A New Sensing Material Based on Tetraaza/SBA15 for Rapid Detection of Copper(II) Ion in Water
Abstract
1. Introduction
2. Materials and Methods
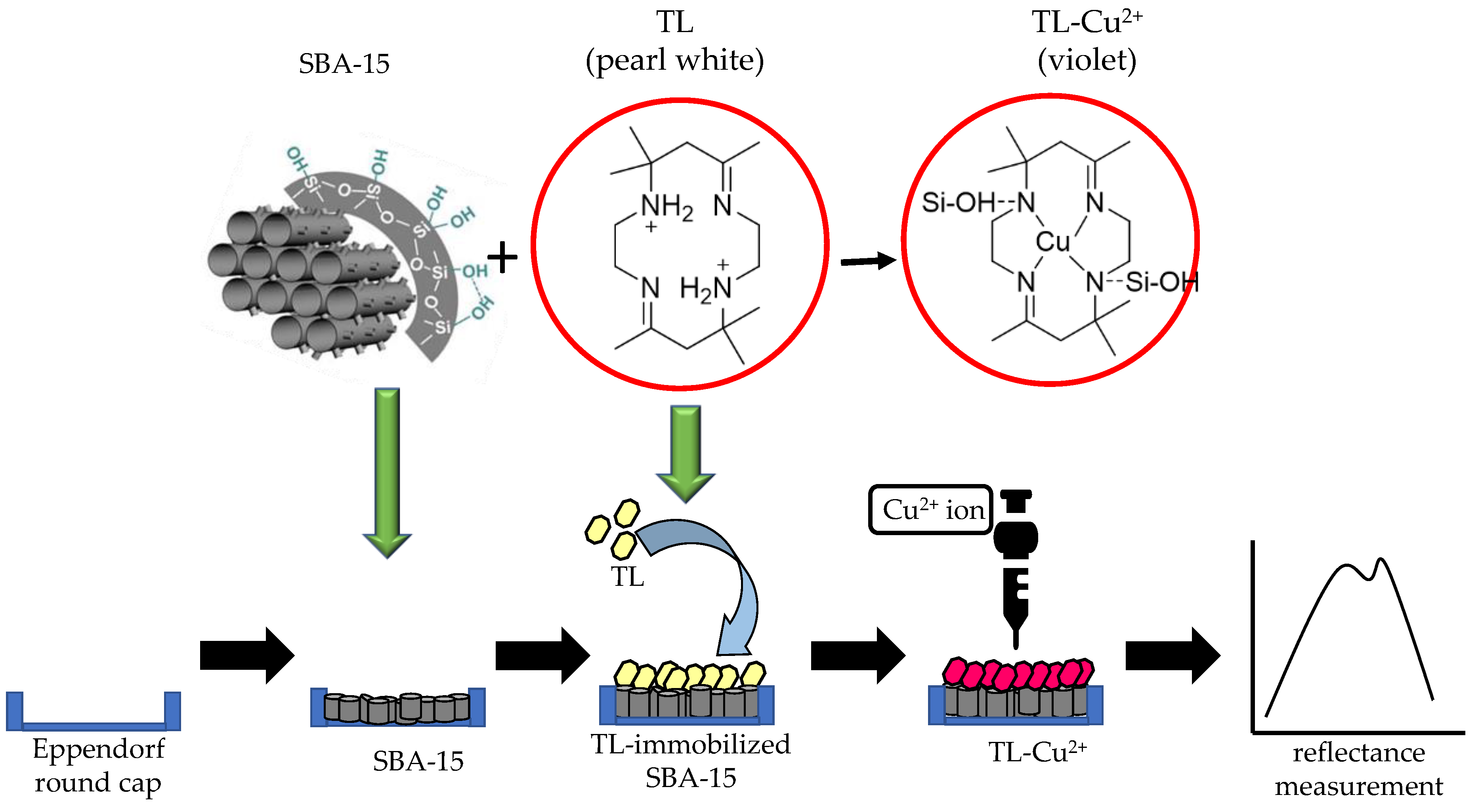
2.1. Synthesis of SBA15 and Tetraaza Compound (TL)
2.2. UV-Vis Solution Study and Binding Titration
2.3. Fabrication of Optical Cu2+ Ion Sensor
2.4. Characterization and Performance Evaluation of Optical Cu2+ Ion Sensor
2.5. Real Samples
3. Results and Discussion
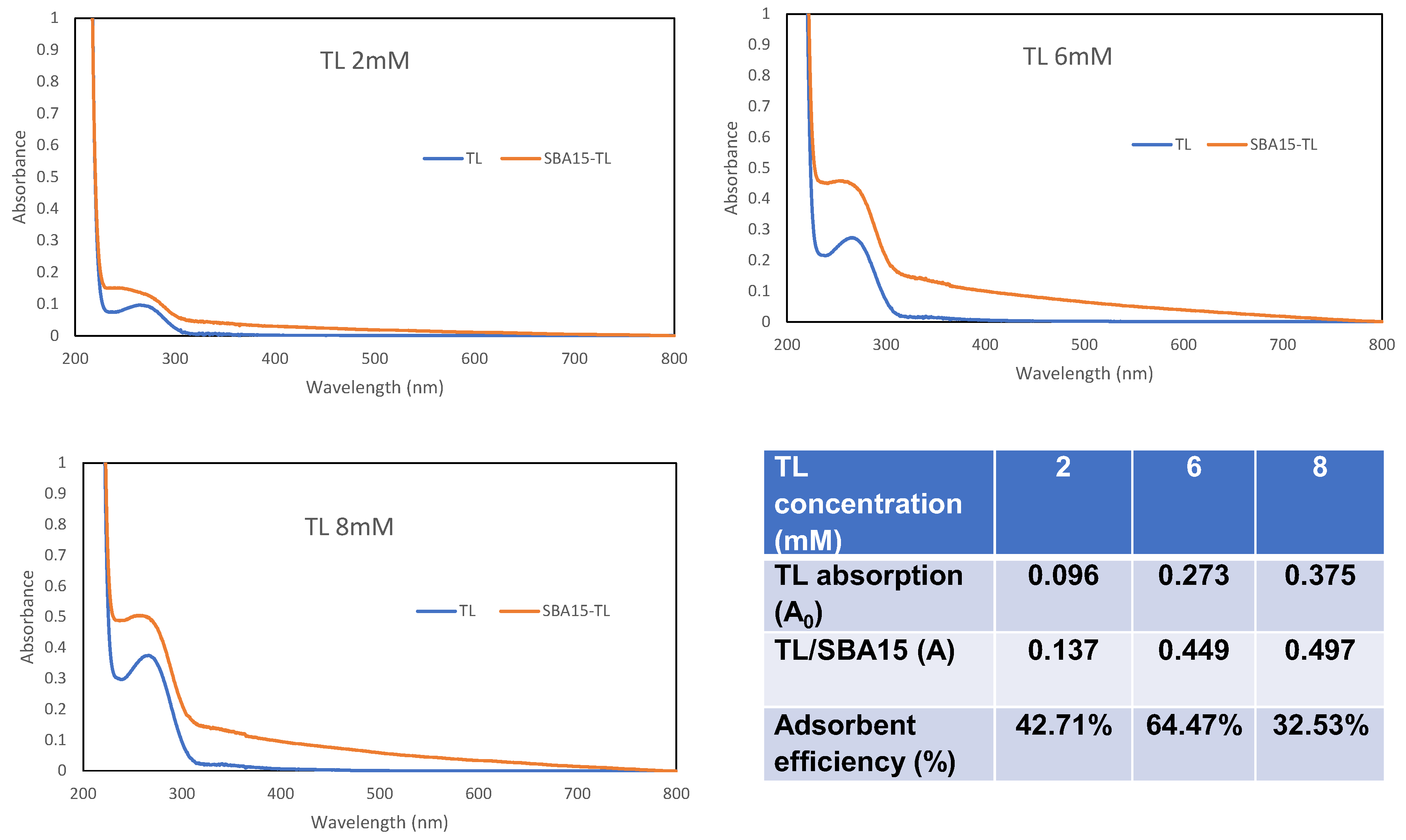
3.1. Metal Screening and UV-Vis Binding Titration Study
3.2. Characterization of Cu2+ Ion Sensor
3.3. Optical Sensor Based on TL-SBA15
3.4. The Optimization of pH and TL Loading
3.5. Analytical Performance of Cu2+ Ion Sensor
3.6. Selectivity of TL–SBA15 Optical Sensor for Cu2+ Ion Detection
3.7. Validation Study of Cu2+ Ion Detection Using TL–SBA15 Optical Sensor in Teabags
3.8. Performance Comparison with Other Reported Cu2+ Ion Optical Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, B.H.; Omer, A.; Aldridge, C.A. Water: Availability and Use; Mississippi State University: Starkville, MS, USA, 2016. [Google Scholar]
- Sawka, M.N.; Cheuvront, S.N.; Carter, R., III. Human waters need. Nutr. Rev. 2005, 63, S30–S39. [Google Scholar] [CrossRef] [PubMed]
- New Hampshire Department of Environmental Services. Environmental Fact Sheet; Minnesota Department of Health, Environmental Health Division: St. Paul, MN, USA, 2013; Available online: https://www.health.state.mn.us (accessed on 31 August 2022).
- Ware, M. Health Benefits and Risks of Copper. 2017. Available online: https://www.medicalnewstoday.com/articles/288165 (accessed on 31 August 2022).
- Hsieh, M.-Y.; Huang, P.-J. Magnetic nanoprobes for rapid detection of copper ion in aqueous environment by surface-enhanced Raman spectroscopy. R. Soc. Chem. 2022, 12, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, G.; Chamjangali, M.A.; Evari, H.S.; Ashrafi, M. Determination of copper(II) by flame atomic absorption spectrometry after its perconcentration by a highly selective and environmentally friendly dispersive liquid—Liquid microextraction technique. J. Anal. Sci. Technol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Sahan, S.; Sahin, U. Determination of Copper(II) Using Atomic Absorption Spectrometry and Eriochrome Blue Black R Loaded Amberlite XAD-1180 Resin. Clean Soil Air Water 2010, 38, 485–491. [Google Scholar] [CrossRef]
- Lee, H.; Choi, Y.; Suh, J.; Lee, S.-H. Mapping Copper and Lead Concentrations at Abandoned Mine Areas Using Element Analysis Data from ICP–AES and Portable XRF Instruments: A Comparative Study. Int. J. Environ. Res. Public Health 2016, 13, 384. [Google Scholar] [CrossRef]
- Burgess, R. Determination of Copper in Green Olives Using ICP-OES. Agilent: Application Note Food and Agriculture. 2018. Available online: www.agilent.com/chem (accessed on 28 August 2022).
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Detection of Mercury and Copper Ions Using Surface Plasmon Resonance Optical Sensor. Sens. Mater. 2011, 23, 325–334. [Google Scholar]
- Razak, N.H.A.; Tan, L.L.; Hasbullah, S.A.; Heng, L.Y. Reflectance chemosensor based on bis-thiourea derivative as ionophore for copper(II) ion detection. Microchem. J. 2020, 153, 104460. [Google Scholar] [CrossRef]
- Ghaedi, M.; Tashkhourian, J.; Montazerozohori, M.; Biyareh, M.N.; Sadeghian, B. Highly selective and sensitive determination of copper ion by two novel optical sensors. Arab. J. Chem. 2013, 10, S2319–S2326. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Yuan, H.; Jia, X.; Dai, B. One-pot synthesis of a natural phenol derived fluorescence sensor for Cu(II) and Hg(II) detection. Dyes Pigments 2018, 155, 100–106. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Yu, Q.; Jia, L.; Wan, L.Y. Poly(aspartic acid) Electrospun Nanofiber Hydrogel MembraneBased Reusable Colorimetric Sensor for Cu(II) and Fe(III) Detection. ACS Omega 2019, 4, 14633–14639. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Zhang, A.; Zhou, G.; Huang, X. Ultra-sensitive optical fiber sensor based on intermodal interference and temperature calibration for trace detection of copper (II) ions. Opt. Express 2021, 29, 22992–23005. [Google Scholar] [CrossRef]
- Pesavento, M.; Profumo, A.; Merli, D.; Cucca, L.; Zeni, L.; Cennamo, N. An Optical Fiber Chemical Sensor for the Detection of Copper(II) in Drinking Water. Sensors 2019, 19, 5246. [Google Scholar] [CrossRef]
- Bowman-James, K. Macrocyclic Ligands. Encycl. Inorg. Bioinorg. Chem. 2006, 1–20. [Google Scholar]
- Yusuf, M.M.; Salga, M.S. Synthesis and study of the efficacies of Tetraaza macrocyclic ligand for the adsorption of heavy metals from wastewater. Bayero J. Pure Appl. Sci. 2018, 11, 126–132. [Google Scholar] [CrossRef]
- Dos Santos, S.M.L.; Nogueira, K.A.B.; de Souza Gama, M.; Lima, J.D.F.; da Silva Júnior, I.J.; de Azevedo, D.C.S. Synthesis and characterization of ordered mesoporous silica (SBA-15 and SBA-16) for adsorption of biomolecules. Microporous Mesoporous Mater. 2013, 180, 284–292. [Google Scholar] [CrossRef]
- Thielemann, J.P.; Girgsdies, F.; Schlögl, R.; Hess, C. Pore structure and surface area of silica SBA-15: Influence of washing and scale-up. Beilstein J. Nanotechnol. 2011, 2, 110–118. [Google Scholar] [CrossRef]
- Tiwari, A.; Demir, M.M. Advanced Sensor and Detection Materials; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Bidhendi, M.E.; Nabi Bidhendi, G.R.; Mehrdadi, N.; Rashedi, H. Modified Mesoporous Silica (SBA-15) with Trithiane as a new effective adsorbent for mercury ions removal from aqueous environment. J. Environ. Health Sci. Eng. 2014, 12, 100. [Google Scholar] [CrossRef]
- Guo, X.; Fenga, Y.; Maa, L.; Gao, D.; Jing, J.; Yu, J.; Sun, H.; Gong, H.; Zhang, Y. Phosphoryl functionalized mesoporous silica for uranium adsorption. Appl. Surf. Sci. 2017, 402, 53–60. [Google Scholar]
- Wu, F.; Ye, G.; Liu, Y.; Yi, R.; Huo, X.; Lu, Y.; Chen, J. New short-channel SBA-15 mesoporous silicas functionalized with polyazamacrocyclic ligands for selective capturing of palladium ions in HNO3 media. RSC Adv. 2016, 6, 66537–66547. [Google Scholar] [CrossRef]
- Saad, A.; Snoussi, Y.; Abderrabba, M.; Chehimi, M.M. Ligand-modified mesoporous silica SBA-15/silver hybrids for the catalyzed reduction of methylene blue. RSC Adv. 2016, 6, 57672–57682. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Nava, R.; Peza-Ledesma, C.L.; Lara-Romero, J.; Alonso-Núez, G.; Pawelec, B.; Rivera-Muñoz, E.M. SBA-15 mesoporous silica as catalytic support for hydrodesulfurization catalysts. Materials 2013, 6, 4139–4167. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, M.F.; Hin, T.Y.Y.; Derawi, D. Synthesis and Characterization of Nickel and Cobalt Species within SBA-15. In Proceedings of the AIP Conference Proceedings 2019, Leuven, Belgium, 8–10 April 2019. [Google Scholar] [CrossRef]
- Ismail, M.W. Sintesis Garam 5,5,7,12,12,14-heksametil-1,4,8,11-tetraazasiklotetradeka-7,14-dienium, Pengkompleksan Dengan logam dan Aktiviti Biologi. Master’s Thesis, UKM Graduate Centre, Bangi, Malaysia, 2012. [Google Scholar]
- Zhang, Z.; Lu, S.; Sha, C.; Xu, D. A single thiourea-appended 1,8-naphthalimide chemosensor for three heavy metal ions: Fe2+, Pb2+ and Hg2+. Sens. Actuators B Chem. 2014, 208, 258–266. [Google Scholar] [CrossRef]
- Kokunesoski, M.; Gulicovski, J.; Matovic, B.; Logar, M.; Milonjic, S.K.; Babic, B. Synthesis and surface characterization of ordered mesoporous silica. Mater. Chem. Phys. 2010, 124, 1248–1252. [Google Scholar] [CrossRef]
- Jangra, S.; Girotra, P.; Chhokar, V.; Tomer, V.K.; Sharma, A.K.; Duhan, S. In-vitro drug release kinetics studies of mesoporous SBA-15-azathioprine composite. J. Porous Mater. 2016, 23, 679–688. [Google Scholar] [CrossRef]
- Paul, L.; Mukherjee, S.; Chatterjee, S.; Bhaumik, A.; Das, D. Organically Functionalized Mesoporous SBA-15 Type Material Bearing Fluorescent Sites for Selective Detection of HgII from Aqueous Medium. ACS Omega 2019, 4, 17857–17863. [Google Scholar] [CrossRef]
- Yusoff, S.F.M.; Huddin, A.A.S.; Yusoff, L.M.; Yamin, B.M.; Leng, O.W. Synthesis, Characterization, and Antibacterial Activity of Cu (II), Ni (II), and Zn (II) Complexes of 14-Membered Macrocyclic Tetraaza Ligand. Orient. J. Chem. 2015, 31, 1751–1758. [Google Scholar] [CrossRef]
- Fazial, F.F.; Tan, L.L.; Zubairi, S.I. Bienzymatic creatine biosensor based on reflectance measurement for real-time monitoring of fish freshness. Sens. Actuators B Chem. 2018, 269, 36–45. [Google Scholar] [CrossRef]
- Khalid, W.E.F.W.; Ahmad, M.; Heng, L.Y. Characterization of a Simple Optical Chemical Sensor for Cu(II) Detection Based on Immobilization of Lipophilized Nitroso-R Reagent in Sol-gel Matrix. Chiang Mai J. Sci. 2014, 42, 383–394. [Google Scholar]
- Sundari, R.; Ahmad, M.; Heng, L.Y. Development of an optical fibre reflectance sensor for copper (II) detection based on immobilised salicylic acid. Sens. Actuators B Chem. 2006, 113, 201–206. [Google Scholar] [CrossRef]
- Kuswandi, B.; Taib, M.N.; Narayanaswamy, R. A new solid-state instrument for optical toxic metal ions sensing. Sens. Actuators A 1999, 776, 183–190. [Google Scholar] [CrossRef]
- Sapari, S.; Razak, N.H.A.; Hasbullah, S.A.; Heng, L.Y.; Chong, K.F.; Tan, L.L. A regenerable screen-printed voltammetric Hg (II) ion sensor based on tris-thiourea organic chelating ligand grafted graphene nanomaterial. J. Electroanal. Chem. 2020, 878, 114670. [Google Scholar] [CrossRef]
- Engineering Service Division Ministry of Health Malaysia. National Standard for Drinking Water Quality Second Version; Engineering Service Division Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2004. [Google Scholar]
- Ling, T.L.; Ahmad, M.; Heng, L.Y. A novel optical ammonia sensor based on reflectance measurements for highly polluted and coloured water. Sens. Actuators B Chem. 2012, 171, 994–1000. [Google Scholar]
- De Souza, J.C.; Pezza, H.R.; Pezza, L. A simple and green analytical method for determination of copper (II) in whisky and sugarcane spirit by diffuse reflectance spectroscopy. Anal. Methods 2016, 8, 1867–1875. [Google Scholar] [CrossRef]
- Baslak, C.; Kursunlu, A.N. A naked-eye fluorescent sensor for copper (II) ions based on a naphthalene conjugate Bodipy dye. Photochem. Photobiol. Sci. 2018, 17, 1091–1097. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Wu, S.; Wei, X.; Fu, Y.; Wu, J.; Wang, P.; Zhang, W. Optically tunable fluorescent carbon nanoparticles and their application in fluorometric sensing copper ions. Nano Res. 2019, 12, 2576–2583. [Google Scholar] [CrossRef]
- Kaewprom, C.; Areerob, Y.; Oh, W.-C.; Ameta, K.L.; Chanthai, S. Simultaneous determination of Hg (II) and Cu (II) in water samples using fluorescence quenching sensor of N-doped and N, K co-doped graphene quantum dots. Arab. J. Chem. 2020, 13, 3714–3723. [Google Scholar] [CrossRef]




| Interference Ion | Relative Intensity of Reflection at Ratio Concentration of Cu2+ Ion and Interference Ion | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| 1:0 | 1:0.1 | 1:1 | 1:10 | 1:100 | |
| Fe2+ | 13,164.35 ± 3.35 | 12,989.85 ± 1.33 | 13,184.10 ± 0.15 | 13,228.72 ± 0.49 | 13,354.87 ± 1.45 |
| Pd2+ | 13,164.35 ± 3.35 | 11,943.23 ± 9.28 * | 12,573.47 ± 4.49 | 12,968.34 ± 1.49 | 13,511.99 ± 2.64 |
| Ni2+ | 13,164.35 ± 3.35 | 12,499.67 ± 4.85 | 12,618.28 ± 4.15 | 13,392.03 ± 1.73 | 13,822.31 ± 5.7 |
| Ir2+ | 13,164.35 ± 3.35 | 12,916.99 ± 1.88 | 13,498.05 ± 2.53 | 13,647.50 ± 3.67 | 13,712.64 ± 4.16 |
| Rh2+ | 13,164.35 ± 3.35 | 12,725.69 ± 3.33 | 13,410.92 ± 1.87 | 13,828.68 ± 4.98 | 15,267.87 ± 15.98 |
| V5+ | 13,164.35 ± 3.35 | 12,635.07 ± 4.02 | 12,852.66 ± 2.37 | 12,916.37 ± 1.88 | 13,512.78 ± 2.65 |
| Co2+ | 13,164.35 ± 3.35 | 12,385.15 ± 4.98 | 12,957.6 ± 1.57 | 13,795.08 ± 4.79 | 13,682.53 ± 3.94 |
| Teabag Sample | Detection of Cu2+ Ion Concentration Using ICP-MS (M) | Detection of Cu2+ Ion Concentration Using Reflectometric TL-SBA15 Sensor (M) | t Values (tcritical = 2.776) |
|---|---|---|---|
| 1 | 1.212 × 10−6 ± 0.00 | 6.51 × 10−7 ± 6.47 × 10−7 | 1.49 |
| 2 | 7.063 × 10−7 ± 0.00 | 9.90 × 10−7 ± 3.60 × 10−7 | 1.36 |
| 3 | 5.790 × 10−7 ± 0.00 | 6.15 × 10−7 ± 1.01 × 10−7 | 0.72 |
| 4 | 2.489 × 10−6 ± 0.00 | 1.47 × 10−6 ± 1.20 × 10−6 | 2.12 |
| 5 | 5.989 × 10−6 ± 0.00 | 6.42 × 10−6 ± 1.72 × 10−6 | 2.66 |
| Immobilization Matrix | Transducer | Linear Range (M) | LOD (M) | Response Time (min) | References |
|---|---|---|---|---|---|
| TL-SBA15 | Reflectance | 1 × 10−7–1 × 10−5 | 1.02 × 10−7 | <1 | This study |
| Nitroso- R reagent-sol gel | Reflectance | 7.87 × 10−5–1.57 × 10−3 | 2.22 × 10−5 | 40 | [35] |
| 1-(2-pyridylazo)-2-naphthol | Reflectance | 7.34 × 10−6–1.56 × 10−4 | 1.88 × 10−6 | 5 | [41] |
| Carbon nanoparticles | Fluorescence | 0–30 × 10−6 | 0.44 × 10−6 | <1 | [43] |
| N-Bodipy | Fluorescence | 2.5 × 10−4–1 × 10−5 | 1.28 × 10−6 | 1–5 | [42] |
| N,K, co-doped graphene quantum dot | Fluorescence | 1–5 × 10−4 | 1.32 × 10−5 | NA | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuhana-Ariffin, E.; Sulaiman, S.S.; Abdul Kadir Jilani, N.; Nokarajoo, D.; Abdul Razak, N.H.; Derawi, D.; Hasbullah, S.A. A New Sensing Material Based on Tetraaza/SBA15 for Rapid Detection of Copper(II) Ion in Water. Membranes 2022, 12, 1152. https://doi.org/10.3390/membranes12111152
Yuhana-Ariffin E, Sulaiman SS, Abdul Kadir Jilani N, Nokarajoo D, Abdul Razak NH, Derawi D, Hasbullah SA. A New Sensing Material Based on Tetraaza/SBA15 for Rapid Detection of Copper(II) Ion in Water. Membranes. 2022; 12(11):1152. https://doi.org/10.3390/membranes12111152
Chicago/Turabian StyleYuhana-Ariffin, Eda, Siti Syahraini Sulaiman, Noraisyah Abdul Kadir Jilani, Devika Nokarajoo, Nurul Hidayah Abdul Razak, Darfizzi Derawi, and Siti Aishah Hasbullah. 2022. "A New Sensing Material Based on Tetraaza/SBA15 for Rapid Detection of Copper(II) Ion in Water" Membranes 12, no. 11: 1152. https://doi.org/10.3390/membranes12111152
APA StyleYuhana-Ariffin, E., Sulaiman, S. S., Abdul Kadir Jilani, N., Nokarajoo, D., Abdul Razak, N. H., Derawi, D., & Hasbullah, S. A. (2022). A New Sensing Material Based on Tetraaza/SBA15 for Rapid Detection of Copper(II) Ion in Water. Membranes, 12(11), 1152. https://doi.org/10.3390/membranes12111152





