A Computer Simulation Study of Thermal and Mechanical Properties of Poly(Ionic Liquid)s
Abstract
:1. Introduction
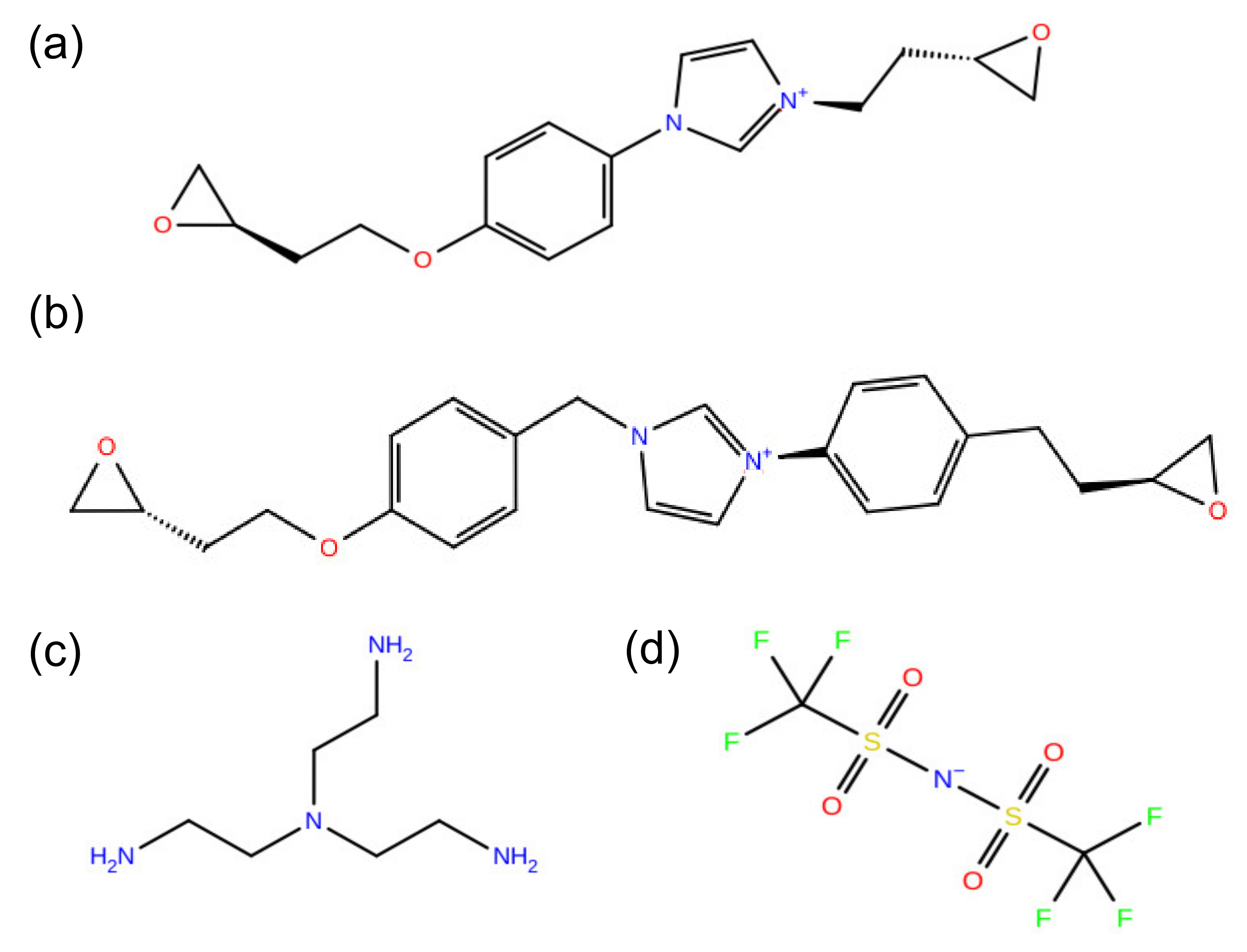
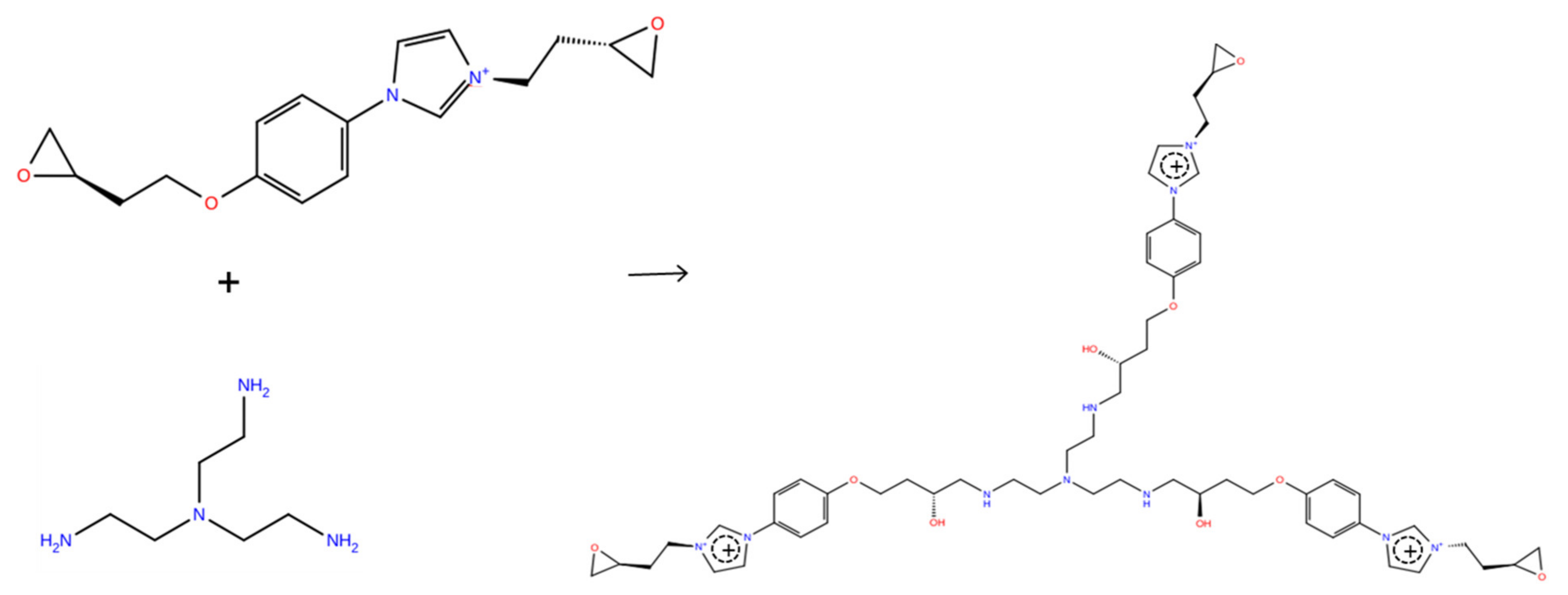
2. Models and Methods
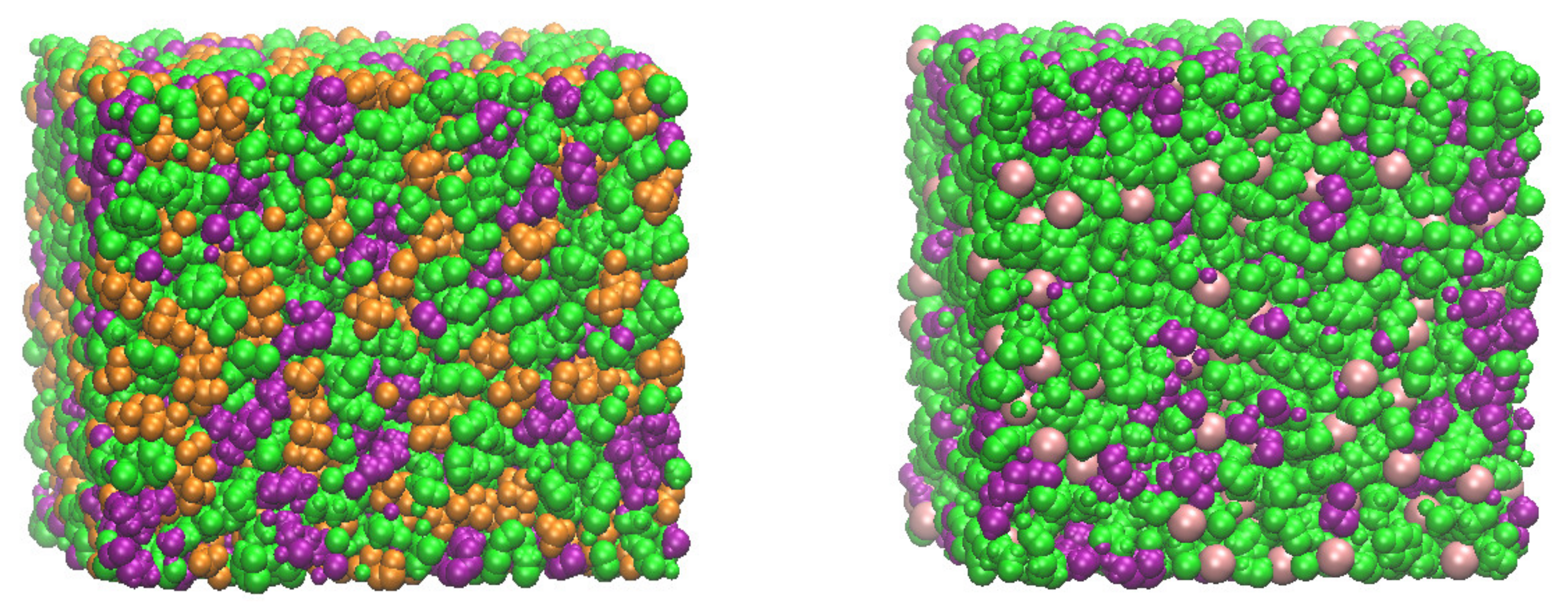
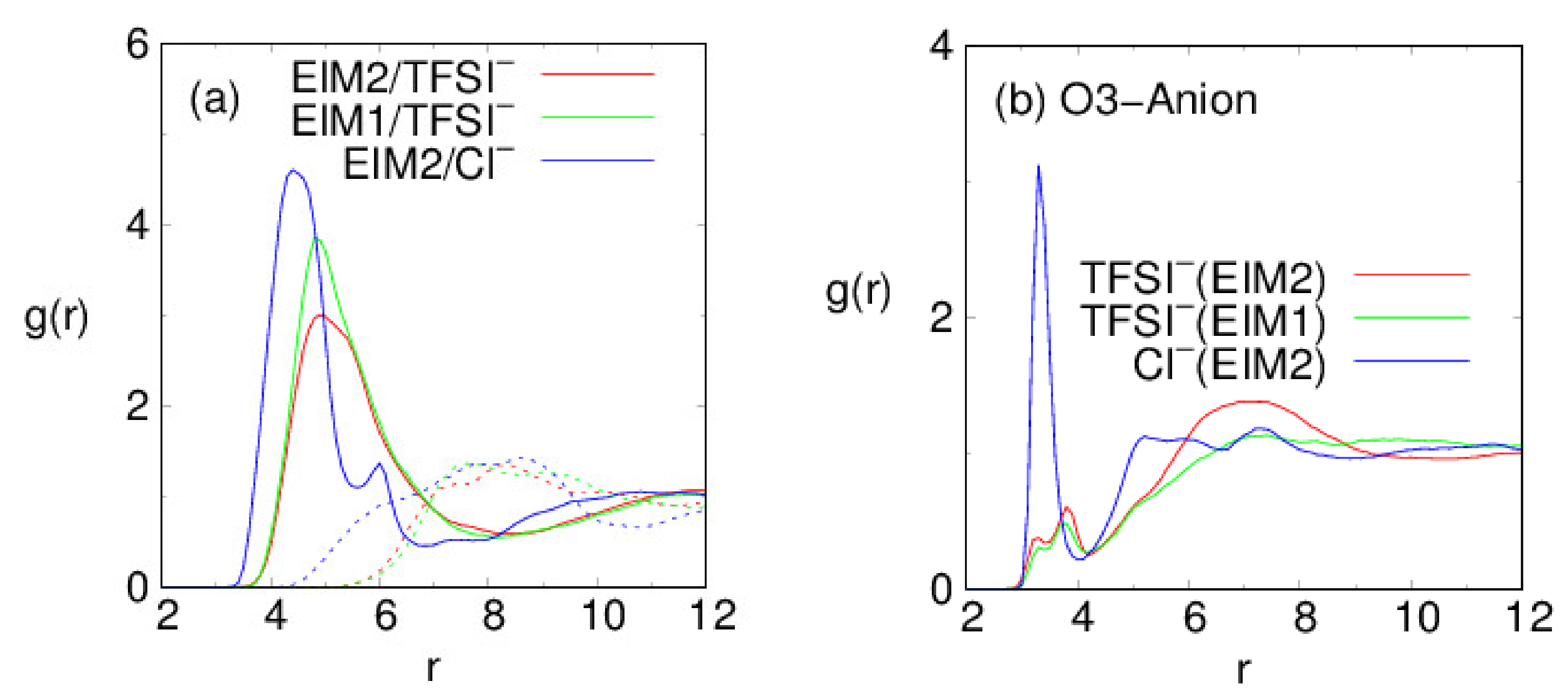
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motokura, K.; Itagaki, S.; Iwasawa, Y.; Miyaji, A.; Baba, T. Silica-supported aminopyridinium halides for catalytic transformations of epoxides to cyclic carbonates under atmospheric pressure of carbon dioxide. Green Chem. 2009, 11, 1876–1880. [Google Scholar] [CrossRef]
- Tharun, J.; Hwang, Y.; Roshan, R.; Ahn, S.; Kathalikkattil, A.C.; Park, D.W. A novel approach of utilizing quaternized chitosan as a catalyst for the eco-friendly cycloaddition of epoxides with CO2. Catal. Sci. Technol. 2012, 2, 1674–1680. [Google Scholar] [CrossRef]
- Wei-Li, D.; Bi, J.; Sheng-Lian, L.; Xu-Biao, L.; Xin-Man, T.; Chak-Tong, A. Polymer grafted with asymmetrical dication ionic liquid as efficient and reusable catalysts for the synthesis of cyclic carbonates from CO2 and epoxides. Catal. Today 2014, 233, 92–99. [Google Scholar] [CrossRef]
- Mousavi, B.; Chaemchuen, S.; Moosavi, B.; Luo, Z.X.; Gholampour, N.; Verpoort, F. Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the conversion of CO2 to cyclic carbonates. New J. Chem. 2016, 40, 5170–5176. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhou, Y.; Guo, Z.J.; Chen, G.J.; Li, J.; Shi, Y.M.; Liu, Y.Q.; Wang, J. Heterogeneous conversion of CO2 into cyclic carbonates at ambient pressure catalyzed by ionothermal-derived meso-macroporous hierarchical poly(ionic liquid)s. Chem. Sci. 2015, 6, 6916–6924. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Chi, Y. Polymer-supported ionic liquids: Imidazolium salts as catalysts for nucleophilic substitution reactions including fluorinations. Angew. Chem. 2004, 116, 489–491. [Google Scholar] [CrossRef]
- Kim, D.W.; Hong, D.J.; Jang, K.S.; Chi, Y. Structural Modification of Polymer-Supported Ionic Liquids as Catalysts for Nucleophilic Substitution Reactions Including Fluorination. Adv. Synth. Catal. 2006, 348, 1719–1727. [Google Scholar] [CrossRef]
- Altava, B.; Burguete, M.I.; Garcia-Verdugo, E.; Karbass, N.; Luis, S.V.; Puzary, A.; Sans, A. Palladium N-methylimidazolium supported complexes as efficient catalysts for the Heck reaction. Tetrahedron Lett. 2006, 47, 2311. [Google Scholar] [CrossRef]
- Karbass, N.; Sans, V.; Garcia-Verdugo, E.; Burguete, M.I.; Luis, S.V. Pd(0) supported onto monolithic polymers containing IL-like moieties. Continuous flow catalysis for the Heck reaction in near-critical EtOH. Chem. Commun. 2006, 3095–3097. [Google Scholar] [CrossRef]
- Lozano, P.; Garcia-Verdugo, E.; Piamtongkam, R.; Karbass, N.; Diego, T.; Butguete, M.I.; Luis, S.V. Bioreactors Based on Monolith-Supported Ionic Liquid Phase for Enzyme Catalysis in Supercritical Carbon Dioxide. Adv. Synth. Catal. 2007, 349, 1077. [Google Scholar] [CrossRef]
- Ohno, H.; Ito, K. Room-temperature molten salt polymers as a matrix for fast ion conduction. Chem. Lett. 1998, 27, 751–752. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, N.; Ohno, H. 15th anniversary of polymerized ionic liquids. Polymer 2014, 55, 3289–3297. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent advances in innovative polymer electrolytes based on poly(ionic liquid)s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Ambrogi, M.; Tiruye, G.A.; Cordella, D.; Fernandes, A.M.; Grygiel, K.; Isik, M.; Patil, N.; Porcarelli, L.; Rocasalbas, G.; et al. Innovative polyelectrolytes/poly(ionic liquid)s for energy and the environment. Polym. Int. 2017, 66, 1119–1128. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin, Germany, 2015; pp. 47–67. [Google Scholar]
- Zhou, D.; Liu, R.; Zhang, J.; Qi, X.; He, Y.-B.; Li, B.; Yang, Q.-H.; Hu, Y.-S.; Kang, F. In situ synthesis of hierarchical poly(ionic liquid)-based solid electrolytes for high-safety lithium-ion and sodium-ion batteries. Nano Energy 2017, 33, 45–54. [Google Scholar] [CrossRef]
- Susan, M.A.B.H.; Kaneko, T.; Noda, A.; Watanabe, M. Ion gels prepared by in situ radical polymerization of vinyl monomers in an ionic liquid and their characterization as polymer electrolytes. J. Am. Chem. Soc. 2005, 127, 4976–4983. [Google Scholar] [CrossRef]
- Carlisle, T.K.; McDanel, W.M.; Cowan, M.G.; Noble, R.D.; Gin, D.L. Vinyl-Functionalized Poly(imidazolium)s: A Curable Polymer Platform for Cross-Linked Ionic Liquid Gel Synthesis. Chem. Mater. 2014, 26, 1294–1296. [Google Scholar] [CrossRef]
- Mogurampelly, S.; Ganesan, V. Ion Transport in Polymerized Ionic Liquid–Ionic Liquid Blends. Macromolecules 2018, 51, 9471–9483. [Google Scholar] [CrossRef]
- Koch, V.R.; Nanjundiah, C.; Appetecchi, G.B. The interfacial stability of Li with two new solvent-free ionic liquids: 1,2-dimethyl-3-propylimidazolium imide and methide. J. Electrochem. Soc. 1995, 142, 116–118. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Athanassov, Y.; Armand, M.; Bonhote, P.; Pettersson, H.; Azam, A.; Grätzel, M. The Performance and Stability of Ambient Temperature Molten Salts for Solar Cell Applications. J. Electrochem. Soc. 1996, 143, 3099–3108. [Google Scholar] [CrossRef]
- Matsumoto, K.; Endo, T. Design and Synthesis of ionic–conductive epoxy–based networked Polymers. React. Funct. Polym. 2013, 73, 278–282. [Google Scholar] [CrossRef]
- Joo, M.; Shin, J.; Kim, J.; You, J.B.; Yoo, Y.; Kwak, M.J.; Oh, M.S.; Gap, S. One-Step Synthesis of Cross-Linked Ionic Polymer Thin Films in Vapor Phase and Its Application to an Oil/Water Separation Membrane. J. Am. Chem. Soc. 2017, 139, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Jiang, B.; Xiao, X.; Xu, M.; Tantai, X.; Wang, B.; Sun, Y.; Zhang, L. Novel Protic Ionic Liquid Composite Membranes with Fast and Selective Gas Transport Nanochannels for Ethylene/Ethane Separation. ACS Appl. Mater. Interfaces 2018, 10, 13963–13974. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Mecerreyes, D.; Forsyth, M.; Zhang, H.; Armand, M. Polymeric ionic liquids for lithium-based rechargeable batteries. Mol. Syst. Des. Eng. 2019, 4, 294–309. [Google Scholar] [CrossRef]
- Matsumoto, K.; Endo, T. Synthesis of Ion Conductive Networked Polymers Based on an Ionic Liquid Epoxide Having a Quaternary Ammonium Salt Structure. Macromolecules 2009, 42, 4580–4584. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, J. Poly(1-Vinyl-1,2,4-triazolium) Poly(Ionic Liquid)s: Synthesis and the Unique Behavior in Loading Metal Ions. Macromol. Rapid Commun. 2016, 37, 1124–1129. [Google Scholar] [CrossRef] [Green Version]
- McDanel, W.M.; Cowan, M.G.; Chisholm, N.O.; Gin, D.L.; Noble, R.D. Fixed-site-carrier facilitated transport of carbon dioxide through ionic-liquid-based epoxy-amine ion gel membranes. J. Membr. Sci. 2015, 492, 303–311. [Google Scholar] [CrossRef]
- McDanel, W.M.; Cowan, M.G.; Barton, J.A.; Gin, D.L.; Noble, R.D. Effect of Monomer Structure on Curing Behavior, CO2 Solubility, and Gas Permeability of Ionic Liquid-Based Epoxy–Amine Resins and Ion-Gels. Ind. Eng. Chem. Res. 2015, 54, 4396–4406. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Obadia, M.M.; Serghei, A.; Livi, S. 1,2,3-Triazolium-Based Epoxy-Amine Networks: Ion-Conducting Polymer Electrolytes. Macromol. Rapid Commun. 2016, 37, 1168–1174. [Google Scholar] [CrossRef]
- McDanel, W.M.; Cowan, M.G.; Carlisle, T.K.; Swanson, A.K.; Noble, R.D.; Gin, D.L. Cross-linked ionic resins and gels from epoxide-functionalized imidazolium ionic liquid monomers. Polymer 2014, 55, 3305–3313. [Google Scholar] [CrossRef]
- Livia, S.; Lins, L.C.; Capeletti, L.B.; Chardin, C.; Halawani, N.; Baudoux, J.; Cardoso, M.B. Antibacterial surface based on new epoxy-amine networks from ionic liquid monomers. Eur. Polym. J. 2019, 116, 56–64. [Google Scholar] [CrossRef]
- Livi, S.; Chardin, C.; Lins, L.C.; Halawani, N.; Pruvost, S.; Duchet-Rumeau, J.; Gérard, J.-F.; Baudoux, J. From Ionic Liquid Epoxy Monomer to Tunable Epoxy–Amine Network: Reaction Mechanism and Final Properties. ACS Sustain. Chem. Eng. 2019, 7, 3602–3613. [Google Scholar] [CrossRef]
- Radchenko, A.V.; Chabane, H.; Demir, B.; Searles, D.J.; Duchet-Rumeau, J.; Gérard, J.-F.; Baudoux, J.; Livi, S. New Epoxy Thermosets Derived from a Bisimidazolium Ionic Liquid Monomer: An Experimental and Modeling Investigation. ACS Sustain. Chem. Eng. 2020, 8, 12208–12221. [Google Scholar] [CrossRef]
- Dzienia, A.; Tarnacka, M.; Koperwas, K.; Maksym, P.; Ziȩba, A.; Feder-Kubis, J.; Kamiński, K.; Paluch, M. Impact of Imidazolium-Based Ionic Liquids on the Curing Kinetics and Physicochemical Properties of Nascent Epoxy Resins. Macromolecules 2020, 53, 6341–6352. [Google Scholar] [CrossRef]
- Radchenko, A.V.; Duchet-Rumeau, J.; Gérard, J.F.; Baudoux, J.; Livi, S. Cycloaliphatic epoxidized ionic liquids as new versatile monomers for the development of shape memory PIL networks by 3D printing. Polym. Chem. 2020, 11, 5475–5483. [Google Scholar] [CrossRef]
- Demir, B.; Perli, G.; Chan, K.Y.; Duchet-Rumeau, J.; Livi, S. Molecular-Level Investigation of Cycloaliphatic Epoxidized Ionic Liquids as a New Generation of Monomers for Versatile Poly(Ionic Liquids). Polymers 2021, 13, 1512. [Google Scholar] [CrossRef]
- D. E. Shaw Research. Materials Science Suite, Desmond; D.E. Shaw Research, LLC.: New York, NY, USA, 2020. [Google Scholar]
- Roos, K.; Wu, C.; Damm, W.; Reboul, M.; Stevenson, J.M.; Lu, C.; Dahlgren, M.K.; Mondal, S.; Chen, W.; Wang, L.; et al. OPLS3e: Extending Force Field Coverage for Drug-Like Small Molecules. J. Chem. Theory Comput. 2019, 15, 1863–1874. [Google Scholar] [CrossRef]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 2, 281–296. [Google Scholar] [CrossRef]
- Estridge, C.E. The effects of competitive primary and secondary amine reactivity on the structural evolution and properties of an epoxy thermoset resin during cure: A molecular dynamics study. Polymer 2018, 141, 12–20. [Google Scholar] [CrossRef]
- Tuckerman, M.; Berne, B.J.; Martyna, G.J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 1992, 97, 1990–2001. [Google Scholar] [CrossRef] [Green Version]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Patrone, P.N.; Dienstfrey, A.; Browning, A.R.; Tucker, S.; Christensen, S. Uncertainty quantification in molecular dynamics studies of the glass transition temperature. Polymer 2016, 87, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Shim, Y.; Choi, M.Y.; Kim, H.J. A molecular dynamics computer simulation study of room-temperature ionic liquids. I. Equilibrium solvation structure and free energetics. J. Chem. Phys. 2005, 122, 044510. [Google Scholar] [CrossRef] [Green Version]
- Chantawansri, T.L.; Yeh, I.-C.; Hsieh, A.J. Investigating the glass transition temperature at the atom-level in select model polyamides: A molecular dynamics study. Polymer 2015, 81, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, A.; Valavala, P.K.; Clancy, T.C.; Wise, K.E.; Odegard, G.M. Molecular modeling of crosslinked epoxy polymers: The effect of crosslink density on thermomechanical properties. Polymer 2011, 52, 2445–2452. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. he Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-Forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Lee, A.; McKenna, G.B. Effect of crosslink density on physical ageing of epoxy networks. Polymer 1988, 29, 1812–1817. [Google Scholar] [CrossRef]
- Garcia, G.; Soares, B.G.; Pita, V.J.R.R.; Sanchez, R.; Rieumont, J. Mechanical properties of epoxy networks based on DGEBA and aliphatic amines. J. Appl. Polym. Sci. 2007, 106, 2047–2055. [Google Scholar] [CrossRef]
- Vignoud, L.; David, L.; Sixou, B.; Vigier, G. Influence of electron irradiation on the mobility and on the mechanical properties of DGEBA/TETA epoxy resins. Polymer 2001, 42, 4657–4665. [Google Scholar] [CrossRef]
- Sindt, O.; Perez, J.; Gerard, J.F. Molecular architecture-mechanical behaviour relationships in epoxy networks. Polymer 1996, 37, 2989–2997. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 2. Variation of Alkyl Chain Length in Imidazolium Cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef]
- Tokuda, H.; Tsuzuki, S.; Susan, M.A.B.H.; Watanabe, M. How Ionic Are Room-Temperature Ionic Liquids? An Indicator of the Physicochemical Properties. J. Phys. Chem. B 2006, 110, 19593–19600. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, Q.; Zhu, S. Comparing ion transport in ionic liquids and polymerized ionic liquids. Sci. Rep. 2020, 10, 7825. [Google Scholar] [CrossRef]
- Mogurampelly, S.; Keith, J.R.; Ganesan, V. Mechanisms Underlying Ion Transport in Polymerized Ionic Liquids. J. Am. Chem. Soc. 2017, 139, 9511–9514. [Google Scholar] [CrossRef]
- Luo, X.; Liu, H.; Paddison, S.J. Molecular Dynamics Simulations of Polymerized Ionic Liquids: Mechanism of Ion Transport with Different Anions. ACS Appl. Polym. Mater. 2021, 3, 141–152. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kim, T.; Jung, B.M.; Lee, S.B.; Choi, U.H. Multifunctional Epoxy-Based Solid Polymer Electrolytes for Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 41. [Google Scholar] [CrossRef]
- Song, Y.H.; Kim, T.; Choi, U.H. Tuning Morphology and Properties of Epoxy-Based Solid-State Polymer Electrolytes by Molecular Interaction for Flexible All-Solid-State Supercapacitors. Chem. Mater. 2020, 32, 3879–3892. [Google Scholar] [CrossRef]
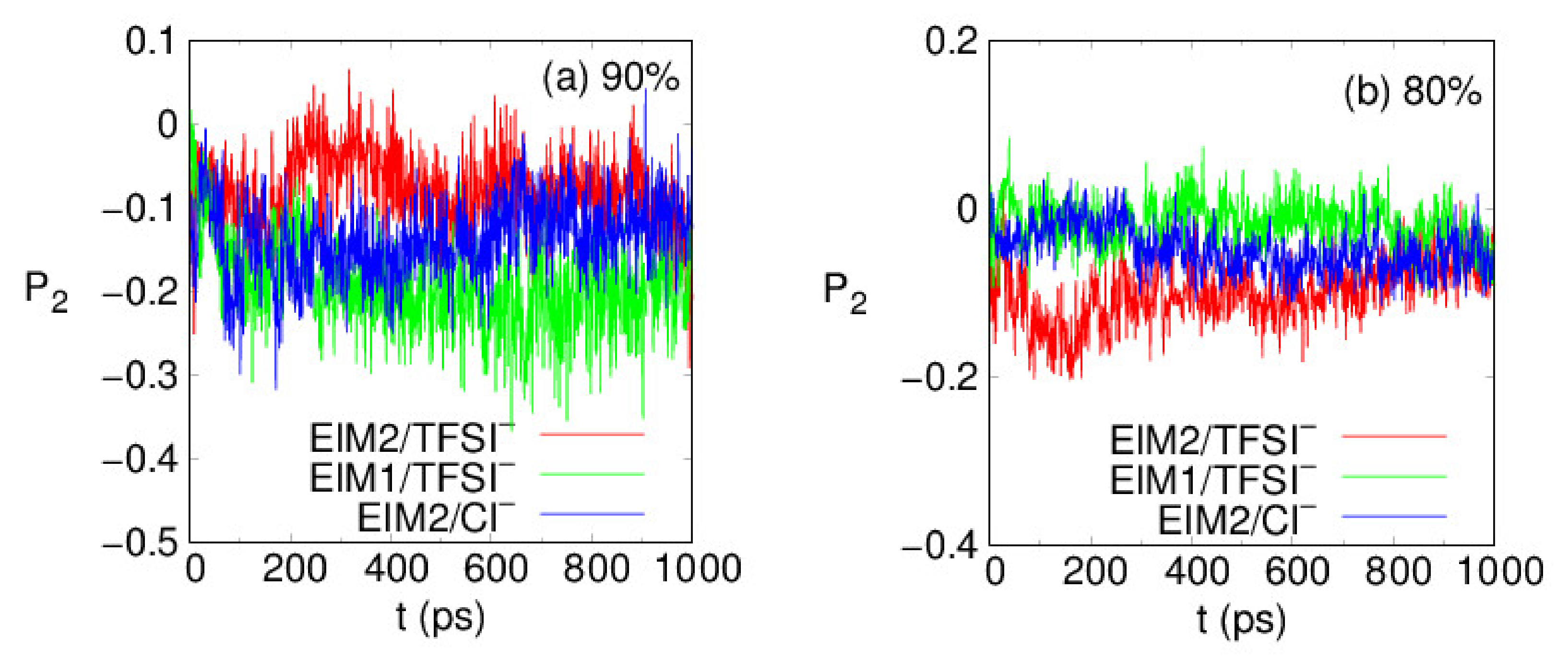
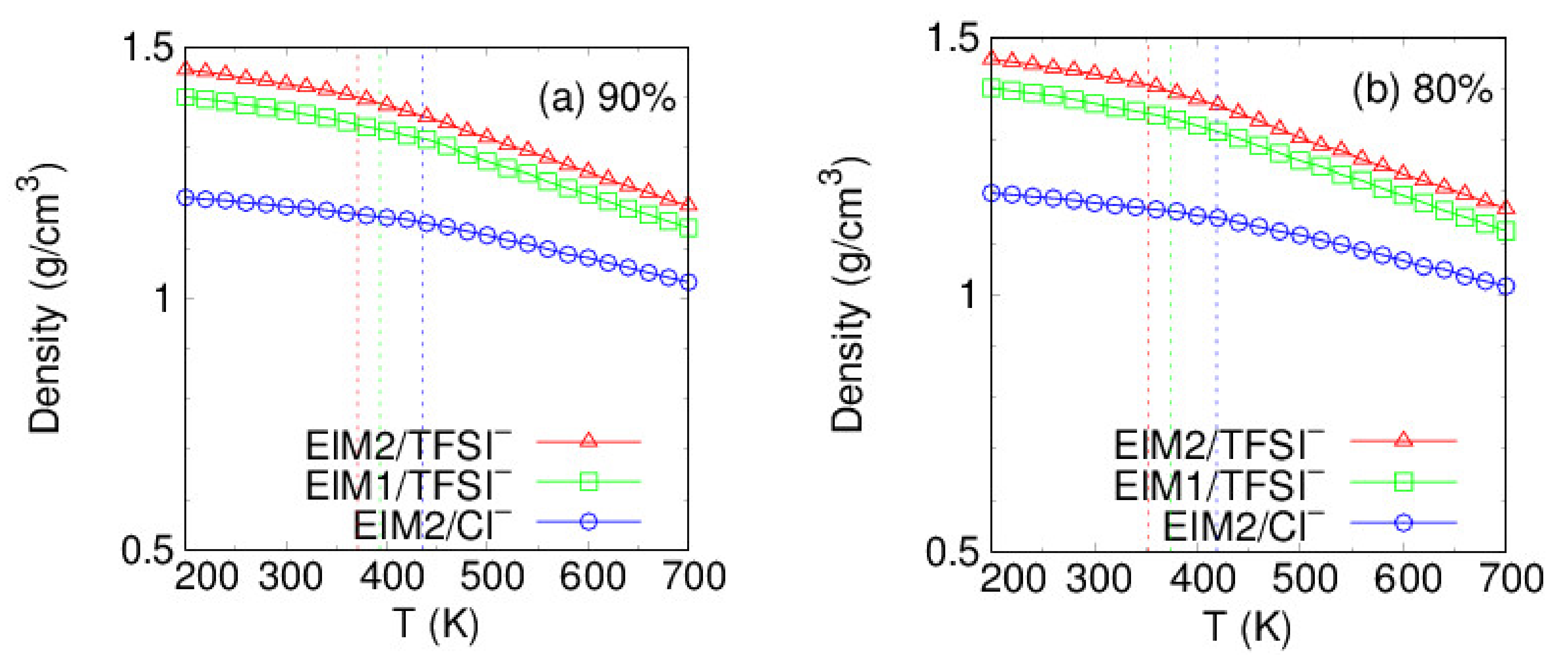
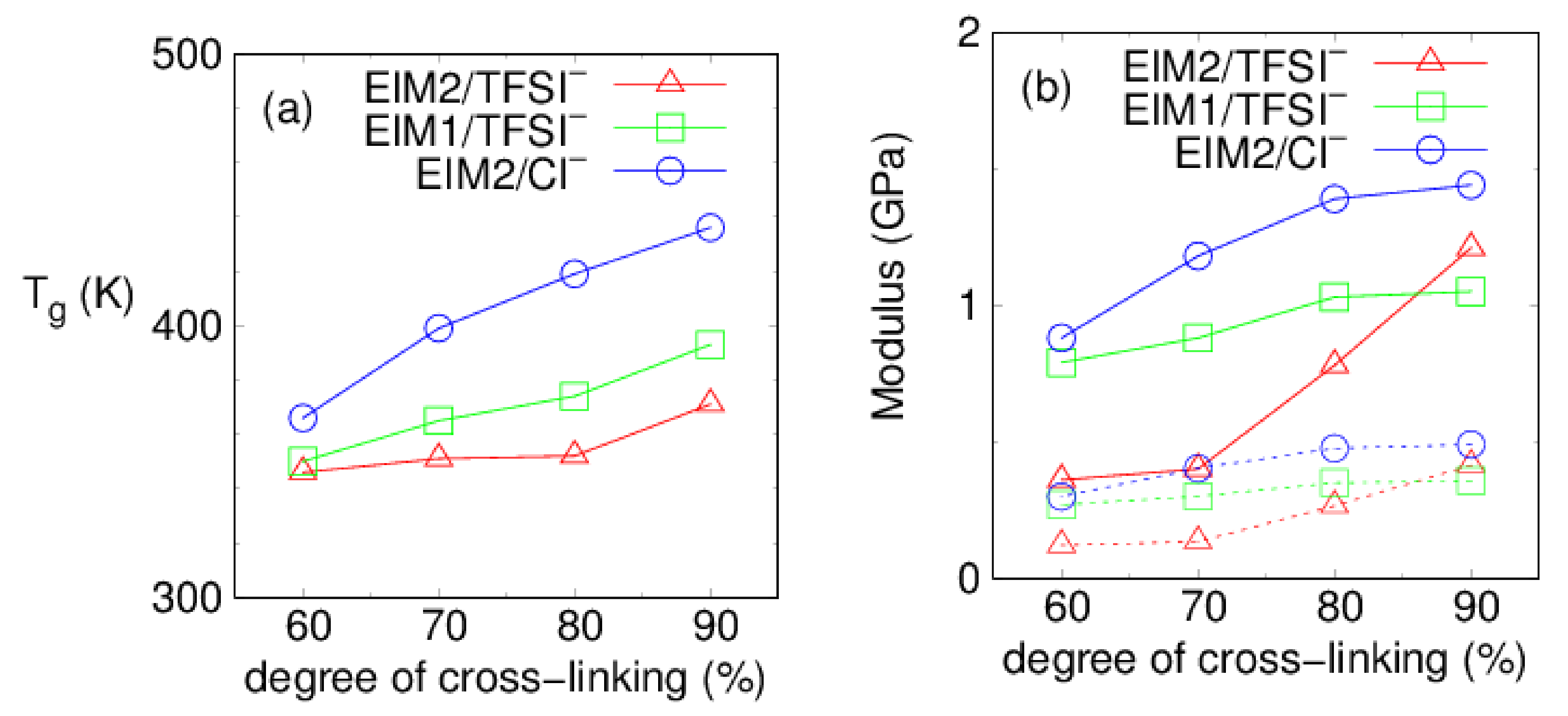
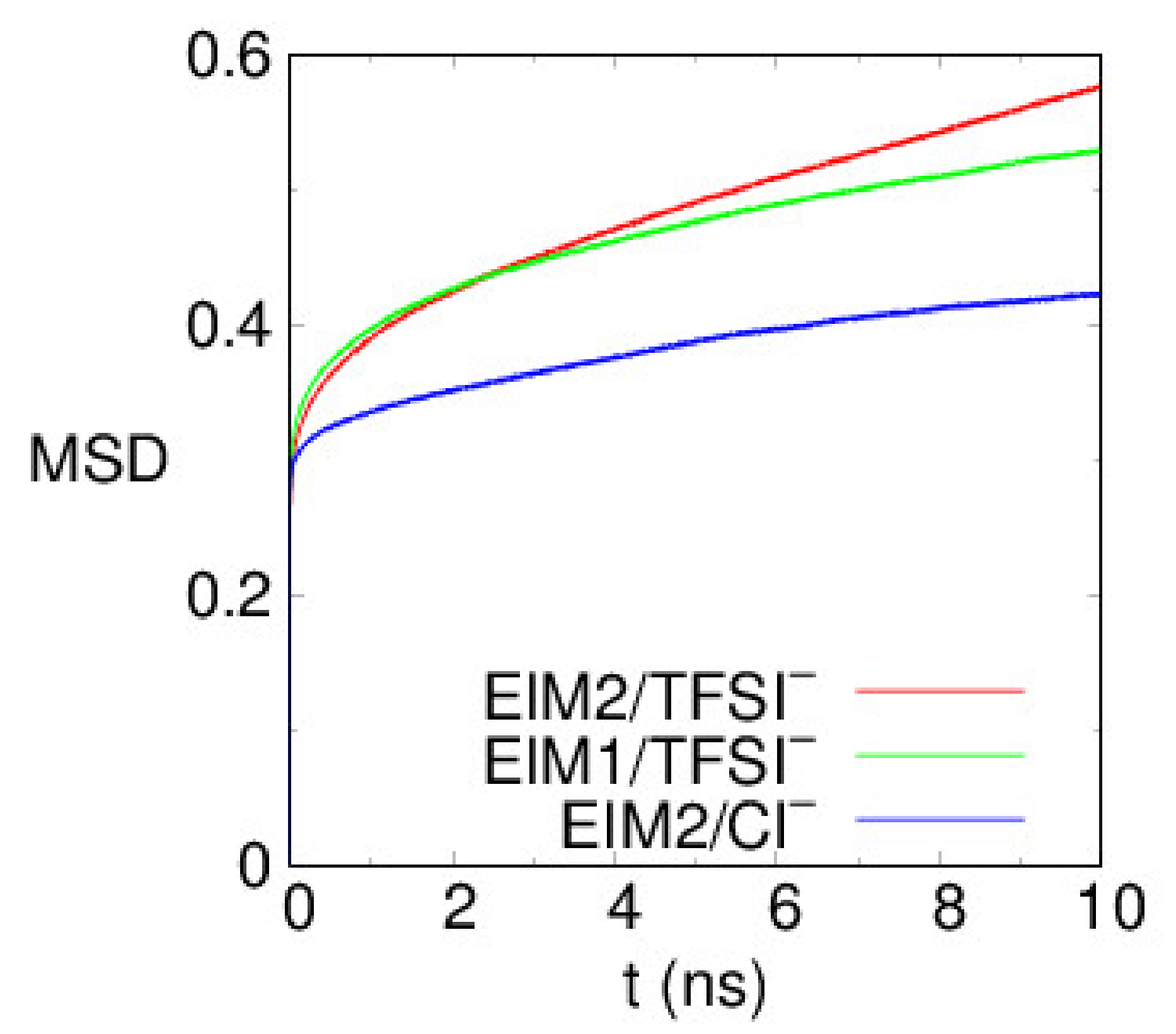
| (%) | Tg (K) | E (GPa) | ||||
|---|---|---|---|---|---|---|
| EIM2/TFSI− | EIM2/Cl− | EIM1/TFSI− | EIM2/TFSI− | EIM2/Cl− | EIM1/TFSI− | |
| 90 | 371 | 436 | 393 | 1.21 | 1.44 | 1.05 |
| 80 | 352 | 419 | 374 | 0.78 | 1.39 | 1.03 |
| 70 | 351 | 399 | 365 | 0.40 | 1.18 | 0.88 |
| 60 | 346 | 366 | 350 | 0.36 | 0.88 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, Y.; Shim, M.; Kim, D.S. A Computer Simulation Study of Thermal and Mechanical Properties of Poly(Ionic Liquid)s. Membranes 2022, 12, 450. https://doi.org/10.3390/membranes12050450
Shim Y, Shim M, Kim DS. A Computer Simulation Study of Thermal and Mechanical Properties of Poly(Ionic Liquid)s. Membranes. 2022; 12(5):450. https://doi.org/10.3390/membranes12050450
Chicago/Turabian StyleShim, Youngseon, Munbo Shim, and Dae Sin Kim. 2022. "A Computer Simulation Study of Thermal and Mechanical Properties of Poly(Ionic Liquid)s" Membranes 12, no. 5: 450. https://doi.org/10.3390/membranes12050450
APA StyleShim, Y., Shim, M., & Kim, D. S. (2022). A Computer Simulation Study of Thermal and Mechanical Properties of Poly(Ionic Liquid)s. Membranes, 12(5), 450. https://doi.org/10.3390/membranes12050450






