Study on the Control of Dichloroacetonitrile Generation by Two-Point Influent Activated Carbon-Quartz Sand Biofilter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Device
2.2. Inoculated Sludge and Influent Water Quality
2.3. Analysis Methods
2.4. Determination of Tyrosine Content
2.5. Determination of Formation Potential of DCAN
2.6. Microbial Community Analysis
3. Results and Discussion
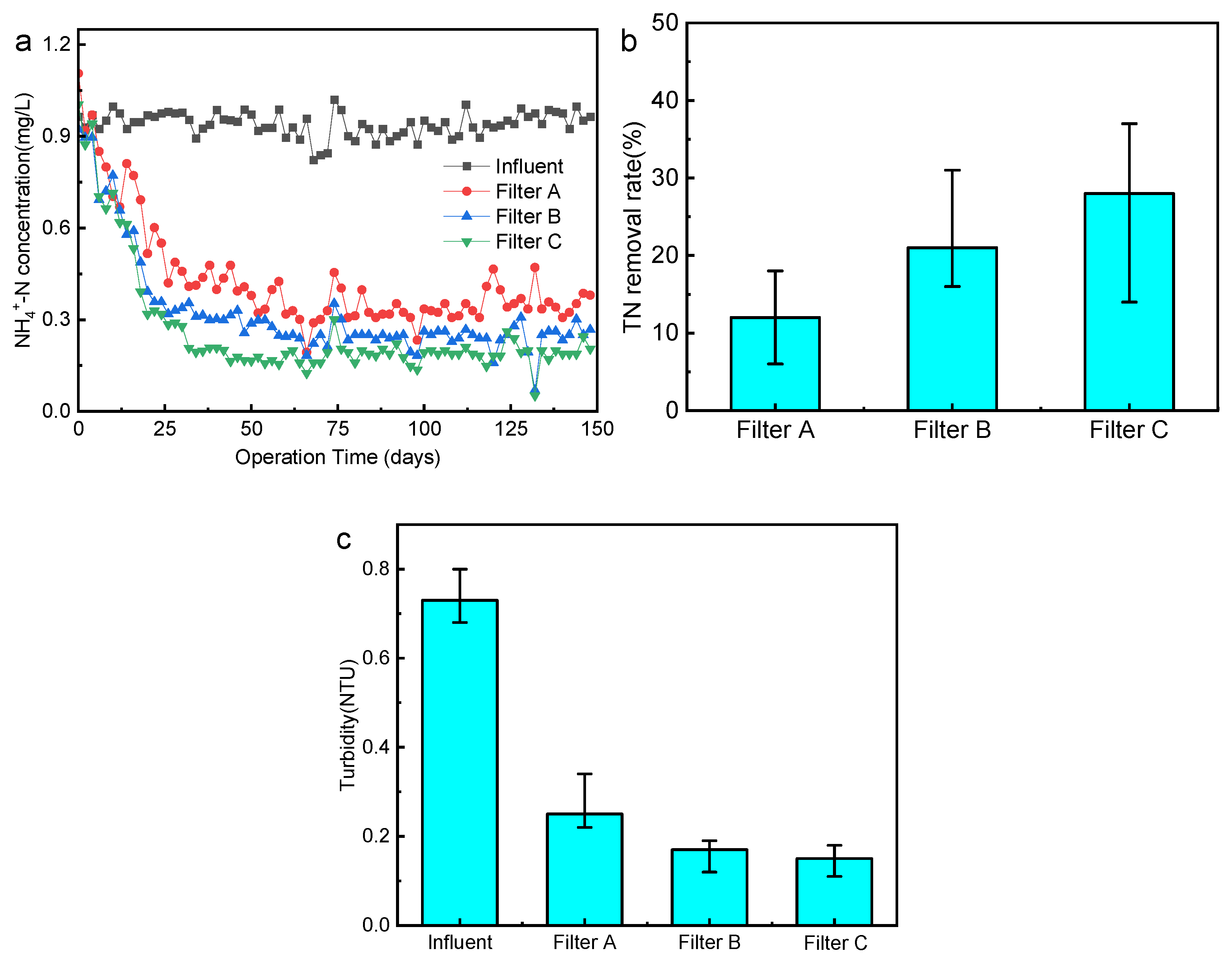
3.1. Analysis of the Removal Effect of Biofilter Pollutants
3.1.1. Removal Effect of Conventional Pollutants
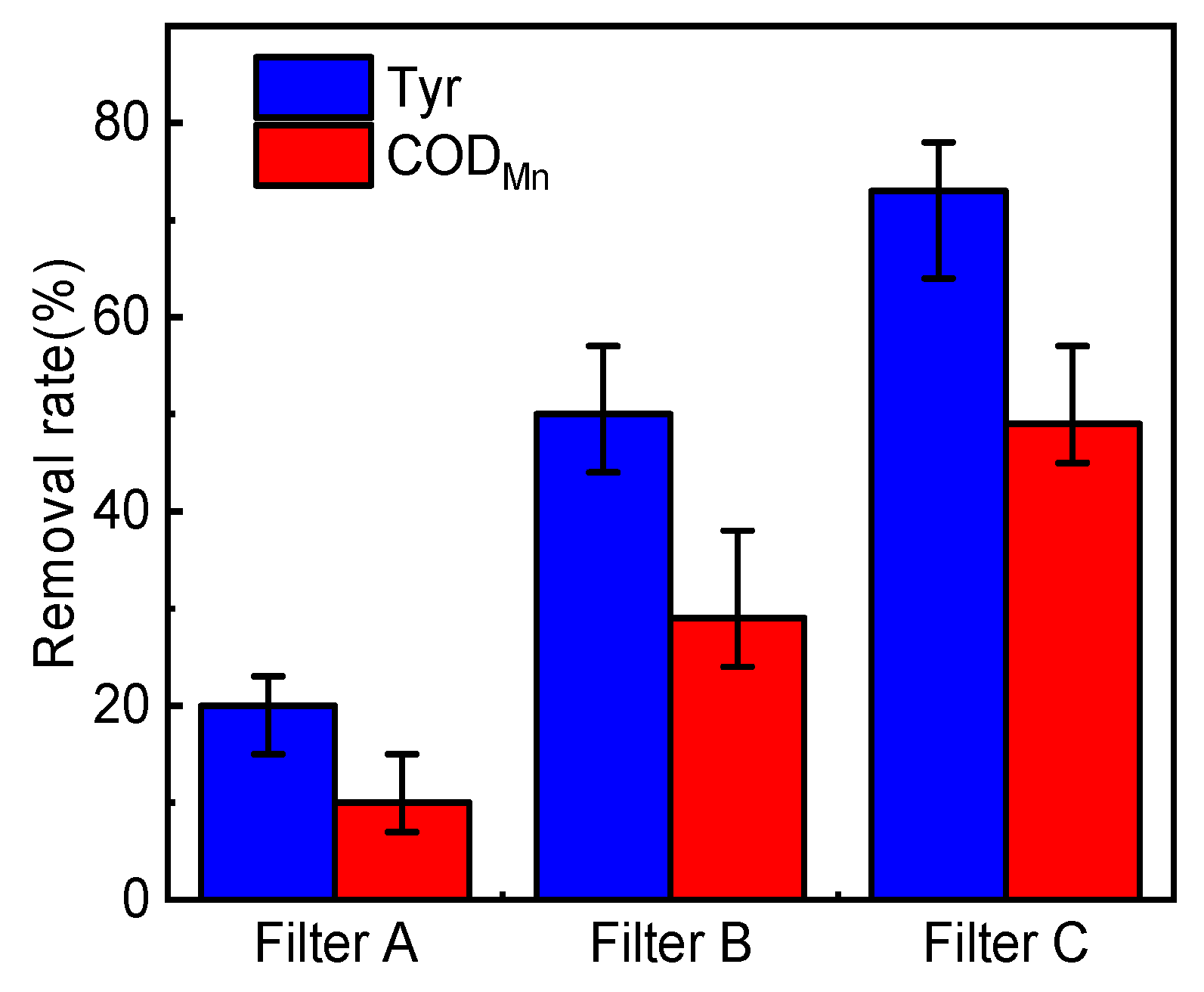
3.1.2. Removal Effect of Tyrosine and COD
3.2. The Formation Potential and Influencing Factors of DCAN in the Effluent of the Biofilter
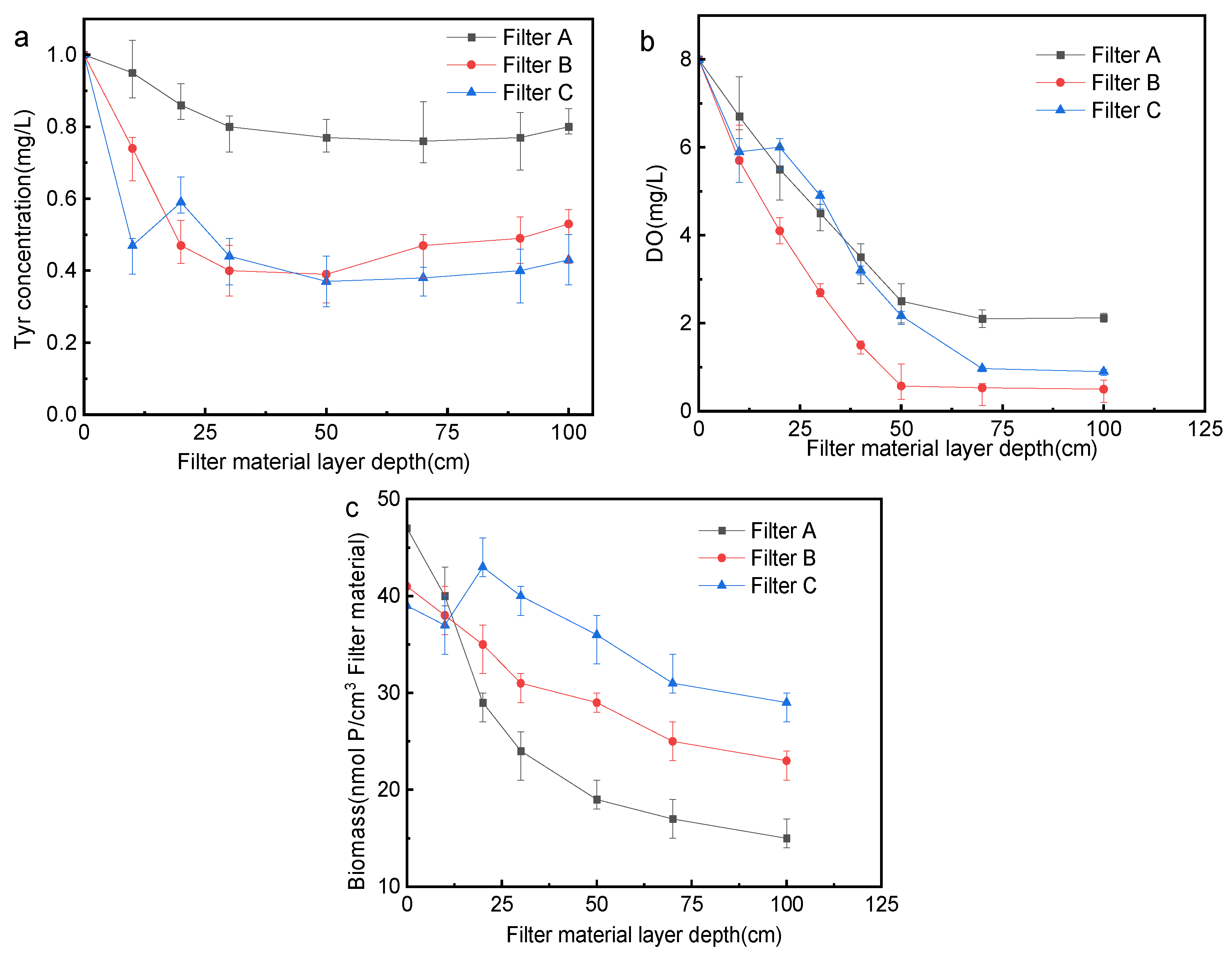
3.3. Analysis of Changes in Biofilter along the Way
3.4. Conversion of Nitrogen in the Biofilter
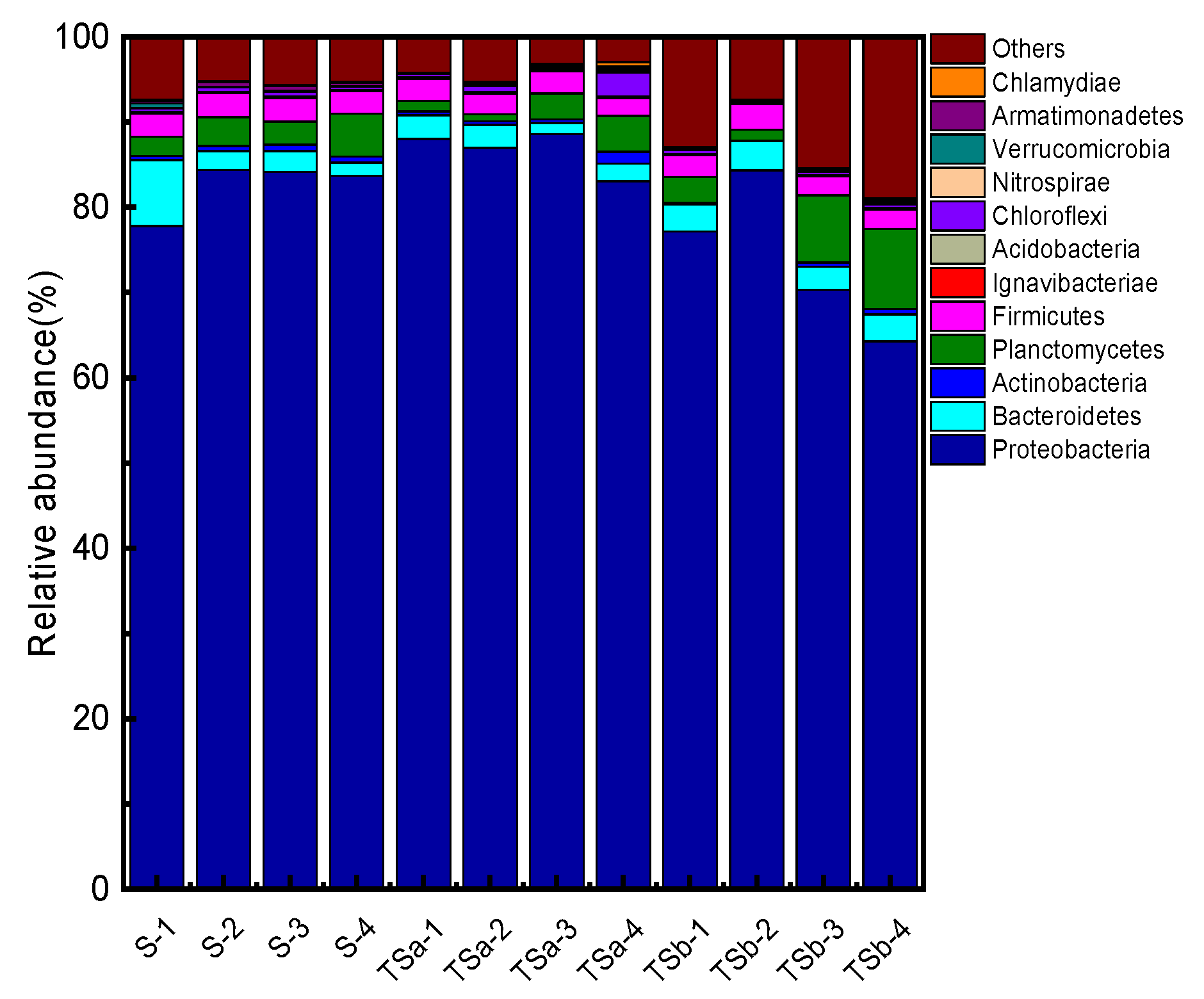
3.5. Analysis of the Microbial Community in the Biofilter
3.5.1. Species Diversity Analysis
3.5.2. Analysis of Microbial Community Structure at the Phylum Level
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res./Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, F.; Shen, H.J.; Lu, R.Z.; Yin, S.Q.; Yang, Q.; Li, Z.F.; Wang, S.H. Evaluation of the water disinfection by-product dichloroacetonitrile-induced biochemical, oxidative, histopathological, and mitochondrial functional alterations: Subacute oral toxicity in rats. Toxicol. Ind. Health 2018, 34, 158–168. [Google Scholar] [CrossRef]
- Ding, S.; Chu, W.H.; Bond, T.; Wang, Q.; Gao, N.Y.; Xu, B.; Du, E. Formation and estimated toxicity of trihalomethanes, haloacetonitriles, and haloacetamides from the chlor(am)ination of acetaminophen. J. Hazard. Mater. 2017, 341, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.H.; Gao, N.Y.; Yin, D.Q. Control Countermeasures of new halogenated nitrogen containing disinfection by-products in drinking water. In Proceedings of the 6th International Conference on Intelligent Systems and Knowledge Engineering, Shanghai, China, 22–24 September 2011. [Google Scholar]
- Chu, W.H.; Gao, N.Y.; Zhao, S.J.; Deng, H.P. Mechanism analysis of chloroform formation by tyrosine chlorination of typical dissolved organic nitrogen in drinking water. J. Chem. 2009, 67, 2505–2510. [Google Scholar]
- Srithep, S.; Phattarapattamawong, S. Kinetic removal of haloacetonitrile precursors by photo-based advanced oxidation processes (UV/H2O2, UV/O3, and UV/H2O2/O3). Chemosphere 2017, 176, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.A.; Kimura, S.Y.; Liberatore, H.K.; Knappe, D.R.U.; Richardson, S.D. GAC to BAC: Does it make chloraminated drinking water safer? Water Res. 2020, 172, 115432. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Lu, X.W.; Yin, L.H.; Yang, F.; Zhu, G.C.; Liu, W.P. Microbial community characterization, activity analysis and purifying efficiency in a biofilter process. J. Environ. Sci. 2013, 25, 677–687. [Google Scholar] [CrossRef]
- Liu, C.; Olivares, C.I.; Pinto, A.J.; Lauderdale, C.V.; Brown, J.; Selbes, M.; Karanfil, T. The control of disinfection byproducts and their precursors in biologically active filtration processes. Water Res. 2017, 124, 630–653. [Google Scholar] [CrossRef]
- Zhang, H.N.; Gu, L.; Liu, B.; Gan, H.H.; Yu, X. An improved biofilter to control the dissolved organic nitrogen concentration during drinking water treatment. Environ. Sci. Pollut. Res. Int. 2016, 23, 18137–18144. [Google Scholar] [CrossRef]
- Thurman, E.M. Organic Geochemistry of Natural Waters. Geochim. Et Cosmochim. Acta. 1986, 50, 2119. [Google Scholar]
- Gu, L.; Liu, B.; Yu, X. Dissolved organic nitrogen (DON) in the processes of polluted source water treatment. Chin. Sci. Bull. 2010, 55, 3098–3101. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.J.; Wang, Z.S. Determination of biomass in biological treatment of drinking water by lipid phosphorus method. Water Supply Drain. 2002, 28, 5. [Google Scholar]
- Compton, O.C.; Jain, B.; Dikin, D.A.; Abouimrane, A.; Amine, K.; Nguyen, S.T. Chemically active reduced graphene oxide with tunable C/O ratios. ACS Nano 2011, 5, 4380–4391. [Google Scholar] [CrossRef]
- Miao, L.; Wang, S.Y.; Li, B.K.; Cao, T.H.; Zhang, F.Z.; Wang, Z.; Peng, Y.Z. Effect of carbon source type on intracellular stored polymers during endogenous denitritation (ED) treating landfill leachate. Water Res. 2016, 100, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Wu, C.D.; Lu, S.M.; Zhang, F. Treatment of micro polluted raw water by GAC quartz sand biofilter. Water Treat. Technol. 2008, 34, 59–62. [Google Scholar]
- Yang, Y.L.; Li, X.; Zhang, Y.; Li, G.B. Experimental study on the efficiency of Biofilter in treating micro polluted water. Ind. Water Treat. 2007, 27, 17–20. [Google Scholar]
- Liu, B.; Gu, L.; Yu, X.; Yu, G.Z.; Zhao, C.M.; Li, Q.F.; Zhai, H.M. Profile of dissolved organic nitrogen (DON) in full-scale ozone and biological activated carbon filter. Desalin. Water Treat. 2015, 55, 2069–2078. [Google Scholar] [CrossRef]
- Jia, A.; Wu, C.; Duan, Y. Precursors and factors affecting formation of haloacetonitriles and chloropicrin during chlor(am)ination of nitrogenous organic compounds in drinking water. J. Hazard. Mater. 2016, 308, 411–418. [Google Scholar] [CrossRef]
- Tan, Y.W.; Lin, T.; Jiang, F.C.; Dong, J.; Chen, W.; Zhou, D.J. The shadow of dichloroacetonitrile (DCAN), a typical nitrogenous disinfection by-product (N-DBP), in the waterworks and its backwash water reuse. Chemosphere 2017, 181, 569–578. [Google Scholar] [CrossRef]
- Lemus-pérez, M.; Susa, M.R. The effect of operational conditions on the disinfection by-products formation potential of exopolymeric substances from biofilms in drinking water—ScienceDirect. Sci. Total Environ. 2020, 748, 141148. [Google Scholar] [CrossRef]
- Liu, B.; Gu, L.; Yu, X.; Zhang, H.N. Dissolved organic nitrogen (DON) in a full-scale drinking water treatment plant. J. Water Supply Res. Technol.—AQUA 2012, 61, 41–49. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Wu, Y.T.; Zhu, S.M.; Sun, J.L.; Bu, L.J.; Dionysiou, D.D. Nitrogen conversion from ammonia to trichloronitromethane: Potential risk during UV/chlorine process. Water Res. 2020, 172, 115508. [Google Scholar] [CrossRef]
- Zhang, H.N.; Zhang, K.F.; Jin, H.X.; Gu, L.; Yu, X. Variations in dissolved organic nitrogen concentration in biofilters with different media during drinking water treatment. Chemosphere 2015, 139, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Liew, D.; Linge, K.L.; Heitz, A.; Joll, C.A. Nitrogenous DBPs in drinking water: Toxicity, regulation, analysis, occurrence and control. Disinfect. By-Prod. Hum. Health 2012, 7, 83–124. [Google Scholar]
- Chu, W.H.; Gao, N.Y.; Deng, Y.; Krasner, S.W. Precursors of Dichloroacetamide, an Emerging Nitrogenous DBP Formed during Chlorination or Chloramination. Environ. Sci. Technol. 2010, 44, 3908–3912. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Mash, H. Dissolved organic nitrogen in drinking water supplies: A review. J. Water Supply Res. Technol.—AQUA 2002, 51, 415–448. [Google Scholar] [CrossRef]
- Krasner, S.W.; Weinberg, H.S.; Richardson, S.D.; Pastor, S.J.; Chinn, R.; Sclimenti, M.J.; Onstad, G.D.; Thruston, A.D. Occurrence of a New Generation of Disinfection Byproducts. Environ. Sci. Technol. 2006, 40, 7175–7185. [Google Scholar] [CrossRef]
- Liu, B.; Fan, H.; Yu, G.Z.; Yu, X.; Zhao, C.M.; Li, Q.F.; Zhang, S.T.; Wei, B. Removal mechanism of ammonia nitrogen in drinking water biofilter. Environ. Sci. 2012, 33, 9. [Google Scholar]
- Fan, Z.H.; Yang, H.L.; Li, S.F.; Yu, X. Tracking and analysis of DBP precursors’ properties by fluorescence spectrometry of dissolved organic matter—ScienceDirect. Chemosphere 2020, 239, 124790. [Google Scholar] [CrossRef] [PubMed]
- Boon, N.; Pycke, B.F.G.; Marzorati, M.; Hammes, F. Nutrient gradients in a granular activated carbon biofilter drives bacterial community organization and dynamics. Water Res. 2011, 45, 6355–6361. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Mendoza-Roca, J.A.; Iborra-Clar, A.; Alonso-Molina, J.L.; Pastor-Alcaniz, L. Comparison of two strategies for the start-up of a biological reactor for the treatment of hypersaline effluents from a table olive packaging industry. Chem. Eng. J. 2015, 273, 595–602. [Google Scholar] [CrossRef]
- Song, W.L.; Lee, L.Y.; You, H.; Shi, X.Q.; Ng, H.Y. Microbial community succession and its correlation with reactor performance in a sponge membrane bioreactor coupled with fiber-bundle anoxic bio-filter for treating saline mariculture wastewater—ScienceDirect. Bioresour. Technol. 2020, 295, 122284. [Google Scholar] [CrossRef] [PubMed]
- Cappello, S.; Volta, A.; Santisi, S.; Morici, C.; Mancini, G.; Quatrini, P.; Genovese, M.; Yakimov, M.M.; Torregrossa, M. Oil-degrading bacteria from a membrane bioreactor (BF-MBR) system for treatment of saline oily waste: Isolation, identification and characterization of the biotechnological potential. Int. Biodeterior. Biodegrad. 2016, 110, 235–244. [Google Scholar] [CrossRef]




| Index | NH4+-N/(mg·L−1) | NO2−-N/(mg·L−1) | NO3−-N/(mg·L−1) | pH | DO/(mg·L−1) | DON/(mg·L−1) |
|---|---|---|---|---|---|---|
| Content | 0.82~1.02 | 0.083~0.125 | 0.87~1.08 | 7.01~8.94 | 6.67~9.53 | 0.61~1.03 |
| Sample | Shannon Index | Chao1 Index | Good’s Coverage |
|---|---|---|---|
| S-1 | 2.64 | 675.56 | 1 |
| S-2 | 2.39 | 616.13 | 1 |
| S-3 | 2.49 | 716.13 | 1 |
| S-4 | 2.28 | 606.36 | 1 |
| TSa-1 | 2.85 | 611.37 | 1 |
| TSa-2 | 3.15 | 788.4 | 1 |
| TSa-3 | 2.91 | 697.33 | 1 |
| TSa-4 | 3.77 | 774.23 | 1 |
| TSb-1 | 2.82 | 584.03 | 1 |
| TSb-2 | 2.3 | 522.67 | 1 |
| TSb-3 | 2.74 | 649.96 | 1 |
| TSb-4 | 3.14 | 680.51 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, X.; Zhang, H.; Ji, B.; Ma, J.; Xu, M.; Li, Y.; Yan, M. Study on the Control of Dichloroacetonitrile Generation by Two-Point Influent Activated Carbon-Quartz Sand Biofilter. Membranes 2022, 12, 137. https://doi.org/10.3390/membranes12020137
Gui X, Zhang H, Ji B, Ma J, Xu M, Li Y, Yan M. Study on the Control of Dichloroacetonitrile Generation by Two-Point Influent Activated Carbon-Quartz Sand Biofilter. Membranes. 2022; 12(2):137. https://doi.org/10.3390/membranes12020137
Chicago/Turabian StyleGui, Xinrui, Huining Zhang, Bixiao Ji, Jianqing Ma, Meijuan Xu, Yan Li, and Ming Yan. 2022. "Study on the Control of Dichloroacetonitrile Generation by Two-Point Influent Activated Carbon-Quartz Sand Biofilter" Membranes 12, no. 2: 137. https://doi.org/10.3390/membranes12020137
APA StyleGui, X., Zhang, H., Ji, B., Ma, J., Xu, M., Li, Y., & Yan, M. (2022). Study on the Control of Dichloroacetonitrile Generation by Two-Point Influent Activated Carbon-Quartz Sand Biofilter. Membranes, 12(2), 137. https://doi.org/10.3390/membranes12020137







