–221. [Google Scholar] [CrossRef]
- Abdullah, N.; Gohari, R.J.; Yusof, N.; Ismail, A.F.; Juhana, J.; Lau, W.J.; Matsuura, T. Polysulfone/hydrous ferric oxide ultrafiltration mixed matrix membrane: Preparation, characterization and its adsorptive removal of lead (II) from aqueous solution. Chem. Eng. J. 2016, 289, 28–37. [Google Scholar] [CrossRef]
- Chan, K.H.; Wong, E.T.; Idris, A.; Yusof, N.M. Modification of PES membrane by PEG-coated cobalt doped iron oxide for improved Cu(II) removal. J. Ind. Eng. Chem. 2015, 27, 283–290. [Google Scholar] [CrossRef]
- Mondal, M.; Dutta, M.; De, S. A novel ultrafiltration grade nickel iron oxide doped hollow fiber mixed matrix membrane: Spinning, characterization and application in heavy metal removal. Sep. Purif. Technol. 2017, 188, 155–166. [Google Scholar] [CrossRef]
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed matrix membranes for water purification applications. Separ. Purif. Rev. 2017, 46, 62–80. [Google Scholar] [CrossRef]
- Kumar, M.; Shevate, R.; Hilke, R.; Peinemann, K.V. Novel adsorptive ultrafiltration membranes derived from polyvinyltetrazole-co-polyacrylonitrile for Cu(II) ions removal. Chem. Eng. J. 2016, 301, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Pu, L.T.; Zou, D.; Cao, M.; Shan, C.; Zhang, Q.X.; Gao, G.D.; Pan, B.C. Removal of model dyes on charged UF membranes: Experiment and Simulation. Chemosphere 2020, 240, 124940. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.L.; Kong, X.; Yuan, J.J.; Shen, Y.J.; Zhu, B.K.; Zhu, L.P.; Yao, Z.K.; Tang, C.Y. Cross-linked PVC/hyperbranched polyester composite hollow fiber. membranes for dye removal. React. Funct. Polym. 2018, 122, 51–59. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, K.; Ma, Z.; Lu, X.; Wang, L.; Wang, Z.; Gao, X. Preparation and characterization of novel polyvinylidene fluoride/2-aminobenzothiazole modified ultrafiltration membrane for the removal of Cr(VI) in wastewater. Polymers 2018, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A. Cationic and anionic dye adsorption by agricultural solid wastes:acomprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Castro, R.S.D.; Caetano, L.; Ferreira, G.; Padilha, P.M.; Saeki, M.J.; Zara, L.F.; Martines, M.A.; Castro, G.R. Banana peel applied to the solid phase extraction of copper and lead from river water: Preconcentration of metalions with a fruit waste. Ind. Eng. Chem. Res. 2011, 50, 3446–3451. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Gung, C.H.; Sun, J.J.; Suen, S.Y. Preparation of polyethersulfone/plantwaste-particles mixed matrix membranes for adsorptive removal of cationic dyes from water. J. Membr. Sci. 2014, 471, 285–298. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B.; Daraei, P.; Madaeni, S.S.; Ghaemi, N.; et al. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water. J. Membr. Sci. 2012, 415, 250–259. [Google Scholar] [CrossRef]
- Parsamanesh, M.; Mansourpanah, Y.; Tehrani, A.D. Improving the efficacy of PES-based mixed matrix membranes incorporated with citric acid-amylose-modified MWCNTs for HA removal from water. Polym. Bull. 2021, 78, 1293–1311. [Google Scholar] [CrossRef]
- Ren, Y.; Li, T.; Zhang, W.M.; Wang, S.; Shi, M.Q.; Shan, C.; Zhang, W.B.; Guan, X.H.; Lv, L.; Hua, M.; et al. MIL-PVDF blend ultrafiltration membranes with ultrahigh MOF loading for simultaneous adsorption and catalytic oxidation of methylene blue. J. Hazard. Mater. 2019, 365, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, W.; Zhai, S.; Gao, G.; Ding, J.; Zhang, W.; Liu, Y.; Zhao, X.; Pan, B.; Lv, L. Efficient removal of nickel(II) from high salinity wastewater by a novel PAA/ZIF-8/PVDF hybrid ultrafiltration membrane. Water Res. 2018, 143, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.P.; Nguyen, M.N.; Wan, G.J.; Xie, J.; Ni, L.H.; Qi, J.W.; Li, J.S. Low pressure operated ultrafiltration membrane with integration of hollow mesoporous carbon nanospheres for effective removal of micropollutants. J. Hazard. Mater. 2020, 397, 122779. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.R. Membrane Separation Principles and Applications: From Material Selection to Mechanisms and Industrial Uses. In Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 361–400. [Google Scholar]
- Rowley, J.Y.; Abu-Zahra, N.H. Synthesis and characterization of polyethersulfone membranes impregnated with (3-aminopropyltriethoxysilane) APTES-Fe3O4 nanoparticles for As(V) removal from water. J. Environ. Chem. Eng. 2019, 7, 102875. [Google Scholar] [CrossRef]
- Li, T.; Ren, Y.; Zhai, S.; Zhang, W.; Zhang, W.; Hua, M.; Lv, L.; Pan, B. Integrating cationic metal-organic frameworks with ultrafiltration membrane for selective removal of perchlorate from Water. J. Hazard. Mater. 2019, 381, 120961. [Google Scholar] [CrossRef]
- Cetinkaya, A.Y. Performance and mechanism of direct As(III) removal from aqueous solution using low-pressure graphene oxide-coated membrane. Chem. Pap. 2018, 72, 2363–2373. [Google Scholar] [CrossRef]
- Ren, Z.; Luo, J.; Wan, Y. Highly permeable biocatalytic membrane prepared by 3D modification: Metal-organic frameworks ameliorate its stability for micropollutants removal. Chem. Eng. J. 2018, 348, 389–398. [Google Scholar] [CrossRef]
- Fang, X.F.; Li, J.S.; Li, X.; Pan, S.L.; Zhang, X.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J. Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal. Chem. Eng. J. 2017, 314, 38–49. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Y.; Min, G. A mini review of multifunctional ultrafiltration membranes for wastewater decontamination: Additional functions of adsorption and catalytic oxidation. Sci. Total. Environ. 2020, 762, 143083. [Google Scholar] [CrossRef]
- Gohari, R.J.; Halakoo, E.N.; Nazri, A.M.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Improving performance and antifouling capability of PES UF membranes via blending with highly hydrophilic hydrous manganese dioxide nanoparticles. Desalination 2014, 335, 87–95. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, J.K.; Fan, R.Q.; Cao, Y.; Lu, H.Y.; Wang, B.W.; Zheng, X.B.; Yin, Y.; Wang, P.; Yang, Y.L. Selective adsorption and detection of p-arsanilic acid on MOF-on-MOF heterostructure induced by nitrogen-rich self-assembly template. Chem. Eng. J. 2021, 427, 131483. [Google Scholar] [CrossRef]
- Lu, B.; Wang, S.Y.; Zhao, L.; Zhou, D.D.; Dong, S.S.; Wang, G. Selective and superior capture of phosphate by using bimetallic bismuth-based metal-organic frameworks. Chem. Eng. J. 2021, 425, 131514. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Pan, F.; He, G.; Fang, C.; Cao, K.; Xing, R.; Jiang, Z. Fabricating graphene oxide-based ultrathin hybrid membrane for pervaporation dehydration via layer-by-layer self-assembly driven by multiple interactions. J. Membr. Sci. 2015, 487, 162–172. [Google Scholar] [CrossRef]
- Fan, H.; Shi, Q.; Yan, H.; Ji, S.; Dong, J.; Zhang, G. Simultaneous spray self- assembly of highly loaded ZIF-8-PDMS nanohybrid membranes exhibiting exceptionally high biobutanol-permselective pervaporation. Angew. Chem. Int. Ed. 2014, 53, 5578–5582. [Google Scholar] [CrossRef]
- Nagaraju, D.; Bhagat, D.G.; Banerjee, R.; Kharul, U.K. In situ growth of metal- organic frameworks on a porous ultrafifiltration membrane for gas separation. J. Mater. Chem. A 2013, 1, 8828–8835. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Yu, F.; Zhao, X.; Wang, Y. Enhanced ethanol recovery of PDMS mixed matrix membranes with hydrophobically modified ZIF-90. Separ. Purif. Technol. 2018, 206, 80–89. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Yang, Y. Improving the pervaporation performance of PDMS membranes for n-butanol by incorporating silane-modified ZIF-8 particles. Sep. Purif. Technol. 2019, 215, 163–172. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y. Poly(vinyl alcohol)/ZIF-8-NH2 Mixed Matrix Membranes for Ethanol Dehydration via Pervaporation. AIChE J. 2016, 62, 1728–1739. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Zhou, J.; Liu, F.; Xu, B.-M.; Pan, Y. Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes 2022, 12, 519. https://doi.org/10.3390/membranes12050519
Yu T, Zhou J, Liu F, Xu B-M, Pan Y. Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes. 2022; 12(5):519. https://doi.org/10.3390/membranes12050519
Chicago/Turabian StyleYu, Tong, Jing Zhou, Feng Liu, Bao-Ming Xu, and Yong Pan. 2022. "Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review" Membranes 12, no. 5: 519. https://doi.org/10.3390/membranes12050519
APA StyleYu, T., Zhou, J., Liu, F., Xu, B.-M., & Pan, Y. (2022). Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes, 12(5), 519. https://doi.org/10.3390/membranes12050519





