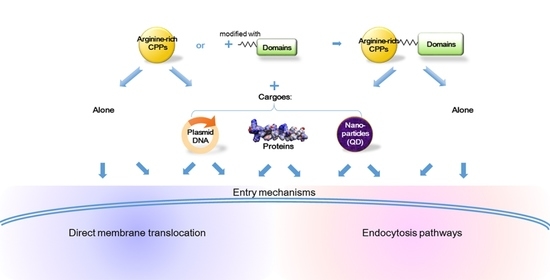
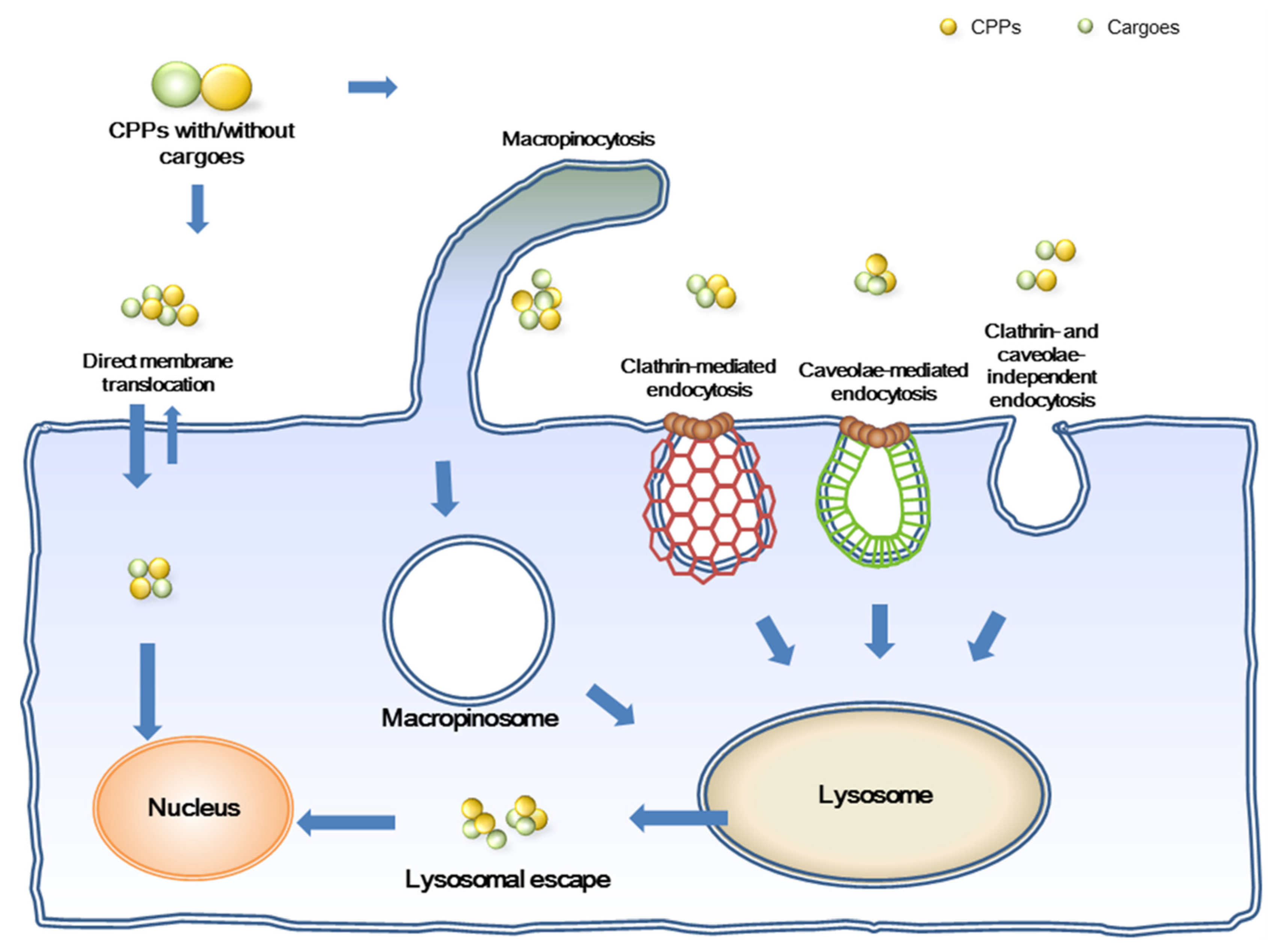
Bio-Membrane Internalization Mechanisms of Arginine-Rich Cell-Penetrating Peptides in Various Species
Abstract
1. Introduction
2. Cell-Penetrating Peptides (CPPs)
3. Mechanisms of Cellular Internalization
3.1. Synthetic Nona-Arginine (SR9)
3.2. Histidine-Rich Nona-Arginine (HR9)
3.3. Pas Nona-Arginine (PR9)
3.4. INF7 Fusion Nona-Arginine (IR9)
4. Evidence of Cellular Internalization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| CPP | Primary Sequence |
|---|---|
| F4R8 | FFFFGRRRRRRRRGC |
| HR9 | CHHHHHRRRRRRRRRHHHHHC |
| IR9 | GLFEAIEGFIENGWEGMIDGWYGRRRRRRRRR |
| L5a | RRWQW |
| NP1 | stearyl-HHHHHHHHHHHHHHHHRRRRRRRR-NH2 |
| Pas2r12 | FFLIGFFLIGRRRRRRRRRRRR |
| PR9 | FFLIPKGRRRRRRRRR |
| SR9 | RRRRRRRRR |
References
- Almeida, P.F. Membrane-active peptides: Binding, translocation, and flux in lipid vesicles. Biochim. Biophys. Acta 2014, 1838, 2216–2227. [Google Scholar] [CrossRef]
- Avci, F.G.; Akbulut, B.S.; Ozkirimli, E. Membrane active peptides and their biophysical characterization. Biomolecules 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zauner, W.; Wagner, E. Application of membrane-active peptides for drug and gene delivery across cellular membranes. Adv. Drug Deliv. Rev. 1998, 34, 21–35. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Rudy, B. Interactions between membranes and cytolytic peptides. Biochim. Biophys. Acta 1986, 864, 123–141. [Google Scholar] [CrossRef]
- Brand, G.D.; Ramada, M.H.S.; Genaro-Mattos, T.C.; Bloch, C., Jr. Towards an experimental classification system for membrane active peptides. Sci. Rep. 2018, 8, 1194. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wang, F. Intracellular transduction and potential of Tat PTD and its analogs: From basic drug delivery mechanism to application. Expert Opin. Drug Deliv. 2012, 9, 457–472. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.; Gautam, A. CPPsite 2.0: A repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2016, 44, D1098–D1103. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Cppsite 2.0: An available database of experimentally validated cell-penetrating peptides predicting their secondary and tertiary structures. J. Mol. Biol. 2021, 433, 166703. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Liu, B.R.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Identification of a short cell-penetrating peptide from bovine lactoferricin for intracellular delivery of DNA in human A549 cells. PLoS ONE 2016, 11, e0150439. [Google Scholar] [CrossRef] [PubMed]
- Stiltner, J.; McCandless, K.; Zahid, M. Cell-penetrating peptides: Applications in tumor diagnosis and therapeutics. Pharmaceutics 2021, 13, 890. [Google Scholar] [CrossRef]
- Yang, W.C.; Patel, K.G.; Lee, J.; Ghebremariam, Y.T.; Wong, H.E.; Cooke, J.P.; Swartz, J.R. Cell-free production of transducible transcription factors for nuclear reprogramming. Biotechnol. Bioeng. 2009, 104, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chou, J.C.; Lee, H.J. Cellular internalization of fluorescent proteins via arginine-rich intracellular delivery peptide in plant cells. Plant Cell Physiol. 2005, 46, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chou, J.C.; Chen, C.P.; Liu, B.R.; Lee, H.J. Noncovalent protein transduction in plant cells by macropinocytosis. New Phytol. 2007, 174, 46–56. [Google Scholar] [CrossRef]
- Chang, M.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Cellular delivery of noncovalently-associated macromolecules by cell-penetrating peptides. Curr. Pharm. Biotechnol. 2014, 15, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Chou, J.C.; Liu, B.R.; Chang, M.; Lee, H.J. Transfection and expression of plasmid DNA in plant cells by an arginine-rich intracellular delivery peptide without protoplast preparation. FEBS Lett. 2007, 581, 1891–1897. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, B.R.; Dai, Y.H.; Lee, C.Y.; Chan, M.H.; Chen, H.H.; Chiang, H.J.; Lee, H.J. A gene delivery system for insect cells mediated by arginine-rich cell-penetrating peptides. Gene 2012, 493, 201–210. [Google Scholar] [CrossRef]
- Dai, Y.H.; Liu, B.R.; Chiang, H.J.; Lee, H.J. Gene transport and expression by arginine-rich cell-penetrating peptides in Paramecium. Gene 2011, 489, 89–97. [Google Scholar] [CrossRef]
- Hou, Y.W.; Chan, M.H.; Hsu, H.R.; Liu, B.R.; Chen, C.P.; Chen, H.H.; Lee, H.J. Transdermal delivery of proteins mediated by non-covalently associated arginine-rich intracellular delivery peptides. Exp. Dermatol. 2007, 16, 999–1006. [Google Scholar] [CrossRef]
- Lee, C.Y.; Li, J.F.; Liou, J.S.; Charng, Y.C.; Huang, Y.W.; Lee, H.J. A gene delivery system for human cells mediated by both a cell-penetrating peptide and a piggyBac transposase. Biomaterials 2011, 32, 6264–6276. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.; Chen, R.L.; Lee, H.J. Induction of apoptosis by gene transfer of human TRAIL mediated by arginine-rich intracellular delivery peptides. Anticancer Res. 2010, 30, 2193–2202. [Google Scholar] [PubMed]
- Liu, B.R.; Chen, H.H.; Chan, M.H.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Three arginine-rich cell-penetrating peptides facilitate cellular internalization of red-emitting quantum dots. J. Nanosci. Nanotechnol. 2015, 15, 2067–2078. [Google Scholar] [CrossRef]
- Liu, B.R.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Comparative mechanisms of protein transduction mediated by cell-penetrating peptides in prokaryotes. J. Membr. Biol. 2015, 248, 355–368. [Google Scholar] [CrossRef]
- Liu, B.R.; Huang, Y.W.; Winiarz, J.G.; Chiang, H.J.; Lee, H.J. Intracellular delivery of quantum dots mediated by a histidine- and arginine-rich HR9 cell-penetrating peptide through the direct membrane translocation mechanism. Biomaterials 2011, 32, 3520–3537. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Lin, M.D.; Chiang, H.J.; Lee, H.J. Arginine-rich cell-penetrating peptides deliver gene into living human cells. Gene 2012, 505, 37–45. [Google Scholar] [CrossRef]
- Liu, B.R.; Liou, J.S.; Chen, Y.J.; Huang, Y.W.; Lee, H.J. Delivery of nucleic acids, proteins, and nanoparticles by arginine-rich cell-penetrating peptides in rotifers. Mar. Biotechnol. 2013, 15, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Liou, J.S.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Intracellular delivery of nanoparticles and DNAs by IR9 cell-penetrating peptides. PLoS ONE 2013, 8, e64205. [Google Scholar] [CrossRef]
- Liu, B.R.; Lo, S.Y.; Liu, C.C.; Chyan, C.L.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Endocytic trafficking of nanoparticles delivered by cell-penetrating peptides comprised of nona-arginine and a penetration accelerating sequence. PLoS ONE 2013, 8, e67100. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Winiarz, J.G.; Moon, J.S.; Lo, S.Y.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Synthesis, characterization and applications of carboxylated and polyethylene-glycolated bifunctionalized InP/ZnS quantum dots in cellular internalization mediated by cell-penetrating peptides. Colloids Surf. B Biointerfaces 2013, 111, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Chiang, H.J.; Huang, Y.W.; Chan, M.H.; Chen, H.H.; Lee, H.J. Cellular internalization of quantum dots mediated by cell-penetrating peptides. Pharm. Nanotechnol. 2013, 1, 151–161. [Google Scholar]
- Xu, Y.; Liu, B.R.; Lee, H.J.; Shannon, K.B.; Winiarz, J.G.; Wang, T.C.; Chiang, H.J.; Huang, Y.W. Nona-arginine facilitates delivery of quantum dots into cells via multiple pathways. J. Biomed. Biotechnol. 2010, 2010, 948543. [Google Scholar] [CrossRef]
- Lu, S.W.; Hu, J.W.; Liu, B.R.; Lee, C.Y.; Li, J.F.; Chou, J.C.; Lee, H.J. Arginine-rich intracellular delivery peptides synchronously deliver covalently and noncovalently linked proteins into plant cells. J. Agric. Food Chem. 2010, 58, 2288–2294. [Google Scholar] [CrossRef]
- Hu, J.W.; Liu, B.R.; Wu, C.Y.; Lu, S.W.; Lee, H.J. Protein transport in human cells mediated by covalently and noncovalently conjugated arginine-rich intracellular delivery peptides. Peptides 2009, 30, 1669–1678. [Google Scholar] [CrossRef]
- Farrera-Sinfreu, J.; Giralt, E.; Castel, S.; Albericio, F.; Royo, M. Cell-penetrating cis-gamma-amino-l-proline-derived peptides. J. Am. Chem. Soc. 2005, 127, 9459–9468. [Google Scholar] [CrossRef]
- Illa, O.; Ospina, J.; Sánchez-Aparicio, J.E.; Pulido, X.; Abengozar, M.; Gaztelumendi, N.; Carbajo, D.; Nogués, C.; Rivas, L.; Maréchal, J.D.; et al. Hybrid cyclobutane/proline-containing peptidomimetics: The conformational constraint influences their cell-penetration ability. Int. J. Mol. Sci. 2021, 22, 5092. [Google Scholar] [CrossRef]
- Ezzat, K.; Andaloussi, S.E.; Zaghloul, E.M.; Lehto, T.; Lindberg, S.; Moreno, P.M.; Viola, J.R.; Magdy, T.; Abdo, R.; Guterstam, P.; et al. PepFect 14, a novel cell-penetrating peptide for oligonucleotide delivery in solution and as solid formulation. Nucleic Acids Res. 2011, 39, 5284–5298. [Google Scholar] [CrossRef]
- Pae, J.; Säälik, P.; Liivamägi, L.; Lubenets, D.; Arukuusk, P.; Langel, Ü.; Pooga, M. Translocation of cell-penetrating peptides across the plasma membrane is controlled by cholesterol and microenvironment created by membranous proteins. J. Control. Release 2014, 192, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Vivès, E.; Brodin, P.; Lebleu, B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Torchilin, V.P. Cell-penetrating peptides: Breaking through to the other side. Trends Mol. Med. 2012, 18, 385–393. [Google Scholar] [CrossRef]
- Layek, B.; Lipp, L.; Singh, J. Cell penetrating peptide conjugated chitosan for enhanced delivery of nucleic acid. Int. J. Mol. Sci. 2015, 16, 28912–28930. [Google Scholar] [CrossRef]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Wadia, J.S.; Stan, R.V.; Dowdy, S.F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004, 10, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I. Revisiting the role of clathrin-mediated endoytosis in synaptic vesicle recycling. Front. Cell. Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Crosio, M.A.; Via, M.A.; Cámara, C.I.; Mangiarotti, A.; Del Pópolo, M.G.; Wilke, N. Interaction of a polyarginine peptide with membranes of different mechanical properties. Biomolecules 2019, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Vazdar, M.; Heyda., J.; Mason, P.E.; Tesei, G.; Allolio, C.; Lund, M.; Jungwirth, P. Arginine “Magic”: Guanidinium like-charge ion pairing from aqueous salts to cell penetrating peptides. Acc. Chem. Res. 2018, 51, 1455–1464. [Google Scholar] [CrossRef]
- Verbeek, S.F.; Awasthi, N.; Teiwes, N.K.; Mey, I.; Hub, J.S.; Janshoff, A. How arginine derivatives alter the stability of lipid membranes: Dissecting the roles of side chains, backbone and termini. Eur. Biophys. J. 2021, 50, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S. Arginine-rich peptides: Potential for intracellular delivery of macromolecules and the mystery of the translocation mechanisms. Int. J. Pharm. 2002, 245, 1–7. [Google Scholar] [CrossRef]
- Melikov, K.; Hara, A.; Yamoah, K.; Zaitseva, E.; Zaitsev, E.; Chernomordik, L.V. Efficient entry of cell-penetrating peptide nona-arginine into adherent cells involves a transient increase in intracellular calcium. Biochem. J. 2015, 471, 221–230. [Google Scholar] [CrossRef][Green Version]
- Almeida, C.; Maniti, O.; Di Pisa, M.; Swiecicki, J.M.; Ayala-Sanmartin, J. Cholesterol re-organisation and lipid de-packing by arginine-rich cell penetrating peptides: Role in membrane translocation. PLoS ONE 2019, 14, e0210985. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Irani, S.; Bolhassani, A.; Sadat, S.M. HR9: An important cell penetrating peptide for delivery of HCV NS3 DNA into HEK-293T cells. Avicenna J. Med. Biotechnol. 2020, 12, 44–51. [Google Scholar] [PubMed]
- Rostami, B.; Irani, S.; Bolhassani, A.; Cohan, R.A. Gene and protein delivery using four cell penetrating peptides for HIV-1 vaccine development. IUBMB Life 2019, 71, 1619–1633. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Wang, F.; Ding, Y.; Wang, T.; Jin, G.; Martz, M.; Gui, Z.; Ouyang, P.; Chen, P. Histidine-rich cell-penetrating peptide for cancer drug delivery and its uptake mechanism. Langmuir 2019, 35, 3513–3523. [Google Scholar] [CrossRef]
- Liu, B.R.; Huang, Y.W.; Korivi, M.; Lo, S.Y.; Aronstam, R.S.; Lee, H.J. The primary mechanism of cellular internalization for a short cell-penetrating peptide as a nano-scale delivery system. Curr. Pharm. Biotechnol. 2017, 18, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Nakase, I.; Michiue, H.; Takeuchi, T.; Tomizawa, K.; Matsui, H.; Futaki, S. Enhanced intracellular delivery using arginine-rich peptides by the addition of penetration accelerating sequences (Pas). J. Control. Release 2009, 138, 128–133. [Google Scholar] [CrossRef]
- Okuda, A.; Futaki, S. Protein delivery to cytosol by cell-penetrating peptide bearing tandem repeat penetration-accelerating sequence. Methods Mol. Biol. 2022, 2383, 265–273. [Google Scholar]
- Okuda, A.; Tahara, S.; Hirose, H.; Takeuchi, T.; Nakase, I.; Ono, A.; Takehashi, M.; Tanaka, S.; Futaki, S. Oligoarginine-bearing tandem repeat penetration-accelerating sequence delivers protein to cytosol via caveolae-mediated endocytosis. Biomacromolecules 2019, 20, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Akita, T.; Kimura, R.; Akaguma, S.; Nagai, M.; Nakao, Y.; Tsugane, M.; Suzuki, H.; Oka, J.I.; Yamashita, C. Usefulness of cell-penetrating peptides and penetration accelerating sequence for nose-to-brain delivery of glucagon-like peptide-2. J. Control. Release 2021, 335, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Hirose, H.; Tanaka, G.; Pujals, S.; Katayama, S.; Nakase, I.; Futaki, S. Effect of the attachment of a penetration accelerating sequence and the influence of hydrophobicity on octaarginine-mediated intracellular delivery. Mol. Pharm. 2012, 9, 1222–1230. [Google Scholar] [CrossRef]
- Algayer, B.; O’Brien, A.; Momose, A.; Murphy, D.J.; Procopio, W.; Tellers, D.M.; Tucker, T.J. Novel pH selective, highly lytic peptides based on a chimeric influenza hemagglutinin peptide/cell penetrating peptide motif. Molecules 2019, 24, 2079. [Google Scholar] [CrossRef]
- Cross, K.J.; Wharton, S.A.; Skehel, J.J.; Wiley, D.C.; Steinhauer, D.A. Studies on influenza haemagglutinin fusion peptide mutants generated by reverse genetics. EMBO J. 2001, 20, 4432–4442. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.S.; Liu, B.R.; Martin, A.L.; Huang, Y.W.; Chiang, H.J.; Lee, H.J. Protein transduction in human cells is enhanced by cell-penetrating peptides fused with an endosomolytic HA2 sequence. Peptides 2012, 37, 273–284. [Google Scholar] [CrossRef]
- Neundorf, I.; Rennert, R.; Hoyer, J.; Schramm, F.; Löbner, K.; Kitanovic, I.; Wölfl, S. Fusion of a short HA2-derived peptide sequence to cell-penetrating peptides improves cytosolic uptake, but enhances cytotoxic activity. Pharmaceuticals 2009, 2, 49–65. [Google Scholar] [CrossRef]
- Habault, J.; Poyet, J.L. Recent advances in cell penetrating peptide-based anticancer therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef]
- Kamei, N.; Morishita, M.; Takayama, K. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides. J. Control. Release 2009, 136, 179–186. [Google Scholar] [CrossRef]
- Morishita, M.; Kamei, N.; Ehara, J.; Isowa, K.; Takayama, K. A novel approach using functional peptides for efficient intestinal absorption of insulin. J. Control. Release 2007, 118, 177–184. [Google Scholar] [CrossRef]
- Nielsen, E.J.B.; Yoshida, S.; Kamei, N.; Iwamae, R.; Khafagy, E.-S.; Olsen, J.; Rahbek, U.L.; Pedersen, B.L.; Takayama, K.; Takeda-Morishita, M. In vivo proof of concept of oral insulin delivery based on a co-administration strategy with the cell-penetrating peptide penetratin. J. Control. Release 2014, 189, 19–24. [Google Scholar] [CrossRef] [PubMed]
| CPPs | Peptide Sequences | Cargoes | Entrance Targets | Mechanisms | References |
|---|---|---|---|---|---|
| SR9 | RRRRRRRRR | — | artificial large unilamellar vesicles (LUVs) in 100 nm diameter | lipid raft | [54] |
| plasmid DNAs | plant tissues | macropinocytosis | [19] | ||
| A549 cells, Sf9 cells, paramecia | unknown | [20,21,23,24,28] | |||
| proteins | A549 cells, plant epidermal cells, mouse skin cells | macropinocytosis | [17,22] | ||
| nanoparticles | A549 cells | multiple pathways | [33,34] | ||
| prokaryotes | macropinocytosis | [26] | |||
| HR9 | CHHHHHRRRRRRRRRHHHHHC | — | A549 cells, rotifers, paramecia | unknown | [21,28,29] |
| plasmid DNAs | A549 cells | direct membrane translocation | [29] | ||
| HEK293T cells, Sf9 cells, rotifers, paramecia, mice | unknown | [20,21,29,55,56] | |||
| proteins | rotifers | unknown | [29] | ||
| nanoparticles | A549 cells | direct membrane translocation | [25,27,33] | ||
| rotifers | unknown | [29] | |||
| PR9 | FFLIPKGRRRRRRRRR | plasmid DNAs | A549 cells, Sf9 cells, paramecia | unknown | [20,21,28,31] |
| nanoparticles | A549 cells | classical endocytosis | [25,27,31,32,33] | ||
| IR9 | GLFEAIEGFIENGWEGMIDGWYGRRRRRRRRR | — | A549 cells | macropinocytosis | [30] |
| rotifers | unknown | [29] | |||
| plasmid DNAs | A549 cells | classical endocytosis | [30] | ||
| rotifers | unknown | [29] | |||
| proteins | rotifers | unknown | [29] | ||
| nanoparticles | A549 cells | classical endocytosis | [30] | ||
| rotifers | unknown | [29] |
| CPP | Additional Modified Domain | Net Charge at pH 7.0 1 | Hydrophilicity 1 | Hydrophobicity 2 | pI 2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Full Sequence | Domain | Full Sequence | Domain | Full Sequence | Domain | Full Sequence | Domain | ||
| SR9 | — | +9.0 | — | 3.0 | — | 0.77 | — | 13.4 | — |
| HR9 | polyhistidine | +9.82 | +0.82 | 0.95 | −0.58 | −25.32 | −6.34 | 12.8 | 7.5 |
| PR9 | reverted cathepsin D | +9.95 | +1.0 | 1.2 | −0.8 | 19.74 | 31.52 | 13.0 | 10.1 |
| IR9 | INF7 domain | +4.0 | −5.0 | 0.6 | −0.34 | 52.25 | 65.73 | 11.9 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.R.; Chiou, S.-H.; Huang, Y.-W.; Lee, H.-J. Bio-Membrane Internalization Mechanisms of Arginine-Rich Cell-Penetrating Peptides in Various Species. Membranes 2022, 12, 88. https://doi.org/10.3390/membranes12010088
Liu BR, Chiou S-H, Huang Y-W, Lee H-J. Bio-Membrane Internalization Mechanisms of Arginine-Rich Cell-Penetrating Peptides in Various Species. Membranes. 2022; 12(1):88. https://doi.org/10.3390/membranes12010088
Chicago/Turabian StyleLiu, Betty Revon, Shiow-Her Chiou, Yue-Wern Huang, and Han-Jung Lee. 2022. "Bio-Membrane Internalization Mechanisms of Arginine-Rich Cell-Penetrating Peptides in Various Species" Membranes 12, no. 1: 88. https://doi.org/10.3390/membranes12010088
APA StyleLiu, B. R., Chiou, S.-H., Huang, Y.-W., & Lee, H.-J. (2022). Bio-Membrane Internalization Mechanisms of Arginine-Rich Cell-Penetrating Peptides in Various Species. Membranes, 12(1), 88. https://doi.org/10.3390/membranes12010088