Simultaneous Production of Aromatics and COx-Free Hydrogen via Methane Dehydroaromatization in Membrane Reactors: A Simulation Study
Abstract
:1. Introduction
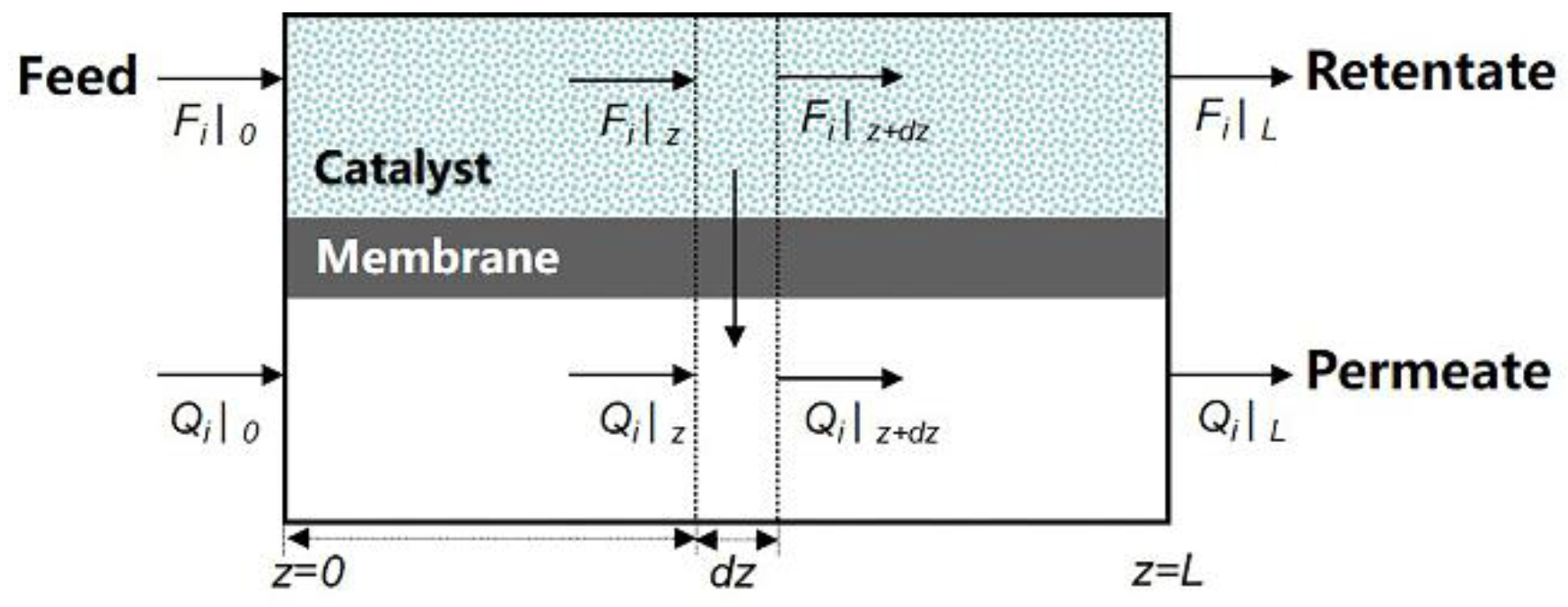
2. Modeling
3. Results and Discussion
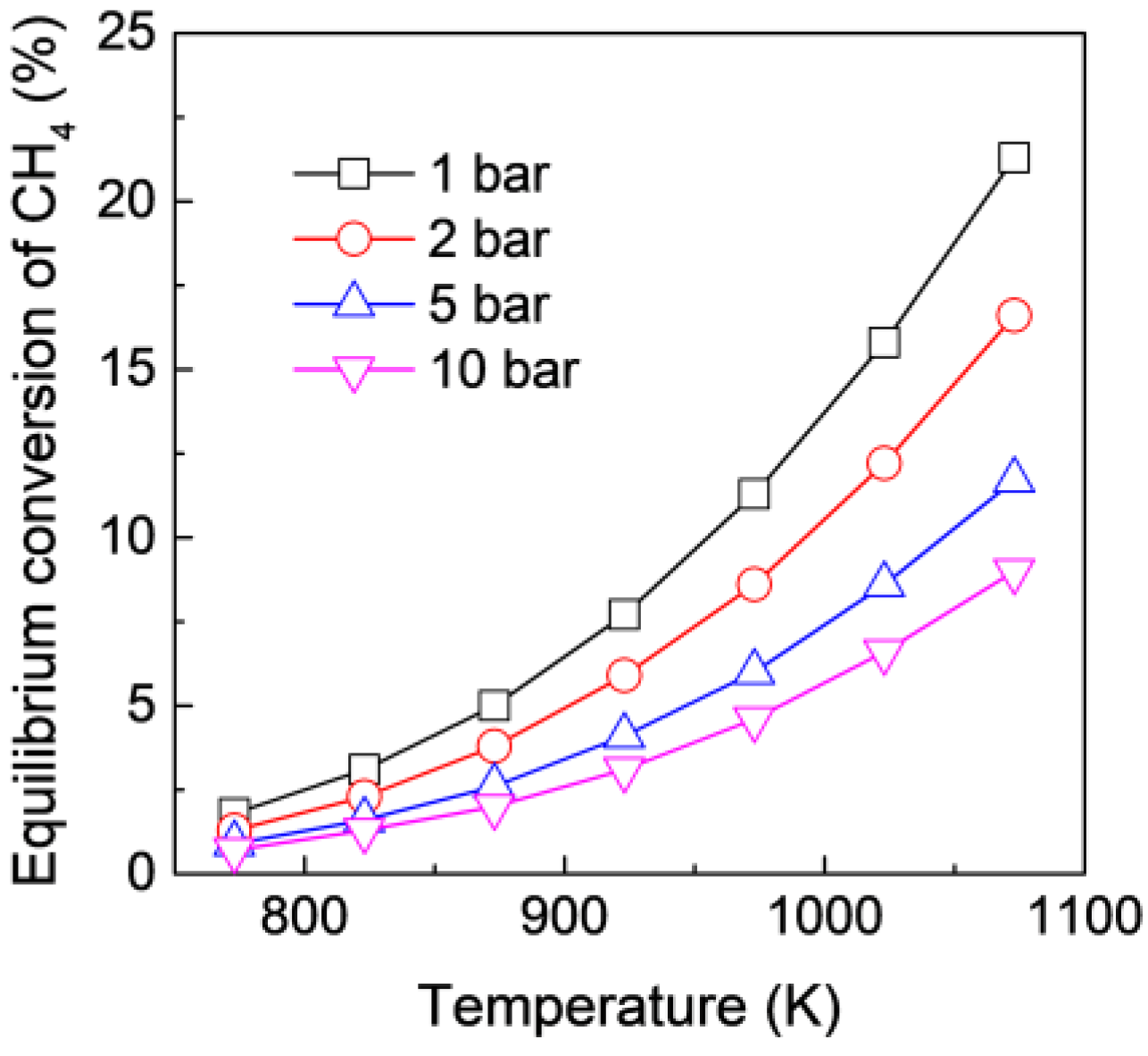
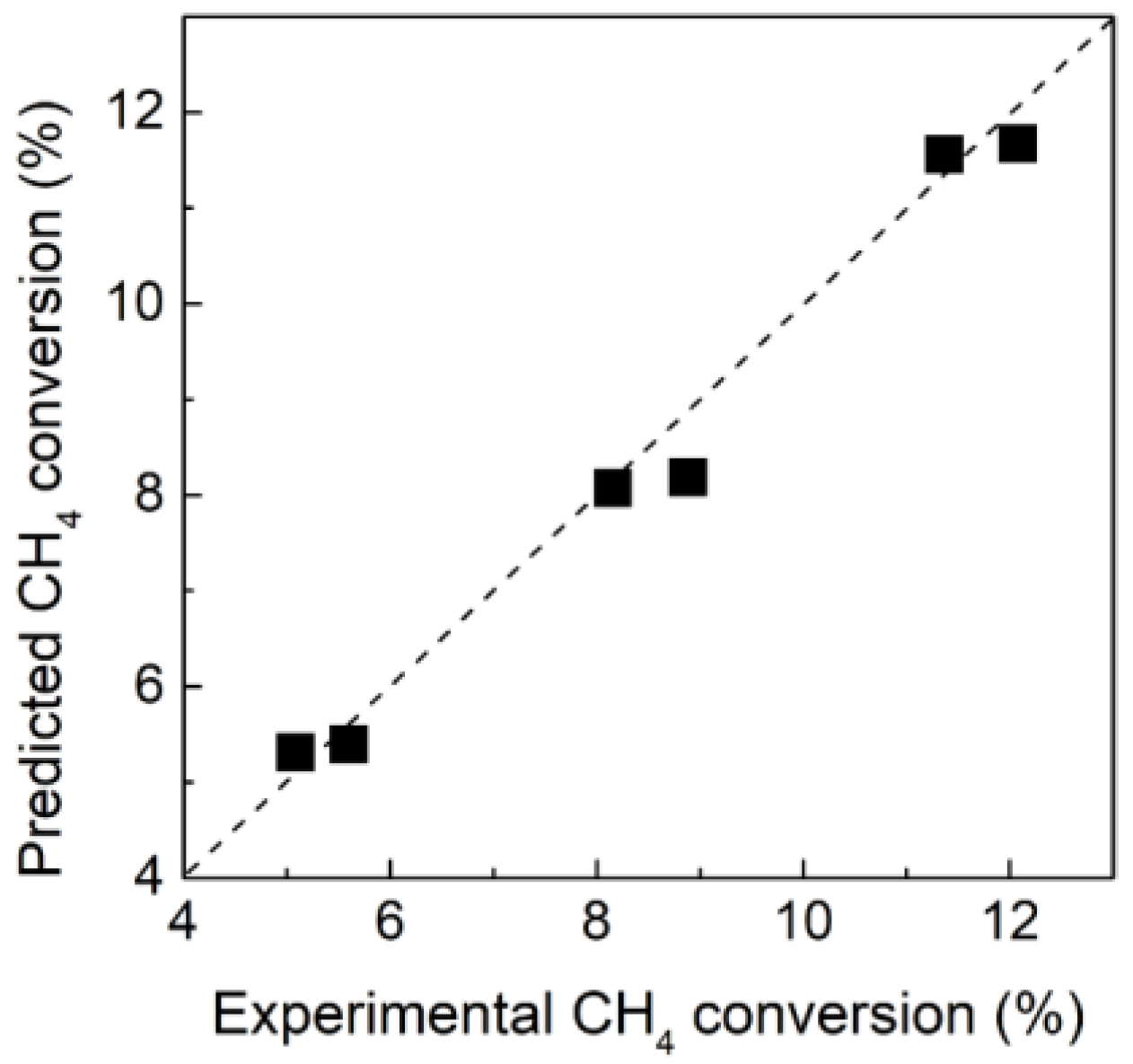
3.1. Thermodynamic Analysis and Model Validation
3.2. Effect of Catalysts on the MR Performance
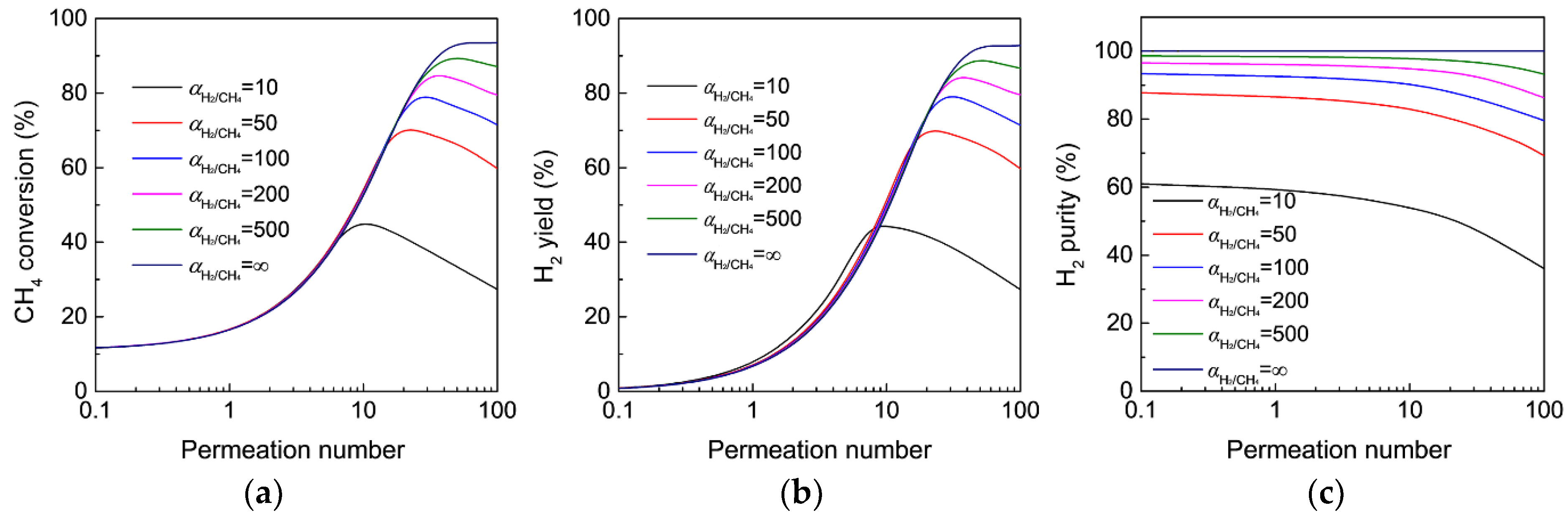
3.3. Effect of Membranes on the MR Performance
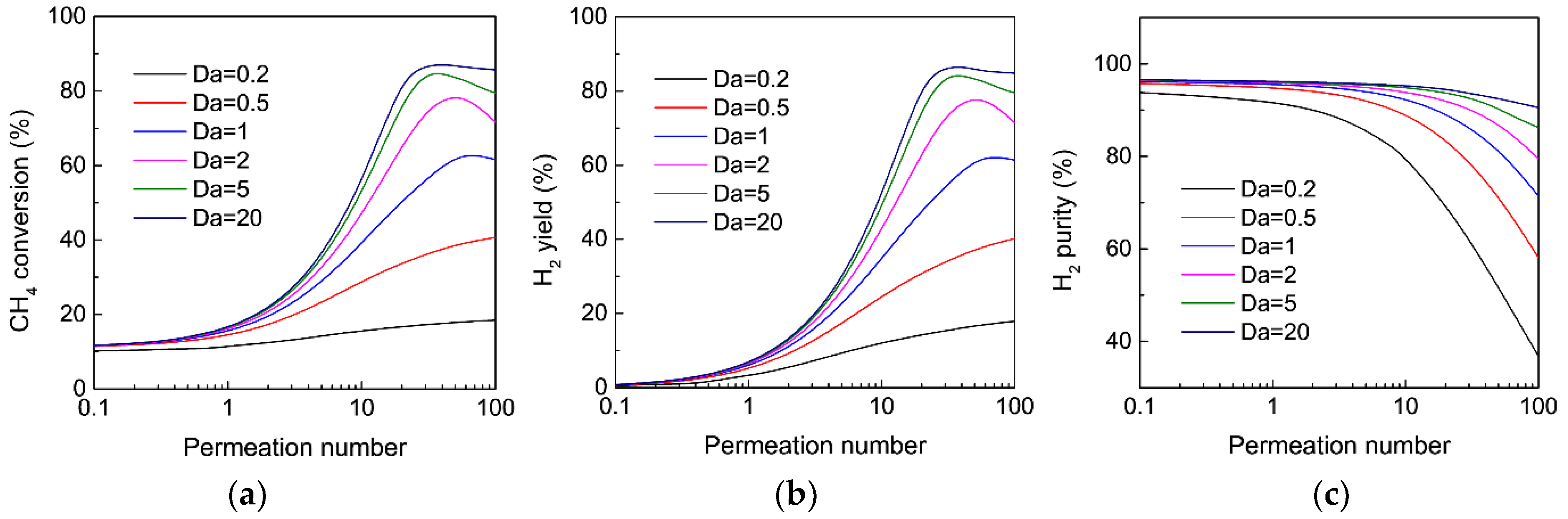
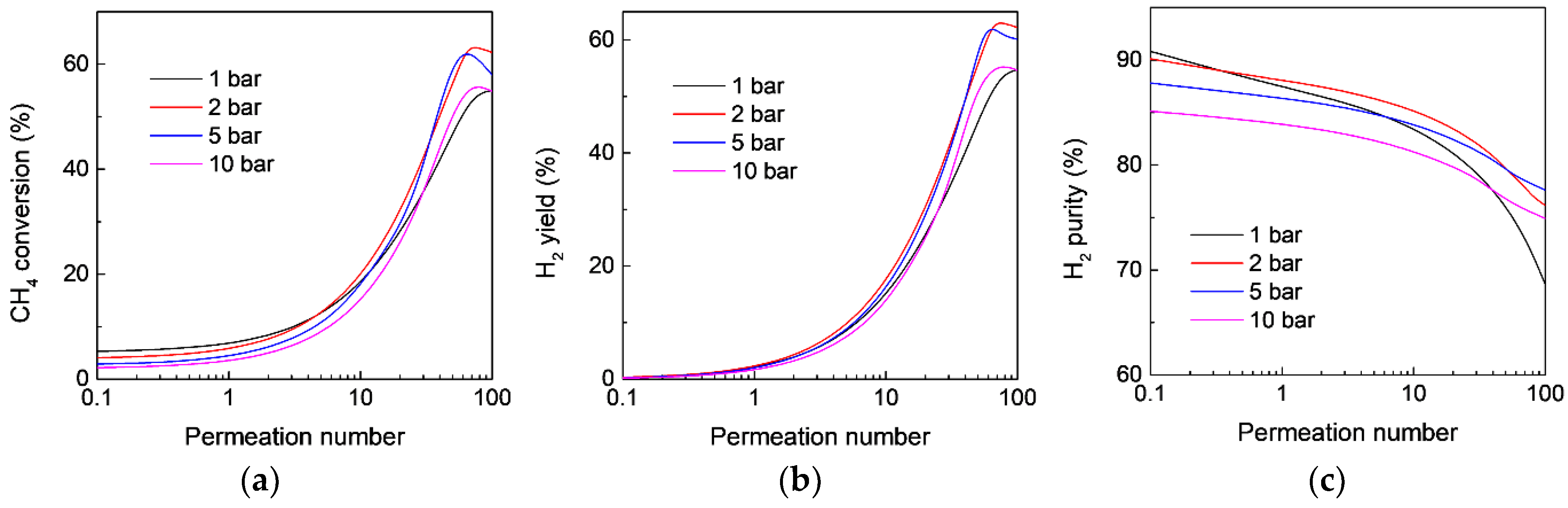
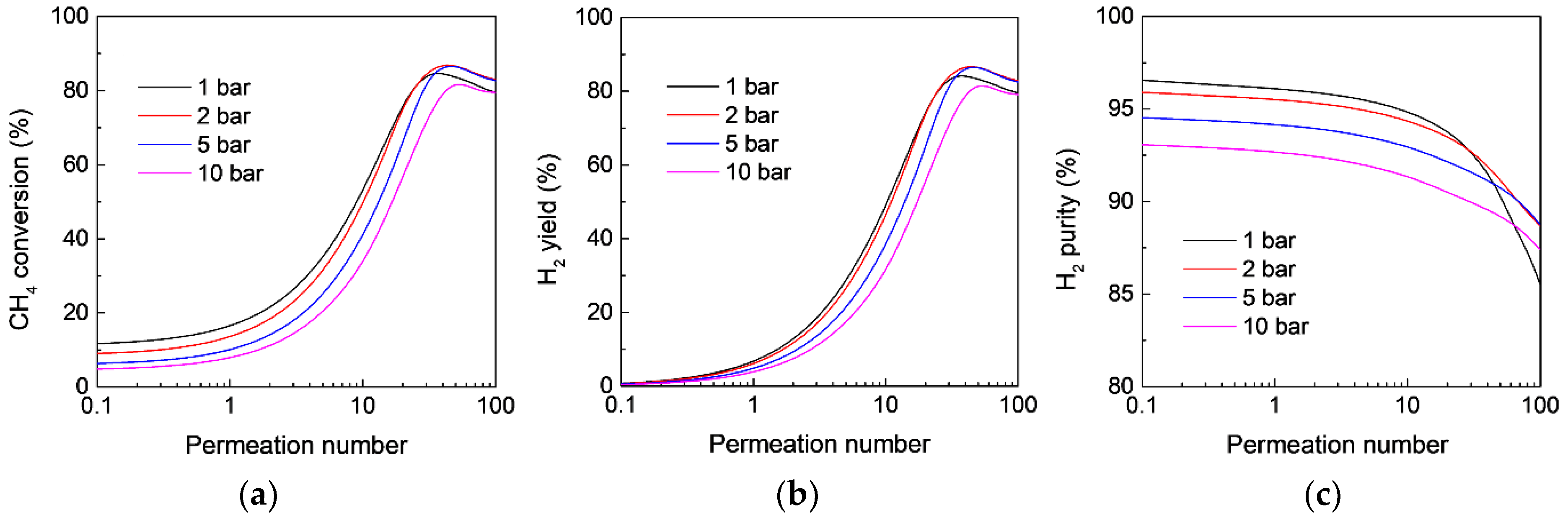
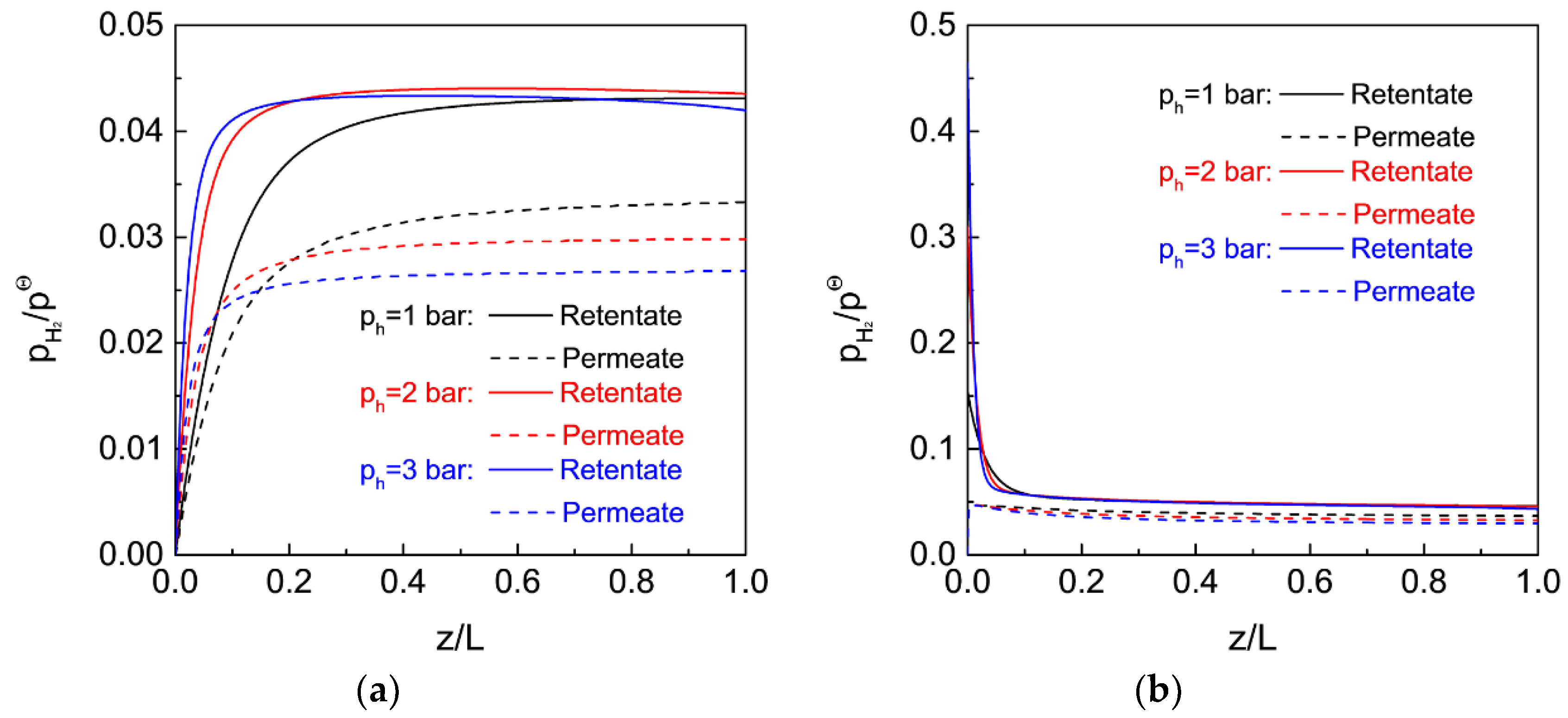
3.4. Effect of Operating Conditions on the MR Performance
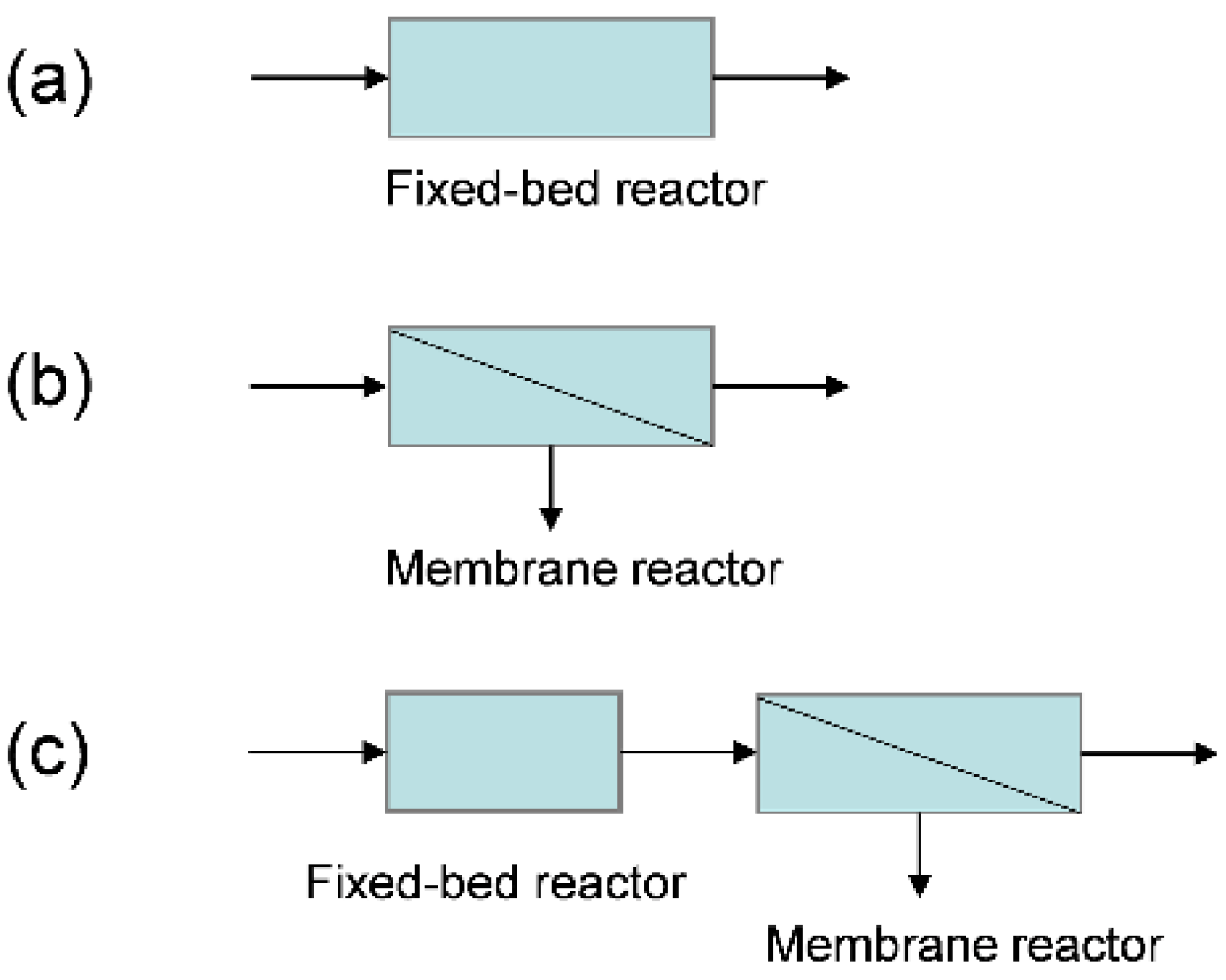
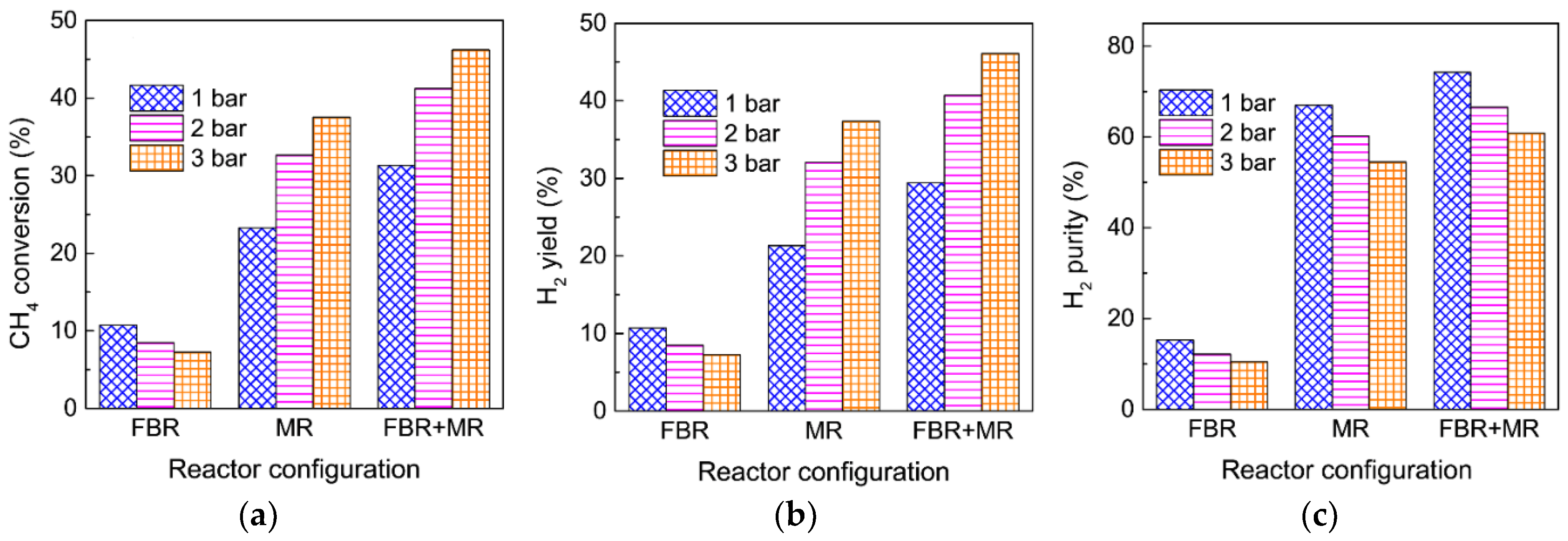
3.5. Integration of an FBR and an MR for Enhanced MDA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Da | Damköhler number, dimensionless |
| f | dimensionless feed-side flow rate, dimensionless |
| F | feed-side molar flow rate, mol h−1 |
| C | purity of hydrogen, dimensionless |
| k | reaction rate constant, mol gcat−1 h−1 bar−1 |
| k2 | reaction rate constant, mol gcat−1 h−1 bar−1 |
| K1 | equilibrium constant, bar−1 |
| K3 | equilibrium constant, bar−1/2 |
| K4 | equilibrium constant, bar1/6 |
| Kp | equilibrium constant, bar2/3 |
| L | membrane reactor length, m |
| p | partial pressure, bar |
| standard pressure, bar | |
| ph | feed-side pressure, bar |
| pl | permeate-side pressure, bar |
| pr | pressure ratio, dimensionless |
| P | gas permeance, mol m−2 h−1 bar−1 |
| q | dimensionless permeate-side flow rate, dimensionless |
| Q | permeate-side molar flow rate, mol h−1 |
| R | reaction rate, mol gcat−1 h−1 |
| Rg | ideal gas constant, J mol−1 K−1 |
| Rmax | maximum reaction rate, mol gcat−1 h−1 |
| R* | dimensionless reaction rate, dimensionless |
| s | membrane area per unit membrane axial length, m2 m−1 |
| T | absolute temperature, K |
| wcat | catalyst weight per unit membrane axial length, gcat m−1 |
| Wcat | catalyst weigh of the membrane module, g |
| x | feed-side mole fraction, dimensionless |
| X | conversion, dimensionless |
| y | permeate-side mole fraction, dimensionless |
| Y | yield, dimensionless |
| z | axial coordinate, m |
| Greek letters | |
| α | permeance ratio, dimensionless |
| θ | permeation number, dimensionless |
| ν | stoichiometric coefficient, dimensionless |
| ζ | dimensionless axial coordinate, dimensionless |
| ΔG | Gibbs free energy, J mol−1 |
| ΔG4 | Gibbs free energy, J mol−1 |
| Subscripts | |
| 0 | inlet of the membrane reactor |
| i | component |
| L | outlet of the membrane reactor |
References
- Schwach, P.; Pan, X.; Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Albarracín-Suazo, S.; Pagán-Torres, Y.; Nikolla, E. Advances in methane conversion processes. Catal. Today 2017, 285, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Ma, C.; Wu, X.; Deng, D.; Wei, M.; et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Li, M.; Allard, L.F.; Lee, S.; Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 2017, 551, 605–608. [Google Scholar] [CrossRef]
- Spivey, J.J.; Hutchings, G. Catalytic aromatization of methane. Chem. Soc. Rev. 2014, 43, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, J.L. Shale gas and hydrofracturing. Environ. Sci. Technol. 2012, 46, 4686. [Google Scholar] [CrossRef]
- Dong, D.; Wang, Y.; Li, X.; Zou, C.; Guan, Q.; Zhang, C.; Huang, J.; Wang, S.; Wang, H.; Liu, H.; et al. Breakthrough and prospect of shale gas exploration and development in China. Nat. Gas Ind. B 2016, 3, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Liu, X.; Christie, P. Emerging shale gas revolution in China. Environ. Sci. Technol. 2012, 46, 12281–12282. [Google Scholar] [CrossRef]
- Striolo, A.; Cole, D.R. Understanding shale gas: Recent progress and remaining challenges. Energy Fuels 2017, 31, 10300–10310. [Google Scholar] [CrossRef]
- Salygin, V.; Guliev, I.; Chernysheva, N.; Sokolova, E.; Toropova, N.; Egorova, L. Global shale revolution: Successes, challenge, and prospects. Sustainability 2019, 11, 1627. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-F.; Ye, J.-L.; Qin, X.-W.; Qiu, H.-J.; Wu, N.-Y.; Lu, H.-L.; Xie, W.-W.; Lu, J.-A.; Peng, F.; Xu, Z.-Q.; et al. The first offshore natural gas hydrate production test in South China Sea. China Geol. 2018, 1, 5–16. [Google Scholar] [CrossRef]
- Ye, J.-L.; Qin, X.-W.; Xie, W.-W.; Lu, H.-L.; Ma, B.-J.; Qiu, H.-J.; Liang, J.-Q.; Lu, J.-A.; Kuang, Z.-G.; Lu, C.; et al. The second natural gas hydrate production test in the South China Sea. China Geol. 2020, 3, 197–209. [Google Scholar] [CrossRef]
- Blumberg, T.; Tsatsaronis, G.; Morosuk, T. On the economics of methanol production from natural gas. Fuel 2019, 256, 115824. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Mevawala, C.; Bai, X.; Kotamreddy, G.; Bhattacharyya, D.; Hu, J. Multiscale modeling of a direct nonoxidative methane dehydroaromatization reactor with a validated model for catalyst deactivation. Ind. Eng. Chem. Res. 2021, 60, 4903–4918. [Google Scholar] [CrossRef]
- Wang, L.; Tao, L.; Xie, M.; Xu, G.; Huang, J.; Xu, Y. Dehydrogenation and aromatization of methane under non-oxidizing conditions. Catal. Lett. 1993, 21, 35–41. [Google Scholar] [CrossRef]
- Alhamdani, Y.A.; Hassim, M.H.; Ng, R.T.L.; Hurme, M. The estimation of fugitive gas emissions from hydrogen production by natural gas steam reforming. Int. J. Hydrog. Energy 2017, 42, 9342–9351. [Google Scholar] [CrossRef]
- Chen, B.; Liao, Z.; Wang, J.; Yu, H.; Yang, Y. Exergy analysis and CO2 emission evaluation for steam methane reforming. Int. J. Hydrog. Energy 2012, 37, 3191–3200. [Google Scholar] [CrossRef]
- Kosinov, N.; Uslamin, E.A.; Coumans, F.J.A.G.; Wijpkema, A.S.G.; Rohling, R.Y.; Hensen, E.J.M. Structure and evolution of confined carbon species during methane dehydroaromatization over Mo/ZSM-5. ACS Catal. 2018, 8, 8459–8467. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Hoffman, M.; Vahi dMohammadi, A.; Smith, J.; Chi, M.; Tatarchuk, B.; Beidaghi, M.; Carrero, C.A. Multilayered two-dimensional V2CTx MXene for methane dehydroaromatization. ChemCatChem 2020, 12, 3639–3643. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, I.; Li, G.; Yarulina, I.; Kosinov, N.; Hensen, E.J.; Houben, K.; Mance, D.; Baldus, M.; Gascon, J.; Kapteijn, F. Relevance of the Mo-precursor state in H-ZSM-5 for methane dehydroaromatization. Catal. Sci. Technol. 2018, 8, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, M.; Cheng, L.; Yang, J.; Liu, L.; Wang, J.; Yin, D.; Lu, J.; Zhang, Y. Facile synthesis and its high catalytic performance of hierarchical ZSM-5 zeolite from economical bulk silicon oxides. Microporous Mesoporous Mater. 2018, 260, 116–124. [Google Scholar] [CrossRef]
- Çağlayan, M.; Paioni, A.L.; Abou-Hamad, E.; Shterk, G.; Pustovarenko, A.; Baldus, M.; Chowdhury, A.D.; Gascon, J. Initial carbon−carbon bond formation during the early stages of methane dehydroaromatization. Angew. Chem. Int. Ed. 2020, 59, 16741–16746. [Google Scholar] [CrossRef]
- Rival, O.; Grandjean, B.P.A.; Guy, C.; Sayari, A.; Larachi, F. Oxygen-free methane aromatization in a catalytic membrane reactor. Ind. Eng. Chem. Res. 2001, 40, 2212–2219. [Google Scholar] [CrossRef]
- Iliuta, M.C.; Grandjean, B.P.A.; Larachi, F. Methane nonoxidative aromatization over Ru-Mo/HZSM-5 at temperature up to 973 K in a palladium-silver/stainless membrane reactor. Ind. Eng. Chem. Res. 2003, 42, 323–330. [Google Scholar] [CrossRef]
- Kee, B.; Karakaya, C.; Zhu, H.; DeCaluwe, S.; Kee, R.J. The influence of hydrogen-permeable membranes and pressure on methane dehydroaromatization in packed-bed catalytic reactor. Ind. Eng. Chem. Res. 2017, 56, 3551–3559. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Means, N.C.; Howard, B.H.; Smith, M.; Abdelsayed, V.; Baltrus, J.P.; Cheng, Y.; Lekse, J.W.; Link, D.; Morreale, B.D. Improved benzene production from methane dehydroaromatization over Mo/HZSM-5 catalysts via hydrogen-permselective palladium membrane reactors. Catal. Sci. Technol. 2015, 5, 5023–5036. [Google Scholar] [CrossRef]
- Kinage, A.K.; Ohnishi, R.; Ichikawa, M. Marked enhancement of the methane dehydrocondensation toward benzene using effective Pd catalytic membrane reactor with Mo/ZSM-5. Catal. Lett. 2003, 88, 199–202. [Google Scholar] [CrossRef]
- Xue, J.; Chen, Y.; Wei, Y.; Feldhoff, A.; Wang, H.; Caro, J. Gas to liquids: Natural gas conversion to aromatic fuels and chemicals in a hydrogen-permeable ceramic hollow fiber membrane reactor. ACS Catal. 2016, 6, 2448–2451. [Google Scholar] [CrossRef]
- Sakbodin, M.; Wu, Y.; Oh, S.C.; Wachsman, E.D.; Liu, D. Hydrogen-permeable tubular membrane reactor: Promoting conversion and product selectivity for non-oxidative activation of methane over an Fe©SiO2 catalyst. Angew. Chem. 2016, 128, 16383–16386. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, H.; Luo, H.; Baumann, S.; Meulenberg, W.A.; Assmann, J.; Mleczko, L.; Liu, Y.; Caro, J. Natural gas to fuels and chemicals: Improved methane aromatization in an oxygen-permeable membrane reactor. Angew. Chem. Int. Ed. 2013, 52, 13794–13797. [Google Scholar] [CrossRef] [PubMed]
- Morejudo, S.H.; Zanón, R.; Escolástico, S.; Yuste-Tirados, I.; Malerød-Fjeld, H.; Vestre, P.K.; Coors, W.G.; Martínez, A.; Norby, T.; Serra, J.M.; et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 2016, 353, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Tsuru, T.; Yamaguchi, K.; Yoshioka, T.; Asaeda, M. Methane steam reforming by microporous catalytic membrane reactors. AIChE J. 2004, 50, 2794–2805. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Ammonia decomposition in catalytic membrane reactors: Simulation and experimental studies. AIChE J. 2013, 59, 168–179. [Google Scholar] [CrossRef]
- Ma, D.; Lu, Y.; Su, L.; Xu, Z.; Tian, Z.; Xu, Y.; Lin, L.; Bao, X. Remarkable improvement on the methane aromatization reaction: A highly selective and coking-resistant catalyst. J. Phys. Chem. B 2002, 106, 8524–8530. [Google Scholar] [CrossRef]
- Wang, N.; Dong, X.; Liu, L.; Cai, D.; Cheng, Q.; Wang, J.; Hou, Y.; Emwas, A.-H.; Gascon, J.; Han, Y. Probing the catalytic active sites of Mo/HZSM-5 and their deactivation during methane dehydroaromatization. Cell Rep. Phys. Sci. 2021, 2, 100309. [Google Scholar] [CrossRef]
- Knonov, S.V.; Dubray, F.; Clatworthy, E.B.; Kouvatas, C.; Gilson, J.-P.; Dath, J.-P.; Minoux, D.; Aquino, C.; Valtchev, V.; Moldovan, S.; et al. Novel strategy for the synthesis of ultra-stable single-site Mo-ZSM-5 zeolite nanocrystals. Angew. Chem. Int. Ed. 2020, 59, 2–10. [Google Scholar]
- Iliuta, M.C.; Iliuta, I.; Grandjean, B.P.A.; Larachi, F. Kinetics of methane nonoxidative aromatization over Ru−Mo/HZSM-5 catalyst. Ind. Eng. Chem. Res. 2003, 42, 3203–3209. [Google Scholar] [CrossRef]
- Kosinov, N.; Hensen, E.J.M. Reactivity, selectivity, and stability of zeolite-based catalysts for methane dehydroaromatization. Adv. Mater. 2020, 32, 2002565. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Qi, X.; Wang, J.; Yang, R.; Li, D.; Hu, X. Innovative non–oxidative methane dehydroaromatization via solar membrane reactor. Energy 2021, 216, 119265. [Google Scholar] [CrossRef]
- Borry III, R.W.; Lu, E.C.; Kim, Y.-H.; Iglesia, E. Non-oxidative catalytic conversion of methane with continuous hydrogen removal. Stud. Surf. Sci. Catal. 1998, 119, 403–410. [Google Scholar]
- Jung, S.H.; Kusakabe, K.; Morooka, S.; Kim, S.-D. Effects of co-existing hydrocarbons on hydrogen permeation through a palladium membrane. J. Membr. Sci. 2000, 170, 53–60. [Google Scholar] [CrossRef]
- Akamatsu, K.; Suzuki, M.; Nakao, A.; Nakao, S. Development of hydrogen-selective dimethoxydimethylsilane-derived silica membranes with thin active separation layer by chemical vapor deposition. J. Membr. Sci. 2019, 580, 268–274. [Google Scholar] [CrossRef]
- Kanezashi, M.; Asaeda, M. Hydrogen permeation characteristics and stability of Ni-doped silica membranes in steam at high temperature. J. Membr. Sci. 2006, 271, 86–93. [Google Scholar] [CrossRef]
- Kageyama, N.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Synthesis and characterization of a silica-alumina composite membrane and its application in a membrane reactor. Sep. Purif. Technol. 2018, 195, 437–445. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, G.; Zhao, J.; Jin, W. Two-dimensional-material membranes: Manipulating the transport pathway for molecular separation. Acc. Mater. Res. 2021, 2, 114–128. [Google Scholar] [CrossRef]
- Pati, S.; Ashok, J.; Dewangan, N.; Chen, T.; Kawi, S. Ultra-thin (~1 μm) Pd–Cu membrane reactor for coupling CO2 hydrogenation and propane dehydrogenation applications. J. Membr. Sci. 2020, 595, 117496. [Google Scholar] [CrossRef]
- Li, G.; Yada, K.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Methylcyclohexane dehydrogenation in catalytic membrane reactors for efficient hydrogen production. Ind. Eng. Chem. Res. 2013, 52, 13325–13332. [Google Scholar] [CrossRef]

| Temperature (K) | k2 (mol gcat−1 h−1 bar−1) | K1 (bar−1) | K3 (bar−1/2) |
|---|---|---|---|
| 873 | 0.00717 | 2.877 | 2.359 |
| 898 | 0.0102 | 2.197 | 2.870 |
| 923 | 0.014 | 1.675 | 3.020 |
| 948 | 0.019 | 1.280 | 3.185 |
| 973 | 0.025 | 1.029 | 3.300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, F.; Fan, S.; Li, W.; Wang, Y.; Lang, X.; Zhang, J.; Li, J.; Li, G. Simultaneous Production of Aromatics and COx-Free Hydrogen via Methane Dehydroaromatization in Membrane Reactors: A Simulation Study. Membranes 2022, 12, 1175. https://doi.org/10.3390/membranes12121175
Ye F, Fan S, Li W, Wang Y, Lang X, Zhang J, Li J, Li G. Simultaneous Production of Aromatics and COx-Free Hydrogen via Methane Dehydroaromatization in Membrane Reactors: A Simulation Study. Membranes. 2022; 12(12):1175. https://doi.org/10.3390/membranes12121175
Chicago/Turabian StyleYe, Feng, Shuanshi Fan, Wenjun Li, Yanhong Wang, Xuemei Lang, Jianli Zhang, Jing Li, and Gang Li. 2022. "Simultaneous Production of Aromatics and COx-Free Hydrogen via Methane Dehydroaromatization in Membrane Reactors: A Simulation Study" Membranes 12, no. 12: 1175. https://doi.org/10.3390/membranes12121175
APA StyleYe, F., Fan, S., Li, W., Wang, Y., Lang, X., Zhang, J., Li, J., & Li, G. (2022). Simultaneous Production of Aromatics and COx-Free Hydrogen via Methane Dehydroaromatization in Membrane Reactors: A Simulation Study. Membranes, 12(12), 1175. https://doi.org/10.3390/membranes12121175






