Light Response and Switching Behavior of Graphene Oxide Membranes Modified with Azobenzene Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Modification of Graphene Oxide
2.2. Membranes Preparation
2.3. Characterization of Composite Membranes
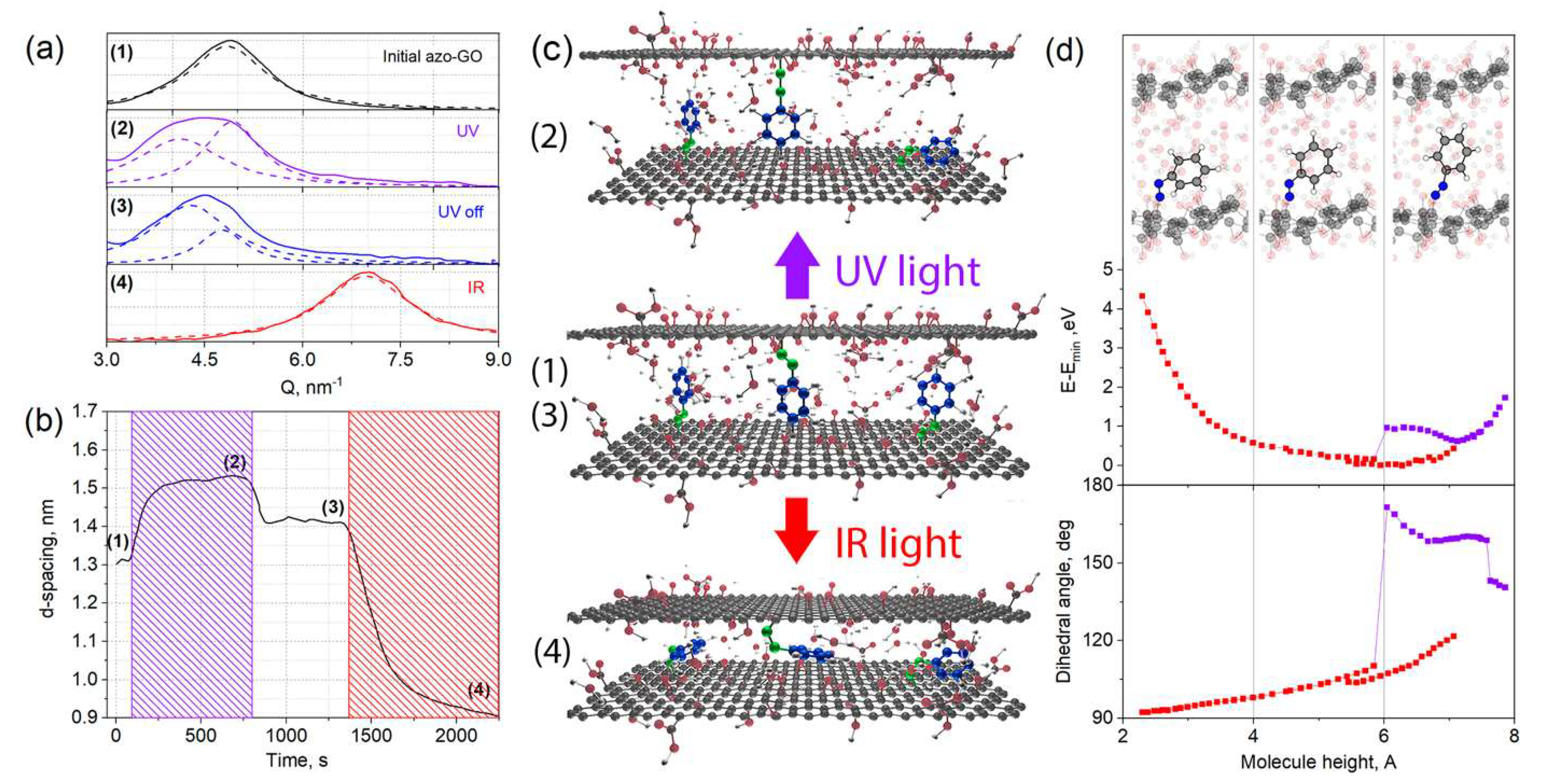
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-Responsive Membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Qian, X.; Wickramasinghe, S.R. Responsive Membranes for Advanced Separations. Curr. Opin. Chem. Eng. 2015, 8, 98–104. [Google Scholar] [CrossRef]
- Vanangamudi, A.; Dumée, L.; Duke, M.; Yang, X. Dual Functional Ultrafiltration Membranes with Enzymatic Digestion and Thermo-Responsivity for Protein Self-Cleaning. Membranes 2018, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Wei, W.; Zhang, H.; Wang, Y.; Cai, G.; Wu, J. PH-Sensitive Membranes with Smart Cleaning Capability for Efficient Emulsion Separation and Pollutant Removal. Membranes 2021, 11, 193. [Google Scholar] [CrossRef]
- Ulbricht, M. Photograft-Polymer-Modified Microporous Membranes with Environment-Sensitive Permeabilities. React. Funct. Polym. 1996, 31, 165–177. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Yue, G.; Wang, N.; Cui, Z.; Hou, L.; Li, J.; Li, Q.; Karton, A.; Cheng, Q.; et al. Thermoresponsive Graphene Membranes with Reversible Gating Regularity for Smart Fluid Control. Adv. Funct. Mater. 2019, 29, 1808501. [Google Scholar] [CrossRef]
- Zhou, K.-G.; Vasu, K.S.; Cherian, C.T.; Neek-Amal, M.; Zhang, J.C.; Ghorbanfekr-Kalashami, H.; Huang, K.; Marshall, O.P.; Kravets, V.G.; Abraham, J.; et al. Electrically Controlled Water Permeation through Graphene Oxide Membranes. Nature 2018, 559, 236–240. [Google Scholar] [CrossRef]
- Himstedt, H.H.; Yang, Q.; Qian, X.; Ranil Wickramasinghe, S.; Ulbricht, M. Toward Remote-Controlled Valve Functions via Magnetically Responsive Capillary Pore Membranes. J. Membr. Sci. 2012, 423–424, 257–266. [Google Scholar] [CrossRef]
- Sadilov, I.S.; Eliseev, A.A.; Eliseev, A.A.; Chumakova, A.V.; Kurtina, D.A.; Vasiliev, R.B.; Petukhov, D.I. The Origin for Hydrocarbons Fast Transport and Photoswitching Permeation Behavior in Grafted Laminar CdTe Membranes. J. Membr. Sci. 2022, 661, 120912. [Google Scholar] [CrossRef]
- Lao, J.; Lv, R.; Gao, J.; Wang, A.; Wu, J.; Luo, J. Aqueous Stable Ti3C2 MXene Membrane with Fast and Photoswitchable Nanofluidic Transport. ACS Nano 2018, 12, 12464–12471. [Google Scholar] [CrossRef]
- Pantuso, E.; de Filpo, G.; Nicoletta, F.P. Light-Responsive Polymer Membranes. Adv. Opt. Mater. 2019, 7, 1900252. [Google Scholar] [CrossRef]
- Weh, K.; Noack, M.; Hoffmann, K.; Schröder, K.-P.; Caro, J. Change of Gas Permeation by Photoinduced Switching of Zeolite-Azobenzene Membranes of Type MFI and FAU. Microporous Mesoporous Mater. 2002, 54, 15–26. [Google Scholar] [CrossRef]
- Weh, K.; Noack, M.; Ruhmann, R.; Hoffmann, K.; Toussaint, P.; Caro, J. Modification of the Transport Properties of a Polymethacrylate-Azobenzene Membrane by Photochemical Switching. Chem. Eng. Technol. 1998, 21, 408–412. [Google Scholar] [CrossRef]
- Lyndon, R.; Konstas, K.; Ladewig, B.P.; Southon, P.D.; Kepert, P.C.J.; Hill, M.R. Dynamic Photo-Switching in Metal-Organic Frameworks as a Route to Low-Energy Carbon Dioxide Capture and Release. Angew. Chem. Int. Ed. 2013, 52, 3695–3698. [Google Scholar] [CrossRef] [PubMed]
- Nocoń-Szmajda, K.; Jankowski, A.; Wolińska-Grabczyk, A.; Konieczkowska, J. Guest-Host and Functionalized Side-Chain Azopolyimide Membranes for Controlled Gas Separation. Polymer 2021, 229, 124012. [Google Scholar] [CrossRef]
- Wang, Z.; Knebel, A.; Grosjean, S.; Wagner, D.; Bräse, S.; Wöll, C.; Caro, J.; Heinke, L. Tunable Molecular Separation by Nanoporous Membranes. Nat. Commun. 2016, 7, 13872. [Google Scholar] [CrossRef]
- Knebel, A.; Sundermann, L.; Mohmeyer, A.; Strauß, I.; Friebe, S.; Behrens, P.; Caro, J. Azobenzene Guest Molecules as Light-Switchable CO2 Valves in an Ultrathin UiO-67 Membrane. Chem. Mater. 2017, 29, 3111–3117. [Google Scholar] [CrossRef]
- Chuah, C.; Lee, J.; Bae, T.-H. Graphene-Based Membranes for H2 Separation: Recent Progress and Future Perspective. Membranes 2020, 10, 336. [Google Scholar] [CrossRef]
- Chernova, E.A.; Gurianov, K.E.; Petukhov, D.I.; Chumakov, A.P.; Valeev, R.G.; Brotsman, V.A.; Garshev, A.V.; Eliseev, A.A. Oxidized Carbon-Based Spacers for Pressure Resistant Graphene Oxide Membranes. Membranes 2022, 12, 934. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Kan, A.S.; Chumakov, A.P.; Konovalov, O.V.; Valeev, R.G.; Eliseev, A.A. MXene-Based Gas Separation Membranes with Sorption Type Selectivity. J. Membr. Sci. 2021, 621, 118994. [Google Scholar] [CrossRef]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.-X.; Wang, S.; Caro, J.; Gogotsi, Y. MXene Molecular Sieving Membranes for Highly Efficient Gas Separation. Nat. Commun. 2018, 9, 155. [Google Scholar] [CrossRef]
- Chernova, E.A.; Petukhov, D.I.; Chumakov, A.P.; Kirianova, A.V.; Sadilov, I.S.; Kapitanova, O.O.; Boytsova, O.V.; Valeev, R.G.; Roth, S.V.; Eliseev, A.A.; et al. The Role of Oxidation Level in Mass-Transport Properties and Dehumidification Performance of Graphene Oxide Membranes. Carbon N. Y. 2021, 183, 404–414. [Google Scholar] [CrossRef]
- Komkova, M.A.; Sadilov, I.S.; Brotsman, V.A.; Petukhov, D.I.; Eliseev, A.A. Facilitated Transport of Ammonia in Ultra-Thin Prussian Blue Membranes with Potential-Tuned Selectivity. J. Membr. Sci. 2021, 639, 119714. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Kapitanova, O.O.; Eremina, E.A.; Goodilin, E.A. Preparation, Chemical Features, Structure and Applications of Membrane Materials Based on Graphene Oxide. Mendeleev Commun. 2021, 31, 137–148. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Hao, L.; Jiang, Y.; van der Bruggen, B.; Sotto, A.; Gao, C.; Shen, J. Thermo- and PH-Responsive Graphene Oxide Membranes with Tunable Nanochannels for Water Gating and Permeability of Small Molecules. J. Membr. Sci. 2019, 587, 117163. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Y.; Zhou, Y.; Jia, M.; Wang, G.; Guo, W.; Jiang, L. Photo-Switchable Two-Dimensional Nanofluidic Ionic Diodes. Chem. Sci. 2017, 8, 4381–4386. [Google Scholar] [CrossRef]
- Pang, W.; Xue, J.; Pang, H. A High Energy Density Azobenzene/Graphene Oxide Hybrid with Weak Nonbonding Interactions for Solar Thermal Storage. Sci. Rep. 2019, 9, 5224. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Spinato, C.; Raya, J.; Bianco, A.; Ménard-Moyon, C. Chemical Reactivity of Graphene Oxide towards Amines Elucidated by Solid-State NMR. Nanoscale 2016, 8, 13714–13721. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Eliseev, A.A. Gas Permeation through Nanoporous Membranes in the Transitional Flow Region. Nanotechnology 2016, 27, 085707. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Chernova, E.A.; Kapitanova, O.O.; Boytsova, O.V.; Valeev, R.G.; Chumakov, A.P.; Konovalov, O.V.; Eliseev, A.A. Thin Graphene Oxide Membranes for Gas Dehumidification. J. Membr. Sci. 2019, 577, 184–194. [Google Scholar] [CrossRef]
- MOPAC. MOPAC2016 Stewart Computational Chemistry; Stewart Computational Chemistry: Colorado Springs, CO, USA, 2016. [Google Scholar]
- Zheng, Y.B.; Payton, J.L.; Chung, C.-H.; Liu, R.; Cheunkar, S.; Pathem, B.K.; Yang, Y.; Jensen, L.; Weiss, P.S. Surface-Enhanced Raman Spectroscopy to Probe Reversibly Photoswitchable Azobenzene in Controlled Nanoscale Environments. Nano Lett. 2011, 11, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kang, J.H.; Min, K.-A.; Hong, S.; Hee Hong, B. Graphene Oxide Catalyzed Cis-Trans Isomerization of Azobenzene. APL Mater. 2014, 2, 092501. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Poyarkov, A.A.; Chernova, E.A.; Eliseev, A.A.; Chumakov, A.P.; Konovalov, O.; Petukhov, D.I. Operando Study of Water Vapor Transport through Ultra-Thin Graphene Oxide Membranes. 2D Mater. 2019, 6, 035039. [Google Scholar] [CrossRef]
- Tiberio, G.; Muccioli, L.; Berardi, R.; Zannoni, C. How Does the Trans-Cis Photoisomerization of Azobenzene Take Place in Organic Solvents? ChemPhysChem 2010, 11, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.P.; Mathies, R.A. Photoexcited Structural Dynamics of an Azobenzene Analog 4-Nitro-4′-Dimethylamino-Azobenzene from Femtosecond Stimulated Raman. Phys. Chem. Chem. Phys. 2012, 14, 6298. [Google Scholar] [CrossRef] [PubMed]
- Fregoni, J.; Granucci, G.; Coccia, E.; Persico, M.; Corni, S. Manipulating Azobenzene Photoisomerization through Strong Light–Molecule Coupling. Nat. Commun. 2018, 9, 4688. [Google Scholar] [CrossRef]


| Sample | Humidity, % | D-Spacing, nm | D-Spacing RSD (Radial FWHM of Reflection), nm−1 | Nanoflakes FWHM. χ |
|---|---|---|---|---|
| GO | 0 | 0.722 | 0.909 | 18.35 |
| GO-azo | 0 | 0.866 | 1.030 | 11.37 |
| GO | 100 | 1.153 | 0.659 | 11.73 |
| GO-azo | 100 | 1.300 | 1.433 | 7.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadilov, I.; Petukhov, D.; Brotsman, V.; Chumakova, A.; Eliseev, A.; Eliseev, A. Light Response and Switching Behavior of Graphene Oxide Membranes Modified with Azobenzene Compounds. Membranes 2022, 12, 1131. https://doi.org/10.3390/membranes12111131
Sadilov I, Petukhov D, Brotsman V, Chumakova A, Eliseev A, Eliseev A. Light Response and Switching Behavior of Graphene Oxide Membranes Modified with Azobenzene Compounds. Membranes. 2022; 12(11):1131. https://doi.org/10.3390/membranes12111131
Chicago/Turabian StyleSadilov, Ilia, Dmitrii Petukhov, Victor Brotsman, Alexandra Chumakova, Artem Eliseev, and Andrei Eliseev. 2022. "Light Response and Switching Behavior of Graphene Oxide Membranes Modified with Azobenzene Compounds" Membranes 12, no. 11: 1131. https://doi.org/10.3390/membranes12111131
APA StyleSadilov, I., Petukhov, D., Brotsman, V., Chumakova, A., Eliseev, A., & Eliseev, A. (2022). Light Response and Switching Behavior of Graphene Oxide Membranes Modified with Azobenzene Compounds. Membranes, 12(11), 1131. https://doi.org/10.3390/membranes12111131








