A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation
Abstract
:1. Introduction
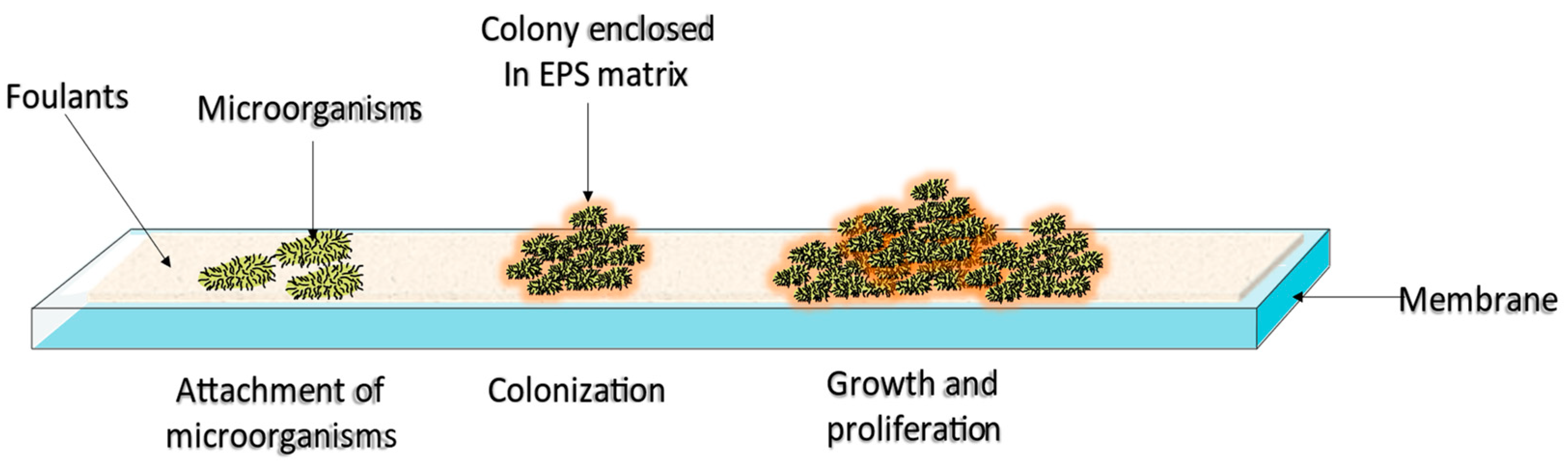
2. Membrane Biofouling Mechanism
- Microorganism: this includes species, population density, growth profile, nutrient status, the hydrophobicity/hydrophilicity of the microorganism, and physiological responses.
- Surface morphology: membrane material, surface charge, hydrophobicity, roughness, and porosity.
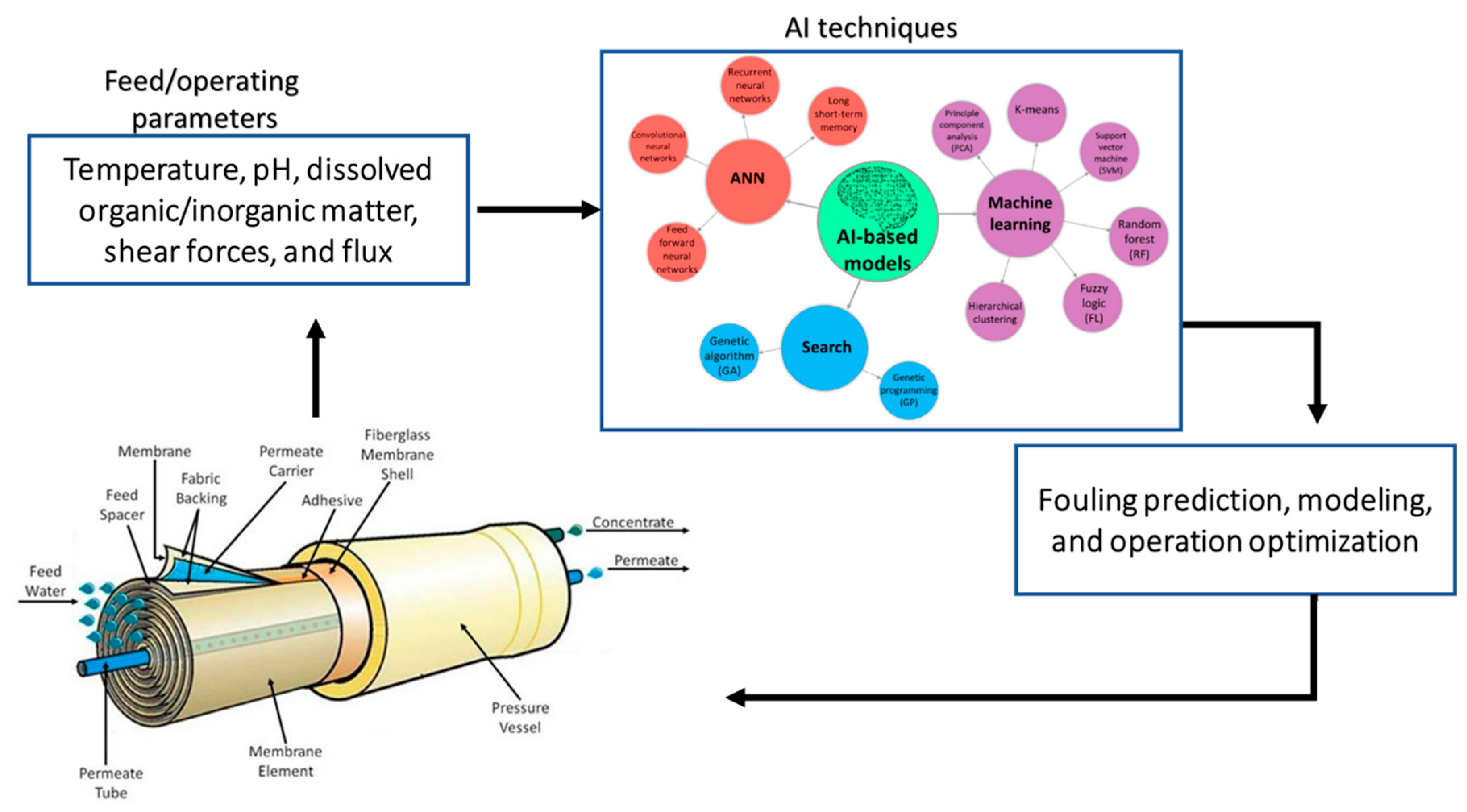
- Feed: temperature, pH, dissolved organic/inorganic matter, shear forces, and flux.
3. Membrane Biofouling Characterization
4. Membrane Biofouling Prediction
| AI Technique | Mode of Operation | Applications | Advantages | Disadvantages |
|---|---|---|---|---|
| k-NN | -Saves all existing data -Classification of new data points based on similarity | -Regression -Classification | -Easy implementation | -Computationally expensive -Memory intensive -Overfitting |
| DT | -Generates a training model to teach simple decision rules | -Regression -Classification | -High accuracy -Easy implementation -Applies to continuous and discrete data | -Instability -Overfitting |
| RF | -Creates DTs on data samples -Makes predictions based on each DT -Uses a voting mechanism to select an optimal solution | -Regression -Classification | -Decreased overfitting -Suitable for large datasets | -Not suitable for imbalanced datasets -Low training speed |
| ANN | -Statistical models built based on human brain neurons | -Pattern recognition -Performs nonlinear computations | -Fast prediction -Good for arbitrary function approximation -Suitable for high-dimensional datasets | -Computationally expensive -Difficulty in interpreting trained models |
| FNN | -Combines fuzzy logic and NNs | -Pattern recognition -Density estimation -Regression -Classification | -Can be used when a mathematical model does not exist for a problem -Easy implementation and interpretation | -Theoretical knowledge necessary -Computationally expensive |
| CNN, FFNN | -Uses convolution instead of matrix multiplication | -Image/video recognition -Classification -Regression -Segmentation | -Accurate results -Good speed | -Computationally expensive -Complex architecture |
| DNN | -Input, output layers -Includes hidden layers | -Learning complex models -High-dimensional data processes | -Best performance if enough data are available -Suitable for nonlinear data -Fast prediction following training | -Computationally expensive -Requires more training data |
| SVM | -Requires labeled training data for each category to identify the next step -Mapping input vector into a high-dimensional feature space | -Classification -Regression -Pattern recognition | -Suitable for high-dimensional datasets -Suitable for linear and nonlinear datasets | -Computationally expensive -Difficult to train -Overfitting -Not suitable for noisy data |
| GA | -Produces the optimal strategy to solve complicated problems under a particular theory | -Regression -Clustering -Classification | -Provides multiple solutions -Supports multi-objective optimization -Suitable for discrete and continuous data | -Difficult to implement -Computationally expensive -Time-consuming |
| PSO | Optimizes a problem by iteratively improving a candidate solution with regard to a given measure of quality | -Clustering -Regression -Classification | -Easy implementation -Parallel computation | -Mathematical background needed for evaluations -Difficult to define initial design parameters |
| Membrane Separation Process | AI Tool | Main Findings | Ref. |
|---|---|---|---|
| Extractive membrane bioreactor | ANN | -ANN was able to interpret complex 2D fluorescence maps. -Properly trained ANN was able to predict process behavior and identify key fluorophores for the prediction of process parameters. | [53] |
| Nanofiltration (NF) | Multivariate projection to latent structures (MPLS) | -Alkalinity, molecular size descriptors, molecular weight, and molar volume were the most relevant contributors to determining foulant rejection. -Adsorption occurred through polar and electrostatic interactions. | [54] |
| Ion-exchange membrane bioreactor | MPLS | -The proposed PLS model accounted for biological contribution to mass transfer. -PLS model predicted anionic fluxes across membranes with ~50% prediction improvement when compared with the simplified mechanistic Donnan dialysis-based transport model. -Transport driving force-related variables were the most important for the anionic transport model. | [55] |
| Osmotic membrane bioreactor | ANN | -The optimal number of hidden layers was 2–6, and the appropriate number of neurons in each layer was 5–30. -pH and conductivity were the most critical parameters for the models. -The ANN models demonstrated good performance, with R2 values of 0.92 and 0.93 reported for the prediction of water flux and membrane fouling simulations, respectively. | [18] |
| Membrane bioreactor | RF, ANN, and long-short-term memory network (LSTM) | -All models provided reliable predictions, while the RF models had the best accuracy. | [56] |
5. Membrane Biofouling Mitigation
5.1. Feedwater Pretreatment
5.2. Nutrient Limitation
5.3. Optimization of Feed Spacer Geometry and Hydrodynamic Conditions
5.4. Membrane Cleaning
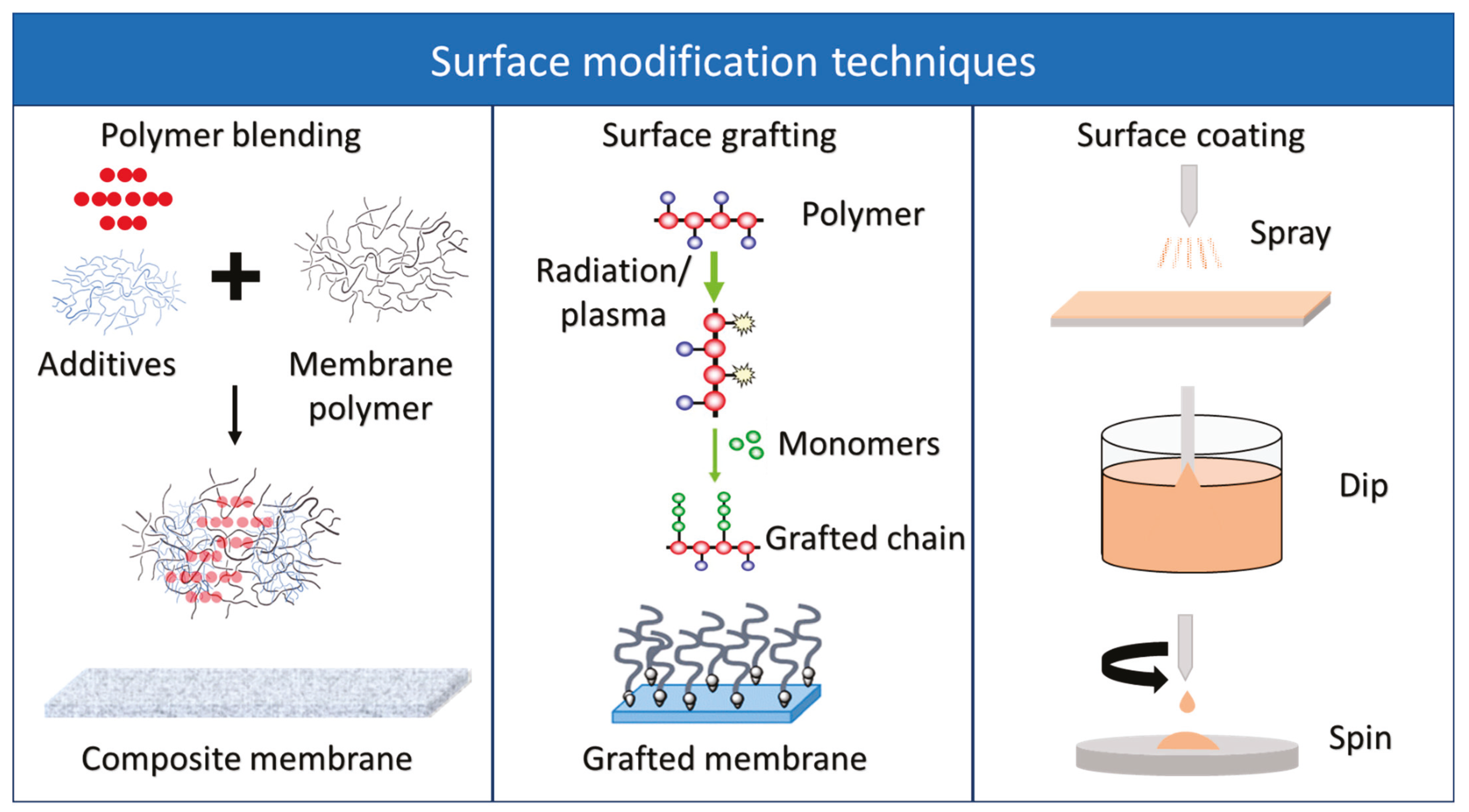
5.5. Surface Modification
5.5.1. Polymer Blending
- Metal and metal oxide nanoparticles (NPs): Metal and metal oxide NPs, such as Titanium oxide (TiO2), silicon oxide (SiO2), and Zinc oxide (ZnO) exhibited excellent hydrophilicity and self-cleaning abilities when added to polymeric membranes. In addition, these NPs can generate free radicals and reactive oxygen species (ROS) and are able to interact with bacterial cells through electrostatic or van der Waals forces, disrupting the cellular membrane structure of microorganisms and inhibiting bacterial growth [20,87,88]. Kusworo et al. [89] doped polysulfone (PSF) membranes with TiO2 NPs. SEM images revealed that the addition of the NPs increased pore size. In addition, the hydrophilicity of the membrane was improved with the water contact angle decreasing from 61.83 to 41.67. The best pollutant removal was achieved with 1 wt% TiO2-PSF doped membranes. In another study, Aoudjit et al. [90] prepared and characterized a 10 wt.% TiO2/PVDF–TrFE nanocomposite membrane to separate Niflumic acid (NFA) from water. The photocatalytic activity of the incorporated TiO2 was tested and the results demonstrated a 91% NFA degradation efficiency after 6 h of solar irradiation at neutral pH. With respect to the reusability of the membrane, an efficiency loss of 9% was observed after three consecutive uses separated by cycles of washing with ultrapure water and drying in the sun. In addition, the authors found that the irradiation time was the most significant parameter affecting the performance of the nanocomposite membrane. Silver (Ag) NPs have also received a great deal of attention for their antibacterial properties and ability to reduce adhesion. The antibacterial properties of Ag NPs originate from the ability of released metal ions (Ag+) to interact with thiol (–SH) groups in microbial membrane cells; this interaction can deactivate certain proteins, which in turn causes the leakage of phospholipids and phosphate in cells, destroys cell DNA replication, and controls the propagation of microorganisms [25]. Spagnol et al. [91] immobilized AgNPs onto cellulose nanowhiskers (CWs) with polyvinyl alcohol (PVA) and poly (N-isopropylacrylamide) (PNIPAAm) as polymeric matrices, and their biological activity was evaluated against Staphylococcus aureus (S. aureus), Bacillus Subtilis (B.subtilis), Escherichia coli (E. coli), and Candida albicans (C. albicans). The properties of the films with CWSAc/AgNPs significantly influenced the antimicrobial activity displayed by each material, and all the films from PVA matrix exhibited the ability to inhibit bacterial growth.
- Microporous materials: Microporous materials such as zeolites and metal-organic frameworks (MOFs) have high porosity and a large surface area that help increase the permeability, hydrophilicity, and anti-fouling behavior of membranes [25,93]. Beisl et al. [94] investigated the antibacterial activity of cellulose acetate/polyvinylpyrrolidone membranes coated with Ag NPs and cellulose acetate/silver ion-exchanged β-Zeolite membranes. The presence of silver ion-loaded zeolites improved the membrane hydrodynamic permeability by 56.3%; in addition, the silver ion-exchanged β-zeolite membrane showed complete Escherichia coli bacterial inactivation after just 210 min of contact time, for the same contact time, the Ag NPs incorporated membrane resulted in 99.95% reduction in bacterial activity indicating that both synthesized membranes possess strong bactericidal properties and are promising for biofouling mitigation. Dehghankar et al. [95] combined hydrophilic zirconium 1,4-dicarboxybenzene (UiO-66) and chromium (III) terephthalate (MIL-101) MOFs and faujasite (FAU) zeolites in a polyvinylidene fluoride (PVDF) polymeric matrix to study the anti-fouling properties of this MMM against bovine serum albumin (BSA). The best anti-fouling behavior was observed from the membrane containing 0.05 wt% UiO-66, 0.1 wt% MIL-101, and 0.1 wt% FAU, with a BSA rejection of 100% and 22.2% irreversible fouling.
- Hydrophilic polymers: Hydrophilic polymers (e.g., polyethylene glycol (PEG), polyethyleneimine (PEI), hyperbranched poly(amido amine) (PAMAM), polydopamine, and dendritic polyamide (PA)) are popular organic additives used to improve the anti-biofouling properties of membranes. Hydrophilic polymers possess a variety of polar groups capable of forming hydrogen bonds with water, which leads to improved membrane hydrophilicity and reduced microorganism adhesion [20,25,77]. In a study conducted by Ma et al. [96], the zwitterionic polymer poly(sulfobetaine methacrylate) (PSBMA) functionalized with graphene oxide (GO) nanocomposites (GO-PSBMA) was incorporated into a polyamide membrane (GO-PSBMA-1h). The synthesized membrane showed improved surface hydrophilicity, and a composition of 0.3 wt% GO-PSBMA-1h exhibited an 80% reduction in Escherichia coli attachment.
5.5.2. Surface Grafting
5.5.3. Surface Coating
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Summary Progress Update 2021: SDG 6—Water and Sanitation for all | UN-Water. Available online: https://www.unwater.org/publications/summary-progress-update-2021-sdg-6-water-and-sanitation-all (accessed on 30 November 2022).
- Kesieme, U.K.; Milne, N.; Aral, H.; Cheng, C.Y.; Duke, M. Economic analysis of desalination technologies in the context of carbon pricing, and opportunities for membrane distillation. Desalination 2013, 323, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Nthunya, L.N.; Bopape, M.F.; Mahlangu, O.T.; Mamba, B.B.; Van der Bruggen, B.; Quist-Jensen, C.A.; Richards, H. Fouling, performance and cost analysis of membrane-based water desalination technologies: A critical review. J. Environ. Manag. 2022, 301, 113922. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Gao, H.; Yu, R.; Gao, L.; Zhan, M. Biological-based control strategies for MBR membrane biofouling: A review. Water Sci. Technol. 2021, 83, 2597–2614. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Yusaf, T. Biofouling in RO system: Mechanisms, monitoring and controlling. Desalination 2012, 302, 1–23. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Stoller, M.; Di Palma, L.; Martínez-Ferez, A. On the optimization of a flocculation process as fouling inhibiting pretreatment on an ultrafiltration membrane during olive mill effluents treatment. Desalination 2016, 393, 151–158. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pichardo-Romero, D.; Garcia-Arce, Z.P.; Zavala-Ramírez, A.; Castro-Muñoz, R. Current advances in biofouling mitigation in membranes for water treatment: An overview. Processes 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Charcosset, C. Classical and Recent Developments of Membrane Processes for Desalination and Natural Water Treatment. Membranes 2022, 12, 267. [Google Scholar] [CrossRef]
- Kalafatakis, S.; Zarebska, A.; Lange, L.; Hélix-Nielsen, C.; Skiadas, I.V.; Gavala, H.N. Biofouling mitigation approaches during water recovery from fermented broth via forward osmosis. Membranes 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, G.; Virtanen, T.; Ferrando, M.; Güell, C.; Lipnizki, F.; Kallioinen, M. A review of in situ real-time monitoring techniques for membrane fouling in the biotechnology, biorefinery and food sectors. J. Memb. Sci. 2019, 588, 117221. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Du, X.; Gong, B.; Jegatheesan, V.; Haq, I.U. Recent Advances in the Prediction of Fouling in Membrane Bioreactors. Membranes 2021, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Bagheri, M.; Akbari, A.; Mirbagheri, S.A. Advanced control of membrane fouling in filtration systems using artificial intelligence and machine learning techniques: A critical review. Process Saf. Environ. Prot. 2019, 123, 229–252. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, Y.M.; Park, H.; Ki, S.; Jeong, K.; Seo, J.; Chae, S.H.; Kim, J.H. Enhancing accuracy of membrane fouling prediction using hybrid machine learning models. Desalin. WATER Treat. 2019, 146, 22–28. [Google Scholar] [CrossRef]
- Viet, N.D.; Jang, A. Development of artificial intelligence-based models for the prediction of filtration performance and membrane fouling in an osmotic membrane bioreactor. J. Environ. Chem. Eng. 2021, 9, 105337. [Google Scholar] [CrossRef]
- Costa, F.C.R.; Ricci, B.C.; Teodoro, B.; Koch, K.; Drewes, J.E.; Amaral, M.C.S. Biofouling in membrane distillation applications—A review. Desalination 2021, 516, 115241. [Google Scholar] [CrossRef]
- Díez, B.; Rosal, R. A critical review of membrane modification techniques for fouling and biofouling control in pressure-driven membrane processes. Nanotechnol. Environ. Eng. 2020, 5, 15. [Google Scholar] [CrossRef]
- Bogler, A.; Lin, S.; Bar-Zeev, E. Biofouling of membrane distillation, forward osmosis and pressure retarded osmosis: Principles, impacts and future directions. J. Memb. Sci. 2017, 542, 378–398. [Google Scholar] [CrossRef]
- Maddah, H.; Chogle, A. Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 2017, 7, 2637–2651. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Liu, C.; Liu, H.; Zhang, J.; Li, H.; Zhang, C. Contemporary antibiofouling modifications of reverse osmosis membranes: State-of-the-art insights on mechanisms and strategies. Chem. Eng. J. 2022, 429, 132400. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, S.; Yang, J.; Ma, A. Advancing Strategies of Biofouling Control in Water-Treated Polymeric Membranes. Polymers 2022, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Inaba, T.; Hori, T.; Aizawa, H.; Ogata, A.; Habe, H. Architecture, component, and microbiome of biofilm involved in the fouling of membrane bioreactors. npj Biofilms Microbiomes 2017, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- De Vries, H.J.; Kleibusch, E.; Hermes, G.D.A.; van den Brink, P.; Plugge, C.M. Biofouling control: The impact of biofilm dispersal and membrane flushing. Water Res. 2021, 198, 117163. [Google Scholar] [CrossRef]
- Pasternak, G.; de Rosset, A.; Tyszkiewicz, N.; Widera, B.; Greenman, J.; Ieropoulos, I. Prevention and removal of membrane and separator biofouling in bioelectrochemical systems: A comprehensive review. iScience 2022, 25, 104510. [Google Scholar] [CrossRef]
- Kerdi, S.; Qamar, A.; Alpatova, A.; Ghaffour, N. An in-situ technique for the direct structural characterization of biofouling in membrane filtration. J. Memb. Sci. 2019, 583, 81–92. [Google Scholar] [CrossRef]
- Benladghem, Z.; Seddiki, S.M.L.; Dergal, F.; Mahdad, Y.M.; Aissaoui, M.; Choukchou-Braham, N. Biofouling of reverse osmosis membranes: Assessment by surface-enhanced Raman spectroscopy and microscopic imaging. Biofouling 2022, 38, 852–864. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Fayzan Shakir, H.M.; Rehan, Z.A.; Javed, T.; Shabbir, R.; Hessien, M.M. Fabrication and Characterization of Sulfonated Graphene Oxide-Doped Polymeric Membranes with Improved Anti-Biofouling Behavior. Membranes 2021, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Masigol, M.; Radaha, E.L.; Kannan, A.D.; Salberg, A.G.; Fattahi, N.; Parameswaran, P.; Hansen, R.R. Polymer Surface Dissection for Correlated Microscopic and Compositional Analysis of Bacterial Aggregates during Membrane Biofouling. ACS Appl. Bio Mater. 2022, 5, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qian, C.; Zhou, K.G.; Yu, H.Q. Molecular Spectroscopic Characterization of Membrane Fouling: A Critical Review. Chem 2018, 4, 1492–1509. [Google Scholar] [CrossRef] [Green Version]
- Chai-Hoon, C.K.O.O.; Mohammad, A.; Suja, F.; Zainal, M. Use and Development of Fouling Index in Predicting Membrane Fouling. Sep. Purif. Rev. 2013, 42, 296–339. [Google Scholar] [CrossRef]
- Sim, L.N.; Chong, T.H.; Taheri, A.H.; Sim, S.T.V.; Lai, L.; Krantz, W.B.; Fane, A.G. A review of fouling indices and monitoring techniques for reverse osmosis. Desalination 2018, 434, 169–188. [Google Scholar] [CrossRef]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- ASTM D4189-07(2014) Standard Test Method for Silt Density Index (SDI) of Water. Available online: https://www.astm.org/Standards/D4189.htm (accessed on 21 June 2020).
- Schippers, J.C.; Verdouw, J. The modified fouling index, a method of determining the fouling characteristics of water. Desalination 1980, 32, 137–148. [Google Scholar] [CrossRef]
- Niu, C.; Li, X.; Dai, R.; Wang, Z. Artificial intelligence-incorporated membrane fouling prediction for membrane-based processes in the past 20 years: A critical review. Water Res. 2022, 216, 118299. [Google Scholar] [CrossRef]
- Gaudio, M.T.; Coppola, G.; Zangari, L.; Curcio, S.; Greco, S.; Chakraborty, S. Artificial Intelligence-Based Optimization of Industrial Membrane Processes. Earth Syst. Environ. 2021, 5, 385–398. [Google Scholar] [CrossRef]
- Lawler, J. Dead-End and Crossflow Microfiltration of Yeast and Bentonite Suspensions: Experimental and Modelling Studies Incorporating the use of Artificial Neural Networks. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2022. [Google Scholar]
- Heidari, S.; Etemadi, H.; Yegani, R. A comprehensive analysis of membrane fouling in microfiltration of complex linear macromolecules based on theoretical modeling and FESEM images. J. Chem. Technol. Biotechnol. 2021, 96, 360–373. [Google Scholar] [CrossRef]
- Duclos-Orsello, C.; Li, W.; Ho, C.C. A three mechanism model to describe fouling of microfiltration membranes. J. Memb. Sci. 2006, 280, 856–866. [Google Scholar] [CrossRef]
- Meskó, B.; Görög, M. A short guide for medical professionals in the era of artificial intelligence. npj Digit. Med. 2020, 3, 126. [Google Scholar] [CrossRef] [PubMed]
- Alam, G.; Ihsanullah, I.; Naushad, M.; Sillanpää, M. Applications of artificial intelligence in water treatment for optimization and automation of adsorption processes: Recent advances and prospects. Chem. Eng. J. 2022, 427, 130011. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef]
- Yokoyama, D.; Suzuki, S.; Asakura, T.; Kikuchi, J. Chemometric Analysis of NMR Spectra and Machine Learning to Investigate Membrane Fouling. ACS Omega 2022, 7, 12654–12660. [Google Scholar] [CrossRef]
- Qamar, A.; Kerdi, S.; Amin, N.; Zhang, X.; Vrouwenvelder, J.; Ghaffour, N. A deep neural networks framework for in-situ biofilm thickness detection and hydrodynamics tracing for filtration systems. Sep. Purif. Technol. 2022, 301, 121959. [Google Scholar] [CrossRef]
- Galinha, C.F.; Crespo, J.G. From Black Box to Machine Learning: A Journey through Membrane Process Modelling. Membranes 2021, 11, 574. [Google Scholar] [CrossRef]
- Li, L.; Rong, S.; Wang, R.; Yu, S. Recent advances in artificial intelligence and machine learning for nonlinear relationship analysis and process control in drinking water treatment: A review. Chem. Eng. J. 2021, 405, 126673. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, T.; Qiao, Z.; Sun, P.; Hao, J.; Yang, Y. Application of artificial intelligence to wastewater treatment: A bibliometric analysis and systematic review of technology, economy, management, and wastewater reuse. Process Saf. Environ. Prot. 2020, 133, 169–182. [Google Scholar] [CrossRef]
- Wolf, G.; Almeida, J.S.; Pinheiro, C.; Correia, V.; Rodrigues, C.; Reis, M.A.M.; Crespo, J.G. Two-dimensional fluorometry coupled with artificial neural networks: A novel method for on-line monitoring of complex biological processes. Biotechnol. Bioeng. 2001, 72, 297–306. [Google Scholar] [CrossRef]
- Sanches, S.; Galinha, C.F.; Barreto Crespo, M.T.; Pereira, V.J.; Crespo, J.G. Assessment of phenomena underlying the removal of micropollutants during water treatment by nanofiltration using multivariate statistical analysis. Sep. Purif. Technol. 2013, 118, 377–386. [Google Scholar] [CrossRef]
- Ricardo, A.R.; Oliveira, R.; Velizarov, S.; Reis, M.A.M.; Crespo, J.G. Multivariate statistical modelling of mass transfer in a membrane-supported biofilm reactor. Process Biochem. 2011, 46, 1981–1992. [Google Scholar] [CrossRef]
- Kovacs, D.J.; Li, Z.; Baetz, B.W.; Hong, Y.; Donnaz, S.; Zhao, X.; Zhou, P.; Ding, H.; Dong, Q. Membrane fouling prediction and uncertainty analysis using machine learning: A wastewater treatment plant case study. J. Memb. Sci. 2022, 660, 120817. [Google Scholar] [CrossRef]
- Bucs, S.; Farhat, N.; Kruithof, J.C.; Picioreanu, C.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Review on strategies for biofouling mitigation in spiral wound membrane systems. Desalination 2018, 434, 189–197. [Google Scholar] [CrossRef]
- Nagaraj, V.; Skillman, L.; Li, D.; Ho, G. Review—Bacteria and their extracellular polymeric substances causing biofouling on seawater reverse osmosis desalination membranes. J. Environ. Manage. 2018, 223, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Mirbagheri, S.A. Critical review of fouling mitigation strategies in membrane bioreactors treating water and wastewater. Bioresour. Technol. 2018, 258, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Bertheas, U.; Majamaa, K.; Arzu, A.; Pahnke, R. Use of DBNPA to control biofouling in RO systems. New Pub Balaban 2012, 3, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.S.; Dudley, L.Y. Biofouling in membrane systems—A review. Desalination 1998, 118, 81–89. [Google Scholar] [CrossRef]
- Marconnet, C.; Houari, A.; Seyer, D.; Djafer, M.; Coriton, G.; Heim, V.; Di Martino, P. Membrane biofouling control by UV irradiation. Desalination 2011, 276, 75–81. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, B.; Lu, Y.; Mei, Y.; Shen, L. Advances in application of ultraviolet irradiation for biofilm control in water and wastewater infrastructure. J. Hazard. Mater. 2022, 421, 126682. [Google Scholar] [CrossRef]
- Feng, J.; Li, X.; Li, H.; Yang, Y. Enhanced filtration performance of biocarriers facilitated gravity-driven membrane (GDM) by vacuum ultraviolet (VUV) pretreatment: Membrane biofouling characteristics and bacterial investigation. J. Memb. Sci. 2022, 660, 120859. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Becker, W.C.; Schorr, P.; Lee, R.G. Evaluating the performance of biologically active rapid filters. J. Am. Water Work. Assoc. 1992, 84, 136–146. [Google Scholar] [CrossRef]
- Bradford, S.M.; Palmer, C.J.; Olson, B.H. Assimilable organic carbon concentrations in Southern California surface and groundwater. Water Res. 1994, 28, 427–435. [Google Scholar] [CrossRef]
- Javier, L.; Farhat, N.M.; Vrouwenvelder, J.S. Enhanced hydraulic cleanability of biofilms developed under a low phosphorus concentration in reverse osmosis membrane systems. Water Res. X 2021, 10, 100085. [Google Scholar] [CrossRef]
- Javier, L.; Pulido-Beltran, L.; Kruithof, J.; Vrouwenvelder, J.S.; Farhat, N.M. Phosphorus Concentration in Water Affects the Biofilm Community and the Produced Amount of Extracellular Polymeric Substances in Reverse Osmosis Membrane Systems. Membranes 2021, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Javier, L.; Farhat, N.M.; Desmond, P.; Linares, R.V.; Bucs, S.; Kruithof, J.C.; Vrouwenvelder, J.S. Biofouling control by phosphorus limitation strongly depends on the assimilable organic carbon concentration. Water Res. 2020, 183, 116051. [Google Scholar] [CrossRef]
- Lin, W.C.; Shao, R.-P.; Wang, X.-M.; Huang, X. Impacts of non-uniform filament feed spacers characteristics on the hydraulic and anti-fouling performances in the spacer-filled membrane channels: Experiment and numerical simulation. Water Res. 2020, 185, 116251. [Google Scholar] [CrossRef]
- Siddiqui, A.; Farhat, N.; Bucs, S.S.; Linares, R.V.; Picioreanu, C.; Kruithof, J.C.; Van Loosdrecht, M.C.M.; Kidwell, J.; Vrouwenvelder, J.S. Development and characterization of 3D-printed feed spacers for spiral wound membrane systems. Water Res. 2016, 91, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Wang, Q.; Sun, L.; Wang, D.; Cabrera, J.; Li, D.; Hu, L.; Jiang, G.; Wang, X.M.; Huang, X. The critical role of feed spacer channel porosity in membrane biofouling: Insights and implications. J. Memb. Sci. 2022, 649, 120395. [Google Scholar] [CrossRef]
- Arnal, J.M.; García-Fayos, B.; Sancho, M. Membrane cleaning. In Expanding Issues in Desalination Edited; Ning, R.Y., Ed.; IntechOpen: London, UK, 2011; pp. 63–84. [Google Scholar] [CrossRef]
- Ehsani, M.; Doan, H.; Lohi, A. A comprehensive review of membrane fouling and cleaning methods with emphasis on ultrasound-assisted fouling control processes. Korean J. Chem. Eng. 2021, 38, 1531–1555. [Google Scholar] [CrossRef]
- Lin, J.C.-T.; Lee, D.-J.; Huang, C. Membrane fouling mitigation: Membrane cleaning. Sep. Sci. Technol. 2010, 45, 858–872. [Google Scholar] [CrossRef]
- Kucera, J. Biofouling of Polyamide Membranes: Fouling Mechanisms, Current Mitigation and Cleaning Strategies, and Future Prospects. Membranes 2019, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Hoek, E.M.V.; Weigand, T.M.; Edalat, A. Reverse osmosis membrane biofouling: Causes, consequences and countermeasures. npj Clean Water 2022, 5, 45. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qiu, L.; Liu, G. Basic characteristics and application of micro-nano bubbles in water treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 510, 42050. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Choi, J.; Jang, A. Enhancement of cleaning-in-place (CIP) of a reverse osmosis desalination process with air micro-nano bubbles. Desalination 2017, 422, 1–4. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.A.; Alhadidi, A.; Ghaffour, N. Membrane backwash cleaning using CO2 nucleation. Water Res. 2019, 165, 114985. [Google Scholar] [CrossRef]
- Shahid, M.K.; Choi, Y. CO2 as an Alternative to Traditional Antiscalants in Pressure-Driven Membrane Processes: An Experimental Study of Lab-Scale Operation and Cleaning Strategies. Membranes 2022, 12, 918. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with surface-enhanced antifouling properties for water purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.; Sharma, A.; Awasthi, K.K.; Awasthi, A. Metal oxides nanocomposite membrane for biofouling mitigation in wastewater treatment. Mater. Today Chem. 2021, 21, 100532. [Google Scholar] [CrossRef]
- Armendariz Ontiveros, M.; Quintero, Y.; Llanquilef, A.; Morel, M.; Argentel Martínez, L.; García García, A.; Garcia, A. Anti-Biofouling and Desalination Properties of Thin Film Composite Reverse Osmosis Membranes Modified with Copper and Iron Nanoparticles. Materials 2019, 12, 2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusworo, T.D.; Ariyanti, N.; Utomo, D.P. Effect of nano-TiO2 loading in polysulfone membranes on the removal of pollutant following natural-rubber wastewater treatment. J. Water Process Eng. 2020, 35, 101190. [Google Scholar] [CrossRef]
- Aoudjit, L.; Salazar, H.; Zioui, D.; Sebti, A.; Martins, P.M.; Lanceros-Méndez, S. Solar Photocatalytic Membranes: An Experimental and Artificial Neural Network Modeling Approach for Niflumic Acid Degradation. Membranes 2022, 12, 849. [Google Scholar] [CrossRef]
- Spagnol, C.; Fragal, E.H.; Pereira, A.G.B.; Nakamura, C.V.; Muniz, E.C.; Follmann, H.D.M.; Silva, R.; Rubira, A.F. Cellulose nanowhiskers decorated with silver nanoparticles as an additive to antibacterial polymers membranes fabricated by electrospinning. J. Colloid Interface Sci. 2018, 531, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Maghami, M.; Abdelrasoul, A.; Maghami, M.; Abdelrasoul, A. Zeolites-Mixed-Matrix Nanofiltration Membranes for the Next Generation of Water Purification. In Nanofiltration; IntechOpen: Rijeka, Croatia, 2018; Available online: https://www.intechopen.com/state.item.id (accessed on 14 October 2022).
- Beisl, S.; Monteiro, S.; Santos, R.; Figueiredo, A.S.; Sánchez-Loredo, M.G.; Lemos, M.A.; Lemos, F.; Minhalma, M.; de Pinho, M.N. Synthesis and bactericide activity of nanofiltration composite membranes—Cellulose acetate/silver nanoparticles and cellulose acetate/silver ion exchanged zeolites. Water Res. 2019, 149, 225–231. [Google Scholar] [CrossRef]
- Dehghankar, M.; Mohammadi, T.; Moghadam, M.T.; Tofighy, M.A. Metal-organic framework/zeolite nanocrystal/polyvinylidene fluoride composite ultrafiltration membranes with flux/antifouling advantages. Mater. Chem. Phys. 2021, 260, 124128. [Google Scholar] [CrossRef]
- Ma, W.; Chen, T.; Nanni, S.; Yang, L.; Ye, Z.; Rahaman, M.S. Zwitterion-Functionalized Graphene Oxide Incorporated Polyamide Membranes with Improved Antifouling Properties. Langmuir 2019, 35, 1513–1525. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Vatanpour, V.; Zoqi, N. Surface modification of commercial seawater reverse osmosis membranes by grafting of hydrophilic monomer blended with carboxylated multiwalled carbon nanotubes. Appl. Surf. Sci. 2017, 396, 1478–1489. [Google Scholar] [CrossRef] [Green Version]
- Isawi, H.; El-Sayed, M.H.; Feng, X.; Shawky, H.; Abdel Mottaleb, M.S. Surface nanostructuring of thin film composite membranes via grafting polymerization and incorporation of ZnO nanoparticles. Appl. Surf. Sci. 2016, 385, 268–281. [Google Scholar] [CrossRef]
- Lee, X.J.; Show, P.L.; Katsuda, T.; Chen, W.H.; Chang, J.S. Surface grafting techniques on the improvement of membrane bioreactor: State-of-the-art advances. Bioresour. Technol. 2018, 269, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Khongnakorn, W.; Bootluck, W.; Jutaporn, P. Surface modification of FO membrane by plasma-grafting polymerization to minimize protein fouling. J. Water Process Eng. 2020, 38, 101633. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Safarpour, M.; Ghadimi, A.; Adabi, J. Synergistic effect of carboxylated-MWCNTs on the performance of acrylic acid UV-grafted polyamide nanofiltration membranes. React. Funct. Polym. 2019, 134, 74–84. [Google Scholar] [CrossRef]
- Yuan, G.; Tian, Y.; Wang, B.; You, X.; Liao, Y. Mitigation of membrane biofouling via immobilizing Ag-MOFs on composite membrane surface for extractive membrane bioreactor. Water Res. 2022, 209, 117940. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar] [CrossRef]
- Bachosz, K.; Vu, M.T.; Nghiem, L.D.; Zdarta, J.; Nguyen, L.N.; Jesionowski, T. Enzyme-based control of membrane biofouling for water and wastewater purification: A comprehensive review. Environ. Technol. Innov. 2022, 25, 102106. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y. Biological control of microbial attachment: A promising alternative for mitigating membrane biofouling. Appl. Microbiol. Biotechnol. 2010, 86, 825–837. [Google Scholar] [CrossRef]
- Malaeb, L.; Le-Clech, P.; Vrouwenvelder, J.S.; Ayoub, G.M.; Saikaly, P.E. Do biological-based strategies hold promise to biofouling control in MBRs? Water Res. 2013, 47, 5447–5463. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xu, H.J.; Yao, L.; Wang, R. A biomimetic antimicrobial surface for membrane fouling control in reverse osmosis for seawater desalination. Desalination 2021, 503, 114954. [Google Scholar] [CrossRef]
- Bao, Q.; Xie, L.; Ohashi, H.; Hosomi, M.; Terada, A. Inhibition of Agrobacterium tumefaciens biofilm formation by acylase I-immobilized polymer surface grafting of a zwitterionic group-containing polymer brush. Biochem. Eng. J. 2019, 152, 107372. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Taheri-Kafrani, A.; Asadnia, M.; Razmjou, A. Bienzymatic modification of polymeric membranes to mitigate biofouling. Sep. Purif. Technol. 2020, 237, 116464. [Google Scholar] [CrossRef]
- Lan, Y.; Hiebner, D.W.; Casey, E. Self-assembly and regeneration strategy for mitigation of membrane biofouling by the exploitation of enzymatic nanoparticles. Chem. Eng. J. 2021, 412, 128666. [Google Scholar] [CrossRef]
- Kamaludin, R.; Abdul Majid, L.; Othman, M.H.D.; Mansur, S.; Sheikh Abdul Kadir, S.H.; Wong, K.Y.; Khongnakorn, W.; Puteh, M.H. Polyvinylidene Difluoride (PVDF) hollow fiber membrane incorporated with antibacterial and anti-fouling by Zinc Oxide for water and wastewater treatment. Membranes 2022, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Căprărescu, S.; Modrogan, C.; Purcar, V.; Dăncilă, A.M.; Orbuleț, O.D. Study of Polyvinyl Alcohol-SiO2 Nanoparticles Polymeric Membrane in Wastewater Treatment Containing Zinc Ions. Polymers 2021, 13, 1875. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Aber, S.; Mahmoodi, N.M. Preparation and characterization of a novel polyethersulfone (PES) ultrafiltration membrane modified with a CuO/ZnO nanocomposite to improve permeability and antifouling properties. Sep. Purif. Technol. 2018, 192, 369–382. [Google Scholar] [CrossRef]
- Nambikkattu, J.; Kaleekkal, N.J.; Jacob, J.P. Metal ferrite incorporated polysulfone thin-film nanocomposite membranes for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 11915–11927. [Google Scholar] [CrossRef]
- Armendáriz-Ontiveros, M.M.; Villegas-Peralta, Y.; Madueño-Moreno, J.E.; Álvarez-Sánchez, J.; Dévora-Isiordia, G.E.; Sánchez-Duarte, R.G.; Madera-Santana, T.J. Modification of Thin Film Composite Membrane by Chitosan–Silver Particles to Improve Desalination and Anti-Biofouling Performance. Membranes 2022, 12, 851. [Google Scholar] [CrossRef]
- Yin, J.; Tang, H.; Xu, Z.; Li, N. Enhanced mechanical strength and performance of sulfonated polysulfone/Tröger’s base polymer blend ultrafiltration membrane. J. Memb. Sci. 2021, 625, 119138. [Google Scholar] [CrossRef]
- Wang, S.Y.; Rolly Gonzales, R.; Zhang, P.; Istirokhatun, T.; Takagi, R.; Motoyama, A.; Fang, L.F.; Matsuyama, H. Surface charge control of poly(methyl methacrylate-co-dimethyl aminoethyl methacrylate)-based membrane for improved fouling resistance. Sep. Purif. Technol. 2021, 279, 119778. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Zhang, Z.; Wang, J.; Wang, S. Surface modification of commercial aromatic polyamide reverse osmosis membranes by graft polymerization of 3-allyl-5,5-dimethylhydantoin. J. Memb. Sci. 2010, 351, 222–233. [Google Scholar] [CrossRef]
- Ren, L.; Ping, M.; Zhang, X. Membrane biofouling control by surface modification of quaternary ammonium compound using atom-transfer radical-polymerization method with silica nanoparticle as interlayer. Membranes 2020, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Funari, R.; Shen, A.Q. Detection and Characterization of Bacterial Biofilms and Biofilm-Based Sensors. ACS Sensors 2022, 7, 347–357. [Google Scholar] [CrossRef]
- Majors, P.D.; McLean, J.S.; Pinchuk, G.E.; Fredrickson, J.K.; Gorby, Y.A.; Minard, K.R.; Wind, R.A. NMR methods for in situ biofilm metabolism studies. J. Microbiol. Methods 2005, 62, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Valladares Linares, R.; Fortunato, L.; Farhat, N.M.; Bucs, S.S.; Staal, M.; Fridjonsson, E.O.; Johns, M.L.; Vrouwenvelder, J.S.; Leiknes, T. Mini-review: Novel non-destructive in situ biofilm characterization techniques in membrane systems. Desalin. Water Treat. 2016, 57, 22894–22901. [Google Scholar] [CrossRef] [Green Version]
- Pengyu, C.; Cui, L.; Zhang, K. Surface-enhanced Raman spectroscopy monitoring the development of dual-species biofouling on membrane surfaces. J. Memb. Sci. 2015, 473, 36–44. [Google Scholar] [CrossRef]
- Virtanen, T.; Reinikainen, S.P.; Lahti, J.; Mänttäri, M.; Kallioinen, M. Visual tool for real-time monitoring of membrane fouling via Raman spectroscopy and process model based on principal component analysis. Sci. Rep. 2018, 8, 11057. [Google Scholar] [CrossRef]
- Czieborowski, M.; Kemperman, A.J.B.; Rolevink, E.; Blom, J.; Visser, T.; Philipp, B. A two-step bioluminescence assay for optimizing antibacterial coating of hollow-fiber membranes with polydopamine in an integrative approach. J. Microbiol. Methods 2022, 196, 106452. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.N.; Anand, S.; Avadhanula, M. Microscopic observation of multispecies biofilm of various structures on whey concentration membranes. J. Dairy Sci. 2010, 93, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Simoska, O.; Gaffney, E.M.; Minteer, S.D.; Franzetti, A.; Cristiani, P.; Grattieri, M.; Santoro, C. Recent trends and advances in microbial electrochemical sensing technologies: An overview. Curr. Opin. Electrochem. 2021, 30, 100762. [Google Scholar] [CrossRef]
- Bjerketorp, J.; Håkansson, S.; Belkin, S.; Jansson, J.K. Advances in preservation methods: Keeping biosensor microorganisms alive and active. Curr. Opin. Biotechnol. 2006, 17, 43–49. [Google Scholar] [CrossRef]
- Qi, X.; Wang, S.; Li, T.; Wang, X.; Jiang, Y.; Zhou, Y.; Zhou, X.; Huang, X.; Liang, P. An electroactive biofilm-based biosensor for water safety: Pollutants detection and early-warning. Biosens. Bioelectron. 2021, 173, 112822. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Pant, D.; Saint, C.P. Bio-analytical applications of microbial fuel cell–based biosensors for onsite water quality monitoring. J. Appl. Microbiol. 2018, 124, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.I.; Shen, J.; Richards, B.S. Renewable energy-powered membrane technology in Tanzanian communities. npj Clean Water 2018, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.I.; Broeckmann, A.; Richards, B.S. Renewable Energy Powered Membrane Technology. 1. Development and Characterization of a Photovoltaic Hybrid Membrane System. Environ. Sci. Technol. 2006, 41, 998–1003. [Google Scholar] [CrossRef]
- Cai, Y.H.; Gopalakrishnan, A.; Deshmukh, K.P.; Schäfer, A.I. Renewable energy powered membrane technology: Implications of adhesive interaction between membrane and organic matter on spontaneous osmotic backwash cleaning. Water Res. 2022, 221, 118752. [Google Scholar] [CrossRef]
- Monnot, M.; Carvajal, G.D.M.; Laborie, S.; Cabassud, C.; Lebrun, R. Integrated approach in eco-design strategy for small RO desalination plants powered by photovoltaic energy. Desalination 2018, 435, 246–258. [Google Scholar] [CrossRef]
- Karavas, C.S.; Arvanitis, K.G.; Papadakis, G. Optimal technical and economic configuration of photovoltaic powered reverse osmosis desalination systems operating in autonomous mode. Desalination 2019, 466, 97–106. [Google Scholar] [CrossRef]
- Shokri, A.; Fard, M.S. A sustainable approach in water desalination with the integration of renewable energy sources. Environ. Adv. 2022, 9, 100281. [Google Scholar] [CrossRef]
- Freire-Gormaly, M.; Bilton, A.M. Design of photovoltaic powered reverse osmosis desalination systems considering membrane fouling caused by intermittent operation. Renew. Energy 2019, 135, 108–121. [Google Scholar] [CrossRef]
- Kim, A.; Hak Kim, J.; Patel, R. Modification strategies of membranes with enhanced Anti-biofouling properties for wastewater Treatment: A review. Bioresour. Technol. 2022, 345, 126501. [Google Scholar] [CrossRef] [PubMed]
- Firouzjaei, M.D.; Seyedpour, S.F.; Aktij, S.A.; Giagnorio, M.; Bazrafshan, N.; Mollahosseini, A.; Samadi, F.; Ahmadalipour, S.; Firouzjaei, F.D.; Esfahani, M.R.; et al. Recent advances in functionalized polymer membranes for biofouling control and mitigation in forward osmosis. J. Memb. Sci. 2020, 596, 117604. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, Y.; Jing, X.; Cai, P.; Igalavithana, A.D.; Tang, C.; Tsang, D.C.W.; Ok, Y.S. Recent advances in mitigating membrane biofouling using carbon-based materials. J. Hazard. Mater. 2020, 382, 120976. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Zhang, L.; Li, S.; Yuan, Y.; Xiao, X.; Fan, X.; Song, C. Carbon-based membrane materials and applications in water and wastewater treatment: A review. Environ. Chem. Lett. 2020, 19, 1457–1475. [Google Scholar] [CrossRef]
| Element Characterized | Technique | Information Obtained |
|---|---|---|
| Biofilm | Epifluorescence microscopy (EFM) | -Morphological observations of biofilm |
| Confocal laser scanning microscopy (CLSM) | -3D structure of the biofilm | |
| Electron microscopy (e.g., SEM and TEM) | -SEM enables imaging complex structures of biofilm -TEM enables the visualization of cross-sectional details of microorganisms -Mapping distribution of macromolecular subcomponents | |
| Atomic force microscopy (AFM) | -Biofilm surface topography | |
| X-ray microscopy | -Revealing the onset of bacterial colonization | |
| FTIR spectroscopy | -Analyzing microbial aggregates -Provides information about the chemical nature of foulants | |
| Nuclear magnetic resonance (NMR) | -Reveals the impact of biofouling on hydrodynamics and mass transport | |
| Microbial community | Epifluorescence microscopy (EFM) with staining | -Microbial activity -Cell counts -2D distribution of bacteria in biofilm |
| Confocal laser scanning microscopy (CLSM) | -3D structure of bacteria | |
| Heterotrophic plate counts (HPCs) | -Monitoring general bacteriological water quality | |
| Flow cytometry | -Species abundance and population dynamics | |
| Extracellular Polymeric Substance (EPS) matrix | Phenol/sulfuric acid assay | -Carbohydrates quantification |
| Lowry/Bicinchoninic acid (BCA) assay | -Protein quantification |
| Fouling Models | Description | Governing Equation(s) |
|---|---|---|
| Resistance-in-series (RIS) | -Enable the determination of the fouling resistance form -Developed for dead-end MF -Vary with fouling mechanism (internal/external) | |
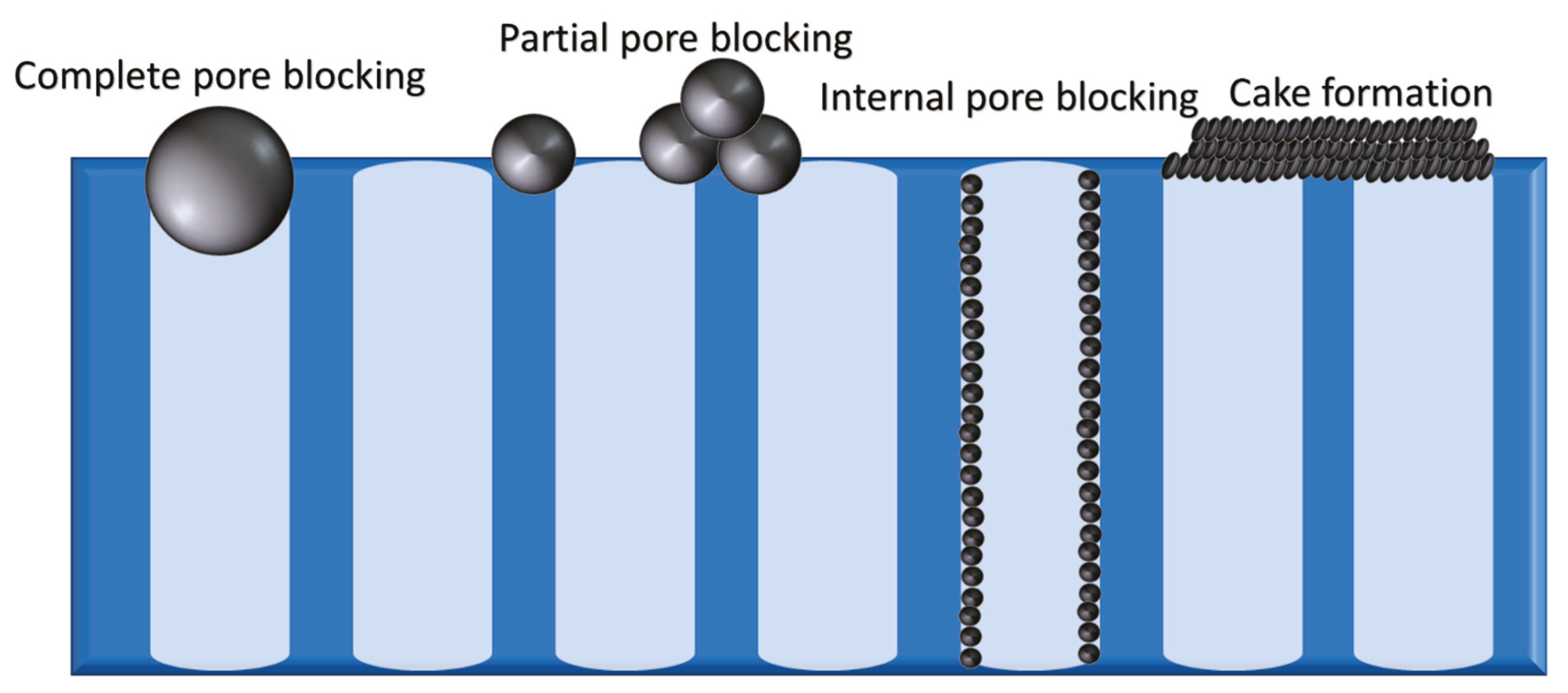
| Pore blockage/Hermia’s models | -Describe the filtrate flux under constant pressure -Four blocking modes: complete pore blocking, standard blocking or pore constriction, intermediate pore blocking, and cake filtration | |
| Complete blocking; n = 2 | ||
| Standard blocking/pore constriction; n = 1.5 | ||
| Intermediate blocking; n = 1 | ||
| Cake filtration; n = 0 | ||
| Combined cake filtration-pore blockage models | -Assume that the fouling occurs in three stages: pore constriction, pore blocking, and cake accumulation |
| Cleaning Method Category | Cleaning Method/Agent | Working Principle | Advantages | Disadvantages |
|---|---|---|---|---|
| Physical | Forward/reverse flushing | Pumping permeate water at high crossflow velocity through the feed side of the membrane (forward). Permeate flush direction alternated in forward and reverse directions (reverse). | 1. Well-established method | 1. Ineffective against irreversible fouling (e.g., pore-clogging with colloidal/dissolved materials) |
| Backwashing | Negative pressure gradient is created across the membrane. | 1. Easy implementation 2. Can be used with chemicals to enhance cleaning | 1. Ineffective against irreversible fouling 2. Possibility of membrane damage | |
| Air flushing/sparging | Flushing along with air bubbles to create turbulence. | 1. Easy to integrate into the membrane system 2. No chemicals involved 3. Low maintenance cost 4. Commonly combined with backwashing to remove biofoulants | 1. Less efficient with hollow-fiber and SWMs 2. Air pumping cost is high | |
| Sponge ball | Sponge ball is used to scrub foulants from the membrane’s surface. | 1. Used for heavily polluted membranes 2. Well-established method | 1. Only applicable for tubular membrane modules | |
| Electrokinetics | The application of an electric field attracts particles from the membrane surface, damages cell membranes of microorganisms, and leads to the generation of oxidizing species. | 1. Enhances efficiency of chemical cleaning | 1. Mutagenic compounds may be created in water 2. Pretreatment required | |
| Chemical | Acids (HCl, HNO3, H3PO4, H2SO4) | Suitable for removing inorganic foulants like salt precipitates or scales and metal oxides | 1. Interfere with the weak electrostatic forces holding the microorganisms to the membrane | 1. Frequent usage can damage the membrane 2. Need to be removed from the stream after cleaning |
| Alkalis (NaOH, KOH, NH4OH) | Hydrolysis and solubilization of proteins and saccharides | 1. Increase solubility of phenolic and carboxylic groups at high pH (~13) 2. Increase the negative charge of humic substances, hence weakening their bond with the membrane | 1. Frequent usage can damage the membrane 2. Need to be removed from the stream after cleaning | |
| Surfactants | Solubilize foulants by enclosing them in micelles | 1. Affect hydrophobic interactions with membrane, hence hindering biofilm formation | 1. Frequent usage can damage the membrane 2. Need to be removed from the stream after cleaning | |
| Nonconventional | Micro-nano bubbles (MNBs) | Foulant detachment due to shear stress generated by the collapse of MNBs, adsorption of foulant on MNBs surface due to hydrophobic interactions, and MNBs can generate hydroxyl radicals when they collapse, leading to the decomposition of organic foulants | 1. Small size and large specific surface area 2. Extended residence time in solution 3. Environmentally friendly and non-chemical cleaning agents | 1. Cavitation effects could lead to membrane damage 2. Cost and large-scale production need further study 3. Stability and storage issues need to be addressed |
| CO2 nucleation | Combines hydraulic and chemical cleaning procedures, i.e., the formation of CO2 bubbles physically removes biofilms off of the membrane and the case a drop in pH acting as an acid-cleaning medium | 1. CO2 gas is highly soluble in water 2. Formation of carbonic acid can facilitate removal of inorganic scaling | 1. Technique still under research 2. Drop in pH may damage the membrane 3. ‘Green’ processes for obtaining CO2 are needed | |
| Ultrasound | US-induced cavitation minimizing foulants deposition and cell disruption | 1. Chemical-free process 2. Can be combined with heat to improve cleaning 3. Membrane can be cleaned while in use 4. Hydroxyl and hydrogen peroxide radicals produced can act as disinfectants | 1. Technique still under research 2. Scale-up of this technique still under study 3. May damage the membrane | |
| Hypersaline backwash | A high-concentration salt solution (hypersaline) is injected into the feed promoting direct osmosis across the membrane, while the reversible flow helps detach the biofilm and other foulants | 1. On-line technique 2. High effectiveness 3. Ease of implementation 4. Chemical free | 1. Technique still under research 2. Pulse concentration and timing need optimization | |
| Rhamnolipids | They act as cleaning agents (biosurfactants) that solubilize and remove the formed biofilms | 1. Lower cost 2. Less toxic than conventional cleaning chemicals 3. Biologically produced | 1. Technique still under research |
| Method | Base Polymer Membrane | Modifier | Main Findings | Ref. |
|---|---|---|---|---|
| Polymer blending | Polysulfone | TiO2 NPs | -Improved hydrophilicity (wetting angle reduction from 61.83 to 41.67) -Increased pore size. -Best pollutant removal with 1 wt% TiO2 NPs dope PSF membranes. | [89] |
| PVDF–TrFE | TiO2 | -91% NFA photocatalytic efficiency was achieved after 6 h of solar irradiation at neutral pH. | [90] | |
| Polyvinylidene Difluoride | ZnO NPs | -Increased ZnO loading (from 2.5 to 7.5 wt%) improved membrane hydrophilicity. -ZnO incorporated membranes achieved BSA rejection of 93.4% ± 0.4 and flux recovery rate of 70.9% ± 2.1. | [112] | |
| Polyvinyl alcohol | SiO2 NPs | -SEM images showed that the SiO2 NPs and polymer matrix were compatible. -SiO2-modified membranes improved zinc ions removal to ~65%. | [113] | |
| Polyethersulfone | CuO/ZnO (CZN) | -Optimal CZN concentration was 0.2 wt% CZN. -SEM images showed the homogenous distribution of NPs in polymeric base. -BSA rejection was around 95% for all nanocomposite membranes. | [114] | |
| Polysulfone | MgFe2O4 and ZnFe2O4 NPs | -Membranes with 0.005 wt.% MgFe2O4 NPs exhibited the highest glucose rejection (96.52 ± 2.35%). | [115] | |
| Polyvinyl alcohol and poly (N-isopropyl acrylamide) | Ag NPs | -The type of polymeric matrix affected the antimicrobial activity. -PVA-based films exhibited the best antibacterial activity. | [91] | |
| Cellulose acetate/polyvinylpyrrolidone | Ag NPs and silver ion-exchanged β-zeolites | -Silver ion exchanged β-zeolites loaded membranes improved permeability by 56.3%, and increased salt rejection to 93%. -Silver ion exchanged β-zeolites loaded membranes showed the best antibacterial activity. | [94] | |
| Polysulfone | Chitosan–Ag NPs | -The modified membrane showed higher bactericidal properties (76% decrease in total cell count) and anti-adhesion capacity (60% less biofilm thickness and 75% less TOC compared to the unmodified membrane). | [116] | |
| Polyvinylidene fluoride | (UiO-66) and MIL-101 MOFs and FAU zeolites | -The optimal anti-fouling results were obtained for the 0.05 wt% UiO-66, 0.1 wt% MIL-101 MOFs, and 0.1 wt% FAU zeolite nanocrystals (~100% BSA rejection). | [95] | |
| Polyamide | GO-PSBMA | - The optimal additive concentration was 0.3 wt% GO-PSBMA. -Bacterial adhesion reduced by 80%. | [96] | |
| Sulfonated polysulfone (SPSf) | Tröger’s base (TB) polymer | -Blending enhanced surface and total porosity. -SPSf/TB blended membranes had slightly lower BSA retention (86.5–94.6%) than pristine SPSf membranes (94.7%). | [117] | |
| Polysulfone | Poly(methyl methacrylate-co-dimethyl aminoethyl methacrylate) (P(MMA-co-DMAEMA)) and 2-carboxyethyl acrylate | -The amount of adhered total E. coli on the membrane surface decreased in the following order for the different blended membranes: PSF > PSF-PMD > PSF-qPMD > PSF-nPMD. -Best antibiofouling behavior achieved by PSF-nPMD because its net charge was close to zero (no electrostatic attractions negatively charged E. coli bacteria). | [118] | |
| Surface grafting | Polyamide | 3-allyl-5,5-dimethylhydantoin (ADMH) | -The modified membrane showed improved microbial adsorption compared to the unmodified membrane. | [119] |
| Cellulose triacetate (CTA) | Acrylic acid (AAc) | -Both plasma gases increased membrane hydrophilicity (water contact angle reduced from 64.0° to 37.1° and 36.4° for CO2 and Ar plasma gases, respectively). -The hydrophilicity increased due to the presence of hydrophilic functional groups such as carboxyl O–C=O and –COOH. -Ar gas generated more free radicals than CO2. -Anti-fouling behavior against proteins was better than polysaccharides. | [101] | |
| Polyamide | AAc and MWCNTs | -Membrane embedded with 0.2 wt% COOH-MWCNTs showed the best water flux improvement (around 30%). -Higher COOH-MWCNTs concentrations reduced the hydrophilicity of the membrane. -All of the COOH-MWCNTs-modified membranes possessed excellent anti-fouling abilities with FRR values of 98–99%. | [102] | |
| Polyvinylidene fluoride (PVDF) | Quaternary ammonium compounds (QACs) and silica NPs | -Approximately 99.9% bacterial inhibition was achieved with the modified membrane. | [120] | |
| Surface coating | Poly (vinyl alcohol) (PVA) | Gum Arabic (GA) | -PVA-GA-5 containing 0.9 wt% GA enhanced the antibacterial properties by 98%, and chlorine resistance by 83%. | [104] |
| Polydimethylsiloxane (PDMS) | Ag NPs and Ag-MOFs | -Both Ag NPs and Ag-MOFs coated membranes exhibited enhanced hydrophilicity and anti-biofouling properties. -The superior anti-biofouling performance of the Ag-MOFs-coated membrane was attributed to the slow and controlled release of silver ions. -The uncoated membrane had 14-times higher protein amounts than the Ag-MOFs-coated membrane (0.004 mg/cm2) on its surface. | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G.A. A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation. Membranes 2022, 12, 1271. https://doi.org/10.3390/membranes12121271
AlSawaftah N, Abuwatfa W, Darwish N, Husseini GA. A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation. Membranes. 2022; 12(12):1271. https://doi.org/10.3390/membranes12121271
Chicago/Turabian StyleAlSawaftah, Nour, Waad Abuwatfa, Naif Darwish, and Ghaleb A. Husseini. 2022. "A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation" Membranes 12, no. 12: 1271. https://doi.org/10.3390/membranes12121271
APA StyleAlSawaftah, N., Abuwatfa, W., Darwish, N., & Husseini, G. A. (2022). A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation. Membranes, 12(12), 1271. https://doi.org/10.3390/membranes12121271






