Study on Treatment of Tiny Pollution Water with PAC-HUM System in Kuitun River
Abstract
1. Introduction
2. Materials and Methods
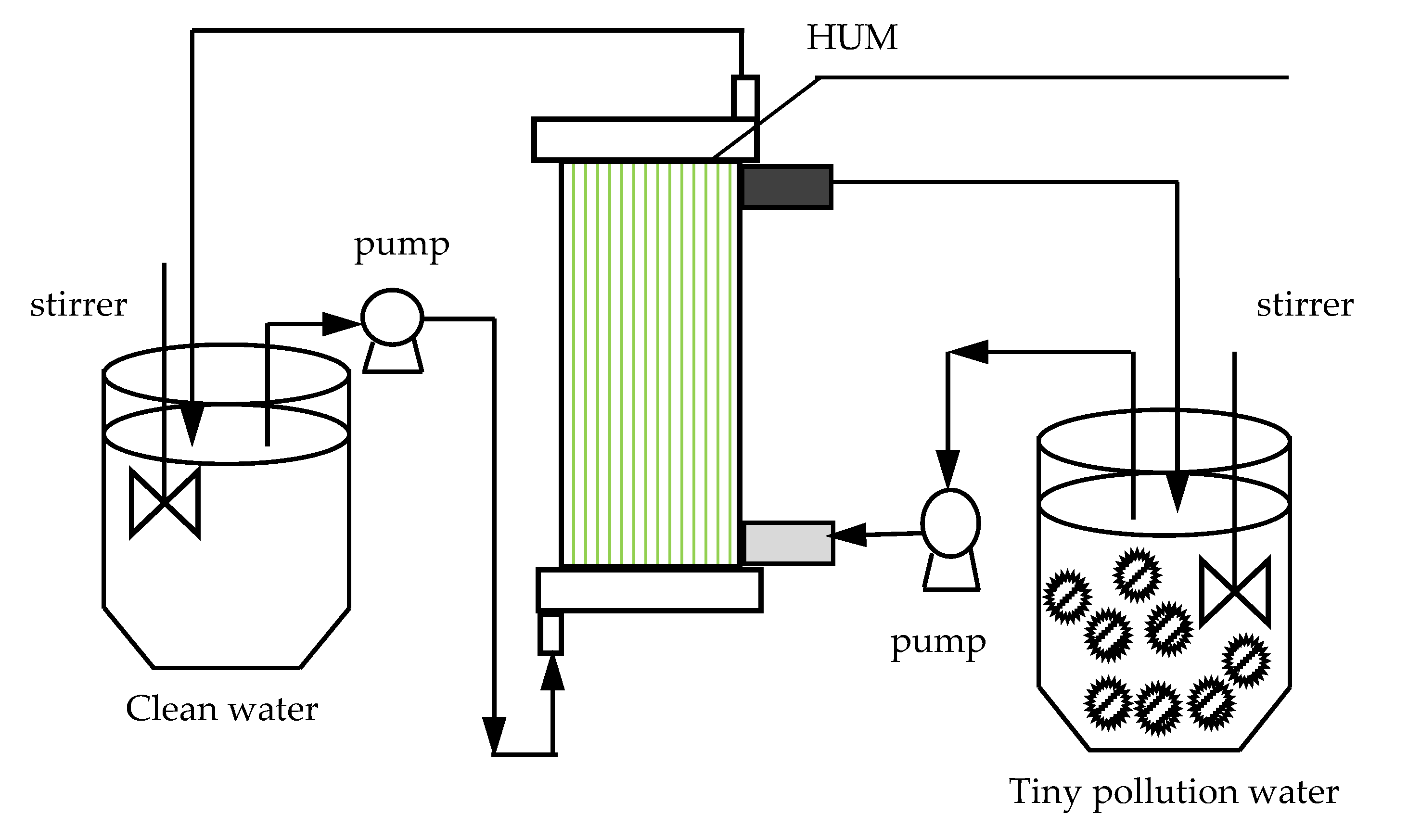
2.1. Experimental Materials
2.2. Experimental Methods
3. Results and Discussion
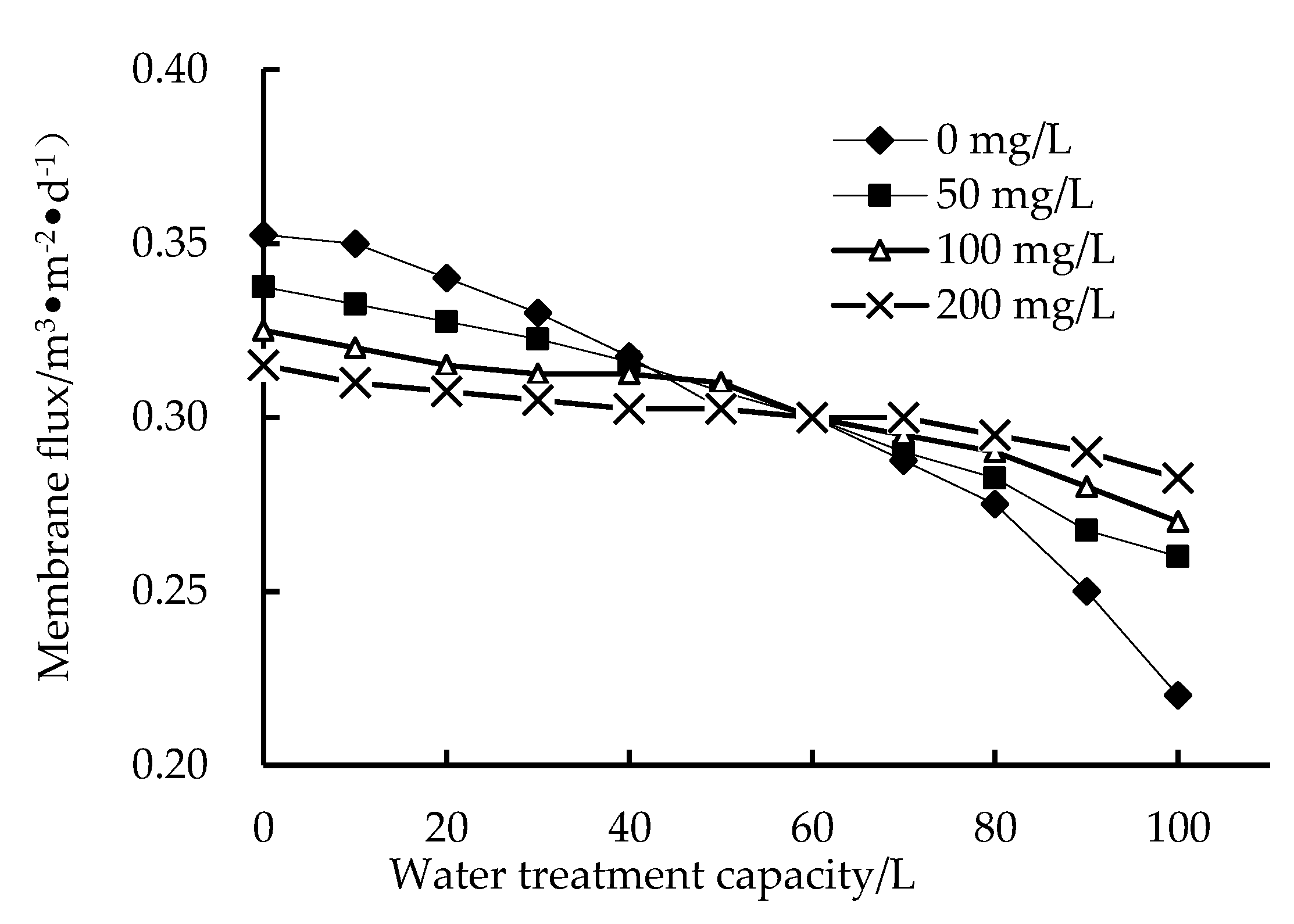
3.1. Effect of PAC on Membrane Flux
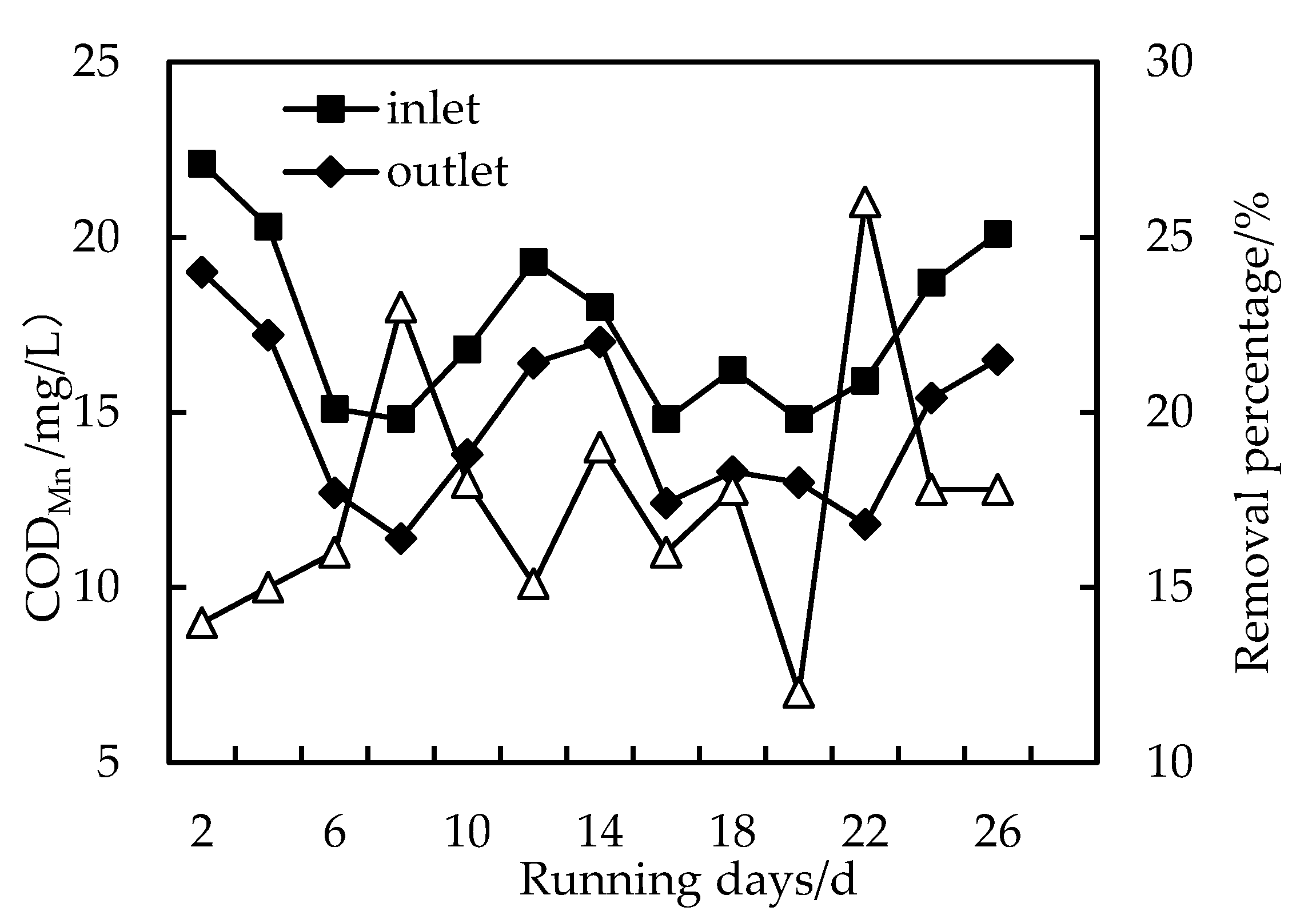
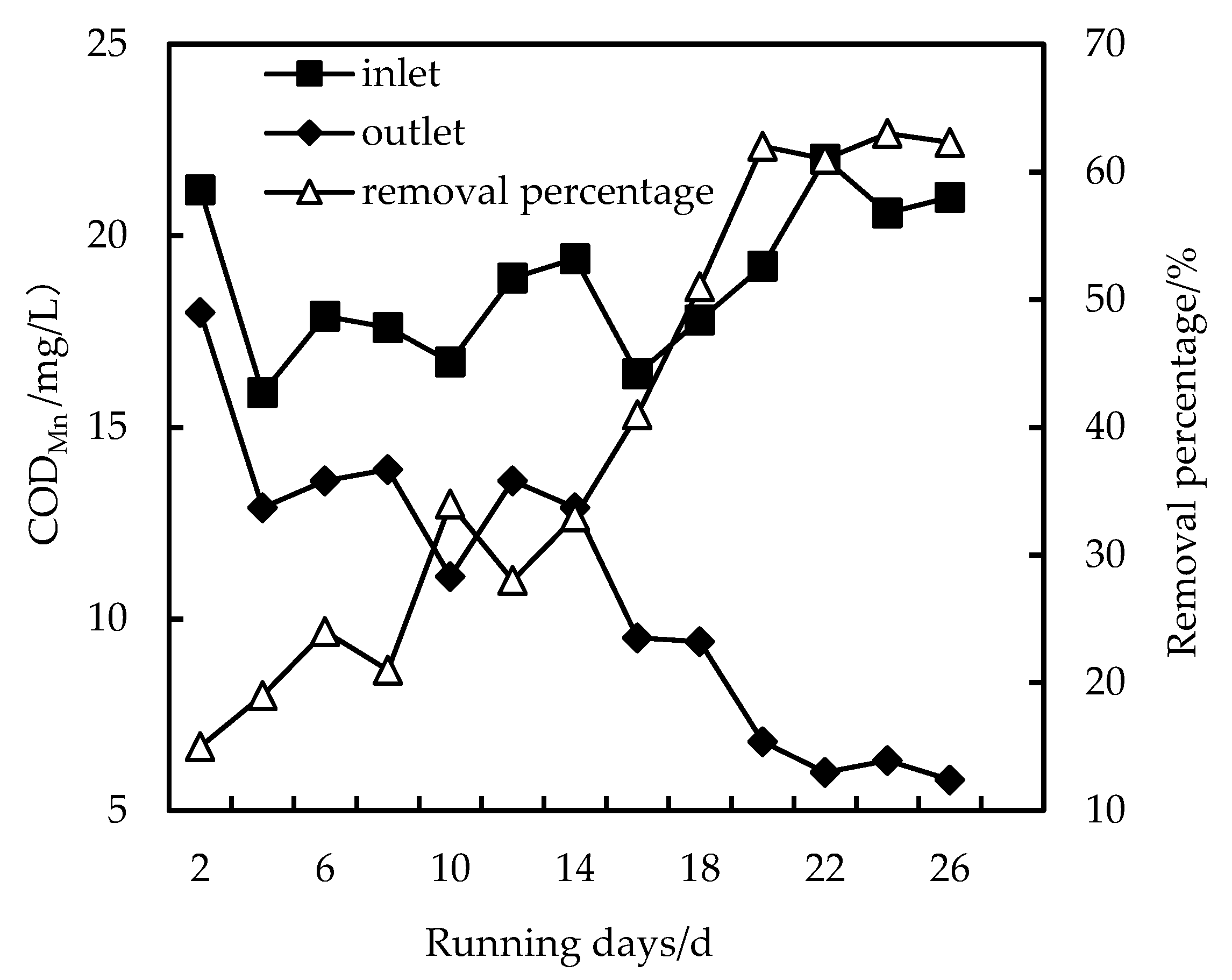
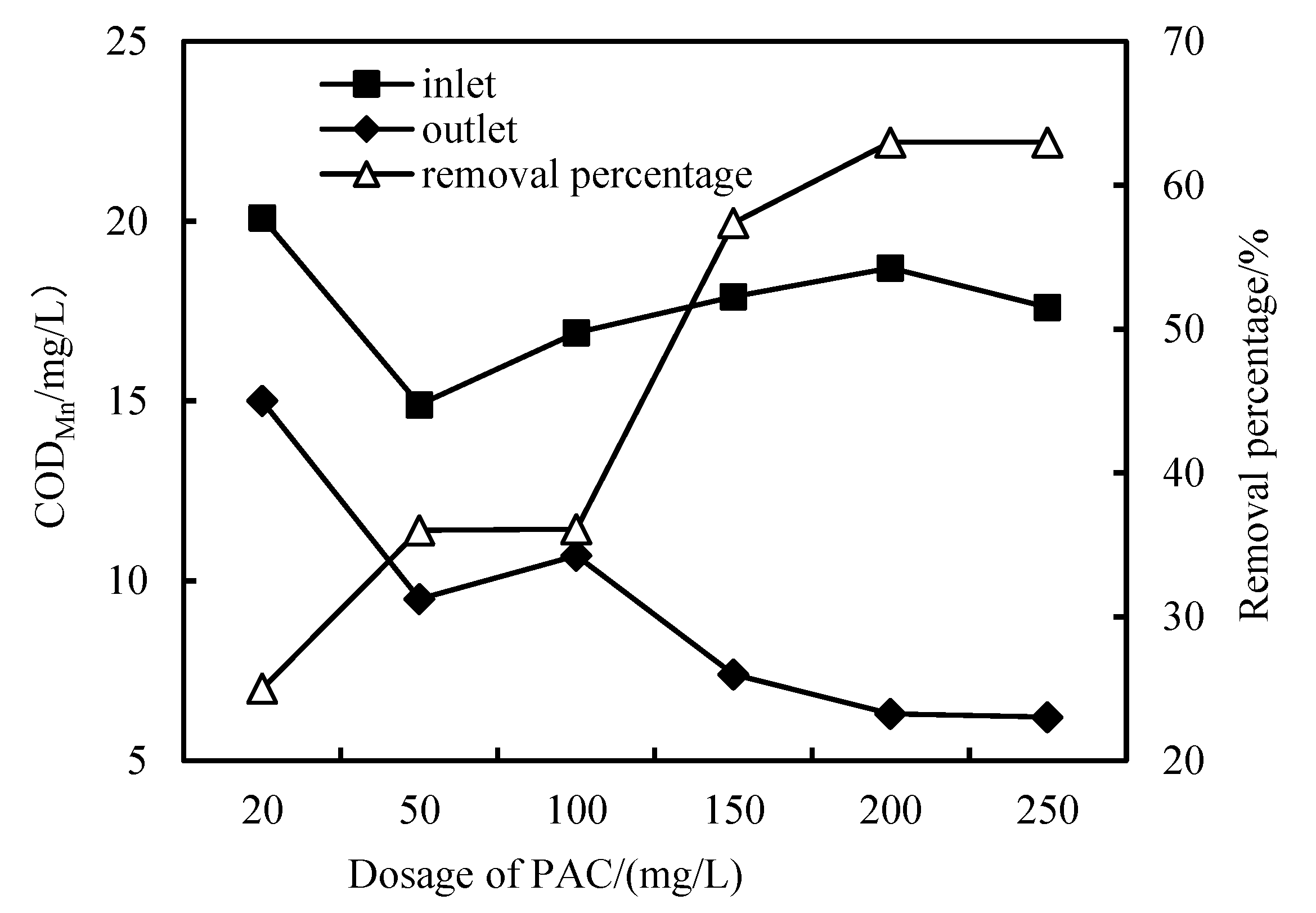
3.2. Removal of CODMn
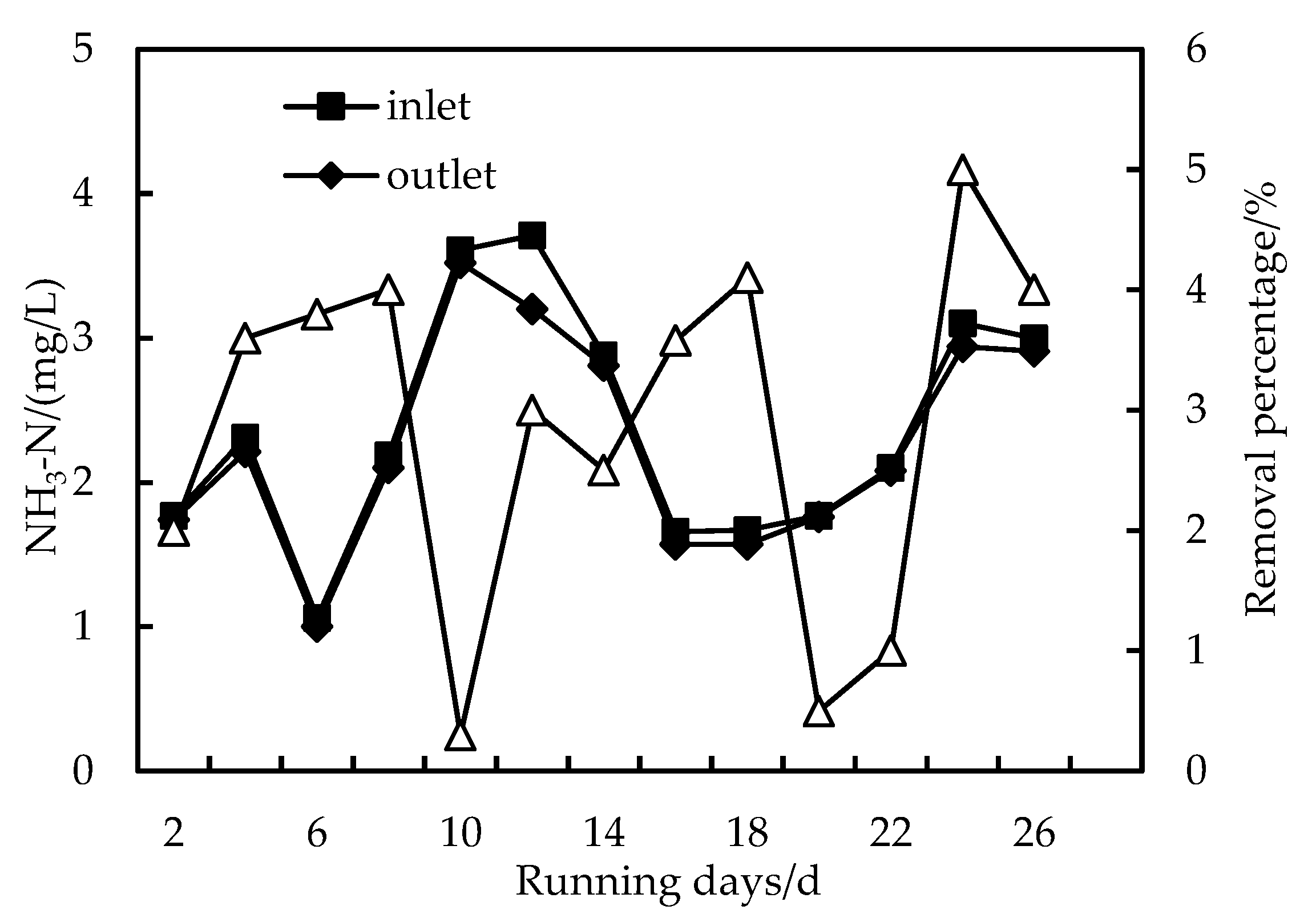
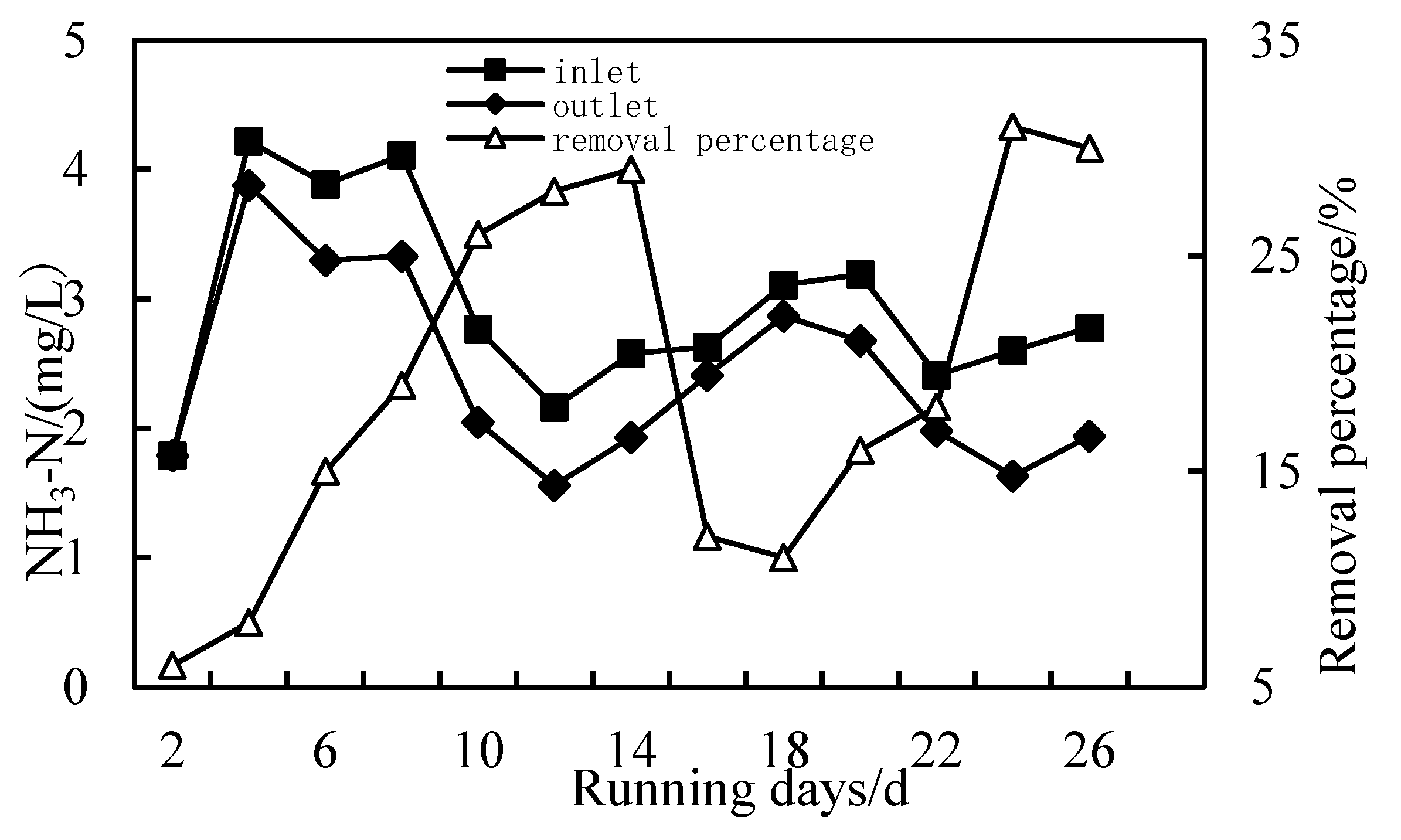
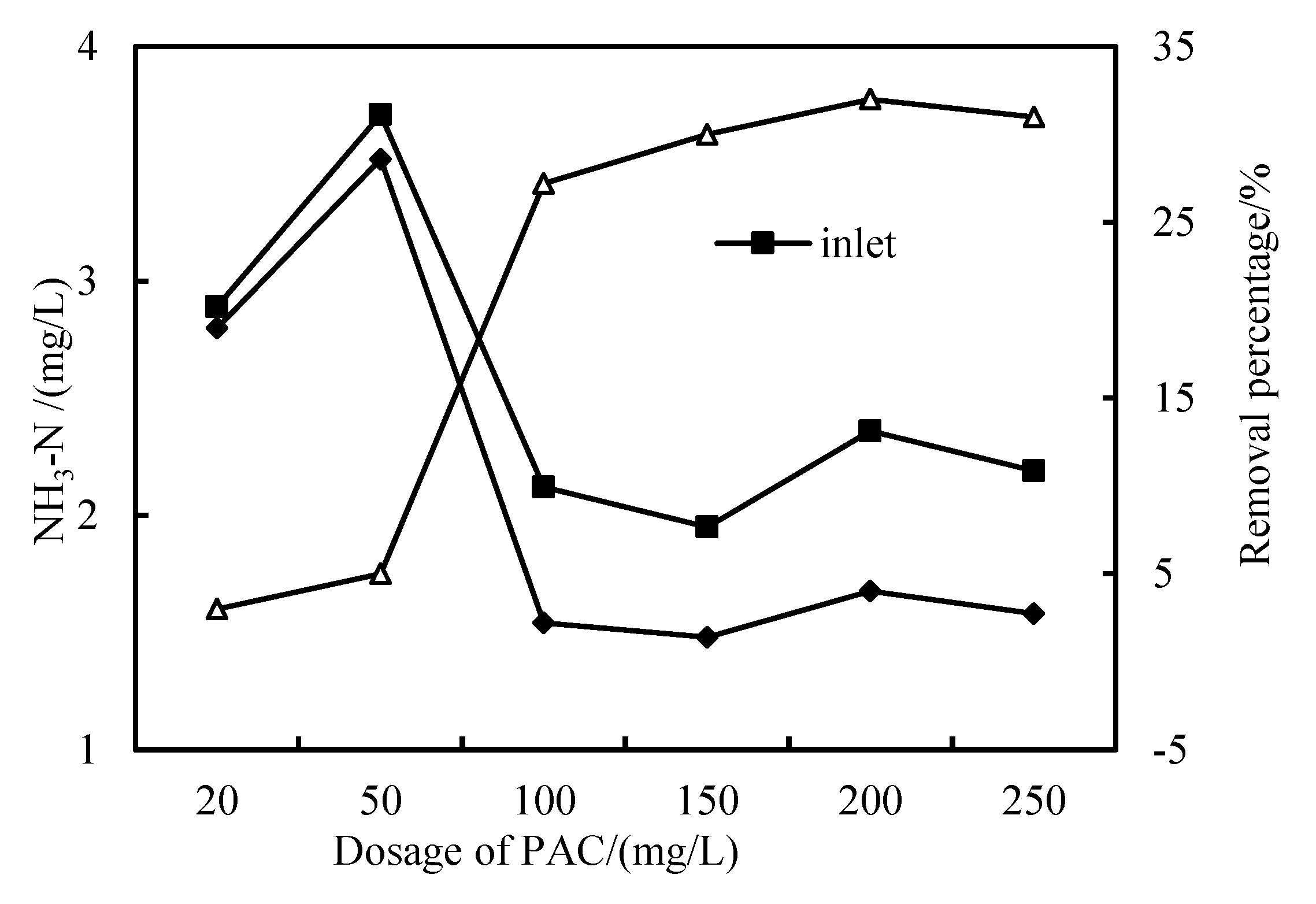
3.3. Removal of NH3-N
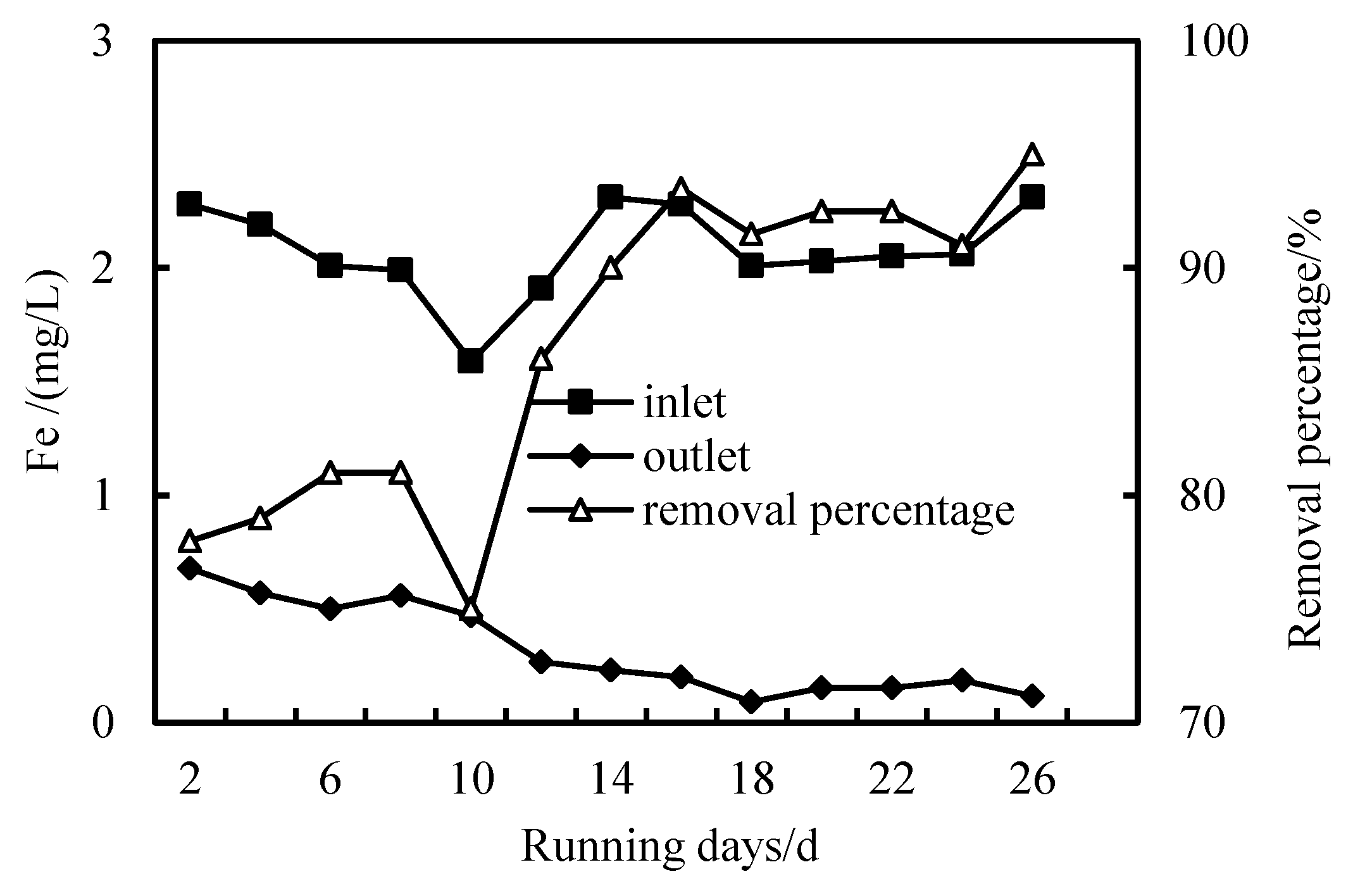
3.4. Removal of Fe Ion
3.5. Removal of SS, Coliform and Turbidity
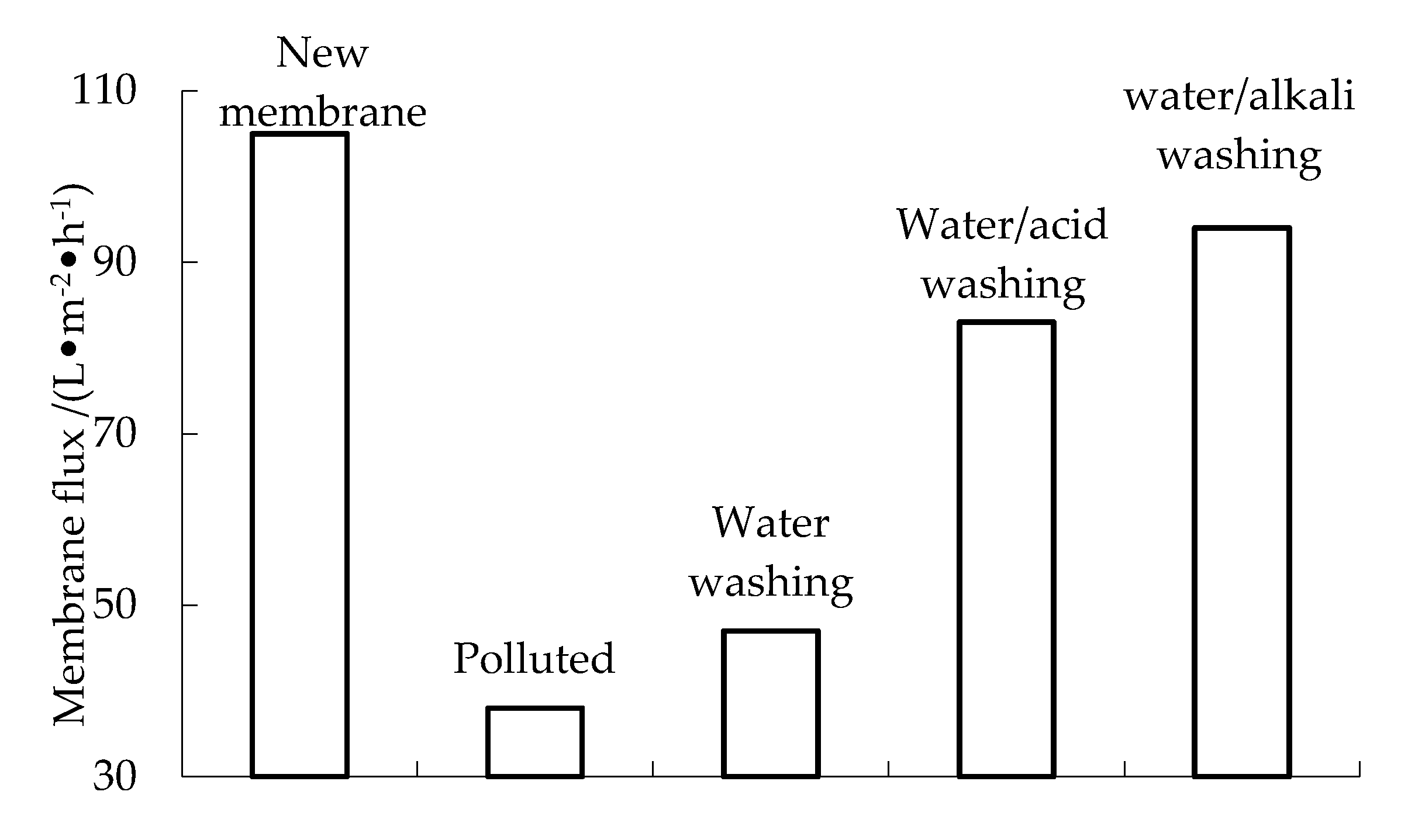
3.6. Membrane Pollution and Cleaning
4. Conclusions
- (1)
- The experimental results show that adding PAC to an HUM system is an effective way to reduce membrane filtration resistance and improve membrane flux.
- (2)
- The removal rates of COD, NH3-N and Fe in wastewater by the combined PAC-HUM process can reach 62%, 32% and 90%, respectively. The best dosage of PAC to achieve a high removal rate is 200 mg/L, 100 mg/L and 150 mg/L for COD, NH3-N and Fe, respectively.
- (3)
- The effluent COD of the combined PAC-HUM process system was about 5.0 mg/L, that of NH3-N was less than 1.5 mg/L, that of Fe was less than 0.5 mg/L, suspended solids (SS) and coliform group were not detected, turbidity was below 0.1 NTU and the water quality was better than the environmental quality standard for surface water (GB3838-2002). The removal effect of organic matter, ammonia nitrogen and iron by PAC combined with HUM was better than that by HUM alone.
- (4)
- For contaminated membranes, water backwashing, water/acid washing and water/alkali washing can restore the membrane flux to 44%, 81% and 88% of that of a new membrane, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chew, C.M.; Aroua, M.K.; Hussain, M.A. Advanced process control for ultrafiltration membrane water treatment system. J. Clean. Prod. 2018, 179, 63–80. [Google Scholar] [CrossRef]
- Qurie, M.; Abbadi, J.; Scrano, L.; Mecca, G.; Bufo, S.A.; Khamis, M.; Karaman, R. Inland Treatment of the Brine Generated from RO Advanced Membrane Wastewater Treatment Plant Using Epuvalisation System This case study investigates the potential of using an epuvalisation system to treat brine generated from an inland RO unit. System 2017, 13, 14. [Google Scholar]
- Qurie, M.; Daghra, S.; Khamis, M.; Kanan, A.; Barghouthi, Z.; Alimari, A.; Karaman, R. Application of the epuvalisation technology for the tertiary treatment of secondary treated effluents using geranium plants. Ann. Agric. Sci. 2019, 64, 237–243. [Google Scholar] [CrossRef]
- Amin, A.; Shaarawy, H.; Hawash, S. Integrated treatment of municipal wastewater using advanced electro-membrane filtration system. SN Appl. Sci. 2019, 1, 1153. [Google Scholar]
- Fan, Y.B.; Wang, J.S.; Jiang, Z.C.; Jiang, M.X.; Xu, K.; Jia, Z.P. Treatment of a dyeing wastewater from a woolen mill using an A/O membrane bio-reactor. J. Environ. Sci. 2000, 12, 344–348. [Google Scholar]
- Iman, Y.E.; Ahmed, N.; Anachh, S.A.M.; Abu Manjur, K. Experimental Investigation on Saline Water Purification Using Reverse Osmosis by a Novus Biomimetic Membrane. J. Biomim. Eng. 2022, 19, 21. [Google Scholar] [CrossRef]
- LaEné, J.M.; Vial, D.; Moulart, P. Status after 10 years of operation-overview of UF technology today. Desalination 2000, 131, 17–25. [Google Scholar] [CrossRef]
- Lipp, P.; Baldauf, G.; Schick, R.; Elsenhans, K.; Stabel, H.-H. Integration of ultrafiltration to conventional drinking water treatment for a better particle removal-efficiency and costs. Desalination 1998, 119, 133–142. [Google Scholar] [CrossRef]
- Yin, H.; Chen, Z.; Chen, Y.; You, Z.; Zhang, J. Pilot Study on Ultrafiltration Treatment of Micro Polluted Reservoir Water. In Paper of the International Seminar on Drinking Water Safety Assurance Technology and Management; Construction Industry Press: Beijing, China, 2005; pp. 525–532. [Google Scholar]
- Jinglun, X. Research on the application of microfiltration and ultrafiltration technology in Macao drinking water treatment. Shanghai Water Aff. 2002, 18, 45–49. [Google Scholar]
- Hagen, K. Removal of particales, bacteria and parasites with ultrafiltration for drinkingwater treatment. Desalination 1998, 119, 85–91. [Google Scholar] [CrossRef]
- Zhifang, F. Experimental study on integrated water purification device of pretreatment+ultrafiltration. J. Shenyang Univ. 2007, 19, 39–42. [Google Scholar]
- Guang, W.; Guangming, Q.; Cuixian, C. Pilot scale experimental study on reuse of reclaimed water in advanced treatment of urban sewage by ultrafiltration membrane. Membr. Sci. Technol. 2004, 24, 40–43. [Google Scholar]
- Ping’an, C.; Tong, L.; Huasheng, Y. Application and research progress of ultrafiltration in drinking water treatment. Ind. Water Wastewater 2006, 37, 7–10. [Google Scholar]
- Rama, K.H.; Rajb, R.E. Mechanical property evaluation of sisal-glass fiber reinforced epoxy polymer composites. Int. J. Mech. Mater. Sci. Res. 2021, 1, 11. [Google Scholar]
- Jacangelo, J.G.; Laîné, J.-M.; Cummings, E.W.; Adham, S.S. UF with Pretreatment for Removing DBP Precursors. J. AWWA 1995, 87, 100–112. [Google Scholar] [CrossRef]
- Xiaolian, W.; Yongzhen, P.; Baozhen, W. Experimental Study on PAC-UF Combined System in Drinking Water Treatment. Water Treat. Technol. 2005, 31, 57–60. [Google Scholar]
- Pei, L.; Dong, B.; Yao, B.H. Drinking Water Treatment by Ultrafiltration and Powdered Activated Carbon Combined Process. Ind. Water Wastewater 2007, 38, 24–27. [Google Scholar]
- Dong, B.Z.; Cao, D.W.; Fan, J.C. Study on the treatment of Huangpu River raw water by powdered activated carbon ultrafiltration membrane. Shanghai Environ. Sci. 2003, 22, 731–737. [Google Scholar]
- Dong, B.Z.; Chen, Y.; Gao, N.Y. Experimental study on micro polluted raw water treatment by powdered activated carbon ultrafiltration membrane. J. Tongji Univ. Nat. Sci. Ed. 2005, 33, 777–780. [Google Scholar]
- Lin, C.-F.; Liu, S.-H.; Hao, O.J. Effect of functional groups of humic substances on UF performance. Water Res. 2001, 35, 395–402. [Google Scholar] [CrossRef]
- Ping, L.I.; Guan, X.J.; Cui-Dan, L.I. Experimental Investigation on Ferrihydrite Promotes the Purification of Slightly Polluted Source Water Containing Arsenic by Wetland Plants. Sci. Technol. Eng. 2018, 312, 10618–10629. [Google Scholar]
- Ba, C. Design of advanced reverse osmosis and nanofiltration membranes for water purification. Diss. Theses Gradworks 2010, 555, 369–378. [Google Scholar]
- Xie, W.; Luo, Z.; Zhou, Y.; Peng, W.; Liu, Q.; Wang, D. Experimental and numerical investigation on opposing plasma synthetic jet for drag reduction. J. Aeronaut. China 2022, 35, 17. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M. Control systems for olive mill wastewater treatment with ultrafiltration and nanofiltration membrane in series based on the boundary flux theory. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2017, 76, 2968. [Google Scholar] [CrossRef] [PubMed]
- Adrian, B. Systems for Treating Water. U.S. Patent EP3148940A4 18 April 2018. [Google Scholar]
- Superuser, S. Bioreactor Systems for Treating Perchlorate-Contaminated Water. In Water Intelligence Online; AwwaRF: Washington, DC, USA, 2005. [Google Scholar]
- Li, Q.; Yan, Z.Q.; Wang, X.L. A poly (sulfobetaine) hollow fiber ultrafiltration membrane for the treatment of oily wastewater. Desalination Water Treat. 2016, 57, 11048–11065. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Chen, S.T.; Sivakumar, M.; Ang, M.B.M.Y.; Patra, T.; Almodovar, J.; Wickramasinghe, S.R.; Hung, W.-S.; Lai, J.-Y. Zwitterionic Polymer Brush Grafted on Polyvinylidene Difluoride Membrane Promoting Enhanced Ultrafiltration Performance with Augmented Antifouling Property. Polymers 2020, 12, 1303. [Google Scholar] [CrossRef]
- Thankappan, H.; Bousquet, G.; Semsarilar, M.; Venault, A.; Chang, Y.; Bouyer, D.; Quemener, D. Development of PVDF Ultrafiltration Membrane with Zwitterionic Block Copolymer Micelles as a Selective Layer. Membranes 2019, 9, 93. [Google Scholar] [CrossRef]
- Li, D.; Sun, X.; Wang, W.; Gao, H.W.; Huang, Y.D.; Gao, C.L. A novel antifouling and thermally stable polysulfone ultrafiltration membranes with sulfobetaine polyimide as porogen. Polym. Adv. Technol. 2020, 32, 945–954. [Google Scholar] [CrossRef]
- GB3838-2002; Environmental Quality Standard for Surface Water of China. Ministry of Environmental Protection of China: Beijing, China, 2002.


| Item | Index |
|---|---|
| True density/(kg/m3) | 2.7 × 1000 |
| Bulk density/(kg/m3) | 0.69 × 1000 |
| Iodine value/(mg/g) | 979 |
| Granularity/% | 94.8 (200 mesh sieve) |
| Particle size/µm | 20~400 |
| Ash content/% | 10.8 |
| Water content/% | 2.3 |
| Item | COD/(mg/L) | TN/(mg/L) | NH3-N/(mg/L) | Turbidity/NTU | Fe/(mg/L) |
|---|---|---|---|---|---|
| Index | 18.1~23.8 | 3.11~7.15 | 2.29~5.01 | 2.12~3.99 | 2.04~4.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, L.; Duo, J. Study on Treatment of Tiny Pollution Water with PAC-HUM System in Kuitun River. Membranes 2022, 12, 1010. https://doi.org/10.3390/membranes12101010
Pei L, Duo J. Study on Treatment of Tiny Pollution Water with PAC-HUM System in Kuitun River. Membranes. 2022; 12(10):1010. https://doi.org/10.3390/membranes12101010
Chicago/Turabian StylePei, Liang, and Jia Duo. 2022. "Study on Treatment of Tiny Pollution Water with PAC-HUM System in Kuitun River" Membranes 12, no. 10: 1010. https://doi.org/10.3390/membranes12101010
APA StylePei, L., & Duo, J. (2022). Study on Treatment of Tiny Pollution Water with PAC-HUM System in Kuitun River. Membranes, 12(10), 1010. https://doi.org/10.3390/membranes12101010






