Modulation of Membrane Microviscosity by Protein-Mediated Carotenoid Delivery as Revealed by Time-Resolved Fluorescence Anisotropy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Purification of Astaxanthin Mono- and Diesters
2.3. Liposomes Synthesis Protocol
2.3.1. Preparation of Nanoemulsion of Soy Lecithin Lipoid S75 Liposomes
2.3.2. Preparation of Egg Yolk Lecithin Liposomes
2.3.3. Assessment of Liposomes Size Distribution and Heterogeneity
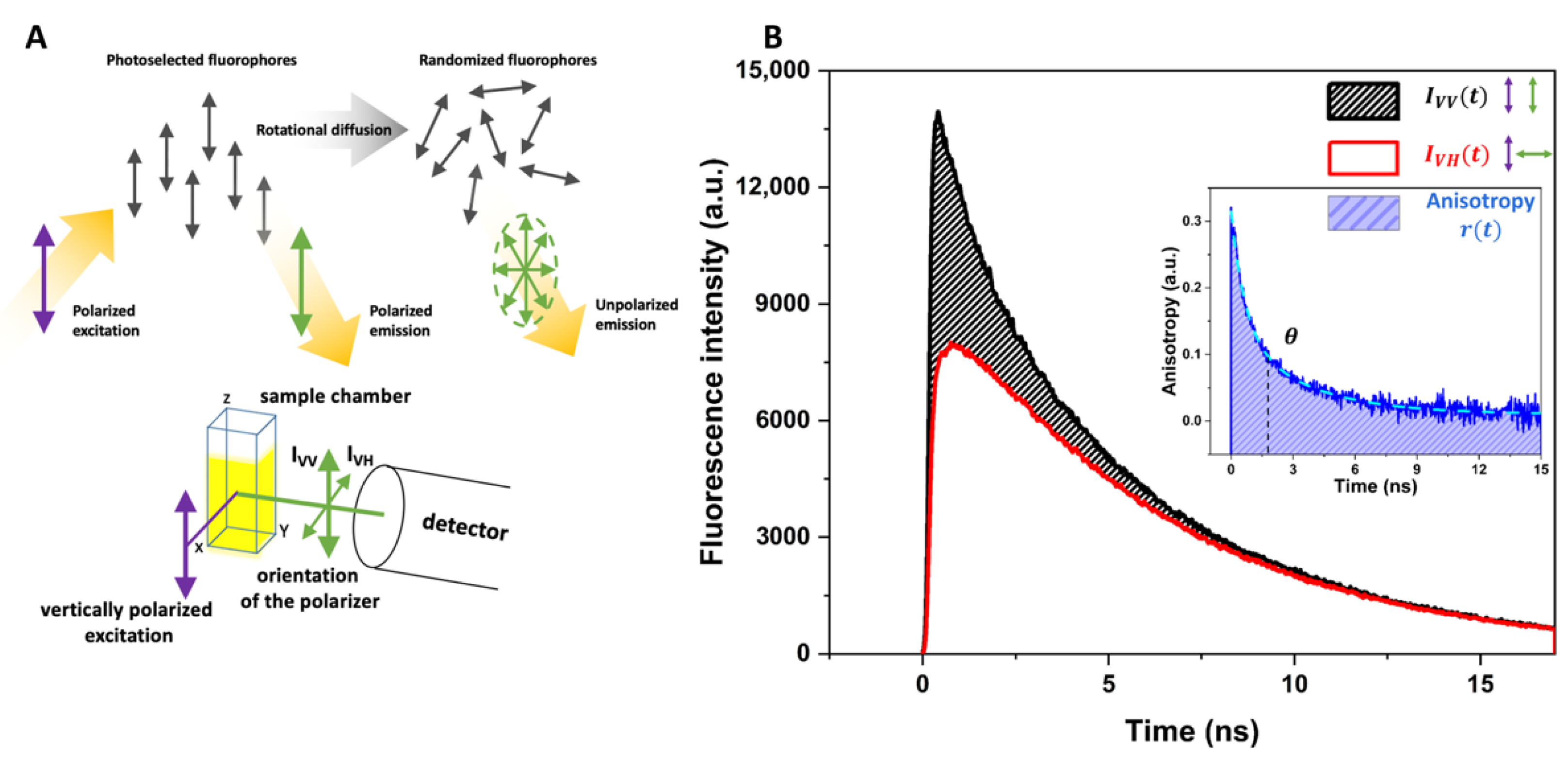
2.4. Picosecond Time-Resolved Fluorescence Anisotropy Measurement
2.5. Molecular Dynamics Modeling In Silico
2.6. Spectroscopy Measurements
2.7. Data Analysis
3. Results
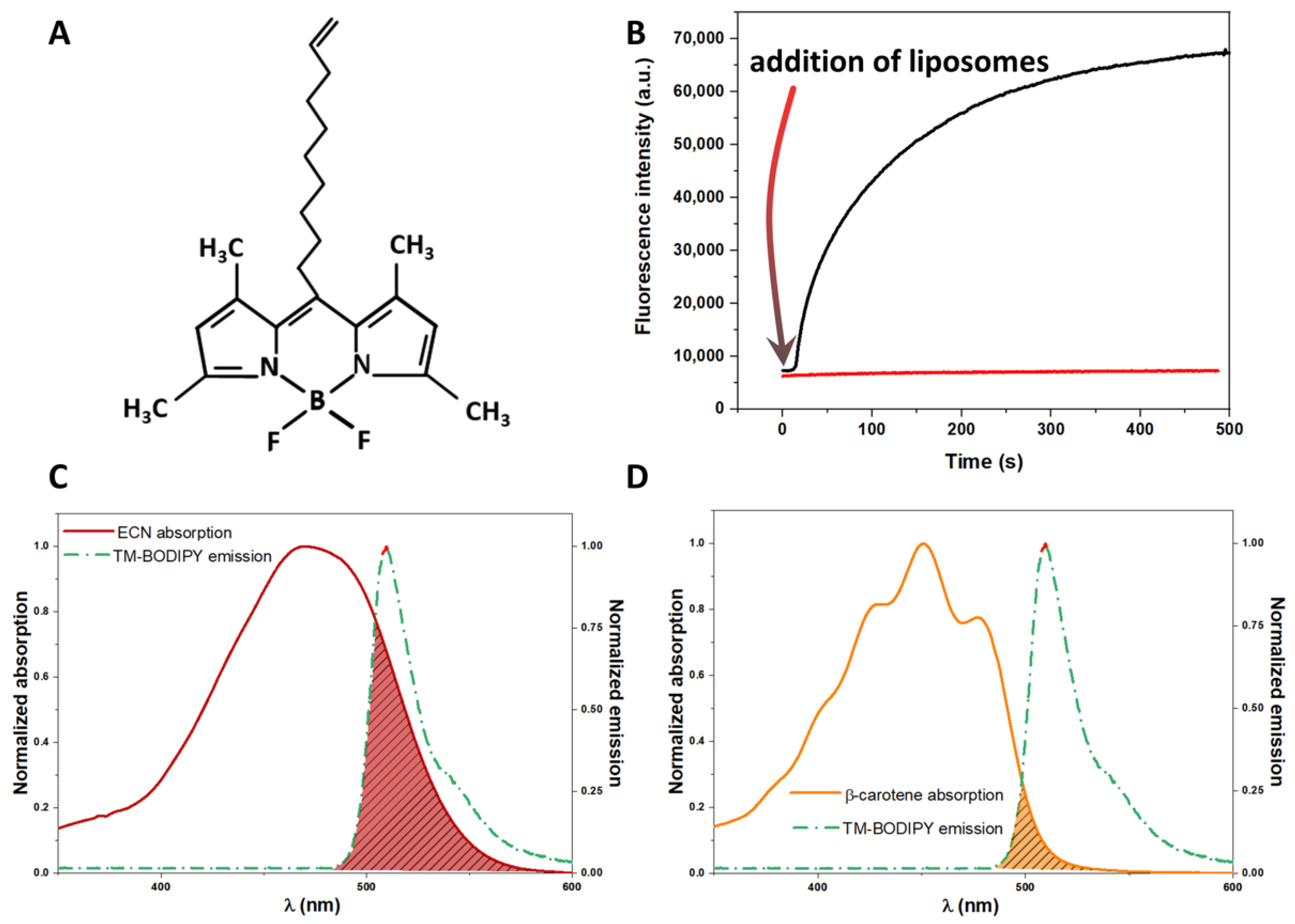
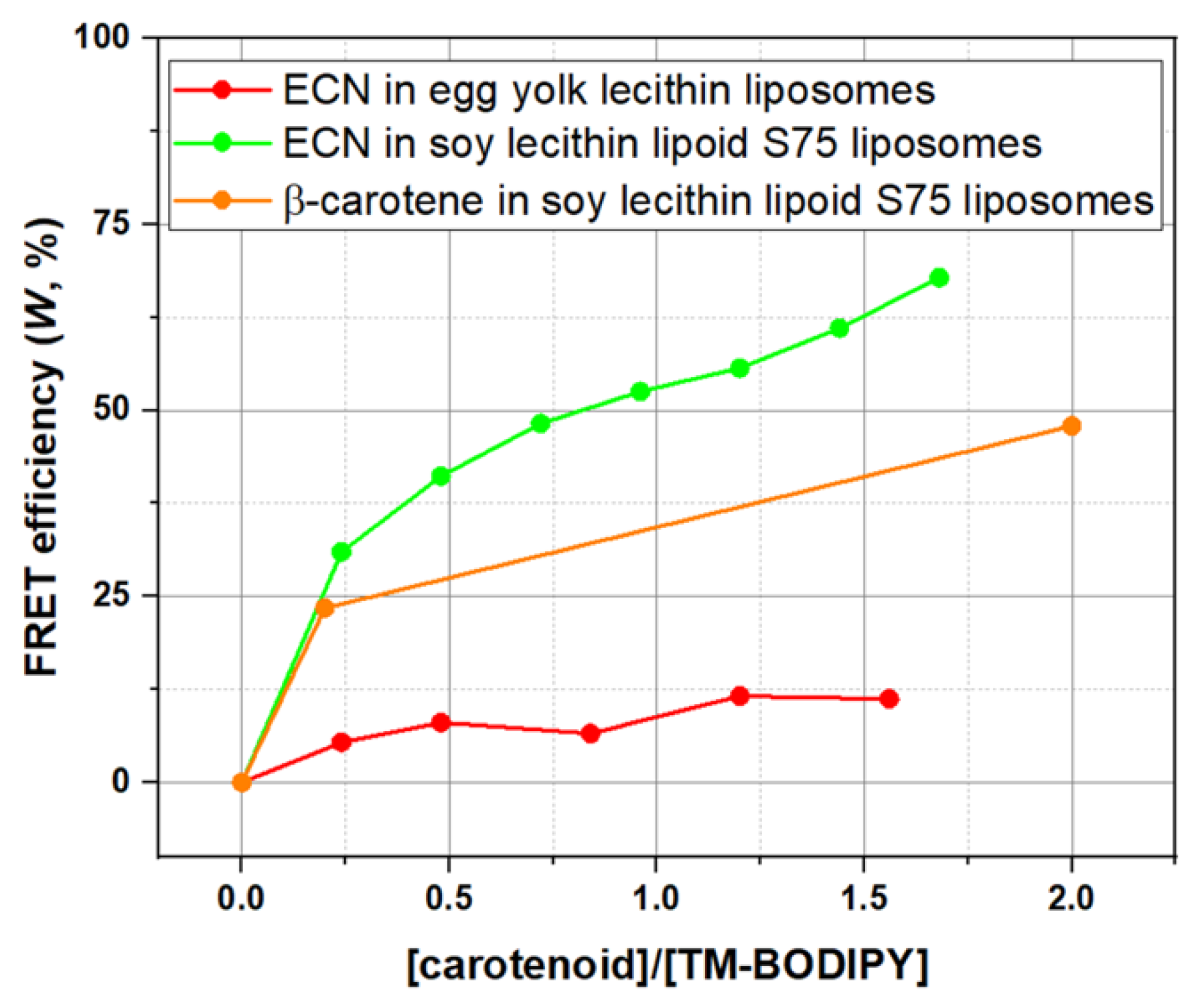
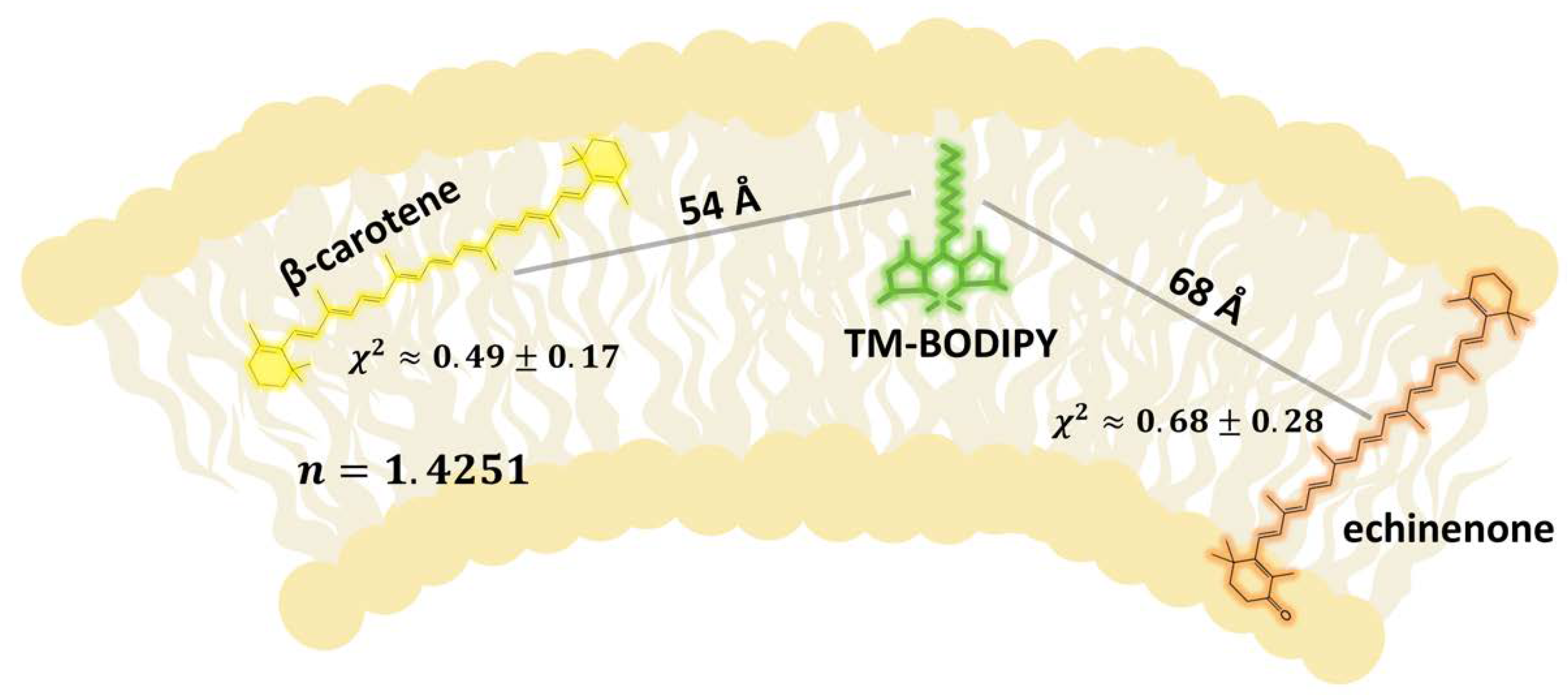
3.1. Delivery of Carotenoid Molecules in the Liposomal Membrane Studied by FRET
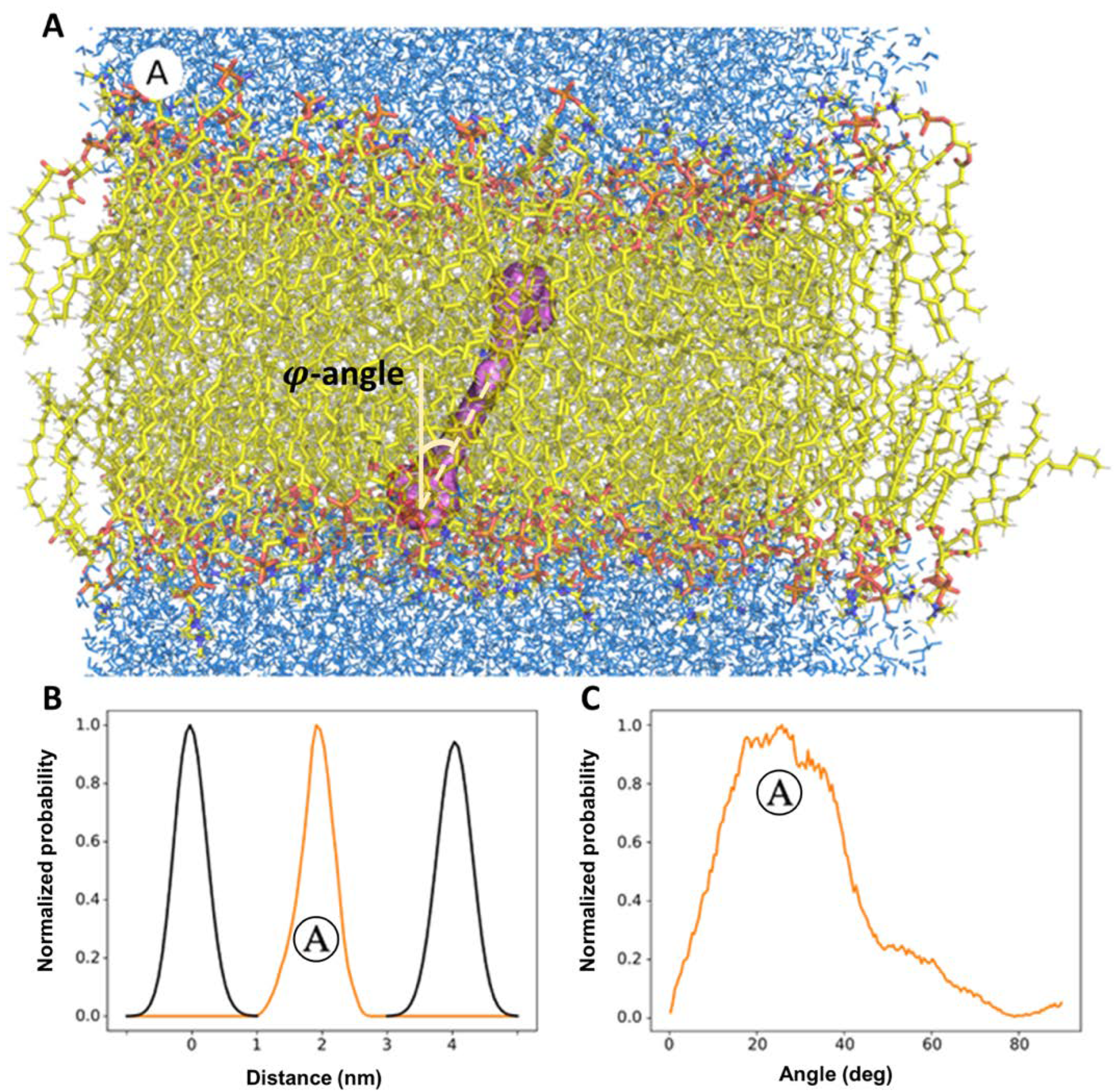
3.2. Molecular Dynamics Simulations of Localization and Distribution of Carotenoids within the Hydrophobic Core of the Model Membrane
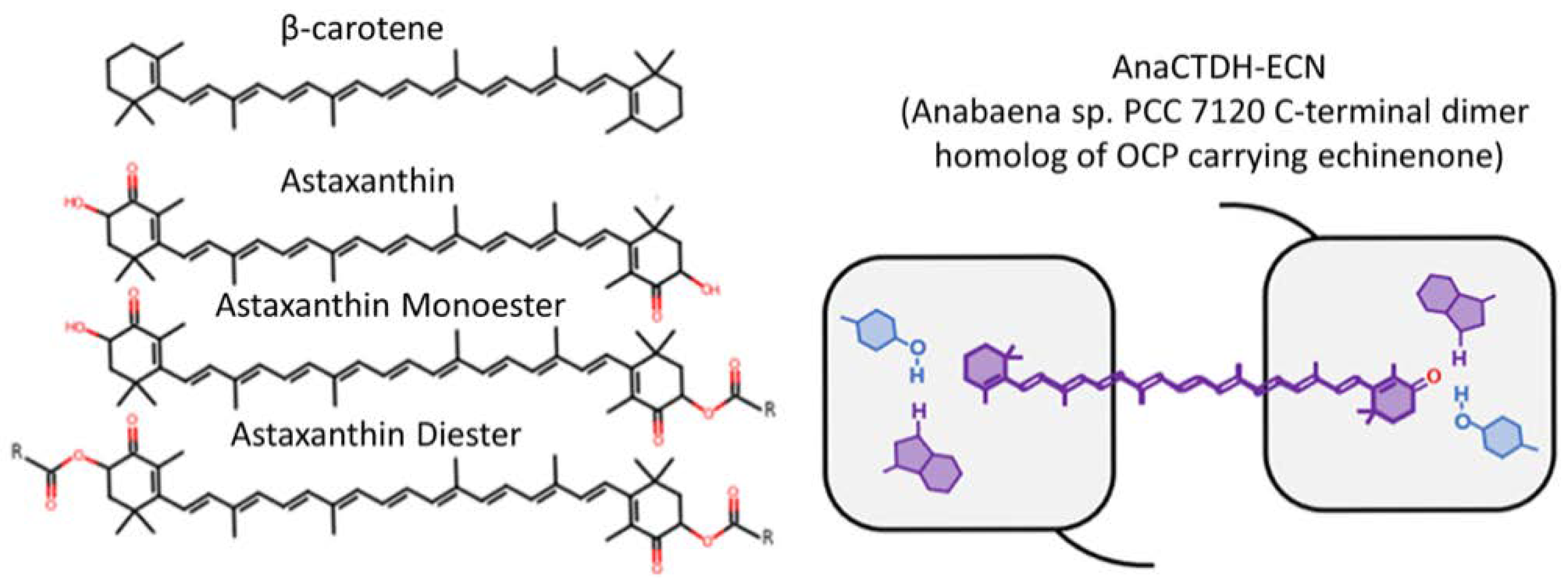
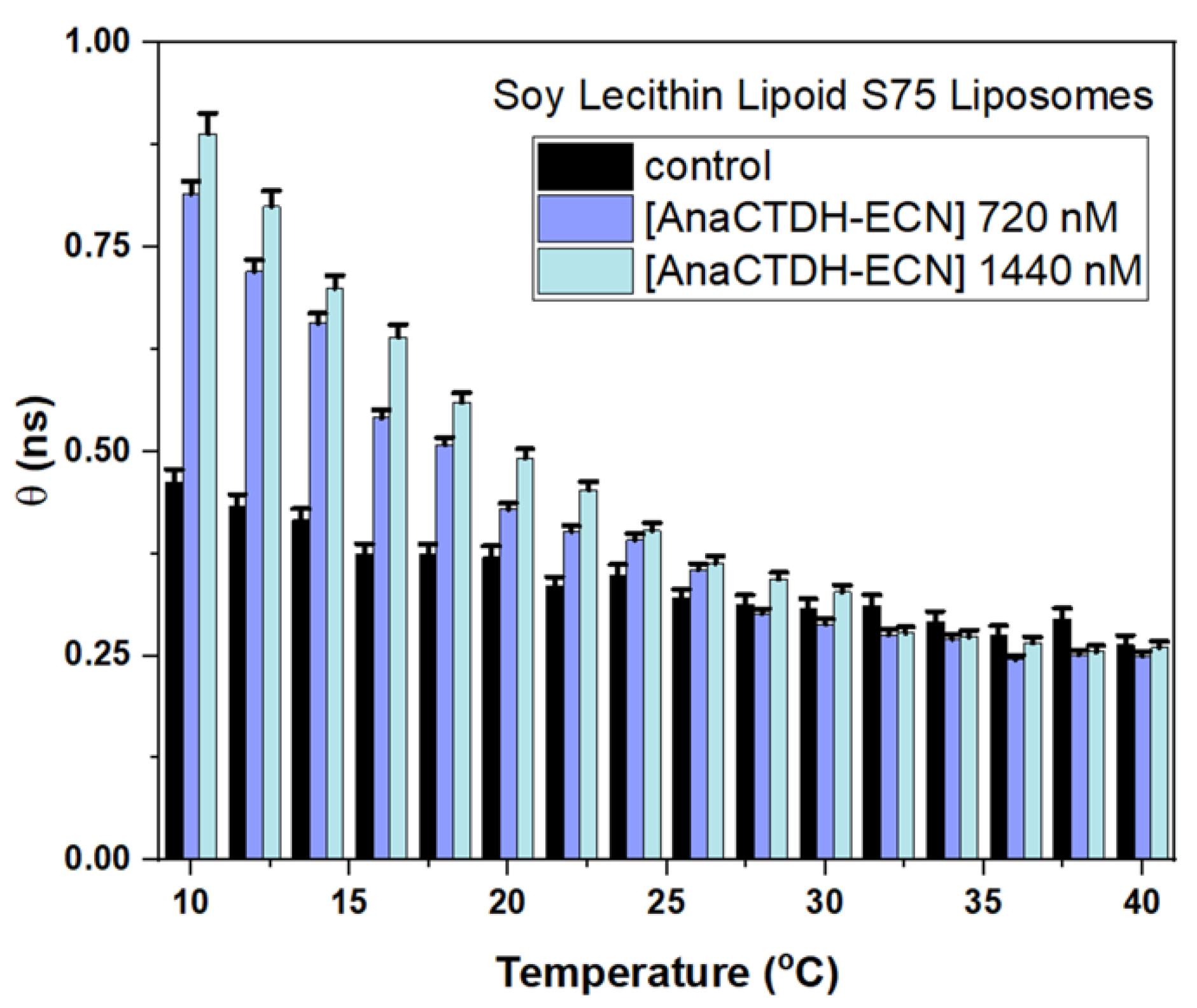
3.3. Targeted Delivery of Carotenoids via Carotenoprotein: Estimation of Efficiency
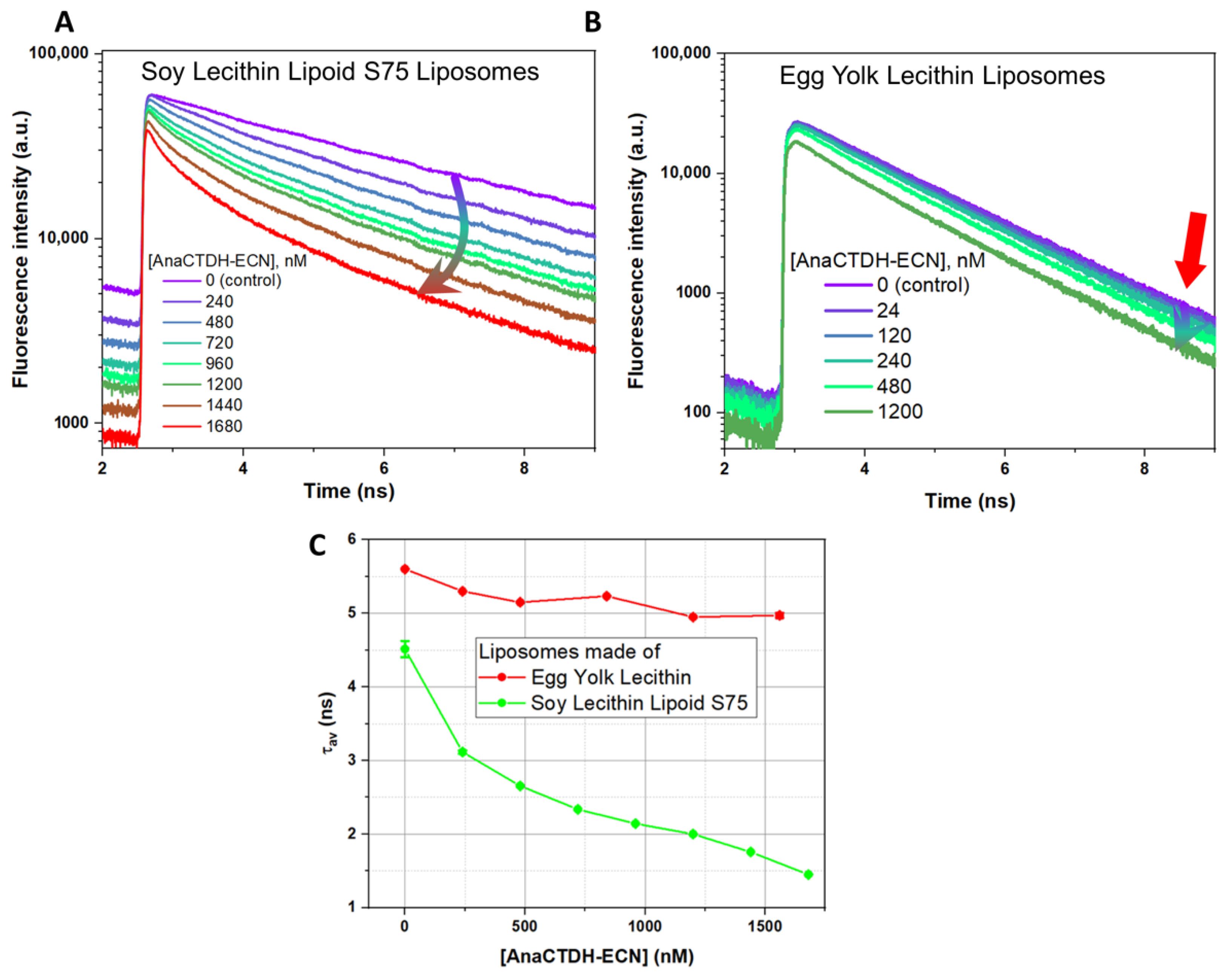
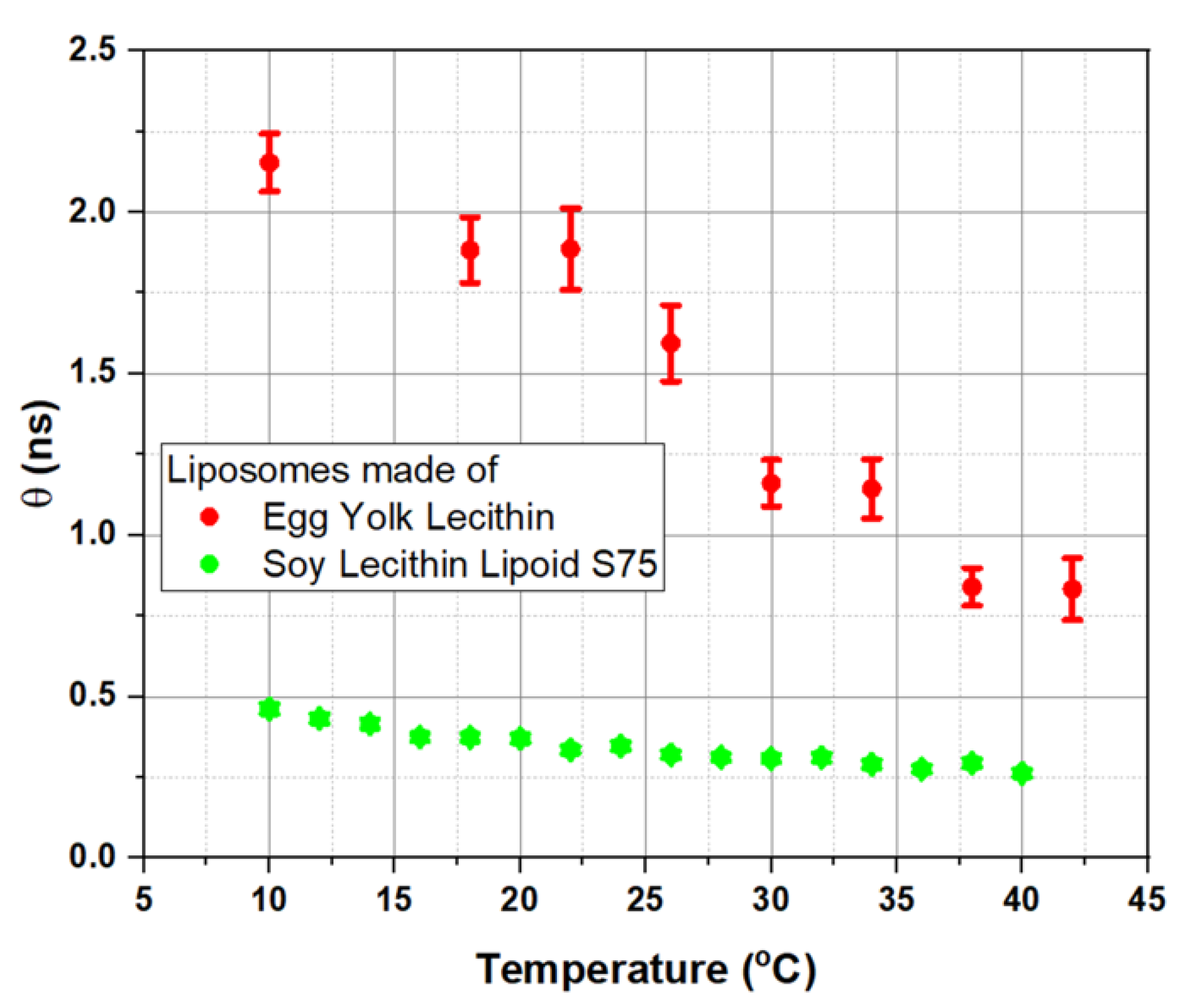
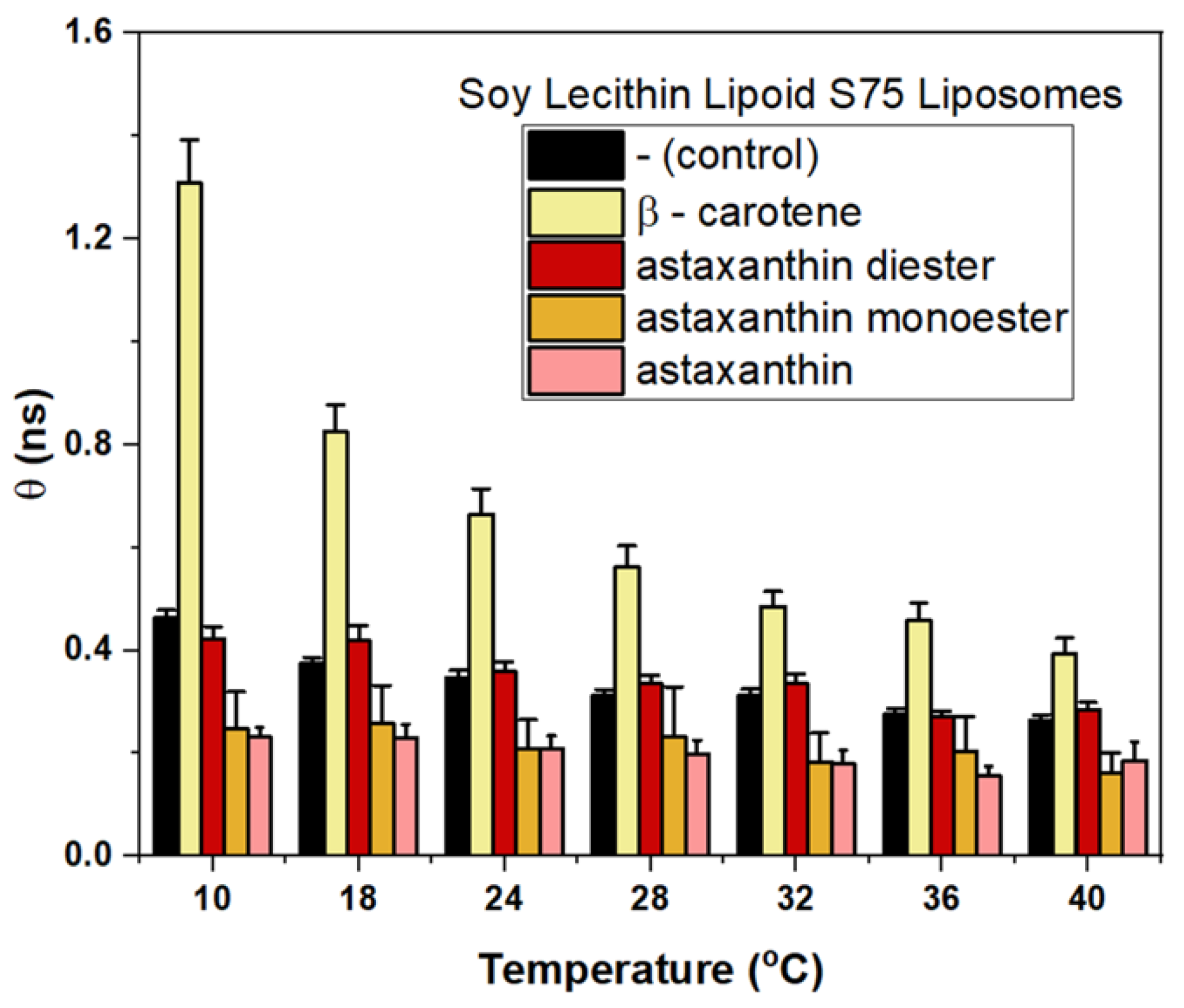
3.4. Effects of Different Carotenoids on Microviscosity of Liposome Membrane Revealed by Fluorescence Anisotropy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerfeld, C.A.; Alexandre, M.; Kirilovsky, D. The Orange Carotenoid Protein of Cyanobacteria. In Carotenoids Physical, Chemical and Biological Functions and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 3–19. [Google Scholar]
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The Role of Carotenoids in the Prevention and Treatment of Cardiovascular Disease–Current State of Knowledge. J. Funct. Foods 2017, 38, 45–65. [Google Scholar] [CrossRef]
- Manochkumar, J.; Doss, C.G.P.; El-Seedi, H.R.; Efferth, T.; Ramamoorthy, S. The neuroprotective potential of carotenoids in vitro and in vivo. Phytomedicine 2021, 91, 153676. [Google Scholar] [CrossRef]
- Darvin, M.; Ey, S.; Brandt, N.N.; Sterry, W. Noninvasive Detection of Beta-Carotene and Lycopene in Human Skin Using Raman Spectroscopy. Laser Phys. 2004, 14, 231–233. [Google Scholar]
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A.; Moore, L.L.; Gomez, C.M. Analysis of Zeaxanthin Distribution within Individual Human Retinas. Methods Enzymol. 1999, 299, 457–467. [Google Scholar]
- Magalingam, K.B.; Somanath, S.D.; Haleagrahara, N.; Selvaduray, K.R.; Radhakrishnan, A.K. Unravelling the neuroprotective mechanisms of carotenes in differentiated human neural cells: Biochemical and proteomic approaches. Food Chem. Mol. Sci. 2022, 4, 100088. [Google Scholar] [CrossRef]
- Craft, N.E.; Haitema, T.B.; Garnett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoid, Tocopherol, and Retinol Concentrations in Elderly Human Brain. J. Nutr. Health Aging 2004, 8, 156–162. [Google Scholar] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.-A.; Biesalski, H.K. β-Carotene Is an Important Vitamin a Source for Humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef]
- Lokstein, H.; Renger, G.; Götze, J.P. Photosynthetic Light-Harvesting (Antenna) Complexes-Structures and Functions. Molecules 2021, 26, 3378. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, R.; Reppert, M.; Zazubovich, V.; Pieper, J.; Reinot, T. Site Selective and Single Complex Laser-Based Spectroscopies: A Window on Excited State Electronic Structure, Excitation Energy Transfer, and Electron–Phonon Coupling of Selected Photosynthetic Complexes. Chem. Rev. 2011, 111, 4546–4598. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Morita, A.; Ohnuki, S.; Hirata, A.; Sekida, S.; Okuda, K.; Ohya, Y.; Kawano, S. Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis. Sci. Rep. 2018, 8, 5617. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, M.; Eisenhut, M.; Hackenberg, C.; Bauwe, H. Pathway and Importance of Photorespiratory 2-Phosphoglycolate Metabolism in Cyanobacteria. In Recent Advances in Phototrophic Prokaryotes; Springer: New York, NY, USA, 2010; Volume 675, ISBN 9781441915276. [Google Scholar]
- Leverenz, R.L.; Jallet, D.; Li, M.-D.; Mathies, R.A.; Kirilovsky, D.; Kerfeld, C.A. Structural and Functional Modularity of the Orange Carotenoid Protein: Distinct Roles for the N- and C-Terminal Domains in Cyanobacterial Photoprotection. Plant Cell 2014, 26, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Slonimskiy, Y.B.; Muzzopappa, F.; Maksimov, E.G.; Wilson, A.F.; Friedrich, T.; Kirilovsky, D.; Sluchanko, N.N. Light-controlled carotenoid transfer between water-soluble proteins related to cyanobacterial photoprotection. FEBS J. 2019, 286, 1908–1924. [Google Scholar] [CrossRef]
- Maksimov, E.G.; Sluchanko, N.N.; Slonimskiy, Y.B.; Mironov, K.S.; Klementiev, K.E.; Moldenhauer, M.; Friedrich, T.; Los, D.A.; Paschenko, V.Z.; Rubin, A.B. The Unique Protein-to-Protein Carotenoid Transfer Mechanism. Biophys. J. 2017, 113, 402–414. [Google Scholar] [CrossRef]
- Moldenhauer, M.; Sluchanko, N.; Buhrke, D.; Zlenko, D.; Tavraz, N.N.; Schmitt, F.-J.; Hildebrandt, P.; Maksimov, E.G.; Friedrich, T. Assembly of photoactive orange carotenoid protein from its domains unravels a carotenoid shuttle mechanism. Photosynth. Res. 2017, 133, 327–341. [Google Scholar] [CrossRef]
- Muzzopappa, F.; Wilson, A.; Yogarajah, V.; Cot, S.; Perreau, F.; Montigny, C.; De Carbon, C.B.; Kirilovsky, D. Paralogs of the C-terminal domain of the cyanobacterial orange carotenoid protein are carotenoid donors to helical carotenoid proteins. Plant Physiol. 2017, 175, 1283–1303. [Google Scholar] [CrossRef]
- Harris, D.; Wilson, A.F.; Muzzopappa, F.; Sluchanko, N.N.; Friedrich, T.; Maksimov, E.G.; Kirilovsky, D.; Adir, N. Structural rearrangements in the C-terminal domain homolog of Orange Carotenoid Protein are crucial for carotenoid transfer. Commun. Biol. 2018, 1, 125. [Google Scholar] [CrossRef]
- Maksimov, E.; Zamaraev, A.; Parshina, E.; Slonimskiy, Y.; Slastnikova, T.; Abdrakhmanov, A.; Babaev, P.; Efimova, S.; Ostroumova, O.; Stepanov, A.; et al. Soluble Cyanobacterial Carotenoprotein as a Robust Antioxidant Nanocarrier and Delivery Module. Antioxidants 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Slonimskiy, Y.B.; Egorkin, N.A.; Friedrich, T.; Maksimov, E.G.; Sluchanko, N.N. Microalgal protein AstaP is a potent carotenoid solubilizer and delivery module with a broad carotenoid binding repertoire. FEBS J. 2021, 289, 999–1022. [Google Scholar] [CrossRef] [PubMed]
- Sakudoh, T.; Sezutsu, H.; Nakashima, T.; Kobayashi, I.; Fujimoto, H.; Uchino, K.; Banno, Y.; Iwano, H.; Maekawa, H.; Tamura, T.; et al. Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the Yellow blood gene. Proc. Natl. Acad. Sci. USA 2007, 104, 8941–8946. [Google Scholar] [CrossRef] [PubMed]
- Slonimskiy, Y.B.; Egorkin, N.A.; Ashikhmin, A.A.; Friedrich, T.; Maksimov, E.G.; Sluchanko, N.N. Reconstitution of the functional Carotenoid-Binding Protein from silkworm in E. coli. Int. J. Biol. Macromol. 2022, 214, 664–671. [Google Scholar] [CrossRef]
- Sluchanko, N.N.; Slonimskiy, Y.B.; Egorkin, N.A.; Varfolomeeva, L.A.; Yu Kleymenov, S.; Minyaev, M.E.; Moysenovich, A.M.; Parshina, Y.; Friedrich, T.; Maksimov, E.G.; et al. Nanocontainer Derived from Silkworm Carotenoprotein for Carotenoid Extraction and Presentation in Biotechnology and Biomedical Applications. bioRxiv 2022. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- During, A.; Hussain, M.M.; Morel, D.W.; Harrison, E.H. Carotenoid uptake and secretion by CaCo-2 cells: β-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 2002, 43, 1086–1095. [Google Scholar] [CrossRef]
- Reboul, E.; Abou, L.; Mikail, C.; Ghiringhelli, O.; André, M.; Portugal, H.; Jourdheuil-Rahmani, D.; Amiot, M.J.; Lairon, D.; Borel, P. Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI). Biochem. J. 2005, 387, 455–461. [Google Scholar] [CrossRef]
- Yang, C.; Hassan, Y.I.; Liu, R.; Zhang, H.; Chen, Y.; Zhang, L.; Tsao, R. Anti-Inflammatory Effects of Different Astaxanthin Isomers and the Roles of Lipid Transporters in the Cellular Transport of Astaxanthin Isomers in Caco-2 Cell Monolayers. J. Agric. Food Chem. 2019, 67, 6222–6231. [Google Scholar] [CrossRef]
- Bandara, S.; Ramkumar, S.; Imanishi, S.; Thomas, L.D.; Sawant, O.B.; Imanishi, Y.; von Lintig, J. Aster proteins mediate carotenoid transport in mammalian cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2200068119. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Chen, D.; Lu, Y. β-Carotene and astaxanthin with human and bovine serum albumins. Food Chem. 2015, 179, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Reszczynska, E.; Welc, R.; Grudzinski, W.; Trebacz, K.; Gruszecki, W.I. Carotenoid binding to proteins: Modeling pigment transport to lipid membranes. Arch. Biochem. Biophys. 2015, 584, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Markowska, E.; Sielewiesiuk, J. Spinlabel studies on phosphatidylcholine-polar carotenoid membranes: Effects of alkyl-chain length and unsaturation. Biochim. Biophys. Acta (BBA)-Biomembr. 1993, 1150, 173–181. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Widomska, J. EPR Spin Labeling in Carotenoid-Membrane Interactions. In Carotenoids Physical, Chemical, and Biological Functions and Properties; CRC Press: Boca Raton, FL, USA, 2010; pp. 189–209. [Google Scholar]
- Socaciu, C.; Jessel, R.; Diehl, H.A. Competitive carotenoid and cholesterol incorporation into liposomes: Effects on membrane phase transition, fluidity, polarity and anisotropy. Chem. Phys. Lipids 2000, 106, 79–88. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic. Biol. Med. 2006, 40, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Shimolina, L.E.; Izquierdo, M.A.; López-Duarte, I.; Bull, J.A.; Shirmanova, M.V.; Klapshina, L.G.; Zagaynova, E.V.; Kuimova, M.K. Imaging tumor microscopic viscosity in vivo using molecular rotors. Sci. Rep. 2017, 7, 41097. [Google Scholar] [CrossRef] [PubMed]
- Shershov, V.E.; Kuznetsova, V.E.; Lapa, S.A.; Spitsyn, M.A.; Guseinov, T.O.; Tkachev, Y.V.; Zasedatelev, A.S.; Chudinov, A.V. Synthesis and characterization of novel zwitterionic heptamethine indocyanine fluorophores. Mendeleev Commun. 2017, 27, 360–362. [Google Scholar] [CrossRef]
- de Bruijn, W.J.; Weesepoel, Y.; Vincken, J.-P.; Gruppen, H. Fatty acids attached to all-trans-astaxanthin alter its cistrans equilibrium, and consequently its stability, upon light-accelerated autoxidation. Food Chem. 2016, 194, 1108–1115. [Google Scholar] [CrossRef]
- Miao, F.; Lu, D.; Li, Y.; Zeng, M. Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. Anal. Biochem. 2006, 352, 176–181. [Google Scholar] [CrossRef]
- Kondratowicz, A.; Neunert, G.; Niezgoda, N.; Bryś, J.; Siger, A.; Rudzińska, M.; Lewandowicz, G. Egg Yolk Extracts as Potential Liposomes Shell Material: Composition Compared with Vesicles Characteristics. J. Food Sci. 2018, 83, 2527–2535. [Google Scholar] [CrossRef]
- Keith, A.D.; Snipes, W. Viscosity of Cellular Protoplasm. Science 1974, 183, 666–668. [Google Scholar] [CrossRef]
- Shinitzky, M.; Barenholz, Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. et Biophys. Acta (BBA)-Rev. Biomembr. 1978, 515, 367–394. [Google Scholar] [CrossRef]
- Puchkov, E.O. Intracellular Viscosity: Methods of Measurements and Role in Metabolism. Biol. Membrany 2014, 31, 3–13. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Fluorescence Anisotropy. In Principles of Luorescence Spectroscopy; Springer: Baltimore, MD, USA, 2006; pp. 372–401. [Google Scholar]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mackerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Woods, R.; Chappelle, R. Restrained electrostatic potential atomic partial charges for condensed-phase simulations of carbohydrates. J. Mol. Struct. THEOCHEM 2000, 527, 149–156. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Palacios, L.E.; Wang, T. Egg-yolk lipid fractionation and lecithin characterization. J. Am. Oil Chem. Soc. 2005, 82, 571–578. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; Van Der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczyńska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and Orientation of Xanthophylls in a Lipid Bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef]
- Van De Ven, M.; Kattenberg, M.; Van Ginkel, G.; Levine, Y. Study of the orientational ordering of carotenoids in lipid bilayers by resonance-Raman spectroscopy. Biophys. J. 1984, 45, 1203–1209. [Google Scholar] [CrossRef]
- Martynov, V.I.; Pakhomov, A.A. BODIPY derivatives as fluorescent reporters of molecular activities in living cells. Russ. Chem. Rev. 2021, 90, 1213–1262. [Google Scholar] [CrossRef]
- Pakhomov, A.A.; Kim, E.E.; Kononevich, Y.N.; Ionov, D.S.; Maksimova, M.A.; Khalchenia, V.B.; Maksimov, E.G.; Anisimov, A.A.; Shchegolikhina, O.I.; Martynov, V.I.; et al. Modulation of the photophysical properties of multi-BODIPY-siloxane conjugates by varying the number of fluorophores. Dye. Pigment. 2022, 203, 110371. [Google Scholar] [CrossRef]
- Oren, A.; Kuhl, M.; Karsten, U. An Endoevaporitic Microbial Mat within a Gypsum Crust: Zonation of Phototrophs, Photopigments, and Light Penetration. Mar. Ecol. Prog. Ser. 1995, 128, 151–159. [Google Scholar] [CrossRef][Green Version]
- Čilek, A.; Čelebi, N.; Tirnaksiz, F. Lecithin-Based Microemulsion of a Peptide for Oral Administration: Preparation, Characterization, and Physical Stability of the Formulation. Drug Deliv. 2006, 13, 19–24. [Google Scholar] [CrossRef]
- Gruszecki, W.I. Carotenoids in Membranes. In The Photochemistry of Carotenoids; Frank, H.A., Young, A.J., Britton, G., Cogdell, R.J., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1999; pp. 363–379. [Google Scholar]
- Strzalka, K.; Gruszecki, W.I. Effect of Beta-Carotene on Structural and Dynamic Properties of Model Phosphatidylcholine Membranes. I. An EPR Spin Label Study. Biochim. Biophys. Acta 1994, 1194, 138–142. [Google Scholar] [CrossRef]
- Budai, L.; Kaszás, N.; Gróf, P.; Lenti, K.; Maghami, K.; Antal, I.; Klebovich, I.; Petrikovics, I.; Budai, M. Liposomes for Topical Use: A Physico-Chemical Comparison of Vesicles Prepared from Egg or Soy Lecithin. Sci. Pharm. 2013, 81, 1151–1166. [Google Scholar] [CrossRef]
- Kondratowicz, A.; Weiss, M.; Juzwa, W.; Majchrzycki, L.; Lewandowicz, G. Characteristics of liposomes derived from egg yolk. Open Chem. 2019, 17, 763–778. [Google Scholar] [CrossRef]
- McNulty, H.; Jacob, R.F.; Mason, R.P. Biologic Activity of Carotenoids Related to Distinct Membrane Physicochemical Interactions. Am. J. Cardiol. 2008, 101, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.; Sujak, A.; Strzalka, K.; Radunz, A.; Schmid, G.H. Organisation of Xanthophyll-Lipid Membranes Studied by Means of Specific Pigment Antisera, Spectrophotometry and Monomolecular Layer Technique Lutein versus Zeaxanthin. Z. Für Nat. C 1999, 54, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I. Carotenoids in Lipid Membranes. In Carotenoids Physical, Chemical and Biological Functions and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 19–30. [Google Scholar]
- Bagatolli, L.; Gratton, E. Two Photon Fluorescence Microscopy of Coexisting Lipid Domains in Giant Unilamellar Vesicles of Binary Phospholipid Mixtures. Biophys. J. 2000, 78, 290–305. [Google Scholar] [CrossRef]
- Juhasz, J.; Davis, J.H.; Sharom, F.J. Fluorescent probe partitioning in GUVs of binary phospholipid mixtures: Implications for interpreting phase behavior. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 19–26. [Google Scholar] [CrossRef]
- Juhasz, J.; Davis, J.H.; Sharom, F.J. Fluorescent probe partitioning in giant unilamellar vesicles of ‘lipid raft’ mixtures. Biochem. J. 2010, 430, 415–423. [Google Scholar] [CrossRef]
- Palozza, P.; Muzzalupo, R.; Trombino, S.; Valdannini, A.; Picci, N. Solubilization and stabilization of β-carotene in niosomes: Delivery to cultured cells. Chem. Phys. Lipids 2006, 139, 32–42. [Google Scholar] [CrossRef]
- Elkholy, N.S.; Shafaa, M.W.; Mohammed, H.S. Cationic liposome-encapsulated carotenoids as a potential treatment for fibromyalgia in an animal model. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166150. [Google Scholar] [CrossRef]
- Peng, C.-H.; Chang, C.-H.; Peng, R.Y.; Chyau, C.-C. Improved membrane transport of astaxanthine by liposomal encapsulation. Eur. J. Pharm. Biopharm. 2010, 75, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, N.S.; Shafaa, M.W.; Mohammed, H.S. Biophysical characterization of lutein or beta carotene-loaded cationic liposomes. RSC Adv. 2020, 10, 32409–32422. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Liposome as a Delivery System for Carotenoids: Comparative Antioxidant Activity of Carotenoids As Measured by Ferric Reducing Antioxidant Power, DPPH Assay and Lipid Peroxidation. J. Agric. Food Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenov, A.N.; Gvozdev, D.A.; Zlenko, D.V.; Protasova, E.A.; Khashimova, A.R.; Parshina, E.Y.; Baizhumanov, A.A.; Lotosh, N.Y.; Kim, E.E.; Kononevich, Y.N.; et al. Modulation of Membrane Microviscosity by Protein-Mediated Carotenoid Delivery as Revealed by Time-Resolved Fluorescence Anisotropy. Membranes 2022, 12, 905. https://doi.org/10.3390/membranes12100905
Semenov AN, Gvozdev DA, Zlenko DV, Protasova EA, Khashimova AR, Parshina EY, Baizhumanov AA, Lotosh NY, Kim EE, Kononevich YN, et al. Modulation of Membrane Microviscosity by Protein-Mediated Carotenoid Delivery as Revealed by Time-Resolved Fluorescence Anisotropy. Membranes. 2022; 12(10):905. https://doi.org/10.3390/membranes12100905
Chicago/Turabian StyleSemenov, Alexey N., Danil A. Gvozdev, Dmitry V. Zlenko, Elena A. Protasova, Anastasia R. Khashimova, Evgenia Yu. Parshina, Adil A. Baizhumanov, Natalia Yu. Lotosh, Eleonora E. Kim, Yuriy N. Kononevich, and et al. 2022. "Modulation of Membrane Microviscosity by Protein-Mediated Carotenoid Delivery as Revealed by Time-Resolved Fluorescence Anisotropy" Membranes 12, no. 10: 905. https://doi.org/10.3390/membranes12100905
APA StyleSemenov, A. N., Gvozdev, D. A., Zlenko, D. V., Protasova, E. A., Khashimova, A. R., Parshina, E. Y., Baizhumanov, A. A., Lotosh, N. Y., Kim, E. E., Kononevich, Y. N., Pakhomov, A. A., Selishcheva, A. A., Sluchanko, N. N., Shirshin, E. A., & Maksimov, E. G. (2022). Modulation of Membrane Microviscosity by Protein-Mediated Carotenoid Delivery as Revealed by Time-Resolved Fluorescence Anisotropy. Membranes, 12(10), 905. https://doi.org/10.3390/membranes12100905






