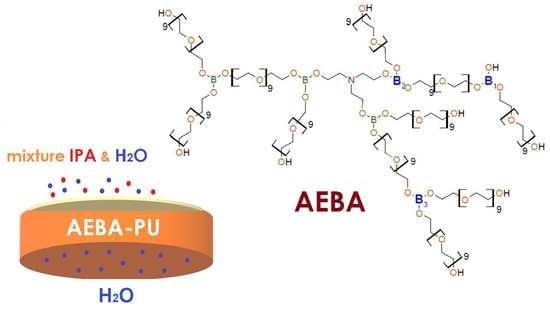
Pervaporation Polyurethane Membranes Based on Hyperbranched Organoboron Polyols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
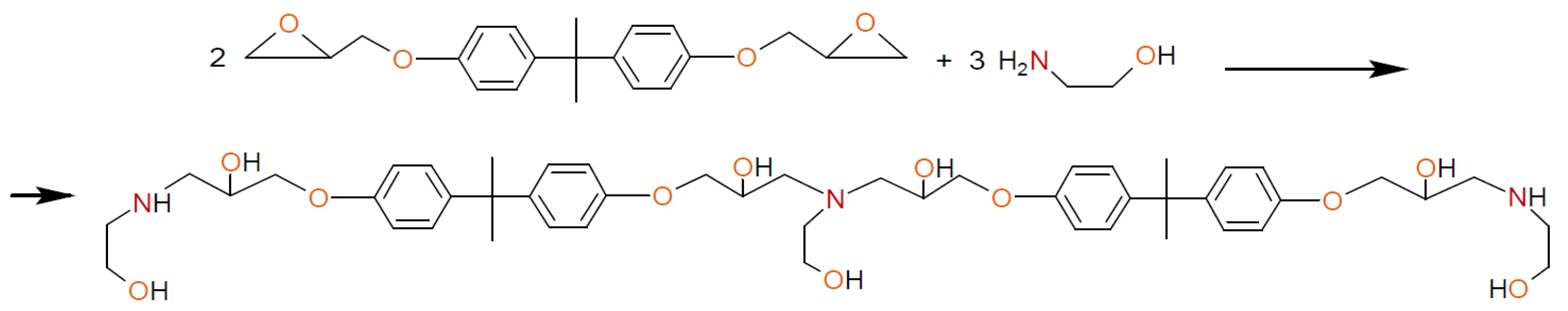
2.2. Procedure for the Synthesis of Ethers and Aminoethers of Boric Acid
2.3. Procedure for the Adducts Synthesis (EM)
2.4. General Procedure for the Synthesis of PUs Based on Aminoethers of Boric Acid
2.5. Manufacturing of Composite Pervaporation Membranes
2.6. Measurements
2.6.1. Dynamic Viscosity and Density Measurements
2.6.2. NMR Spectroscopy
2.6.3. Water Concentration Measurement
2.6.4. Fourier-Transform Infrared Spectroscopy Analysis (FTIR)
2.6.5. Tensile Stress-Strain Measurements
2.6.6. Dynamic Light Scattering (DLS)
2.6.7. Water Vapor Permeability (WVP) Measurements
2.6.8. Contact Angle Measurements
2.6.9. Water Absorption Measurements
2.6.10. Pervaporation Processes
3. Results
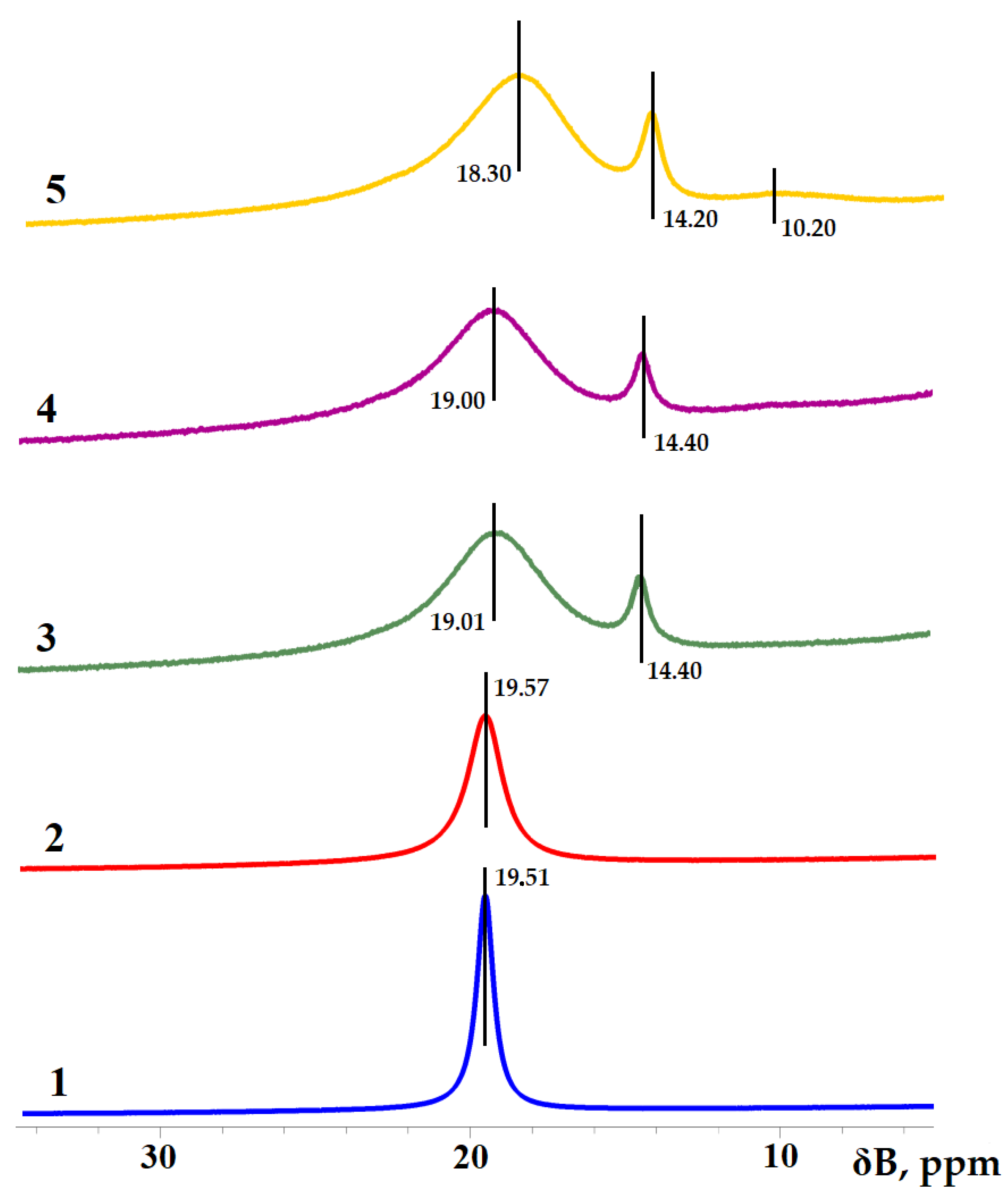
3.1. Boric Acid Etherification
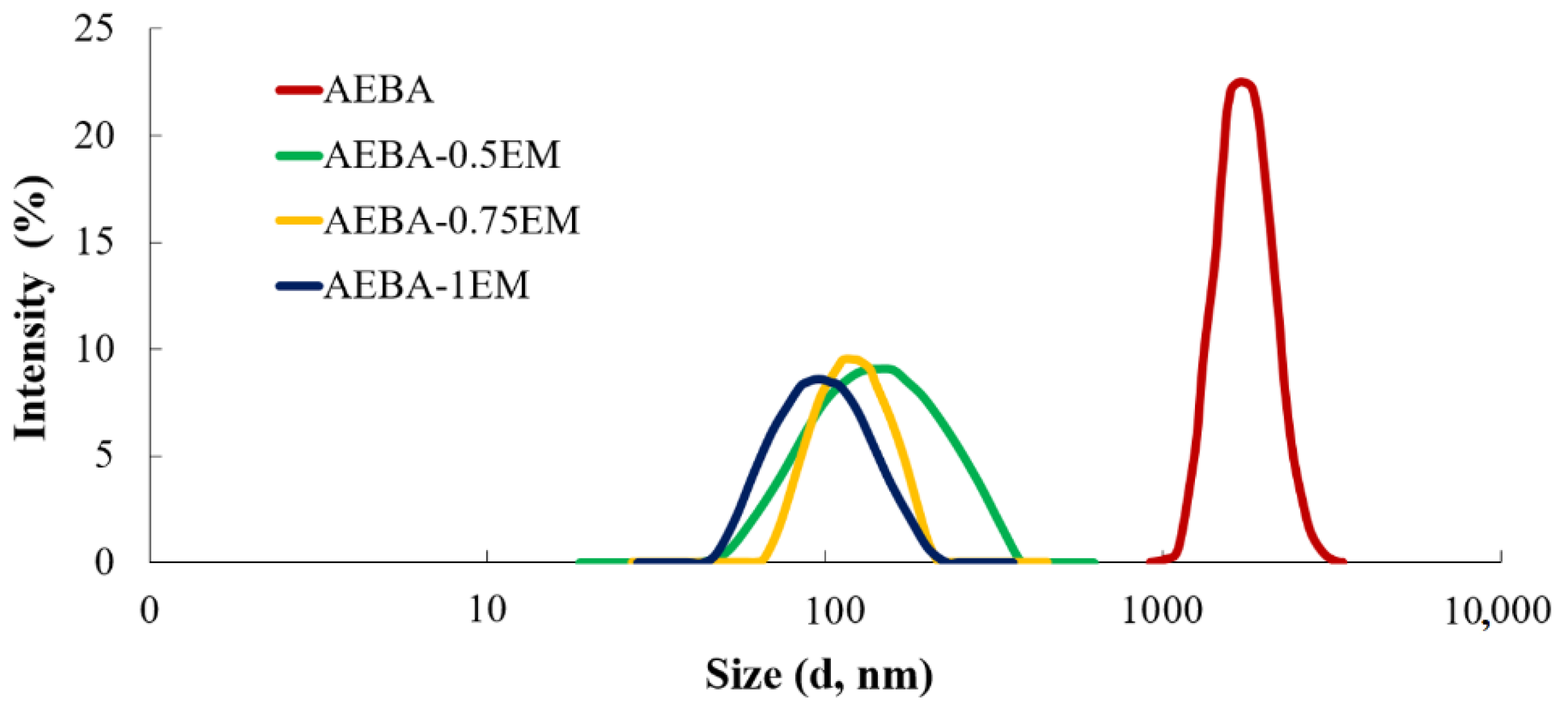
3.2. Synthesis of Polyurethanes Based on AEBA and AEBA-EM
3.3. Vapor Permeability and Pervaporation Characteristics of AEBA-PU and AEBA-EM-PU Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, K. Separation of liquid mixtures/pervaporation. In Handbook of Industrial Membranes, 2nd ed.; Elsevier: Oxford, UK, 1995; pp. 331–351. [Google Scholar]
- Burts, K.S.; Plisko, T.V.; Bildyukevich, A.V.; Li, G.; Kujawa, J.; Kujawski, W. Development of dynamic PVA/PAN membranes for pervaporation: Correlation between kinetics of gel layer formation, preparation conditions, and separation performance. Chem. Eng. Res. Des. 2022, 182, 544–557. [Google Scholar] [CrossRef]
- Xi, T.; Lu, Y.; Ai, X.; Tang, L.; Yao, L.; Hao, W.; Cui, P. Ionic liquid copolymerized polyurethane membranes for pervaporation separation of benzene/cyclohexane mixtures. Polymer 2019, 185, 121948. [Google Scholar] [CrossRef]
- Amaral, R.A.; Habert, R.A.; Borges, C.P. Activated carbon polyurethane membrane for a model fuel desulfurization by pervaporation. Mater. Lett. 2014, 137, 468–470. [Google Scholar] [CrossRef]
- Das, S.; Banthia, A.K.; Adhikari, B. Porous polyurethane urea membranes for pervaporation separation of phenol and chlorophenols from water. Chem. Eng. J. 2008, 138, 215–223. [Google Scholar] [CrossRef]
- Tonga, Z.; Liu, X.; Zhang, B. Sulfonated graphene oxide based membranes with enhanced water transport capacity for isopropanol pervaporation dehydration. J. Membr. Sci. 2020, 612, 118446. [Google Scholar] [CrossRef]
- Tseng, C.; Liu, Y.-L. Creation of water-permeation pathways with matrix-polymer functionalized carbon nanotubes in polymeric membranes for pervaporation desalination. J. Membr. Sci. Lett. 2022, 2, 100027. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, B.; Liu, X.; Tong, Z. Manipulation of homogeneous membranes with nano-sized spherical polyelectrolyte complexes for enhanced pervaporation performances in isopropanol dehydration. Sep. Purif. Technol. 2020, 234, 116093. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, G.; Cheng, G.; Wang, X.; Liu, G.; Jin, W. Emerging membranes for separation of organic solvent mixtures by pervaporation or vapor permeation. Sep. Purif. Technol. 2022, 299, 121729. [Google Scholar] [CrossRef]
- Kamtsikakis, A.; Delepierre, G.; Weder, C. Cellulose nanocrystals as a tunable nanomaterial for pervaporation membranes with asymmetric transport properties. J. Membr. Sci. 2021, 635, 119473. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Z.; Zhu, X.; Zhao, Q. A poly(ionic liquid) complex membrane for pervaporation dehydration of acidic water-isopropanol mixtures. J. Membr. Sci. 2019, 576, 59–65. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wang, Y.; Chung, T.-S.; Qiao, X.Y.; Lai, J.-Y. Polyimides membranes for pervaporation and biofuels separation. Prog. Polym. Sci. 2009, 34, 1135–1160. [Google Scholar] [CrossRef]
- Ong, Y.K.; Wang, H.; Chung, T.-S. A prospective study on the application of thermally rearranged acetate-containing polyimide membranes in dehydration of biofuels via pervaporation. Chem. Eng. Sci. 2012, 79, 41–53. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Missyul, A.; Ermakov, S.; Roizard, D. Development and Characterization of New Pervaporation PVA Membranes for the Dehydration Using Bulk and Surface Modifications. Polymers 2018, 10, 571. [Google Scholar] [CrossRef] [Green Version]
- Toth, A.J.; Andre, A.; Haaz, E.; Mizsey, P. New horizon for the membrane separation: Combination of organophilic and hydrophilic pervaporations. Sep. Purif. Technol. 2015, 156, 432–443. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y.M. Liquid Separation by Membrane Pervaporation: A Review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Hung, W.-S.; Chang, S.-M.; Lecaros, R.L.G.; Ji, Y.-L.; An, Q.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Fabrication of hydrothermally reduced graphene oxide/chitosan composite membranes with a lamellar structure on methanol dehydration. Carbon 2017, 117, 112–119. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y. Poly(vinyl alcohol)/ZIF-8-NH2mixed matrix membranes for ethanol dehydration via pervaporation. AIChE J. 2016, 62, 1728–1739. [Google Scholar] [CrossRef]
- Xia, L.L.; Li, C.L.; Wang, Y. In-situ crosslinked PVA/organosilica hybrid membranes for pervaporation separations. J. Membr. Sci. 2016, 498, 263–275. [Google Scholar] [CrossRef]
- Le, N.L.; Wang, Y.; Chung, T.S. Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation. J. Membr. Sci. 2011, 379, 174–183. [Google Scholar] [CrossRef]
- Le, N.L.; Tang, Y.P.; Chung, T.-S. The development of high-performance 6FDA-NDA/DABA/POSS/Ultem dual-layer hollow fibers for ethanol dehydration via pervaporation. J. Membr. Sci. 2013, 447, 163–176. [Google Scholar] [CrossRef]
- Król, P.; Król, B. Structures, properties and applications of the polyurethane ionomers. J. Mater. Sci. 2020, 55, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Yu, K.C.; Houng, S.L.; Lai, J.Y. Gas transport properties of HTPB based polyurethane/cosalen membrane. J. Membr. Sci. 2000, 173, 99–106. [Google Scholar] [CrossRef]
- Gupta, T.; Pradhan, N.C.; Adhikari, B. Synthesis and performance of a novel polyurethaneurea as pervaporation membrane for the selective removal of phenol from industrial waste water. Bull. Mater. Sci. 2002, 25, 533–536. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.H.; Huang, S.; Liu, S.; Chen, C.; Chen, P.; Chen, S. Synthesis and properties of poly(urethane-imide) interpenetrating network membranes. Desalination 2008, 233, 191–200. [Google Scholar] [CrossRef]
- Nunez, C.M.; Chiou, B.S.; Andrady, A.; Khan, S.A. Solution rheology of hyperbranched polyesters and their blends with linear polymers. Macromolecules 2000, 33, 1720–1726. [Google Scholar] [CrossRef]
- Liou, Y.J.; Chin, T.M.; Kuo, Y.M.; Chao, D.Y. Water vapor-permeable polyurethane ionomer. J. Appl. Polym. Sci. 2006, 101, 3767–3773. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Huang, S.; Chang, P.; Tsai, M.; Chang, H. Properties and pervaporation performances of crosslinked HTPB-based polyurethane membranes. Sep. Purif. Technol. 2007, 56, 63–70. [Google Scholar] [CrossRef]
- Chao, M.-S.; Huang, S.-L. Epoxidized HTPB-based polyurethane membranes for pervaporation separation. J. Chin. Chem. Soc. 2005, 52, 287–294. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Huang, S.-L.; Chang, P.-H.; Chen, C.-J. Properties and pervaporation separation of hydroxyl-terminated polybutadiene-based polyurethane/poly(methyl metharcylate) interpenetrating networks membranes. J. Appl. Polym. Sci. 2007, 106, 4277–4286. [Google Scholar] [CrossRef]
- Das, S.; Sarkar, S.; Basak, P.; Adhikari, B. Dehydration of alcohols by pervaporation using hydrophilic polyether urethane membranes. J. Sci. Ind. Res. India. 2008, 67, 219–227. [Google Scholar]
- Tsai, L.; Chen, Y. Hyperbranched Poly(fluorenevinylene)s Obtained from Self-Polymerization of 2,4,7-Tris(bromomethyl)-9,9-dihexylfluorene. Macromolecules 2008, 41, 5098–5106. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Hobzova, R.; Vakhtangishvili, L.V.; Schwarz, G. Multicyclic Poly(ether ketone)s Obtained by Polycondensation of 2,6,4-Trifluorobenzophenone with Various Diphenols. Macromolecules 2005, 38, 4630–4637. [Google Scholar] [CrossRef]
- Satija, J.; Karunakaran, B.; Mukherji, S. A dendrimer matrix for performance enhancement of evanescent wave absorption-based fiber-optic biosensors. RSC Adv. 2014, 4, 15841–15848. [Google Scholar] [CrossRef]
- Irfan, M.; Seiler, M. Encapsulation using hyperbranched polymers: From research and technologies to emerging applications. Ind. Eng. Chem. Res. 2010, 49, 1169–1196. [Google Scholar] [CrossRef]
- Deng, Y.; Saucier-Sawyer, J.K.; Hoimes, C.J.; Zhang, J.; Seo, Y.-E.; Andrejecsk, J.W.; Saltzman, W.M. The effect of hyperbranched polyglycerol coatings on drug delivery using degradable polymer nanoparticles. Biomaterials 2014, 35, 6595–6602. [Google Scholar] [CrossRef] [Green Version]
- Fritz, T.; Hirsch, M.; Richter, F.C.; Müller, S.S.; Hofmann, A.M.; Rusitzka, K.A.; Markl, J.; Massing, U.; Frey, H.; Helm, M. Click modification of multifunctional liposomes bearing hyperbranched polyether chains. Biomacromolecules 2014, 15, 2440–2448. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Fazlyev, A.R.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Polyurethane ionomers based on amino ethers of orto-phosphoric acid. RSC Adv. 2019, 9, 18599–18608. [Google Scholar] [CrossRef] [Green Version]
- Davletbaeva, I.M.; Sazonov, O.O.; Nikitina, E.A.; Kapralova, V.M.; Nizamov, A.A.; Akhmetov, I.G.; Arkhipov, A.V.; Sudar, N.T. Dielectric Properties of Organophosphorus Polyurethane Ionomers. J. Appl. Polym. Sci. 2022, 139, 51751. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, W.; Xia, W. Environmental-Sensitive Hyperbranched Polymers as Drug Carriers. Des. Monomers Polym. 2008, 11, 105–122. [Google Scholar] [CrossRef]
- Zhang, K.; Li, L.; Yu, W.; Hu, M.; Zhou, Y.; Fan, X. Synthesis of HTPB based PU-PS IPN membrane for pervaporation recovery of butanol. J. Wuhan Univ. Technol. Mater. Sci. Edit. 2017, 32, 1280–1286. [Google Scholar] [CrossRef]
- Huang, J.; Meagher, M.M. Pervaporative recovery of n-butanol from aqueous solutions and ABE fermentation broth using thin-film silicalite-filled silicone composite membranes. J. Membr. Sci. 2001, 192, 231–242. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Hoshi, M.; Kogure, M.; Saitoh, T.; Nakagawa, T. Separation of aqueous phenol through polyurethane membranes by pervaporation. J. Appl. Polym. Sci. 1997, 65, 469–479. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Zakirov, I.N.; Gumerov, A.M.; Klinov, A.V.; Fazlyev, A.R.; Malygin, A.V. Organophosphorus polyurethane ionomers as water vapor permeable and pervaporation membranes. Polymers 2021, 13, 1442. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Nurgaliyeva, G.R.; Akhmetshina, A.I.; Davletbaev, R.S.; Atlaskin, A.A.; Sazanova, T.S.; Efimov, S.V.; Klochkov, V.V.; Vorotyntsev, I.V. Porous polyurethanes based on hyperbranched amino ethers of boric acid. RSC Adv. 2016, 6, 111109–111119. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Dulmaev, S.E.; Sazonov, O.O.; Gumerov, A.M.; Davletbaev, R.S.; Valiullin, L.R.; Ibragimov, R.G. Polyurethane based on modified aminoethers of boric acid. Polym. Sci. Ser. B 2020, 62, 295–305. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Emelina, O.Y.; Vorotyntsev, I.V.; Davletbaev, R.S.; Grebennikova, E.S.; Petukhov, A.N.; Ahkmetshina, A.I.; Sazanova, T.S.; Loskutov, V.V. Synthesis and properties of novel polyurethanes based on amino ethers of boric acid for gas separation membranes. RSC Adv. 2015, 5, 65674–65683. [Google Scholar] [CrossRef]
- Aydoğmuş, N.; Köse, D.A.; Beckett, M.A.; Karan, B.Z. Organic biomolecules bind to phosphate through borate linkages in aqueous solutions. Turk. J. Chem. 2014, 38, 617–628. [Google Scholar] [CrossRef]
- Tevyashova, A.N.; Chudinov, M.V. Progress in the medicinal chemistry of organoboron compounds. Russ. Chem. Rev. 2021, 90, 451–487. [Google Scholar] [CrossRef]
- Bondarenko, S.N.; Khohlova, T.V.; Orlova, S.A.; Tuzhikov, O. Synthesis of oligomeric boron-containing phosphopolyols. Russ. J. Gen. Chem. 2006, 76, 1055–1058. [Google Scholar] [CrossRef]
- Shteinberg, L.Y. A study of the kinetics and mechanism of amidation of 4-nitrobenzoic acid with ammonia, catalyzed by boric acid in the presence of polyethylene glycol PEG-400. Russ. J. Appl. Chem. 2011, 84, 815–819. [Google Scholar] [CrossRef]
- Androshchuk, A.; Lenskii, M.A.; Belousov, A.M. The Interaction of Polyesters and Polymethylene Esters of Phenols and Boric Acid with Epoxy Resin. Int. Polym. Sci. Technol. 2011, 38, 33–36. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, J.; Zhang, Y.; Han, T.; Yang, B.; Xiao, W. Improved fracturing fluid using: Organic amino boron composite crosslinker with B-N covalent bond. J. Appl. Polym. Sci. 2019, 136, 47675. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Advanced luminescent materials based on organoboron polymers. Macromol. Rapid Commun. 2012, 33, 1235–1255. [Google Scholar] [CrossRef]
- Vedelago, J.; Mattea, F.; Triviño, S.; Montesinos, M.D.; Keil, W.; Valente, M.; Romero, M.R. Smart material based on boron crosslinked polymers with potential applications in cancer radiation therapy. Sci. Rep. 2021, 11, 12269. [Google Scholar] [CrossRef]
- Dai, K.; Zheng, Y.; Wei, W. Organoboron-Containing Polymer Electrolytes for High-Performance Lithium Batteries. Adv. Funct. Mater. 2021, 31, 2008632. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czuprynski, B.; Liszkowska, J. Boron-containing fire retardant rigid polyurethane-polyisocyanurate foams: Part I—Polyol precursors based on boric acid and di(hydroxymethyl)urea derivatives. J. Fire Sci. 2015, 33, 37–47. [Google Scholar] [CrossRef]
- Shirke, A.G.; Dholakiya, B.Z.; Kuperkar, K. Modification of tung oil-based polyurethane foam by anhydrides and inorganic content through esterification process. J. Appl. Polym. Sci. 2017, 135, 45786. [Google Scholar] [CrossRef]
- Liu, X.; Hong, W.; Chen, X. Continuous Production of Water-Borne Polyurethanes: A Review. Polymers 2020, 12, 2875. [Google Scholar] [CrossRef]
- Zarzyka, I. Preparation and characterization of rigid polyurethane foams with carbamide and borate groups. Polym. Int. 2016, 65, 1430–1440. [Google Scholar] [CrossRef]
- Czupryński, B.; Paciorek, J.W. The effect of tri(2-hydroxypropyl) borate on the properties of rigid polyurethane-polyisocyanurate foams. Polimery 1999, 44, 552–554. [Google Scholar] [CrossRef]
- Łukasiewicz, B.; Lubczak, J. Polyurethane foams with purine ring and boron. J. Cell. Plast. 2014, 50, 337–359. [Google Scholar] [CrossRef]
- Chmiel, E.; Lubczak, J. Polyurethane foams with 1,3,5-triazine ring, boron and silicon. J. Cell. Plast. 2019, 56, 187–205. [Google Scholar] [CrossRef]
- Qiu, F.; Zhao, W.; Han, S.; Zhuang, X.; Lin, H.; Zhang, F. Recent Advances in Boron-Containing Conjugated Porous Polymers. Polymers 2016, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chew, S.L.; Kerton, F.M.; Yan, N. Direct conversion of chitin into a N-containing furan derivative. Green Chem. 2014, 16, 2204–2212. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Lim, H.; Fitzkee, N.C.; Cosovic, B.; Jeremic, D. Feasibility of Manufacturing Strand-Based Wood Composite Treated with β-Cyclodextrin–Boric Acid for Fungal Decay Resistance. Polymers 2020, 12, 274. [Google Scholar] [CrossRef] [Green Version]
- Reid, R.C.; Prausnitz, J.M.; Sherwood, T.K. The Properties of Gases and Liquids, 3rd ed.; McGraw-Hill: New York, NY, USA, 1977; p. 688. [Google Scholar] [CrossRef]



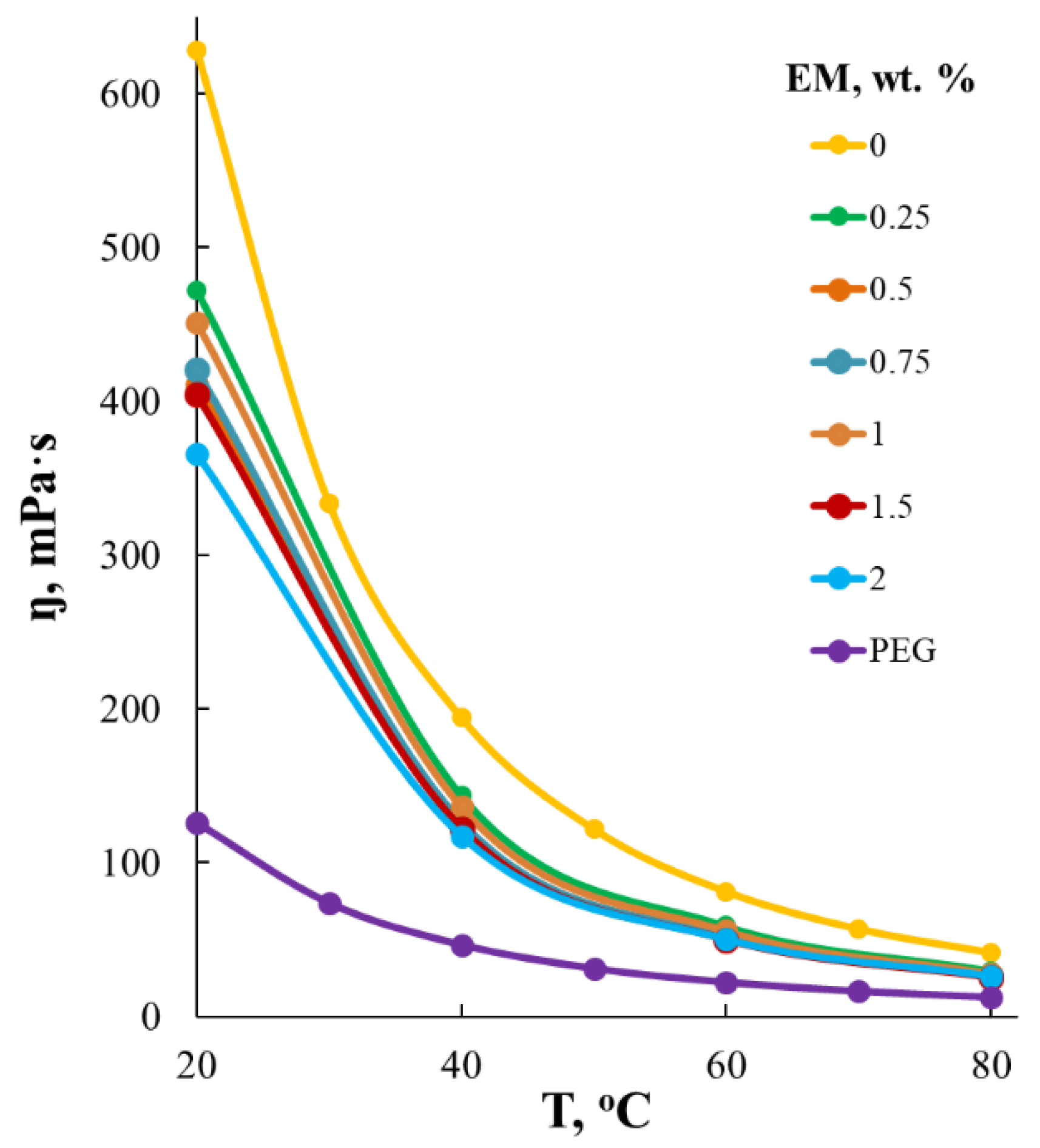
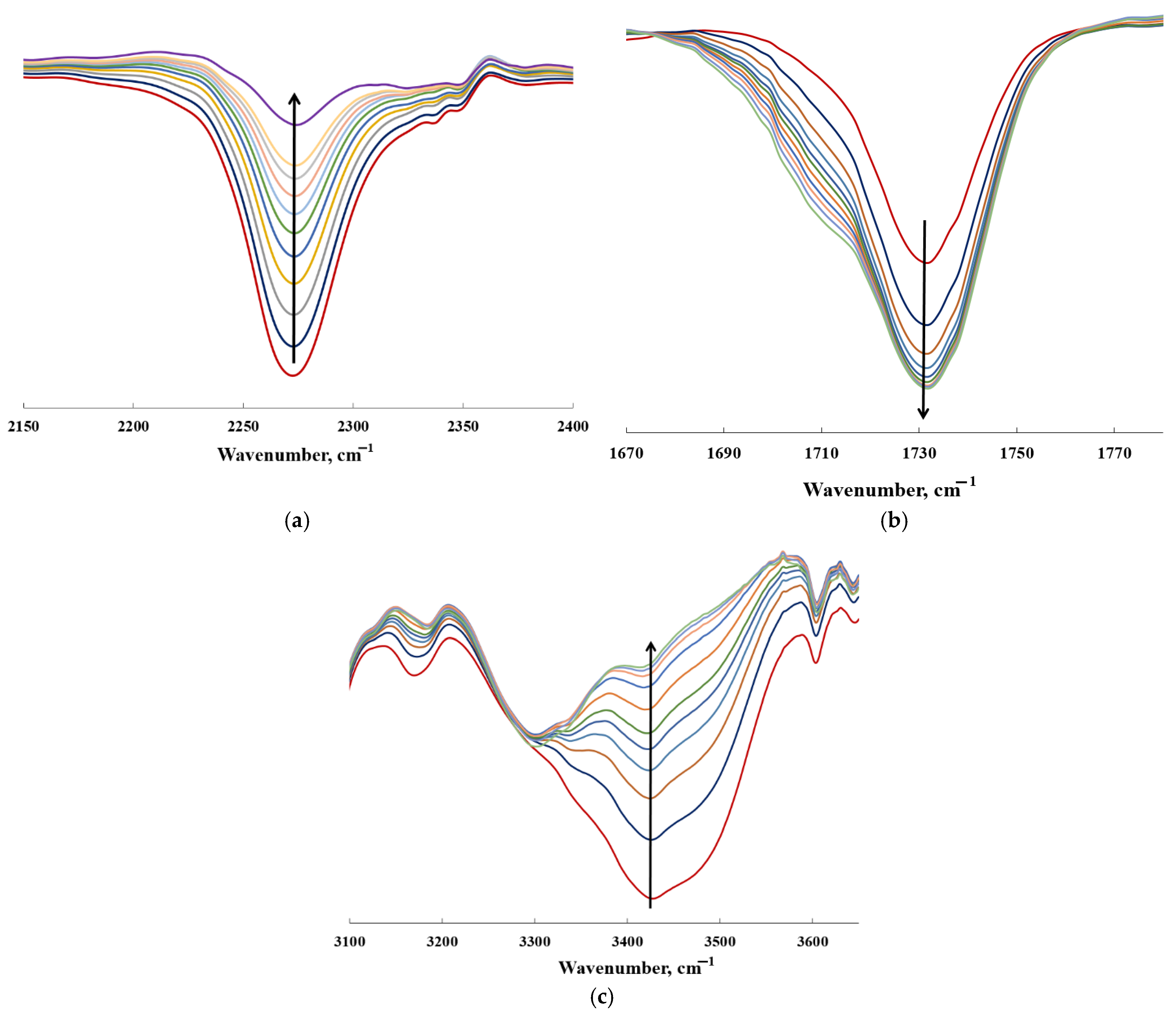

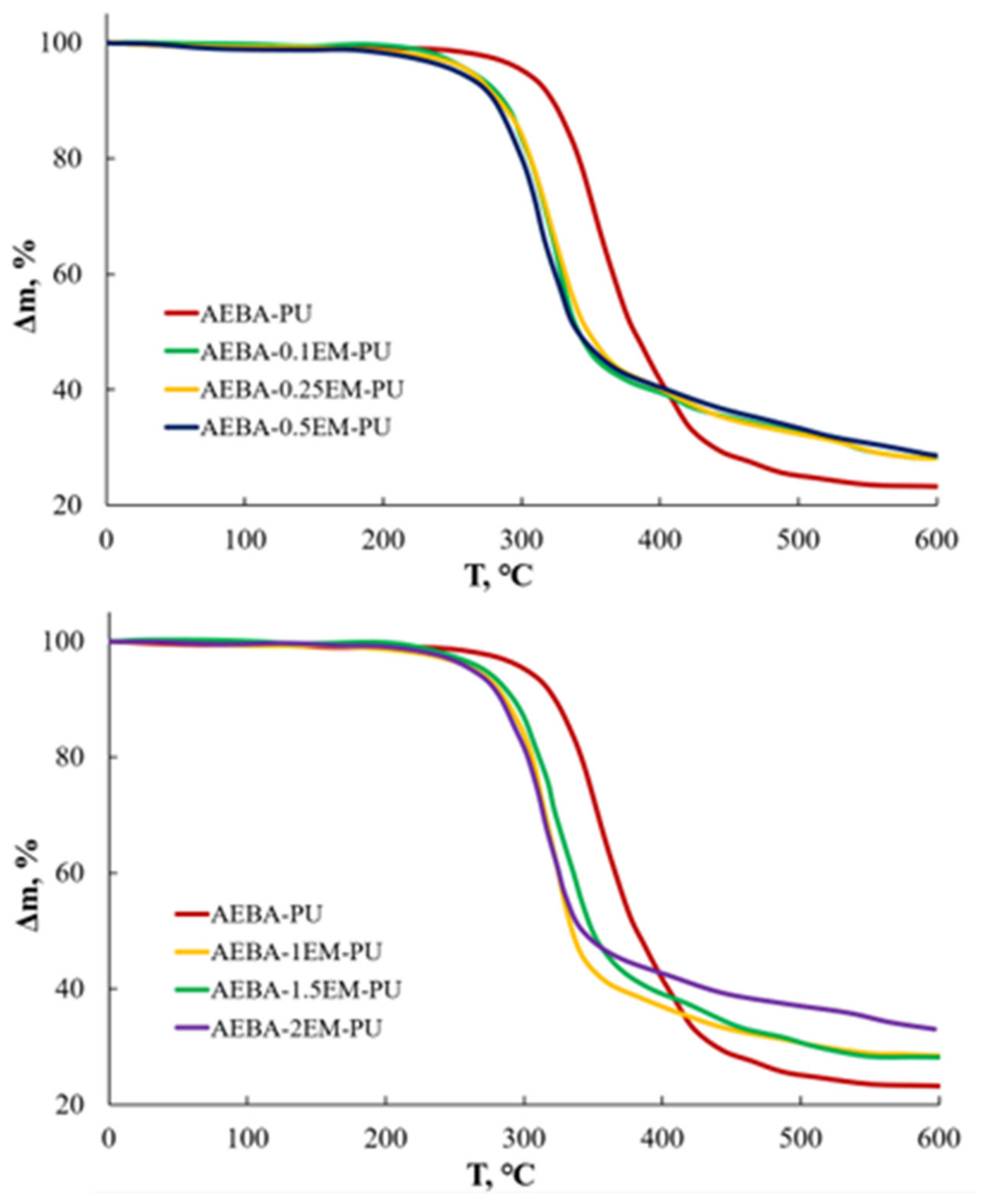
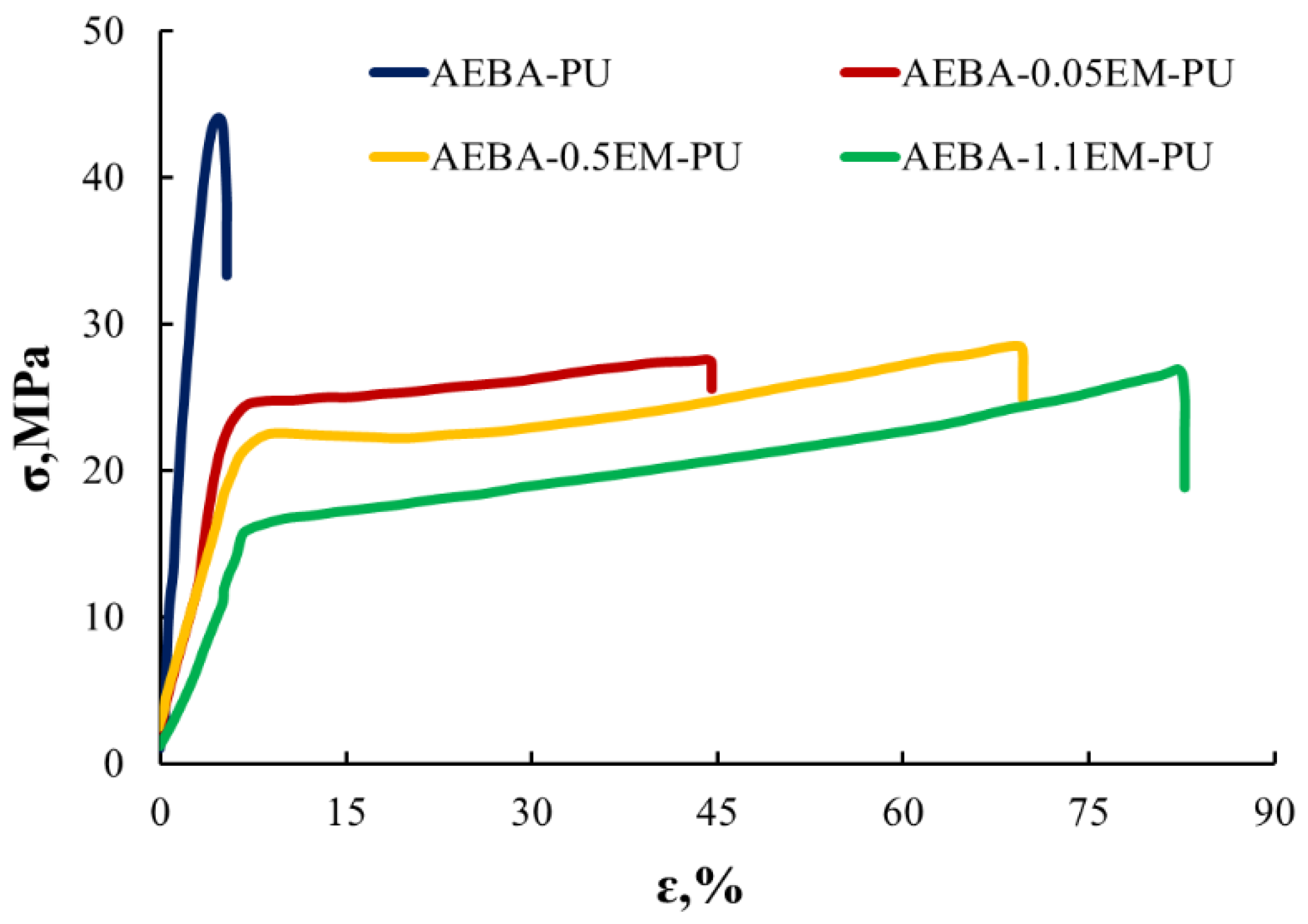
| EM Content, wt.% | Water Contact Angle | Water Absorption, wt.% |
|---|---|---|
| 0.0 | 85 | 12.0 |
| 0.05 | 78 | 13.5 |
| 0.1 | 66 | 14.3 |
| 0.25 | 45 | 15.0 |
| 0.5 | 43 | 15.5 |
| 1.0 | 70 | 16.5 |
| 1.5 | 72 | 17.2 |
| 2.0 | 72 | 17.5 |
| Polyurethane | Concentration IPA, wt.% | IPA in Permeate, wt.% | * Flux, g/m²∙h | Separation Factor | Activity, | KW, g/(m∙s∙Pa) |
|---|---|---|---|---|---|---|
| Feed temperature, 40 °C | ||||||
| AEBA-PU | 95 | 6.0 | 21 | 295 | 4.09 | 0.29 |
| 85 | 2.9 | 44 | 190 | 3.21 | 0.19 | |
| AEBA-0.25EM-PU | 95 | 17.4 | 128 | 90 | 4.09 | 1.47 |
| 85 | 13.6 | 352 | 36 | 3.21 | 1.36 | |
| Feed temperature, 60 °C | ||||||
| AEBA-PU | 95 | 21.7 | 77 | 69 | 3.62 | 0.20 |
| 85 | 11.4 | 148 | 44 | 2.94 | 0.19 | |
| AEBA-0.25EM-PU | 95 | 30.7 | 449 | 43 | 3.62 | 1.03 |
| 85 | 16.2 | 1067 | 29 | 2.94 | 1.30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletbaeva, I.M.; Sazonov, O.O.; Dulmaev, S.E.; Klinov, A.V.; Fazlyev, A.R.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Pervaporation Polyurethane Membranes Based on Hyperbranched Organoboron Polyols. Membranes 2022, 12, 1247. https://doi.org/10.3390/membranes12121247
Davletbaeva IM, Sazonov OO, Dulmaev SE, Klinov AV, Fazlyev AR, Davletbaev RS, Efimov SV, Klochkov VV. Pervaporation Polyurethane Membranes Based on Hyperbranched Organoboron Polyols. Membranes. 2022; 12(12):1247. https://doi.org/10.3390/membranes12121247
Chicago/Turabian StyleDavletbaeva, Ilsiya M., Oleg O. Sazonov, Sergey E. Dulmaev, Alexander V. Klinov, Azat R. Fazlyev, Ruslan S. Davletbaev, Sergey V. Efimov, and Vladimir V. Klochkov. 2022. "Pervaporation Polyurethane Membranes Based on Hyperbranched Organoboron Polyols" Membranes 12, no. 12: 1247. https://doi.org/10.3390/membranes12121247
APA StyleDavletbaeva, I. M., Sazonov, O. O., Dulmaev, S. E., Klinov, A. V., Fazlyev, A. R., Davletbaev, R. S., Efimov, S. V., & Klochkov, V. V. (2022). Pervaporation Polyurethane Membranes Based on Hyperbranched Organoboron Polyols. Membranes, 12(12), 1247. https://doi.org/10.3390/membranes12121247