Discharge Characteristics, Plasma Electrolytic Oxidation Mechanism and Properties of ZrO2 Membranes in K2ZrF6 Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. PEO Membrane Preparation
2.2. Evolution of the Surface of the Substrate
2.3. Test of the PEO Membranes and Electrolytes
3. Results and Discussion
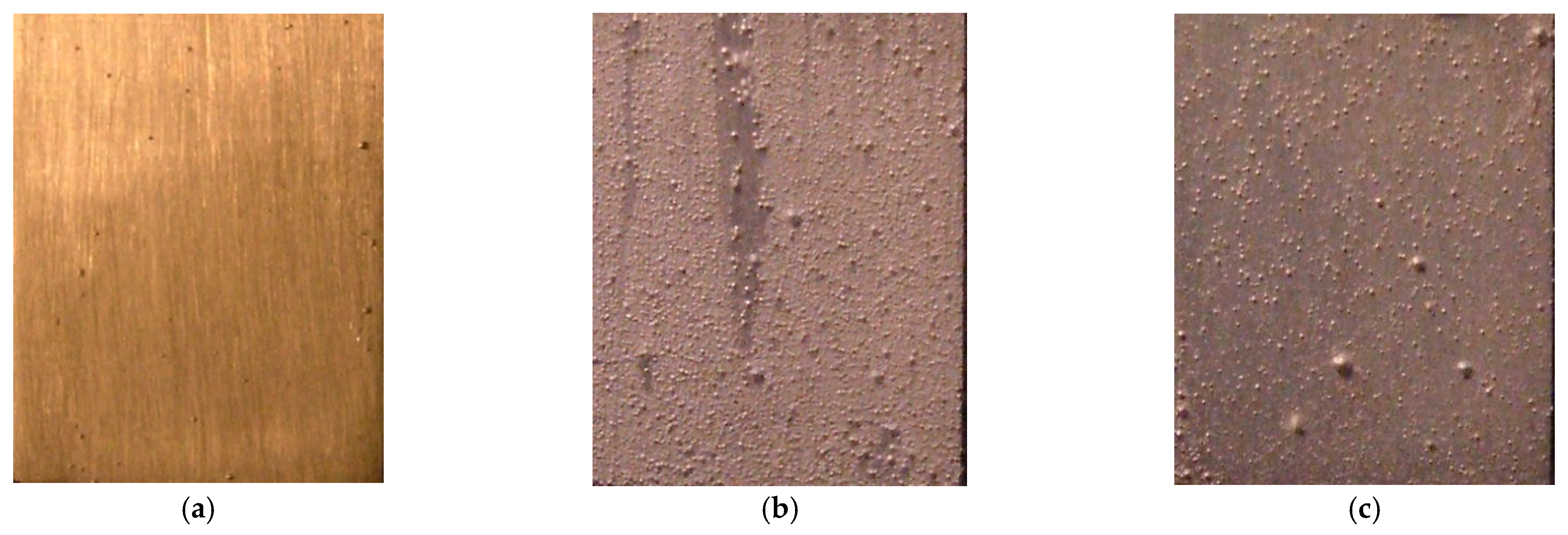
3.1. Discharge Characteristics and Surface Variation during the PEO Process
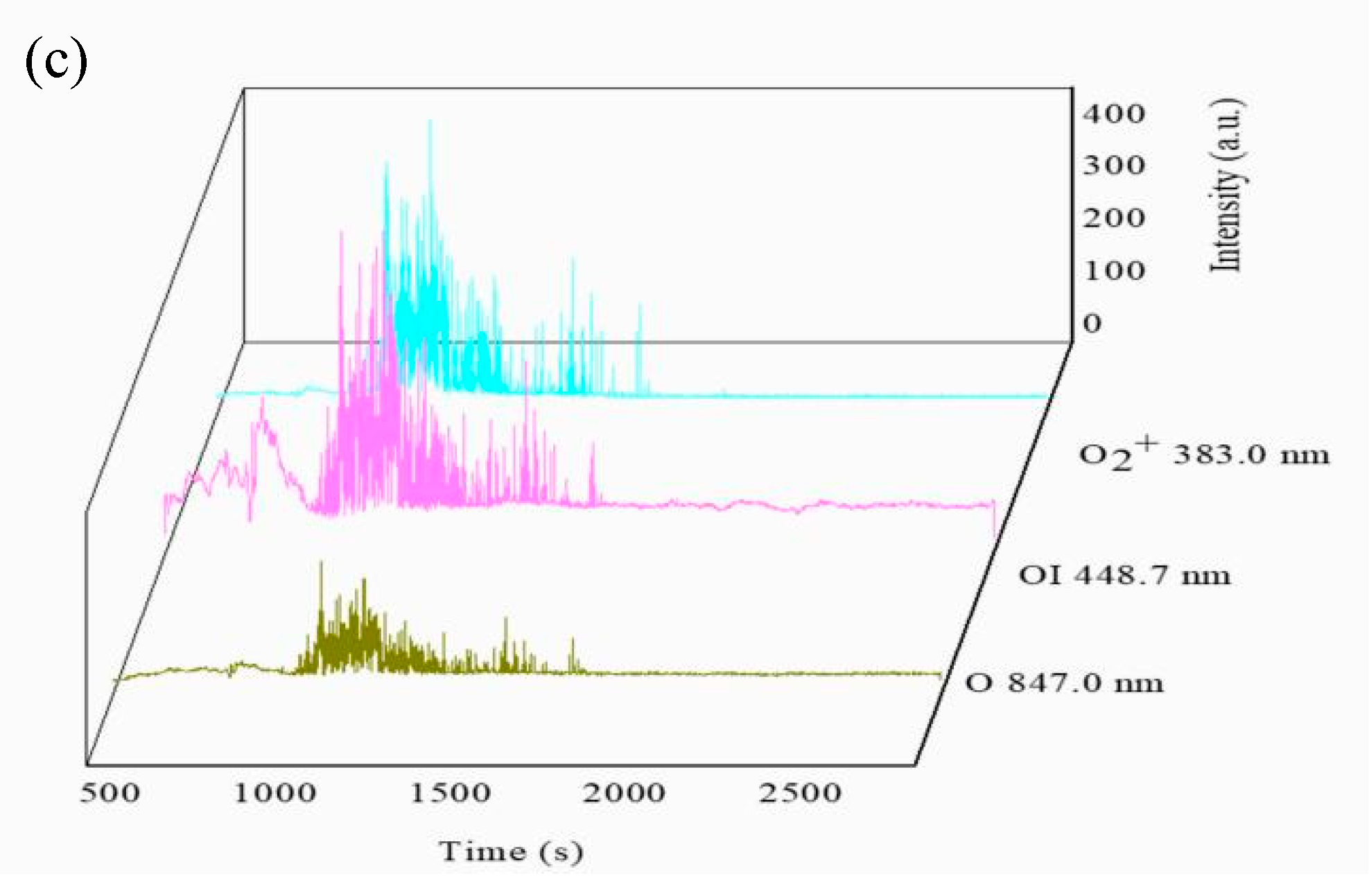
3.2. Variation in the Spectroscopy during the PEO Process
3.3. The Spectra Intensity Variation of Each Active Species
3.4. The Temperature of the Active Species
3.5. Ion Transfer
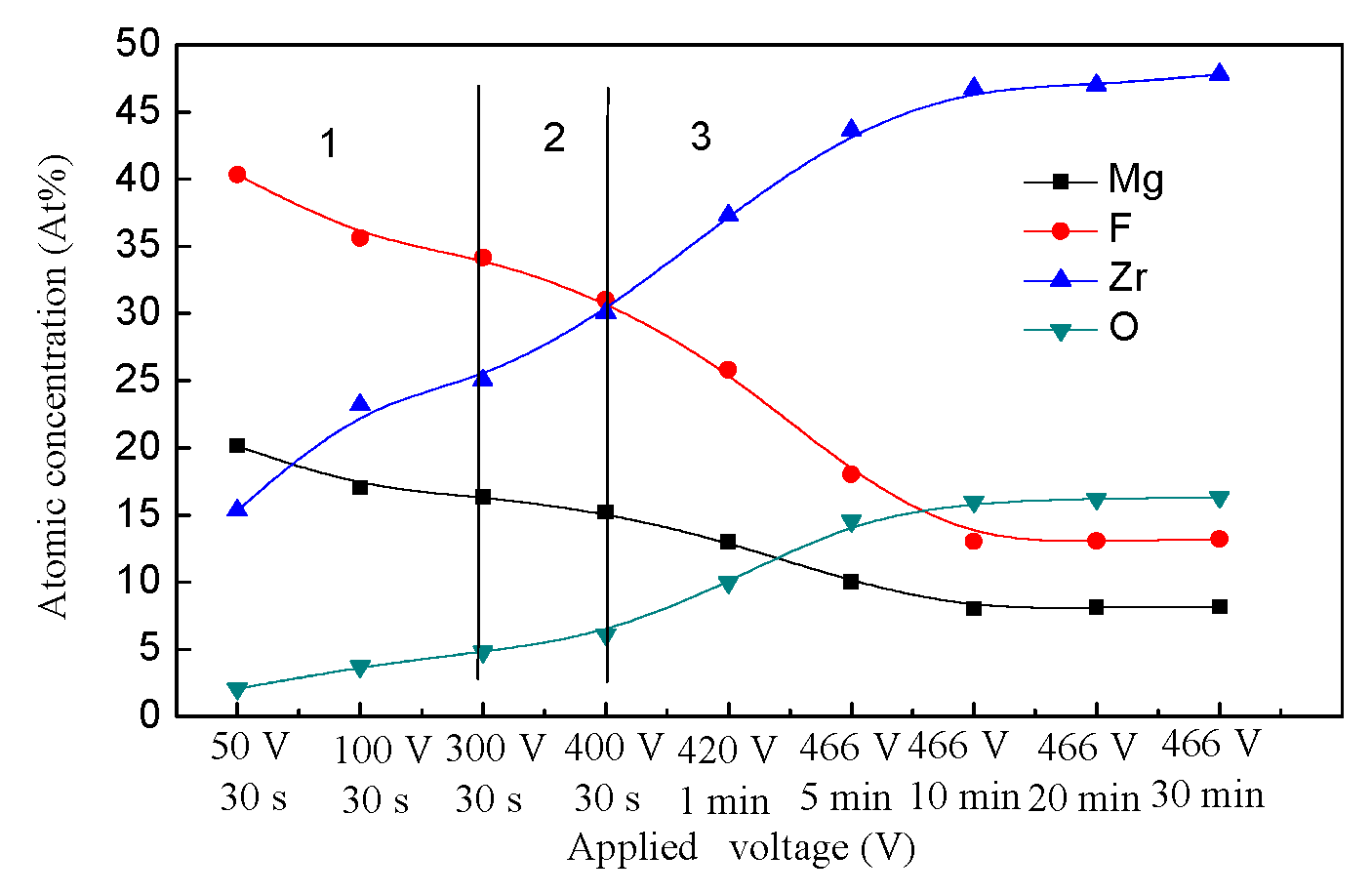
3.5.1. The Ion Migration of the Substrate
3.5.2. The Ion Migration of the Electrolyte
3.6. PEO Mechanism
3.6.1. The Heat- and Mass-Transfer Models during the PEO Process
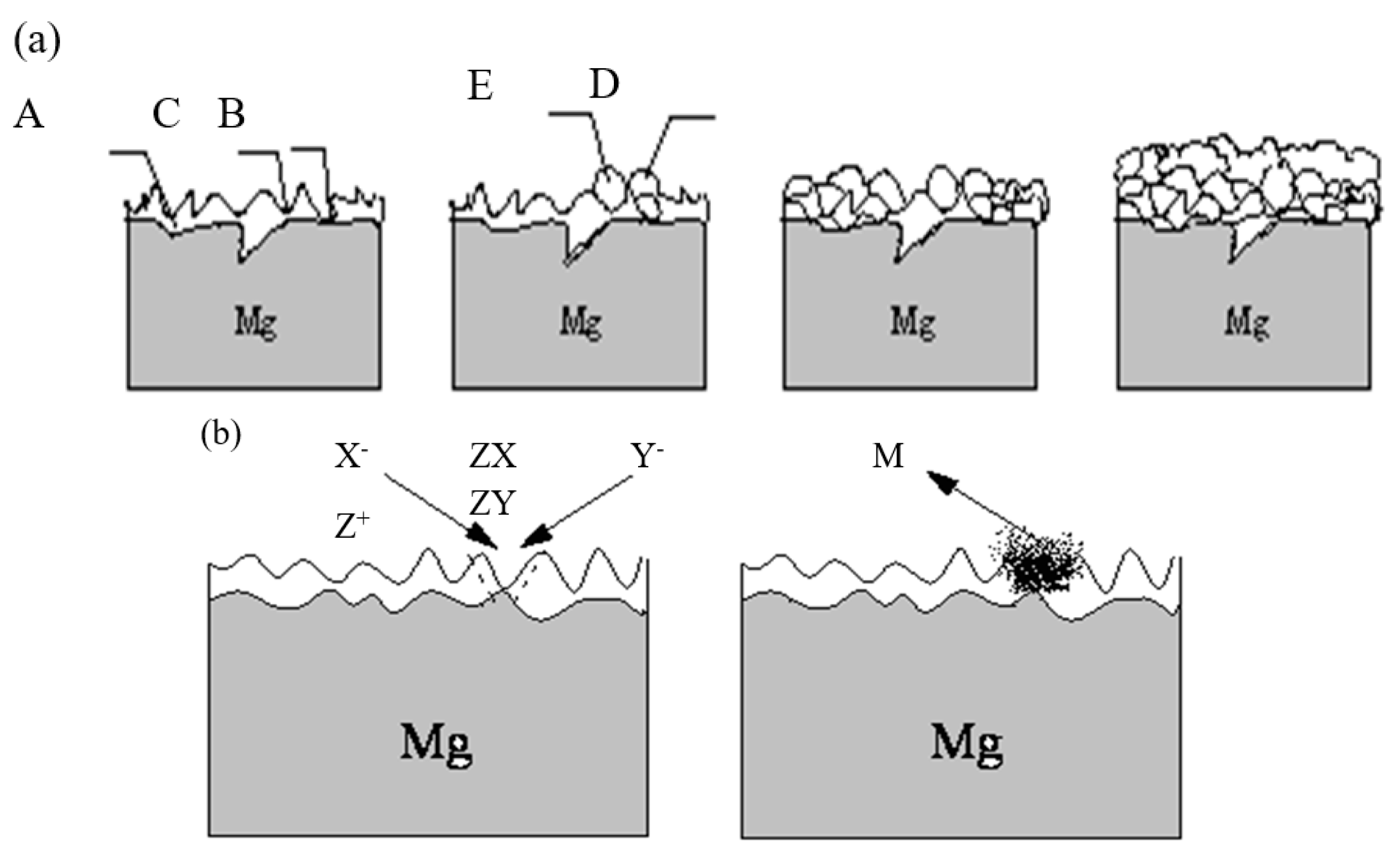
3.6.2. PEO Membrane Growth Mechanism
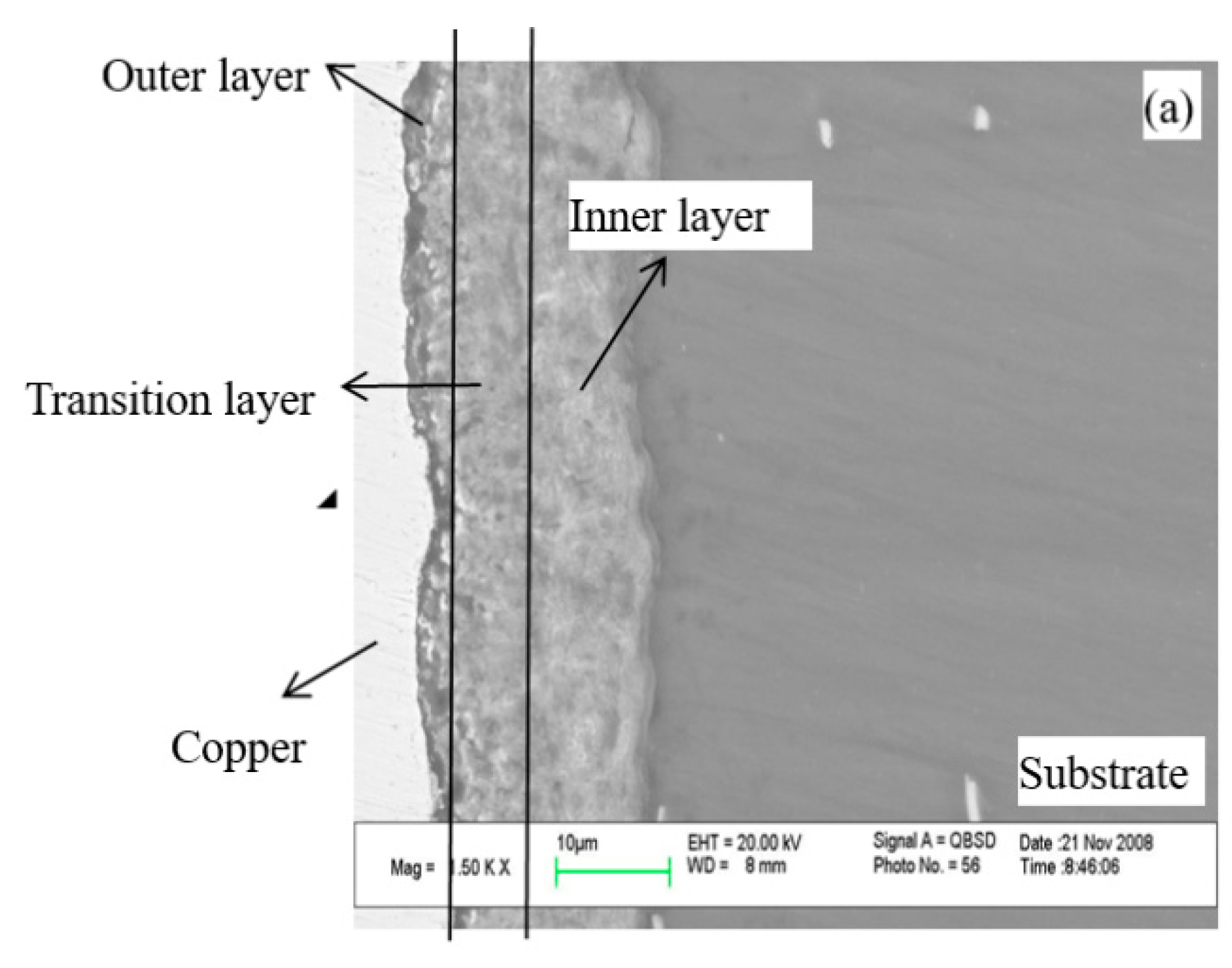
3.6.3. SEM of Cross Section
3.7. PEO Membrane Properties
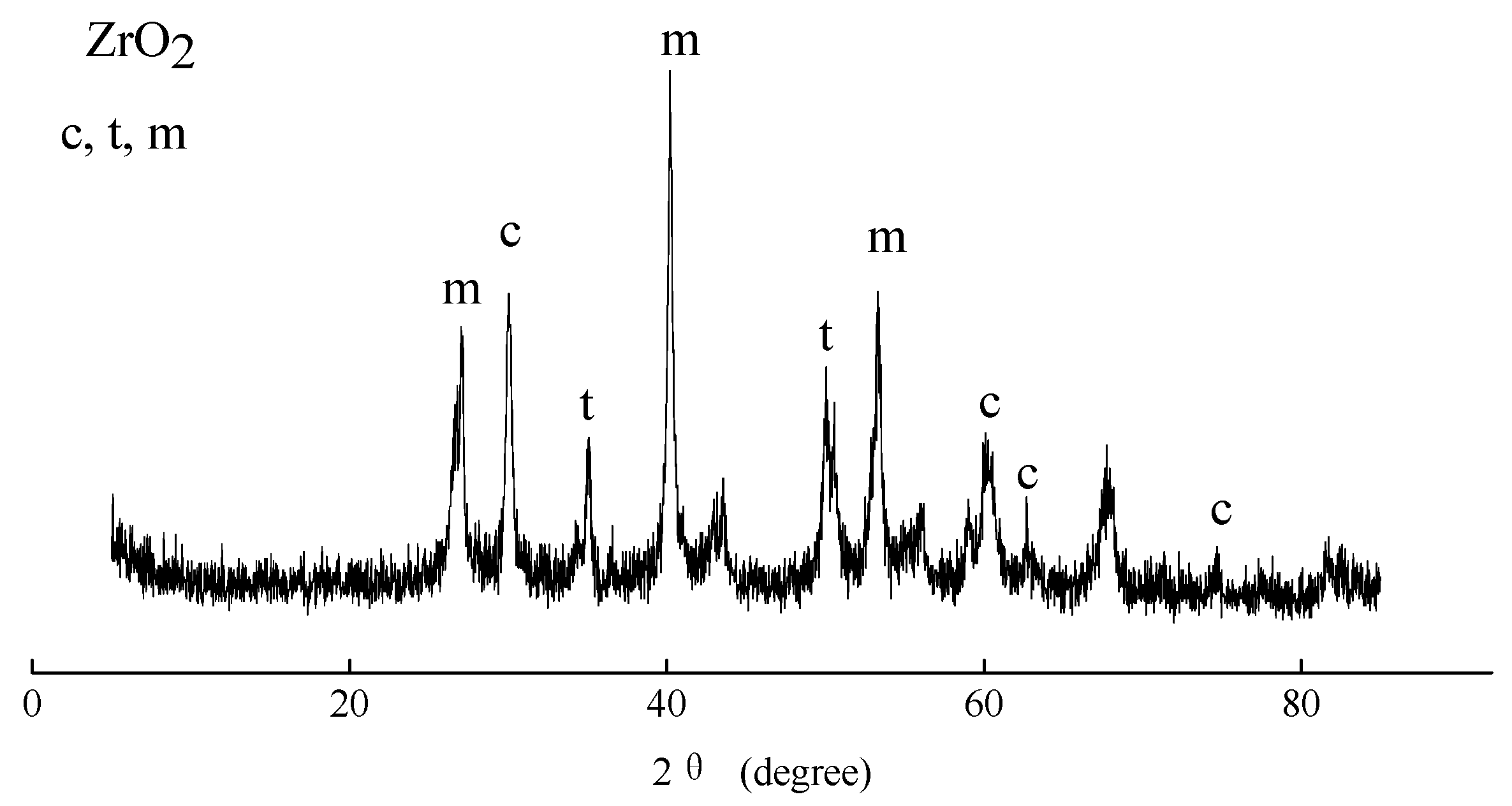
3.7.1. Crystal Structure of PEO Membranes
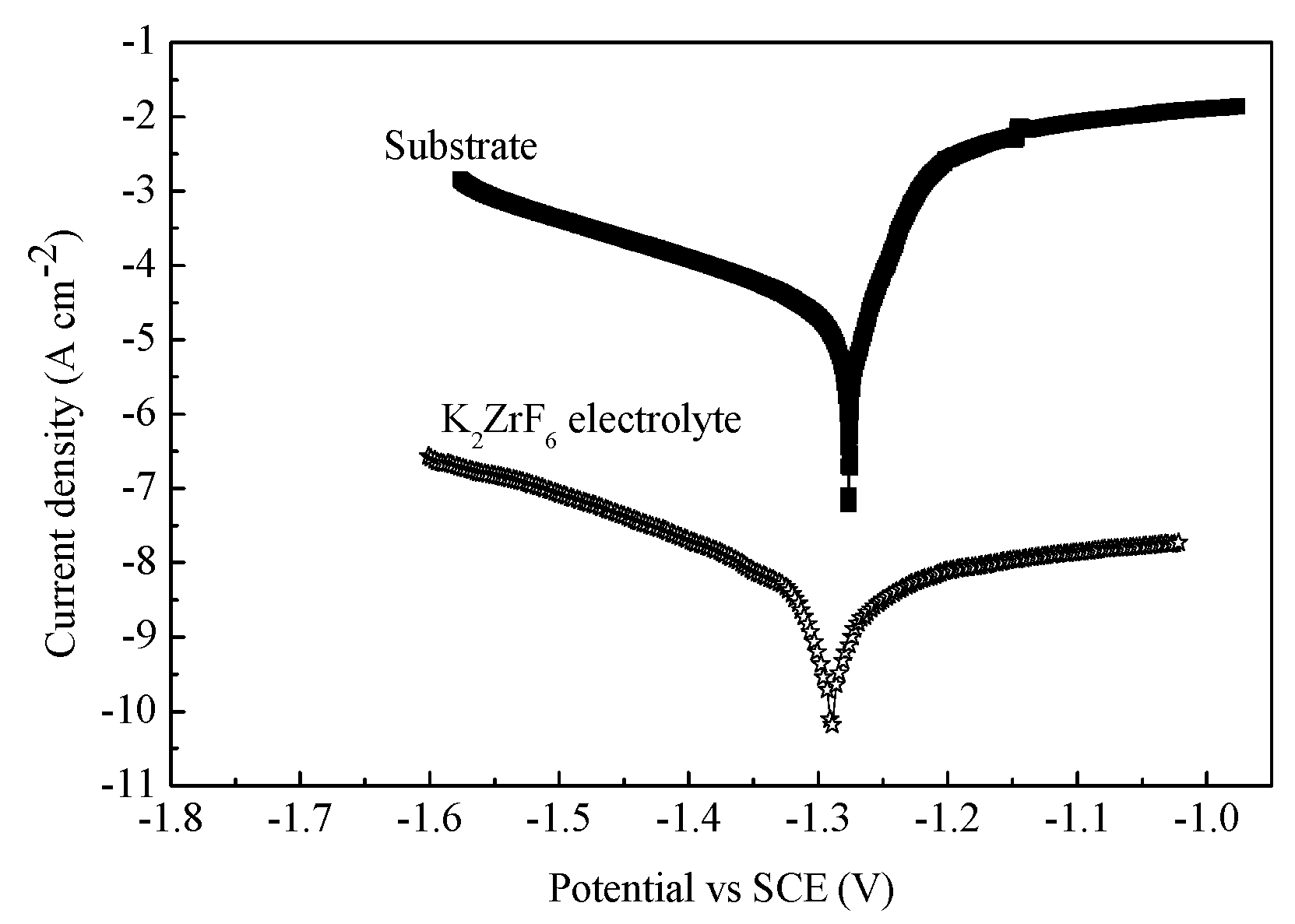
3.7.2. PEO Membrane Corrosion Performance
4. Conclusions
- (1)
- The cations had the highest spectra intensity related to the lowest excitation energy with the higher plasma concentration. Although the anion of the electrolyte does not contribute to the composition of the plasma active species, it plays an important role in the charge balance of the entire system and the composition of the membrane layer. The intensity of active species during the PEO process is related to the energy state of the working electrode’s surface. The more energy there is, the more likely it is that the active species will be excited to generate energy level transitions.
- (2)
- The heat and mass transfer during the PEO process were analyzed, and the PEO films’ growth mechanism was also proposed. The ion transfer at different stages exhibited different tendencies. At the conventional oxidation stage and transition stage, the migration resistance of the ions was low and increased gradually. At the initial discharge stage, the migration resistance was the highest because the highest membrane growth rate occurred at this stage. At the later discharge stage, the migration resistance tended to be stable, which is ascribed to a dynamic equilibrium PEO membrane growth rate.
- (3)
- The prepared PEO ceramic membranes had a uniform surface with many different inner discharge channels distributed in the outside discharge channel. This proves that the PEO membranes grow layer by layer from the inner layer to the outer layer.
- (4)
- The corrosion current density of the ZrO2 ceramic membrane was improved by six orders of magnitude compared with the AZ31B substrate, a result attributed to the high-temperature phase formation of the cubic, tetragonal, and monoclinic ZrO2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wen, F.; Wang, S.Q.; Li, J. Plasma electrolytic oxidation coatings in KOH electrolyte and its discharge characteristics. J. Alloys Compd. 2014, 594, 27–31. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Nenad, T.; Nenad, R.; Boško, G.; Rastko, V. CdS particles modified TiO2 coatings formed by plasma electrolytic oxidation with enhanced photocatalytic activity. Surf. Coat. Technol. 2018, 344, 528–533. [Google Scholar] [CrossRef]
- Masoud, R.; Arash, F.A.; Seyed, O.G.; Mohsen, K.K. Study of the effect of ZnO nanoparticles addition to PEO coatings on pure titanium substrate: Microstructural analysis, antibacterial effect and corrosion behavior of coatings in Ringer’s physiological solution. J. Alloys Compd. 2018, 740, 330–345. [Google Scholar]
- Tran, Q.P.; Chin, T.S.; Kuo, Y.C.; Jin, C.-X.; Trung, T.; Van Tuan, C.; Dang, D.Q. Diamond powder incorporated oxide layers formed on 6061 Al alloy by plasma electrolytic oxidation. J. Alloys Compd. 2018, 751, 289–298. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Fu, W. Optical emission spectroscopy studies of discharge mechanism and plasma characteristics during plasma electrolytic oxidation of magnesium in different electrolytes. Surf. Coat. Technol. 2010, 205, 1651–1658. [Google Scholar] [CrossRef]
- Yao, Z.; Su, P.; Shen, Q.; Ju, P.; Wu, C.; Zhai, Y.; Jiang, Z. Preparation of thermal control coatings on Ti alloy by plasma electrolytic oxidation in K2ZrF6 solution. Surf. Coat. Technol. 2015, 269, 273–278. [Google Scholar] [CrossRef]
- Marti-Catlatayud, M.C.; Garcia-Gabaldon, M.; Perez-Herranz, V.; Sales, S.; Mestre, S. Ceramic anion-exchange membrances based on microporous supports infifiltrated with hydrated zirconium dioxide. RSC Adv. 2015, 5, 46348–46358. [Google Scholar] [CrossRef] [Green Version]
- Albella, J.M.; Montero, I.; Martinez-Duart, J.M. Anodization and breakdown model of Ta2O5 films. Thin Solid Film. 1985, 125, 57–62. [Google Scholar] [CrossRef]
- Dzyazko, Y.; Rozhdestveskaya, L.; Zmievskii, Y.; Zakharov, V.; Myronchuk, V. Composite inorganic anion exchange membrane for electrodialytic desalination of milky whey. Mater. Today Proc. 2019, 6, 250–259. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminum. J. Phys. D Appl. Phys. 2003, 36, 2110–2120. [Google Scholar] [CrossRef]
- Wail, A.Z.; Addul, W.A.; Bassem, A.; Ko, Y.G. Toward two-dimensional hybrid organic-inorganic materials based on a I-PE/UHV-PVD system for exceptional corrosion protection. Appl. Mater. Today 2021, 24, 101142. [Google Scholar]
- Wail, A.Z.; Nisa, N.; Abdelkarim, C.; Ko, Y.G. Self-assembled molecular network formed by controlling molecular deposition of organic compounds. FlatChem 2021, 29, 100270. [Google Scholar]
- Yu, J.-M.; Cho, H.-R.; Choe, H.-C. Electrochemical characteristics of Sr/Si-doped hydroxyapatite coating on the Ti alloy surface via plasma electrolytic oxidation. Thin Solid Film. 2022, 746, 139124. [Google Scholar] [CrossRef]
- Mehri, H.; Keyvan, R.; Fakhreddin, A.; Hakimizad, A.; Santamaria, M. Incorporation mechanism of colloidal TiO2 nanoparticles and their effect o properties of coatings grown on 7075 Al alloy from silicate-based solution using plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. China 2021, 12, 3659–3676. [Google Scholar]
- Sun, M.; Wu, J.; Lu, P.; Zhang, Z.; Zhang, Y.; Li, D. Sphere-like MoS2 and porous TiO2 composites film on Ti foil as lithium-ion battery anode synthesized by plasma electrolytic oxidation an magnetron sputtering. J. Alloys Compd. 2021, 892, 162075–162084. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Petkovic, M.; Nedic, Z.; Kasalica, B.; Belca, I.; Zekovic, L. Luminescence properties of oxide films formed by anodization of aluminum in 12-tungstophosphoric acid. Electrochim. Acta 2010, 55, 3857–3863. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Belca, I.; Tadic, M. Structural and luminescence characterizaiton of porous anodic oxide films on aluminum formed in sulfamic acid solution. Appl. Surf. Sci. 2008, 255, 2845–2850. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Belca, I.; Tadic, M.; Kasalica, B.; Nedic, Z.; Zekovic, L. Galvanoluminescence properties of porous oxide films formed by anodization of aluminum in malonic acid. J. Electroanal. Chem. 2008, 619-620, 125–130. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Belca, I.; Kasalica, B.; Zekovic, L.; Tadic, M. The galvanoluminescence spectra of barrier oxide films on aluminum formed in inorganic electrolytes. Electrochem. Commun. 2006, 8, 1621–1624. [Google Scholar] [CrossRef]
- Shimizu, K.; Tajima, S. Localized nature of the luminescence during galvanostatic anodizing of high purity aluminium in inorganic electrolytes. Electrochim. Acta 1980, 25, 259–266. [Google Scholar] [CrossRef]
- Wang, L.; Wen, F.; Li, C. Evolution of active species and discharge sparks in Na2SiO3 electrolyte during PEO process. J. Alloys Compd. 2011, 509, 7652–7656. [Google Scholar] [CrossRef]
- Gu, B.; Jin, N.Q.; Wang, Z.P.; Zeng, X.H. Calculation of the transition spectra of sodium atom via TDDFT. Acta Phys. Sin. 2005, 54, 4648–4653. [Google Scholar]
- Holmlid, L.; Menon, P.G. Emission and loss of potassium promoter from styrene catalysts: Studies by ultrahigh vacuum/molecular-beam and laser techniques. Appl. Catal. A Gen. 2001, 212, 247–255. [Google Scholar] [CrossRef]
- Liu, X.P.; Huang, S.R. Inorganic Chemistry, 2nd ed.; Science Press: Beijing, China, 2005; pp. 127–128. [Google Scholar]
- Paulmier, T.; Bell, J.M.; Fredericks, P.M. Development of a novel plasma/electrolytic deposition technique Part 2: Physico-chemical analysis of the plasma discharge. Surf. Coat. Technol. 2007, 201, 8771–8781. [Google Scholar] [CrossRef] [Green Version]
- Didenko, Y.T.; Nastich, D.N.; Pugach, S.P. The effect of bulk solution temperature on the intensity and spectra of water sonoluminescence. Ultrasonics 1994, 32, 71–76. [Google Scholar] [CrossRef]
- Posakony, G.J.; Greenwood, L.R.; Ahmed, S. Stable multibubble sonoluminescence bubble patterns. Ultrasonics 2006, 44, 445–449. [Google Scholar] [CrossRef]
- Chang, L.M. Growth regularity of ceramic coating on magnesium alloy by plasma electrolytic oxidation. J. Alloys Compd. 2009, 468, 462–465. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.C. The influence of additives on the stability behavior of electrolyte, discharge and PEO films characteristics. J. Alloys Compd. 2010, 493, 445–452. [Google Scholar] [CrossRef]








| Species | λ (nm) | Transition | ∆E(eV) |
|---|---|---|---|
| O2 | 686.7 | - | |
| O2 | 627.6 | - | |
| O2 | 538.0 | - | |
| O | 847.0 | - | |
| OH | 309.3 | - | |
| OH | 512.3 | - | |
| OH | 882.9 | - | |
| H2O | 716.4 | (3 0 1)–(0 0 0) | - |
| H2O+ | 659.4 | (0 7 0)–(0 0 0) | - |
| O2+ | 383.0 | - | |
| Na | 589.0 | 3p-3s | 2.101 [22] |
| K | 769.9 | 4s-3d | 2.650 [23] |
| Al | 309.3 | 3d-3p | 3.613 [24] |
| Mg | 519.4 | - | 7.654 [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Fu, W.; Yi, G.; Chen, Z.; Gao, Z.; Pan, Q. Discharge Characteristics, Plasma Electrolytic Oxidation Mechanism and Properties of ZrO2 Membranes in K2ZrF6 Electrolyte. Membranes 2022, 12, 516. https://doi.org/10.3390/membranes12050516
Wang L, Fu W, Yi G, Chen Z, Gao Z, Pan Q. Discharge Characteristics, Plasma Electrolytic Oxidation Mechanism and Properties of ZrO2 Membranes in K2ZrF6 Electrolyte. Membranes. 2022; 12(5):516. https://doi.org/10.3390/membranes12050516
Chicago/Turabian StyleWang, Li, Wen Fu, Guangkun Yi, Ziyang Chen, Zhitin Gao, and Qingyu Pan. 2022. "Discharge Characteristics, Plasma Electrolytic Oxidation Mechanism and Properties of ZrO2 Membranes in K2ZrF6 Electrolyte" Membranes 12, no. 5: 516. https://doi.org/10.3390/membranes12050516
APA StyleWang, L., Fu, W., Yi, G., Chen, Z., Gao, Z., & Pan, Q. (2022). Discharge Characteristics, Plasma Electrolytic Oxidation Mechanism and Properties of ZrO2 Membranes in K2ZrF6 Electrolyte. Membranes, 12(5), 516. https://doi.org/10.3390/membranes12050516






