pH and Design on n–Alkyl Alcohol Bulk Liquid Membranes for Improving Phenol Derivative Transport and Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Methods
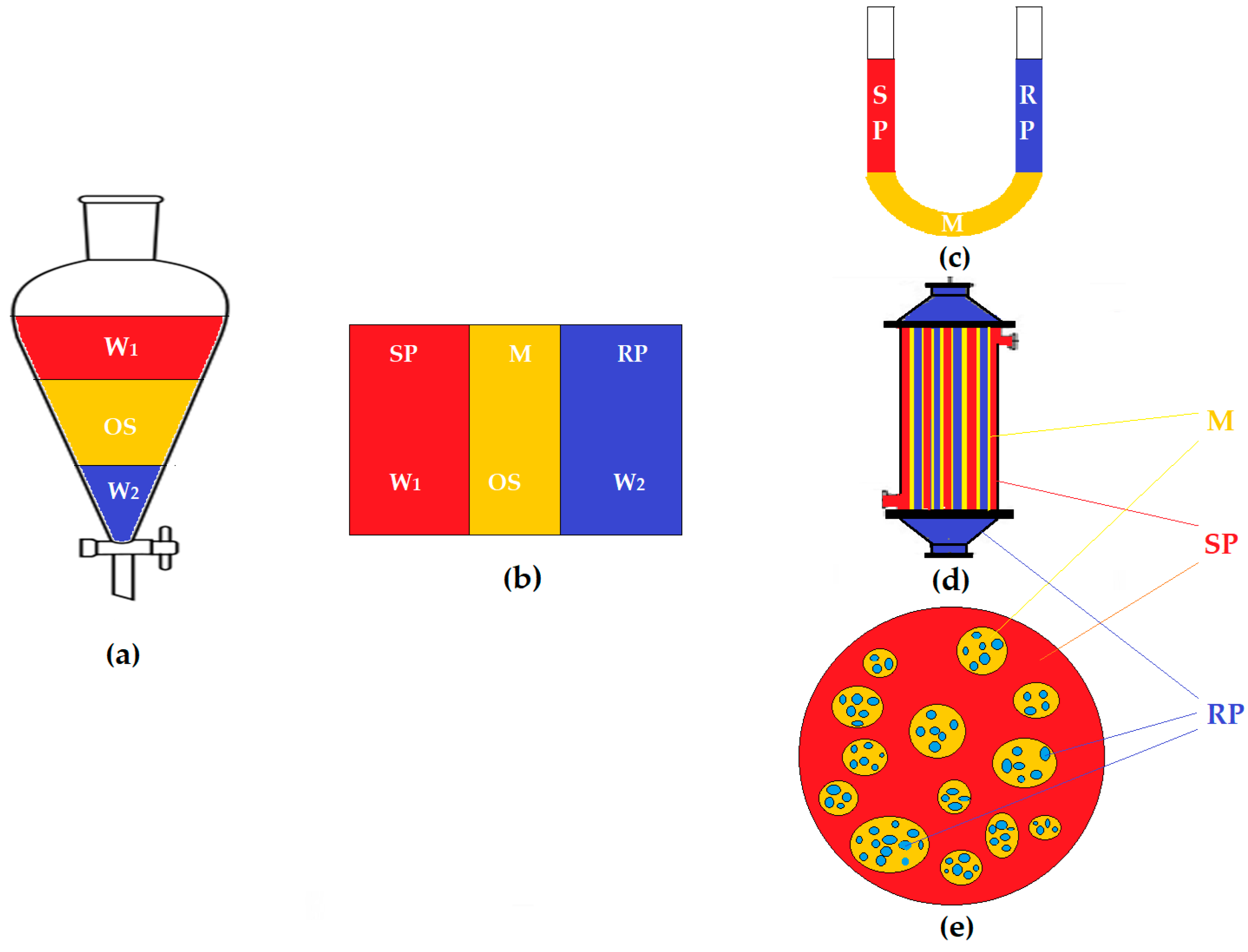
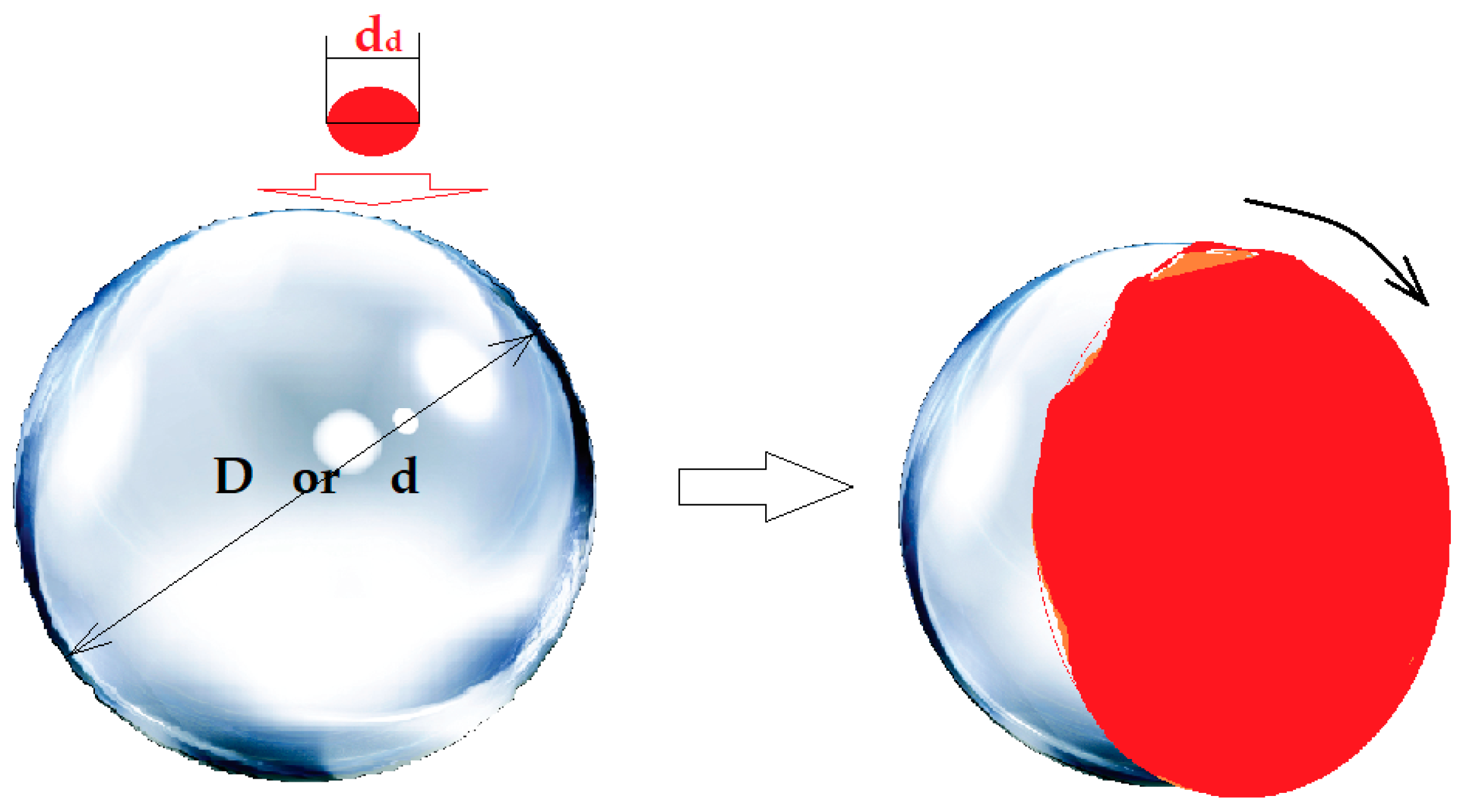
2.2.1. Design of the Experimental Installation
2.2.2. Transport and Separation Experiments of the Phenol Derivatives
2.3. Equipment
3. Results and Discussion
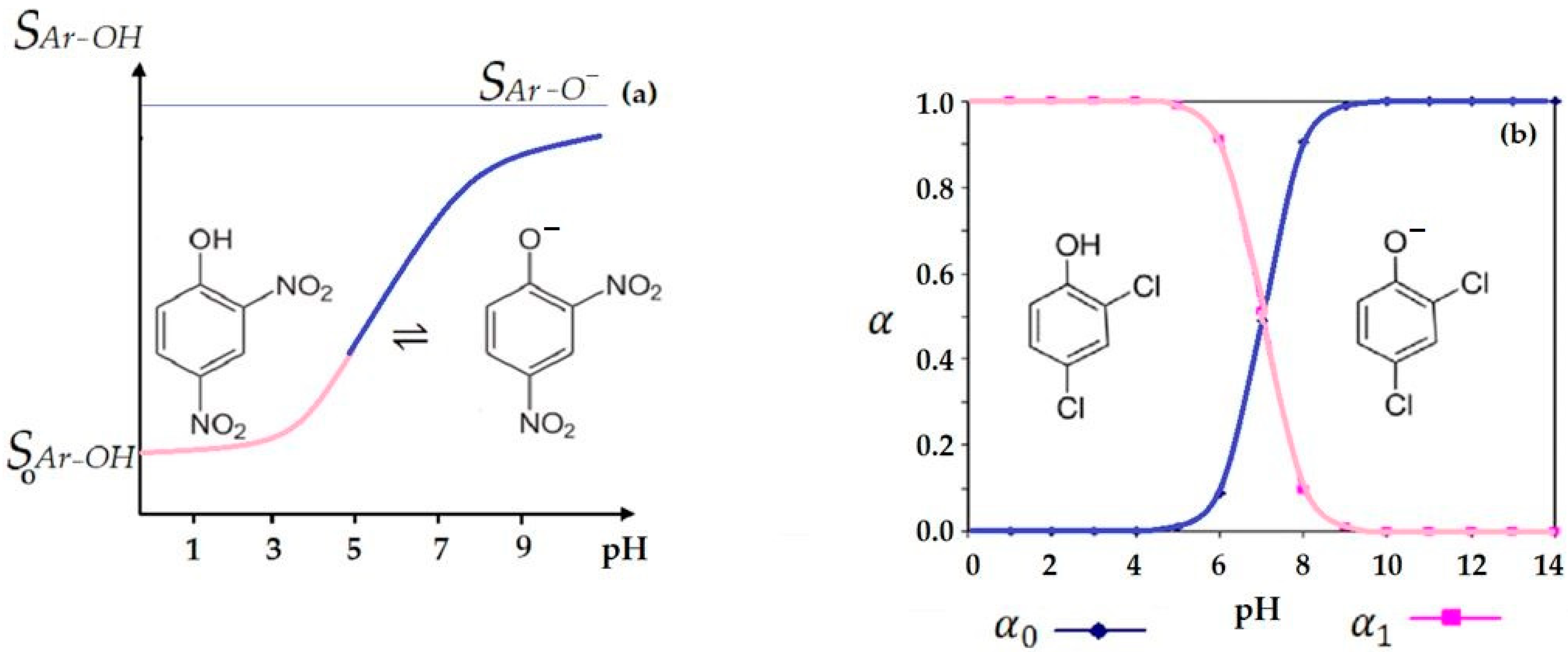
3.1. Brief Argument for Choosing the pH of Aqueous Phases for the Membrane Transport of Weakly Acidic Phenolic Chemical Species
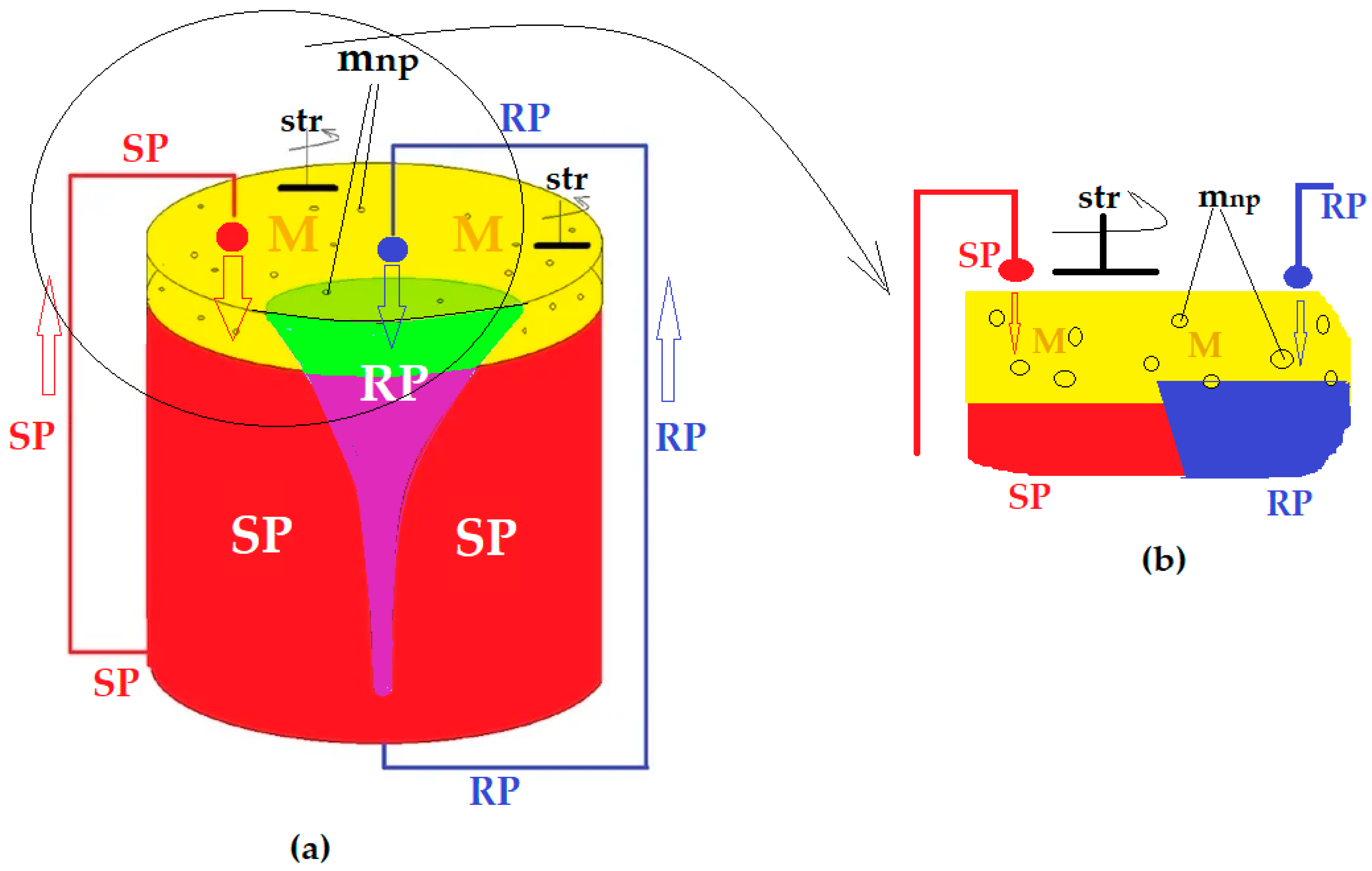
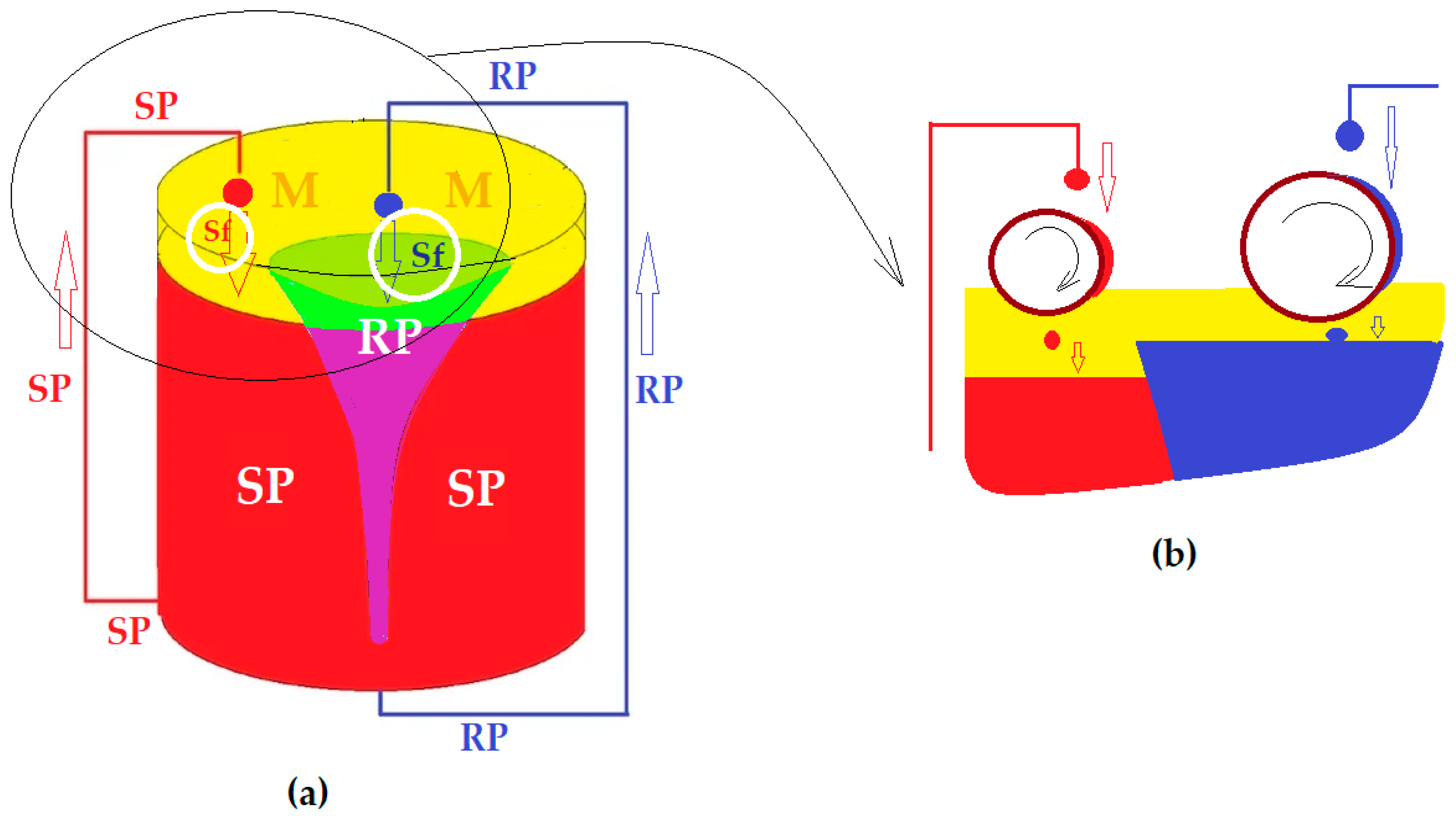
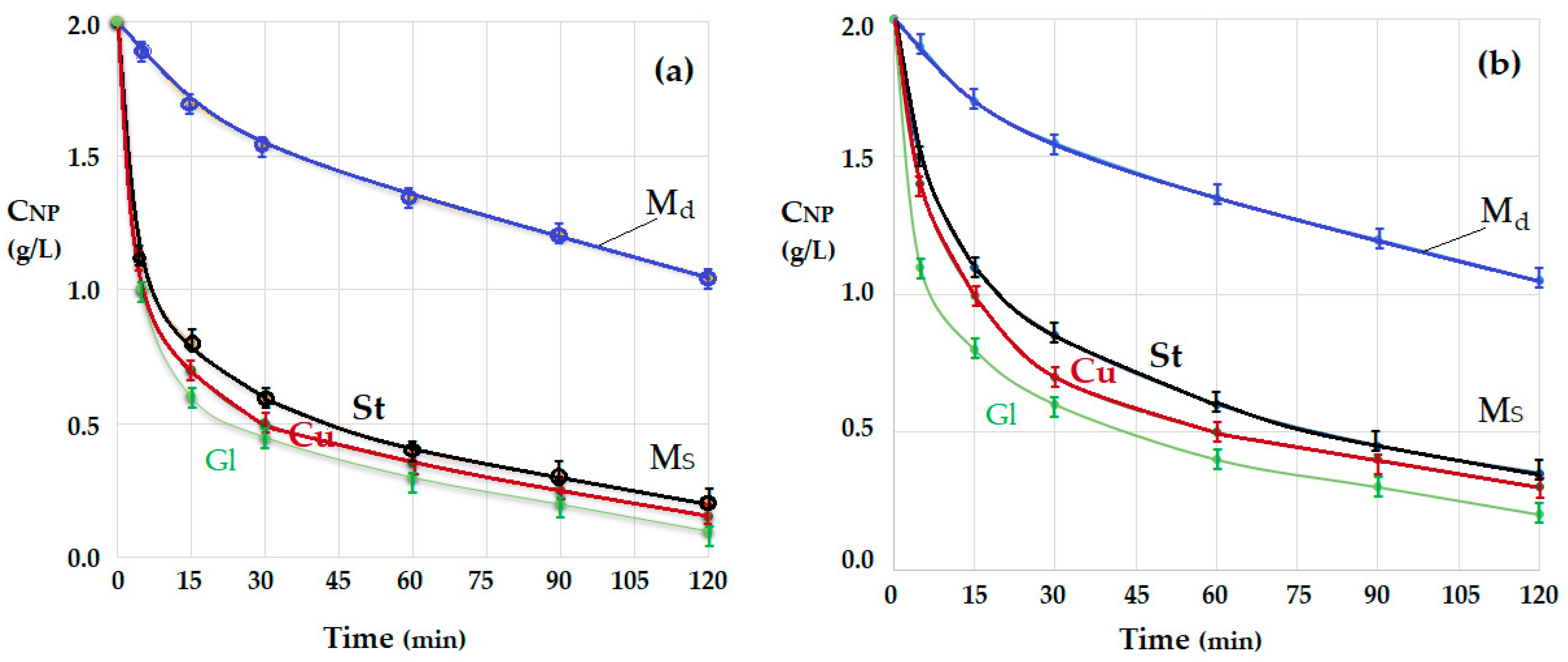
3.2. Transport of p-Nitrophenol in the Free-Rotating Spherical Membrane System
3.2.1. The Influence of the Source Phase pH and of the Nature of the Membrane
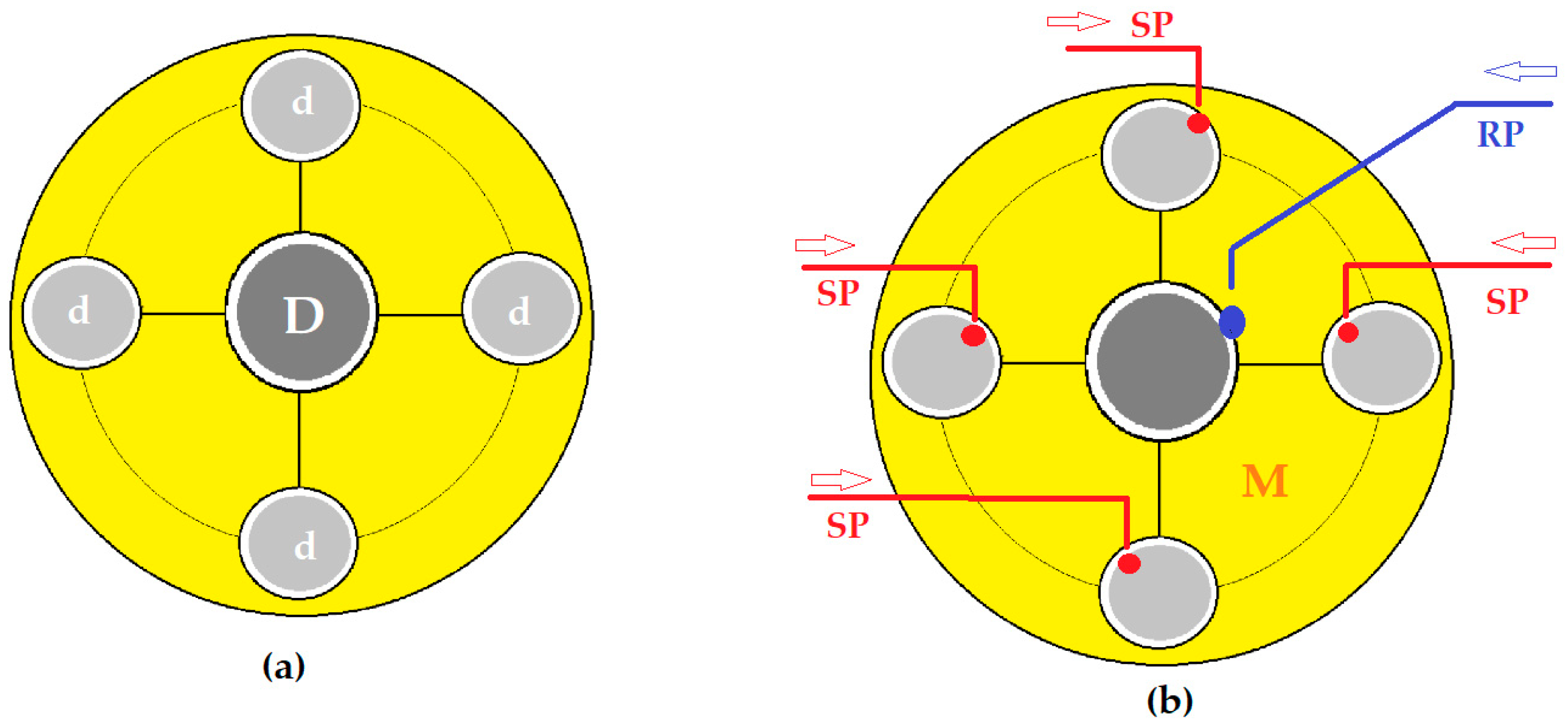
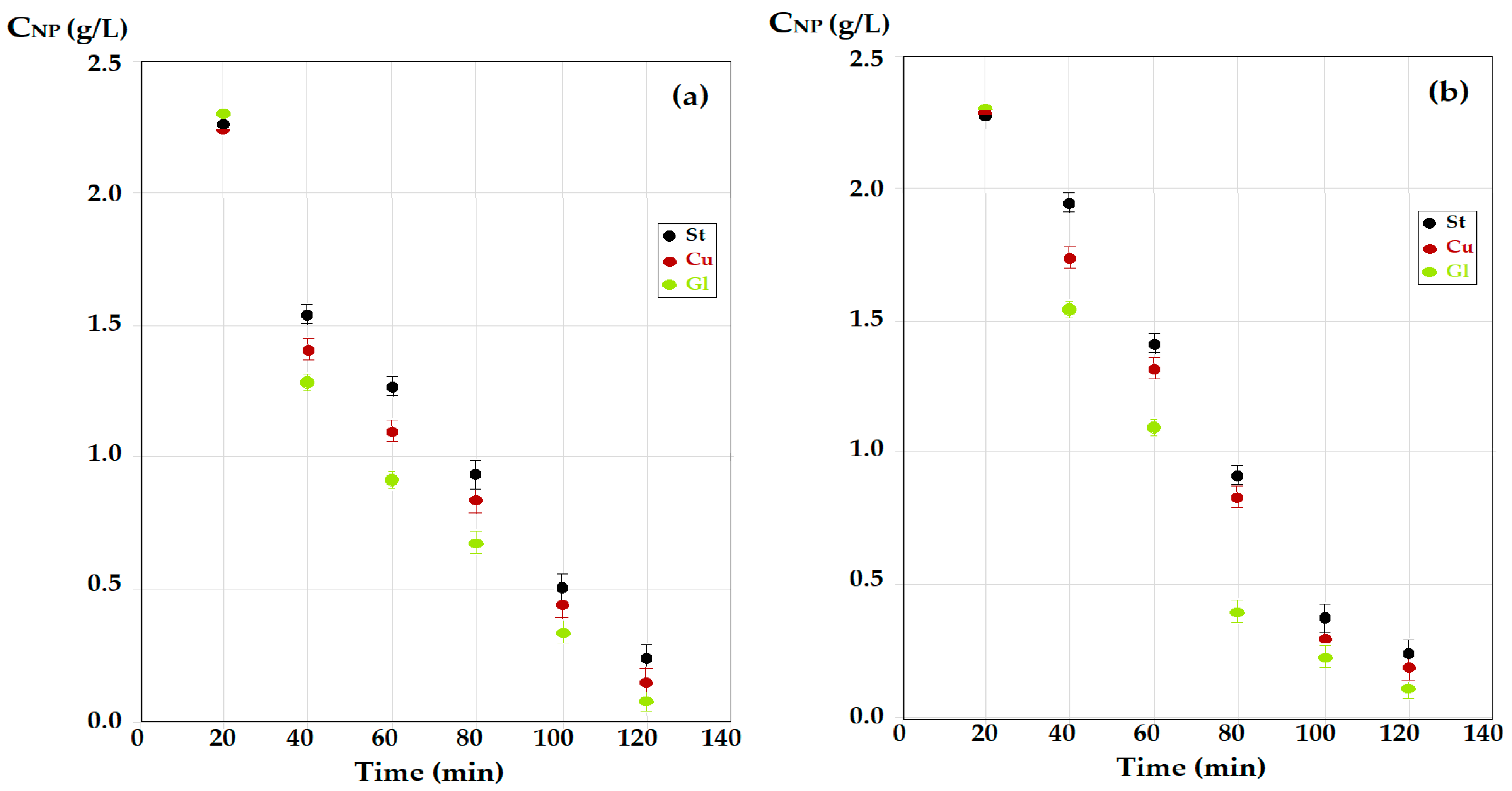
3.2.2. Influence of the Nature of the Material for Making Contact Spheres
3.2.3. Influence of Sodium Chloride Concentration on p–Nitrophenol Extraction Efficiency
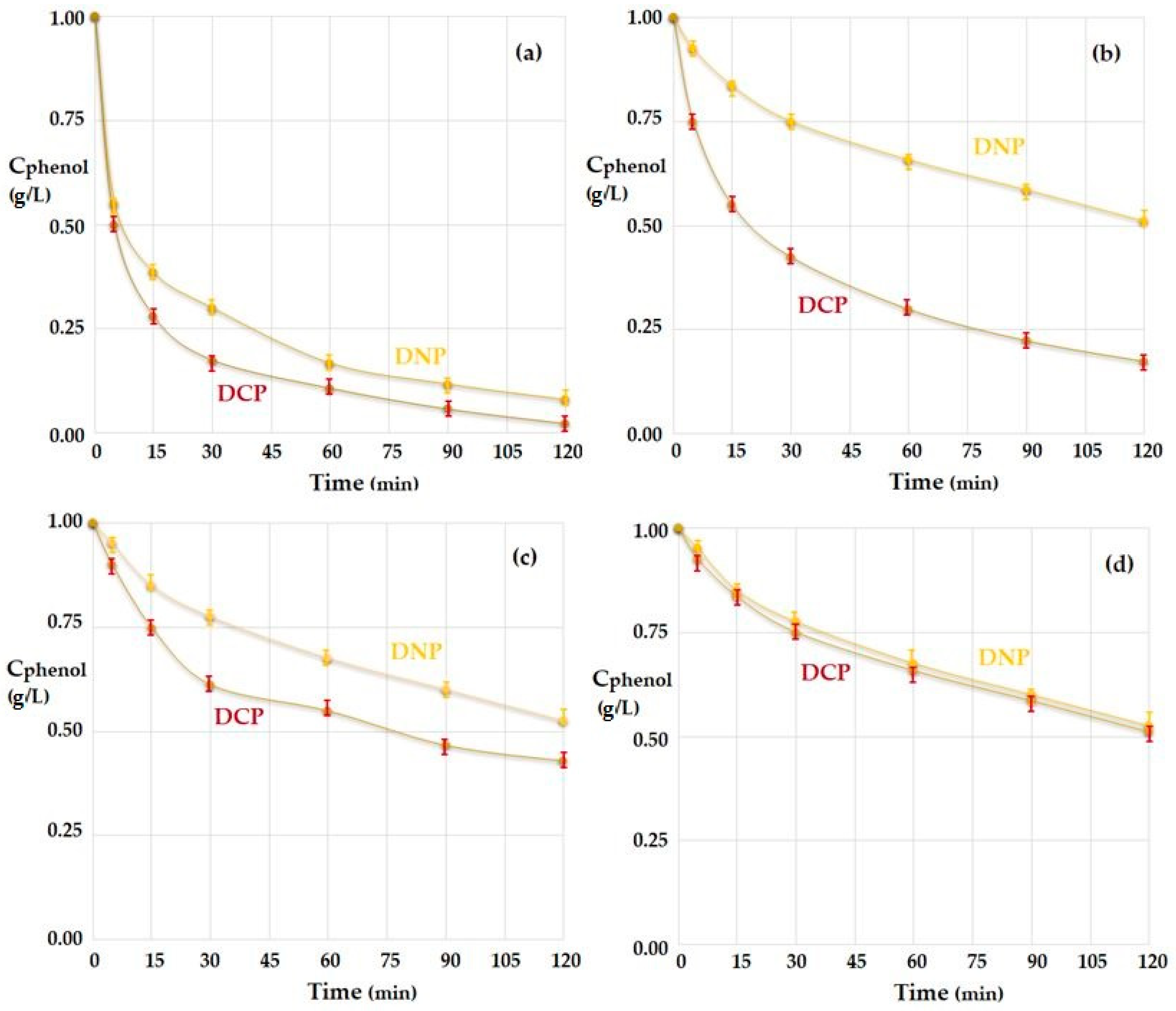
3.3. The Transport and Separation of the Phenol Derivatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Žitek, T.; Borjan, D.; Golle, A.; Knez, Ž.; Knez, M. Optimization of Extraction of Phenolic Compounds with Antimicrobial Properties from Origanum vulgare. Processes 2021, 9, 1032. [Google Scholar] [CrossRef]
- Malapert, A.; Reboul, E.; Dangles, O.; Thiéry, A.; Sylla, N.; Tomao, V. One-Step Extraction of Olive Phenols from Aqueous Solution Using β-Cyclodextrin in the Solid State, a Simple Eco-Friendly Method Providing Photochemical Stability to the Extracts. Molecules 2021, 26, 4463. [Google Scholar] [CrossRef] [PubMed]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef]
- Fuentes, J.A.M.; López-Salas, L.; Borrás-Linares, I.; Navarro-Alarcón, M.; Segura-Carretero, A.; Lozano-Sánchez, J. Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds. Foods 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Drioli, E.; Romano, M. Progress and new perspectives on integrated membrane operations for sustainable industrial growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Moldovan, C.; Mocan, A.; Mare, A.D.; Ciurea, C.N.; Man, A.; Muntean, D.-L.; Tanase, C. Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts. Plants 2022, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Sirkar, K.K. Membrane-Based Solvent Extraction. In Membrane Handbook; Ho, W.S.W., Sirkar, K.K., Eds.; Springer: Boston, MA, USA, 1992. [Google Scholar] [CrossRef]
- Bartsch, R.A.; Way, J.D. Chemical Separations with Liquid Membranes; ACS Symposium Series Volume; ACS: Washington, DC, USA, 1996; Volume 642, pp. 208–221. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.L.; Mohd Harun, M.H.Z.; Akmal Jasni, M.K.; Zaulkiflee, N.D. Removal of Ibuprofen at Low Concentration Using a Newly Formulated Emulsion Liquid Membrane. Membranes 2021, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Nishiki, T.; Osaki, K.; Muto, A. A practicable process for phenol removal with liquid surfactant membrane permeation column. Sep. Sci. Technol. 1997, 32, 1447–1462. [Google Scholar] [CrossRef]
- Sirkar, K.K.; Shanbhag, P.V.; Kovvali, A.S. Membrane in a Reactor: A Functional Perspective. Ind. Eng. Chem. Res. 1999, 38, 3715–3737. [Google Scholar] [CrossRef]
- Kislik, V.S. Liquid Membrane: Principles and Applications in Chemical Separations & Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Gębura, K.; Wieczorek, P.P.; Poliwoda, A. Determination of Glyphosate and AMPA in Food Samples Using Membrane Extraction Technique for Analytes Preconcentration. Membranes 2022, 12, 20. [Google Scholar] [CrossRef]
- San Román, M.F.; Bringas, E.; Ibañez, R.; Ortiz, I. Liquid membrane technology: Fundamentals and review of its applications. J. Chem. Technol. Biotechnol. 2010, 85, 2–10. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Bildyukevich, A.V.; Volkov, A.V. Gas-liquid hollow fiber membrane contactors for different applications. Fibers 2018, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Schlosser, Š.; Sabolová, E.; Kertész, R.; Kubišová, L. Factors influencing transport through liquid membranes and membrane based solvent extraction. J. Sep. Sci. 2001, 24, 509–518. [Google Scholar] [CrossRef]
- Xiujuan, Z.; Jianghong, L.; Oiongjia, F.; Oingtang, L.; Xingtai, Z. Industrial application of liquid membrane separation for phenolic waste water treatment. Technol. Water Treat. 1987, 5. [Google Scholar]
- Kislik, V.S.; Eyal, A.M. Hybrid liquid membrane (HLM) system in separation technologies. J. Membr. Sci. 1996, 111, 259–272. [Google Scholar] [CrossRef]
- Majumdar, S.; Sirkar, K.K. Hollow-fiber contained liquid membrane. In Membrane Handbook; Ho, W.S.W., Sirkar, K.K., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1992; pp. 764–808. [Google Scholar]
- Schlosser, S.; Sabol, E. Three-phase contactor with distributed U-shaped bundles of hollow-fibers for pertraction. J. Membr. Sci. 2002, 210, 331–347. [Google Scholar] [CrossRef]
- Eyal, A.; Kislik, V. Aqueous hybrid liquid membrane a novel system for separation of solutes using water-soluble polymers as carriers. J. Membr. Sci. 1999, 161, 207–221. [Google Scholar] [CrossRef]
- Boyadzhiev, L.; Benenshek, E.; Lazarova, Z. Removal of phenol from wastewater by double emulsion membranes and creeping film pertraction. J. Membr. Sci. 1984, 21, 137–144. [Google Scholar] [CrossRef]
- Bazhenov, S.; Kristavchuk, O.; Kostyanaya, M.; Belogorlov, A.; Ashimov, R.; Apel, P. Interphase Surface Stability in Liquid-Liquid Membrane Contactors Based on Track-Etched Membranes. Membranes 2021, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, A.; Nikzad, A.; Maleki, R.; Asadnia, M.; Razmjou, A. Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection. Membranes 2022, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Gadekar, P.T.; Mukkolath, A.V.; Tiwari, K.K. Recovery of nitrophenols from aqueous solutions by a liquid emulsion membrane system. Sep. Sci. Technol. 1992, 27, 427–445. [Google Scholar] [CrossRef]
- Kujawski, W.; Warszawski, A.; Ratajczak, W.; Porebski, T.; Capala, W.; Ostrowska, I. Removal of phenol from wastewater by different separation techniques. Desalination 2004, 163, 287–296. [Google Scholar] [CrossRef]
- Szczepański, P.; Diaconu, I. Transport of p-nitrophenol through an agitated bulk liquid membrane. Sep. Sci. Technol. 2012, 47, 1725–1732. [Google Scholar] [CrossRef]
- Dimulescu, I.A.; Nechifor, A.C.; Bǎrdacǎ, C.; Oprea, O.; Paşcu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungău, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, G.; Păncescu, F.M.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Ioan, M.-R.; Nechifor, A.C. Transport and Separation of the Silver Ion with n–decanol Liquid Membranes Based on 10–undecylenic Acid, 10–undecen–1–ol and Magnetic Nanoparticles. Membranes 2021, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Ferencz, A.; Grosu, A.R.; Al-Ani, H.N.A.; Nechifor, A.C.; Tanczos, S.-K.; Albu, P.C.; Crăciun, M.E.; Ioan, M.-R.; Grosu, V.-A.; Nechifor, G. Operational Limits of the Bulk Hybrid Liquid Membranes Based on Dispersion Systems. Membranes 2022, 12, 190. [Google Scholar] [CrossRef]
- Diaconu, I.; Nechifor, G.; Nechifor, A.C.; Totu, E.E.; Ruse, E. The transport of nitrophenols through liquid membranes. Rev. Chim. 2009, 60, 1243–1246. [Google Scholar]
- Miron, A.R.; Nechifor, A.C.; Rikabi, A.A.K.K.; Tanczos, S.K. Separation of aromatic intermediates of biological interest using emulsion liquid membranes. Environ. Eng. Manag. J. 2015, 14, 373–379. [Google Scholar] [CrossRef]
- Diaconu, I.; Nechifor, G.; Nechifor, A.C.; Ruse, E.; Totu, E.E. Membranary techniques used at the separation of some phenolic compounds from aqueous media. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2009, 71, 39–46. [Google Scholar]
- Diaconu, I.; Gîrdea, R.; Cristea, C.; Nechifor, G.; Ruse, E.; Totu, E.E. Removal and recovery of some phenolic pollutants using liquid membranes. Rom. Biotechnol. Lett. 2010, 15, 5702–5708. [Google Scholar]
- Kakoi, T.; Goto, M.; Natsukawa, S.; Lkemizu, K.; Nakashio, F. Recovery of phenols using liquid surfactant membranes prepared with newly synthesized surfactants. Sep. Sci. Technol. 1996, 31, 107–124. [Google Scholar] [CrossRef]
- Eurachem/CITAC Guide on “Assessment of Performance and Uncertainty in Qualitative Chemical Analysis” Terminology and Content of the Eurachem/CITAC Guide Session 1. Available online: https://www.eurachem.org/index.php/publications/guides/performance-and-uncertainty-in-qualitative-analysis (accessed on 17 February 2022).
- Szczepański, P.; Tanczos, K.S.; Ghindeanu, D.L.; Wódzki, R. Transport of p-nitrophenol in an agitated bulk liquid membrane system–Experimental and theoretical study by network analysis. Sep. Pur. Technol. 2014, 132, 616–626. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. [Google Scholar] [CrossRef]
- Florea-Spiroiu, M.; Olteanu, M.; Stanescu, V.; Nechifor, G. Surface tension components of plasma treated polysulphone membranes. An. Univ. Bucureşti–Chim. Anul XVII 2008, 2, 13–18. [Google Scholar]
- Luca, C. pH-ul si Aplicatiile Lui; Editura Tehnica: Bucuresti, Romania, 1964; p. 236. [Google Scholar]
- Luca, C.; Duca, A.; Crişan, I.A. Chimie Analitica si Analiza Instrumentala; Editura Didactică şi Pedagogică: Bucureşti, Romania, 1983; p. 416. [Google Scholar]
- Gheorghe, E.; Barbu, L.; Nechifor, G.; Luca, C. The Pb2+ cations transport through liquid membrane with macro cycle benzo-18-crown-6. Rev. Chim. 2006, 57, 940–944. [Google Scholar]
- Diaconu, I.; Aboul-Enein, H.Y.; Al-Omar, M.A.; Nechifor, G.; Ruse, E.; Bunaciu, A.A.; Totu, E.E. Separation of nitrophenols. Equilibriums in bi-and tri-phasic systems. Arab. J. Chem. 2011, 4, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Din, I.S.; Cimbru, A.M.; Al Ani, H.N.A.; Nafliu, I.M.; Tanczos, S.K.; Nechifor, G. Iono-molecular Separation with Composite Membranes, V. Nitro-phenol separation on n-alkyl alcohols supported liquid membranes. Rev. Chim. 2018, 69, 1084–1088. [Google Scholar] [CrossRef]
- Din, I.S.; Cimbru, A.M.; Rikabi, A.A.K.K.; Tanczos, S.K.; Ticu, S.; Nechifor, G. Iono-molecular Separation with Composite Membranes VI. Nitro-phenol separation through sulfonated polyether ether ketone on capillary polypropylene membranes. Rev. Chim. 2018, 69, 1603–1607. [Google Scholar] [CrossRef]
- Nafliu, I.M.; Al Ani, H.N.A.; Grosu, A.R.; Tanczos, S.K.; Maior, I.; Necifor, A.C. Iono-molecular Separation with Composite Membranes. VII. Nitrophenols pertraction on capillary polypropylene S-EPDM composite membranes. Mater. Plast. 2018, 55, 511–516. [Google Scholar] [CrossRef]
- Nafliu, I.M.; Al Ani, H.N.A.; Grosu, A.R.; Albu, P.C.; Nechifor, G. Iono-molecular separation with composite membranes. VIII. Recuperative aluminium ions separation on capilary polypropylene s-epdm composite membranes. Mater. Plast. 2019, 56, 32–36. [Google Scholar] [CrossRef]
- Diaconu, I.; Ruse, E.; Aboul-Enein, H.Y.; Bunaciu, A.A. Analytical applications of transport through bulk liquid membranes. Crit. Rev. Anal. Chem. 2016, 46, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, A.C.; Pîrțac, A.; Albu, P.C.; Grosu, A.R.; Dumitru, F.; Dimulescu, I.A.; Oprea, O.; Pașcu, D.; Nechifor, G.; Bungău, S.G. Recuperative Amino Acids Separation through Cellulose Derivative Membranes with Microporous Polypropylene Fiber Matrix. Membranes 2021, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen, V.K.; Pham, T.T.H.; Pham, T.T.; Nguyen, T.D. Effects of Surface Modification with Stearic Acid on the Dispersion of Some Inorganic Fillers in PE Matrix. J. Compos. Sci. 2021, 5, 270. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, H.; Jia, X.; Yan, L.; Jiang, B. Study on the Hydrophobic Modification of MTES/NH3 Vapor Surface Treatment for SiO2 Broadband Anti-Reflection Coating. Materials 2022, 15, 912. [Google Scholar] [CrossRef]
- Craciun, M.E.; Mihai, M.; Nechifor, G. Characteristics of double jet immobilized membrane. Environ. Eng. Manag. J. 2009, 8, 771–776. [Google Scholar]
- Zogorski, J.S.; Faust, S.D. The effect of phosphate buffer on the adsorption of 2,4-dichlorophenol and 2, 4-dinitrophenol. J. Environ. Sci. Health 1976, 11, 501–515. [Google Scholar] [CrossRef]
- Simsek, E.B.; Aytas, B.; Duranoglu, D.; Beker, U.; Trochimczuk, A.W. A comparative study of 2-chlorophenol, 2,4-dichlorophenol, and 2,4,6-trichlorophenol adsorption onto polymeric, commercial, and carbonaceous adsorbents. Desalination Water Treat. 2016, 57, 9940–9956. [Google Scholar] [CrossRef]
- Wu, G.-Q.; Sun, X.-Y.; Hui, H.; Zhang, X.; Yan, J.; Zhang, Q.-S. Adsorption of 2,4-dichlorophenol from aqueous solution by activated carbon derived from moso bamboo processing waste. Desalination Water Treat. 2013, 51, 4603–4612. [Google Scholar] [CrossRef]
- Yusof, N.A.; Zakaria, N.D.; Maamor, N.A.M.; Abdullah, A.H.; Haron, M.J. Synthesis and Characterization of Molecularly Imprinted Polymer Membrane for the Removal of 2,4-Dinitrophenol. Int. J. Mol. Sci. 2013, 14, 3993–4004. [Google Scholar] [CrossRef]



| Phenol Derivative (Ar-OH) | Name and Symbol | Molar Mass (g/mol) | Absorbance λ (nm) | Solubility in Water (g/L) | pKa | |
|---|---|---|---|---|---|---|
| Ar-OH | Ar-O– | |||||
 | 2,4–dinitro phenol (DNP) | 185.106 | 358 | 361 | 17.0 | 4.11 |
 | 4–nitrophenol (NP) | 139.110 | 317 | 404 | 18.1 | 7.01 |
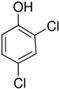 | 2,4–dichloro phenol (DCP) | 161.964 | 208 | 284 | 4.5 | 7.89 |
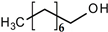 | n–octanol (nO) | 130.230 | 197 | 0.300 | 15.21 | |
 | n–decanol (nD) | 158.280 | 192 | 0.037 | 16.84 | |
| Sphere Material | Steel (St) | Copper (Cu) | Glass (Gl) |
|---|---|---|---|
| Contact angle (θ°) | 83 ± 2 | 58 ± 2 | 36 ± 2 |
| Atom ratio (Fe/O, Cu/O, and Si/O) | 1:1.12 | 1:1.47 | 1:4.22 |
| Liquid Membrane Module | Bulk or Supported [31,40,41,42,43,44] | Drop by Drop | Free Rotating Spheres |
|---|---|---|---|
| Flow (J) (mol/(m2 s)) | (1–100) 10−11 | (2.1–5.8) 10−8 | (5.3–10.6) 10−8 |
| NaCl (%) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| EE (%) | 83 ± 3 | 86 ± 3 | 90 ± 3 | 93 ± 3 | 95 ± 3 |
| pH Source Phase | 2 | 4 | 6 | 8 | |
|---|---|---|---|---|---|
| EE (%) | DNP | 91 ± 3 | 49 ± 3 | 48 ± 3 | 47 ± 3 |
| DCP | 93 ± 3 | 86 ± 3 | 61 ± 3 | 49 ± 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albu, P.C.; Tanczos, S.-K.; Ferencz, A.; Pîrțac, A.; Grosu, A.R.; Pașcu, D.; Grosu, V.-A.; Bungău, C.; Nechifor, A.C. pH and Design on n–Alkyl Alcohol Bulk Liquid Membranes for Improving Phenol Derivative Transport and Separation. Membranes 2022, 12, 365. https://doi.org/10.3390/membranes12040365
Albu PC, Tanczos S-K, Ferencz A, Pîrțac A, Grosu AR, Pașcu D, Grosu V-A, Bungău C, Nechifor AC. pH and Design on n–Alkyl Alcohol Bulk Liquid Membranes for Improving Phenol Derivative Transport and Separation. Membranes. 2022; 12(4):365. https://doi.org/10.3390/membranes12040365
Chicago/Turabian StyleAlbu, Paul Constantin, Szidonia-Katalin Tanczos, Andreea Ferencz (Dinu), Andreia Pîrțac, Alexandra Raluca Grosu, Dumitru Pașcu, Vlad-Alexandru Grosu, Constantin Bungău, and Aurelia Cristina Nechifor. 2022. "pH and Design on n–Alkyl Alcohol Bulk Liquid Membranes for Improving Phenol Derivative Transport and Separation" Membranes 12, no. 4: 365. https://doi.org/10.3390/membranes12040365
APA StyleAlbu, P. C., Tanczos, S.-K., Ferencz, A., Pîrțac, A., Grosu, A. R., Pașcu, D., Grosu, V.-A., Bungău, C., & Nechifor, A. C. (2022). pH and Design on n–Alkyl Alcohol Bulk Liquid Membranes for Improving Phenol Derivative Transport and Separation. Membranes, 12(4), 365. https://doi.org/10.3390/membranes12040365









