Abstract
In this research, electrolyte-insulator-semiconductor (EIS) capacitors with Sb2O3 sensing membranes were fabricated. The results indicate that Mg doping and Ti-doped Sb2O3 membranes with appropriate annealing had improved material quality and sensing performance. Multiple material characterizations and sensing measurements of Mg-doped and Ti doping on Sb2O3 sensing membranes were conducted, including of X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM). These detailed studies indicate that silicate and defects in the membrane could be suppressed by doping and annealing. Moreover, compactness enhancement, crystallization and grainization, which reinforced the surface sites on the membrane and boosted the sensing factor, could be achieved by doping and annealing. Among all of the samples, Mg doped membrane with annealing at 400 °C had the most preferable material properties and sensing behaviors. Mg-doped Sb2O3-based with appropriate annealing are promising for future industrial ionsensing devices and for possible integration with Sb2O3-based semiconductor devices.
1. Introduction
Over the past fifty years, growing attention has been paid to the development of the chemical sensing of ion concentrations in various solutions. Measurements of ion concentrations, such as pH sensing, are crucial to monitor human health, food safety and environmental pollution. Owing to rapid detection, fast response and reliable long-term operations, semiconductor-based ion sensitive devices have been proposed, such as ion-sensitive field-effect transistors (ISFETs), electrolyte-insulator -semiconductor (EIS) capacitors and light-addressable potentiometric sensors (LAPS-) [1,2]. Among these devices, EIS capacitors, with their advantages of compact size, low cost and simple fabrication, have been demonstrated as multianalyte ion and solute sensing devices [3,4]. In an EIS capacitor, the key component is the sensing membrane, capable of detecting ions in solutions. For conventional EIS devices, SiO2 is commonly used as the dielectric for the membrane, though materials such as Ta2O5 [5], Gd2O3 [6] and Zr2O3 have also been studied as possible replacements for traditional SiO2. In order to further boost the EIS capacitor’s performance and provide the possibility for future device integration, it is worthwhile to explore new materials and treatments for the membrane material in order to fabricate EIS capacitors with pH-sensing behaviors. Based on previous reports [7,8], Sb2O3 has been used for transparent conducting films, varistors and photocatalysts. In addition, the usage of Sb2O3 can improve electrical and optical device properties. Based on recent studies [9,10,11,12,13,14,15,16,17,18,19] Sb2O3 has been used for gas and liquid solution sensors. However, Sb2O3 EIS sensors have not been clearly reported yet. Compared with conventional semiconductor materials, Sb2O3 with a wide band gap of around 3 eV [20] can withstand high breakdown voltage. As for new treatments, incorporating various atoms such as F [21], N [22], Ti [23] and Mg [24] by plasma treatment [25] or co-sputtering [26] into EIS membranes to strengthen their material quality has been intensively investigated. For foreign atoms dopings, Ti doping and Mg doping via cosputtering can optimize the EIS sensor’s sensing behaviors because the incorporated atoms can passivate defects. Moreover, Mg doping could further boost the sensitivity and reliability of EIS sensors, owing to the decrease of the double-layer capacitance in the solution and sensing factor enhancement. Until now, however, comparison of the effects of Mg doping and Ti doping on EIS membranes has not been demonstrated. In this study, Mg-doped and Ti-doped Sb2O3 were fabricated as membranes with excellent sensing performance. However, EIS capacitors with Mg-doped or Ti-doped Sb2O3 [27,28,29,30,31,32,33,34,35] as the membrane material have not been clearly reported yet. Since Mg has low electron affinity and low electronegativity, and the radius of Mg2+ is similar to that of Sb3+, Mg can perfectly replace the lattice site of Sb [36,37,38,39,40,41,42,43]. In addition, many studies have also shown that Mg doping can increase the energy gap and reduce redundant oxygen vacancies. Moreover, annealing treatment in an O2 ambient can further improve sensing performance and device reliability. This is because filling the oxygen vacancy and reducing the defects in an oxygen environment repairs the dangling bonds and releases the strain bonds, while more oxygen atoms are added to the surface and the oxygen vacancies in the lattice are filled. Therefore, Mg-doped Sb2O3 EIS sensors with annealing at 400 °C can achieve a high sensitivity of 60.17 mV/pH, which is above the Nernst limit [44]. To gain insight into the effects of annealing, multiple material analyses including X-ray diffraction (XRD), X-ray photoelectron sptroscopy (XPS) and field-effect scanning electron microscopy (FESEM) were used to examine the surface morphologies and material properties of Sb2O3 membranes. Material characterizations reveal that annealing at an appropriate temperature can enhance Sb2O3 crystallization and Ti doping of the Sb2O3, and Mg doped of the Sb2O3 can suppress the formation of silicate grainization. Therefore, the EIS pH-sensing capability could be boosted and reliability issues such as hysteresis and drift voltage can be mitigated. Mg-doped Sb2O3-based EIS capacitors are promising for versatile integration in chemical ion-sensing applications with other Sb2O3-based devices [45,46,47,48].
2. Experimental
To fabricate Sb2O3-based EIS capacitors, the films were deposited on 4-inch n-type (100) wafers with a resistivity of 5–10 Ω-cm. The Sb2O3 was sputtered, and Mg or Ti was co-sputtered on the wafers in an Ar:O2 = 20:5 ambient, respectively. The RF power was l00 W and the pressure inside the chamber was l0 mTorr. Based on the measurements, the thickness of the undoped Sb2O3 was 61.53 nm, the thickness of Mg-doped Sb2O3 was 63.40 nm and the thickness of the Ti-doped Sb2O3 was 69.25 nm. The rapid thermal annealing (RTA) process was carried out for 30 s in O2 ambient at temperatures of 400, 500 and 600 °C, respectively. Then, a 300 nm aluminum film was deposited on the backside of the wafer. The backside aluminum film was grown by e-beam evaporation. After that, an epoxy bond was used to determine the sensing area. Finally, silver gel was used to attach samples on the copper wires of the printed circuit board (PCB). The detailed EIS structure is shown in Figure 1.
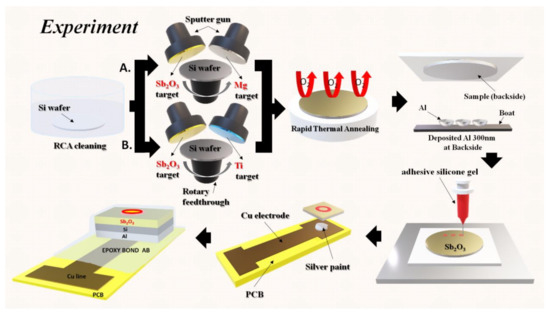
Figure 1.
A diagram of the fabrication process including, RCA cleaning, co-sputtering, rapid thermal annealing, aluminum film backside deposition, sensing area definition and the attachment of the sensing chip onto the PCB board.
Analyses using CV measurements were performed. Using a reference capacitance of 0.4 Cmax in the CV curves, the correlation between the substrate bias and the electrolyte concentration was calculated. In addition, the change of substrate bias voltage variation caused by the change of electrolyte concentration can be illustrated by the site-binding model [49,50], with the flat band voltage shift proportional to the electrolyte concentration, as in the following equation:
is the reference electrode potential, and is the surface dipole potential of the solution. is the work function of silicon, and is the liquid junction potential difference. All of the terms in the equation are constant, except for , which makes the membrane sensitive to the electrolyte due to polarization and forms the potential barrier. Furthermore, is closely related to the surface sites on the membrane.
Moreover, the hydrogen ionic reaction with the membrane interface is illustrated in the site-binding model shown in Equation (2) [51,52,53]. The surface potential (ψ) can be related to the membrane parameter β. k is Boltzmann’s constant, q is the elementary charge, T is the temperature, pHpzc is the pH value with zero charge on the surface and β is a factor that points to the sensitivity of the gate membrane.
Furthermore, β is closely related to the density of surface hydroxyl groups, as shown in (3). Ns is the number of surface sites per unit surface area and CDL is the double layer capacitance, according to the Gouy–Chapman–Stern model [54].
In this research, Mg-doped Sb2O3-based EIS capacitors were demonstrated as the best pH-sensing devices, as shown in Figure 2. The tighter inner layer becomes the Helmholtz layer, which causes the positive charge to be absorbed by the Helmholtz layer and is not affected by the potential difference.
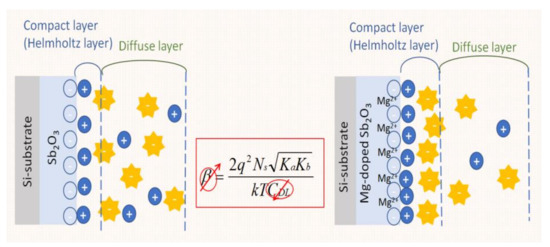
Figure 2.
An illustration of the Mg co-sputtered on the Sb2O3-based EIS capacitor.
Therefore, the outer layer is still a diffusion layer, and the resulting capacitance CDL is reduced. It is known from the Moss-Berstein effect that, when the capacitance CDL decreases, the β value increases, so, the higher β is, the better the sensing capability will be [55,56].
Based on (3), the higher the surface site density of Ns is, the higher β is and the better the sensing capability will be. The grainization, crystallization and compactness of the material structure on the sensing membrane may reinforce the quality and quantity of the surface sites.
3. Results and Discussion
To investigate the effects of Ti doping and Mg doping with annealing on a Sb2O3 membrane, XRD was used to monitor the crystalline phases of the differently treated films. The XRD patterns of the undoped, Ti-doped and Mg-doped samples are shown in Figure 3a–c, respectively. As shown in Figure 3a–c, Sb2O3 (222), (400) and (440) phases can be observed in the XRD patterns of all of the samples. Among the undoped samples, the film annealed at 500 °C had the strongest crystalline phases. In addition, among Ti-doped and Mg-doped samples, the samples annealed at 400 °C had the strongest crystallized phases. Furthermore, Ti-doped and Mg-doped samples had stronger XRD peaks than the undoped samples, indicating that the combination of doping could further enhance the crystallization. However, as the anneal temperature increased to 400 and 500 °C, all of the Sb2O3 peaks increased, indicative of the crystalline phases strengthening and the crystallization enhancing. As the annealing temperature increased to 600 °C, the Sb2O3 decreased, and the crystalline structures might be deteriorated in undoped, Ti-doped and Mg-doped samples owing to the RTA pH-sensing devices at 600 °C.
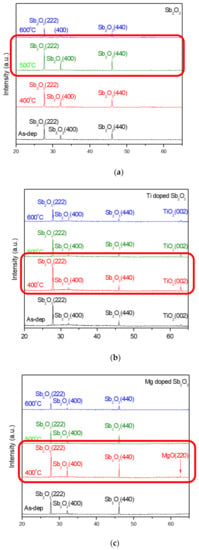
Figure 3.
XRD patterns of (a) the undoped Sb2O3 films, (b) Ti-doped Sb2O3 films and (c) Mg-doped Sb2O3 films annealed at various temperatures in O2 ambient for 30 s.
Furthermore, to monitor the chemical bindings and element compositions, the O 1s XPS analysis was performed on the undoped, Ti-doped and Mg-doped samples, as shown in Figure 4a–c, respectively. Figure 4a reveals that an RTA at an appropriate temperature of 500 °C could effectively suppress the formation of silicate and optimize the sensing device’s performance. However, as the annealing temperature further increased to 600 °C, the amount of silicate increased again. Similarly, annealing at 400 °C could effectively suppress the formation of silicate, as shown in the XPS spectra of Ti-doped and Mg-doped samples, as shown in Figure 4b,c. Moreover, annealing could strengthen Sb-O-Ti bonds and Sb-O-Mg bonds in Ti-doped and Mg-doped samples, respectively. Furthermore, the Sb 3d XPS analysis was performed on the undoped, Ti doped and Mg doped samples, as shown in Figure 4d–f, respectively. The Sb-O doublet peaks can be observed on these spectra. The Sb 3d XPS spectra also show that the Sb-O bonds could be strengthened with an appropriate annealing temperature of 500 °C for the undoped sample, as shown in Figure 4d. In addition, Mg doping could further enhance the Sb-O bonds in terms of Sb-O doublet peaks compared with the undoped samples. Consistent with the XRD patterns, silicate could be mitigated and chemical bindings could be enhanced by annealing and Mg doping [57]. Since XPS could complement the XRD analysis and was the technique used to identify the presence of the Mg and Ti bindings, the measurements of the Ti 2p XPS spectra for Ti-doped samples, as shown in Figure 4g, and the Mg 2p spectra for Mg-doped samples, as shown in Figure 4h, were performed, respectively. The results indicate that the XPS peaks for Ti and Mg did not vary a lot in various annealing conditions, but the peaks did represent the presence of Mg and Ti binding.
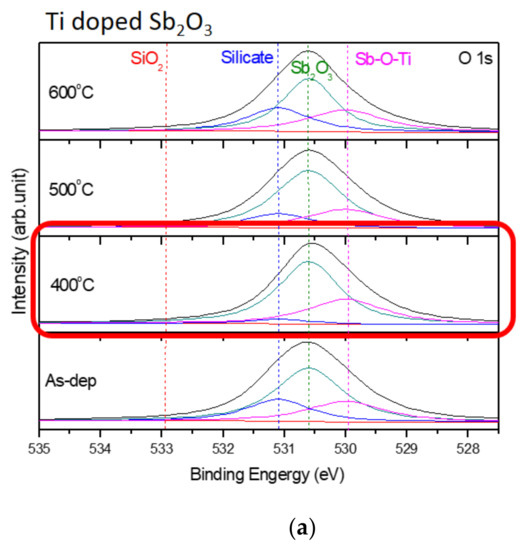
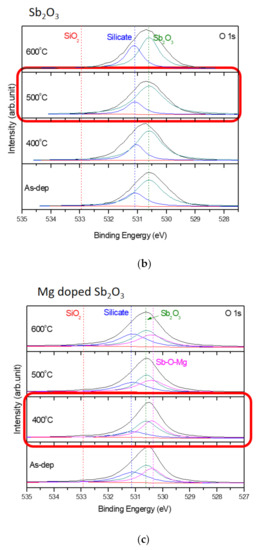
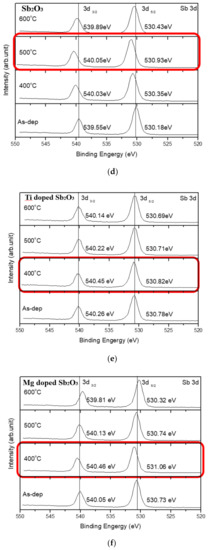
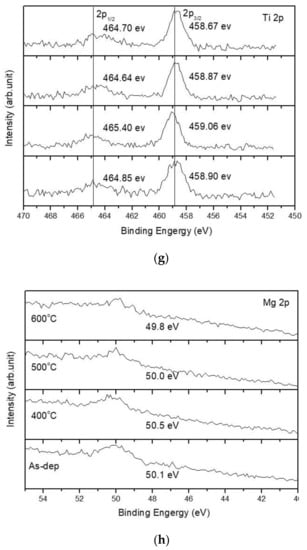
Figure 4.
The O 1s XPS spectra of (a) the undoped Sb2O3 film, (b) the Ti-doped Sb2O3 films and (c) the Mg-doped Sb2O3 films. The Sb 3d XPS spectra of (d) the undoped Sb2O3 film, (e) the Ti-doped Sb2O3 films and (f) the Mg-doped Sb2O3 films annealed at various temperatures in O2 ambient for 30 s. (g) The Ti 2p XPS spectra of the Ti doped Sb2O3 film. (h) The Mg 2p XPS spectra of the Mg doped Sb2O3 film.
To examine the nanostructures of the Sb2O3 samples, TEM and HRTEM were used to analyze the film in nanometer scale [58]. The TEM and HRTEM images of the as-deposited Sb2O3, the Ti-doped Sb2O3 sample annealed at 400 °C, the as-deposited Mg-doped Sb2O3 sample and the Mg-doped Sb2O3 sample annealed at 400 °C are shown in Figure 5a–d, respectively. The crystalline interval of the as-deposited sample is not clear. As for the annealed Ti-doped sample, the interval spacing can be observed on the TEM image, as shown in Figure 5b. In addition, the enlarged HRTEM image of the interval spacing reveals that the width interval spacing is 0.327 nm, which is close to the 0.322 nm of Sb2O3 (222) in a HRTEM image based on a previous report [59]. In addition, the interval spacing of the as-deposited Ti-doped sample can be seen in both the TEM and HRTEM images of Figure 5c. The morphology of the interval spacing of the annealed Ti-doped sample and the as-deposited Mg-doped sample are similar. Compared with the annealed Ti-doped sample and the as-deposited Mg-doped sample, the Mg-doped annealed sample exhibits clearer interval spacing in the TEM and HRTEM images, as shown in Figure 5d. Corresponding with the XRD patterns, as shown in Figure 2, the Mg-doped film annealed at 400 °C had the strongest Sb2O3 (222) phases, implying that the combination of Mg doping and annealing could optimize the film’s material quality.
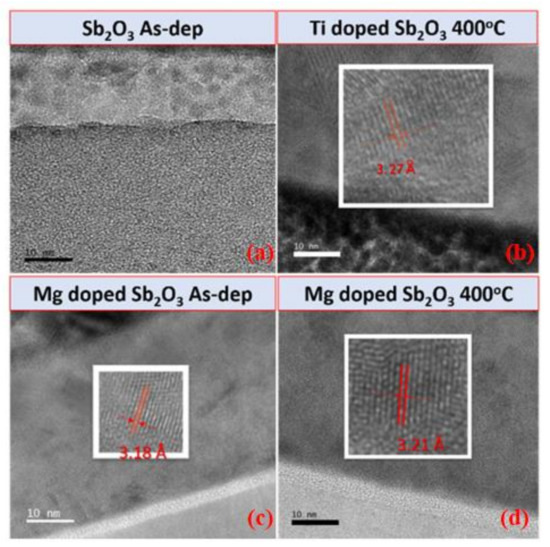
Figure 5.
TEM and HRTEM images of (a) the as-deposited Sb2O3 films, (b) the Ti doped Sb2O3 film annealed at 400 °C, (c) the as-deposited Mg-doped Sb2O3 film and (d) the Mg-doped Sb2O3 film annealed at 400 °C.
To measure the sensitivity and linearity of EIS capacitors, a Ketheley 2400 Source Meter was used to evaluate the C-V curves of the samples treated in various conditions. With 0.4 Cmax set as the reference capacitance, the sensitivity and linearity could be calculated by extracting the points of various pH values with this reference capacitance. Figure 6a–f show the C-V curves of the EIS capacitors with the as-deposited Sb2O3 film and the Sb2O3 film annealed at 500 °C, the as-deposited Ti-doped Sb2O3 film, the Ti-doped Sb2O3 film annealed at 400 °C, the as-deposited Mg-doped film and the Mg-doped Sb2O3 film annealed at 400 °C, respectively.
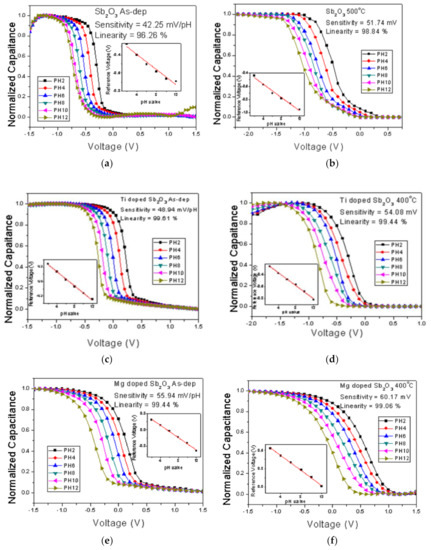
Figure 6.
C–V curves of (a) the as-deposited Sb2O3 sensing membrane, (b) the Sb2O3 sensing membrane annealed at 500 °C, (c) the as-deposited Ti-doped Sb2O3 sensing membrane, (d) the Ti-doped Sb2O3 sensing membrane annealed at 400 °C, (e) the as-deposited Mg-doped sensing membrane and (f) the Mg-doped O3 sensing membrane annealed at 400 °C.
Consistent with the material characterizations, the pH sensitivity and linearity of the as-deposited membrane could be improved by annealing at 500 °C, as shown in Figure 6a,b. Moreover, the sensitivity and linearity could also be boosted by Ti doping and Mg doping without annealing, as shown in Figure 6c,e. In addition, appropriate annealing at 500 °C could further enhance the sensitivity of the Ti-doped samples from 48.94 to 54.08 mV/pH and the Mg-doped samples from 55.94 to 60.17 mV/pH, respectively, as shown in Figure 6d,f. All of the linearity and sensitivity data of the undoped, the Ti-doped and the Mg-doped samples treated with various annealing conditions are presented in Figure 7a–c.
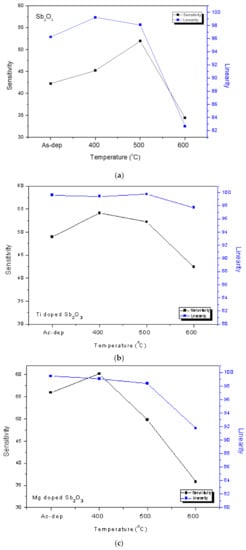
Figure 7.
The pH sensitivity and linearity of (a) the undoped Sb2O3 membrane, (b) the Ti-doped Sb2O3 membrane and (c) the Mg-doped Sb2O3 membranes treated with different RTA temperatures in O2 ambient.
As for the undoped samples, annealing at the appropriate temperatures of 400 °C and 500 °C effectively enhanced the sensitivity and linearity of the membrane, as shown in Figure 8a. Moreover, as shown in Figure 7b,c, in line with all of the material analyses, the combination of doping and appropriate annealing at 400 °C superpositionally increased both sensitivity and linearity for the Ti-doped and Mg-doped samples. Annealing with a high annealing temperature of 600 °C would degrade the material quality and the sensing behaviors of the doped Sb2O3 membranes. Material quality improvements enhanced the device sensing properties. Among all of the membranes, the Mg-doped membrane annealed at 400 °C with the strongest crystallization, grainization, and chemical bindings had the best pH sensitivity of 60.17 mV/pH and a high linearity of 99.06% [60,61].
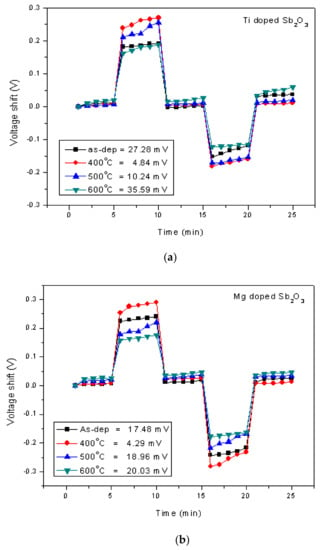
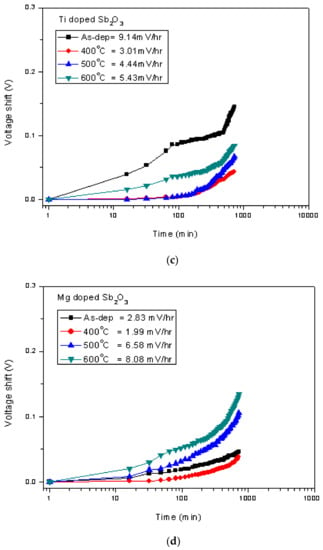
Figure 8.
The hysteresis of (a) the Ti-doped Sb2O3 EIS membranes and (b) the Mg-doped Sb2O3 EIS membranes annealed in different conditions during a pH loop of 7→4→7→10. The drift voltage measurements of (c) the Ti-doped Sb2O3 EIS membranes and (d) the Mg-doped Sb2O3 EIS membranes annealed in different conditions.
To study stability and long-term reliability issues, the hysteresis voltages and the drift voltages were assessed. To calculate the hysteresis voltages, the samples were immersed in solutions with various pH values of 7, 4, 7, 10 in an alternating cycle with an immersion time of 5 min. The hysteresis voltage of the Ti-doped and Mg-doped samples with various annealing treatments are shown in Figure 8a,b, respectively. Consistent with the material characterizations and sensing measurements, the hysteresis voltages for the Ti-doped sample and the Mg-doped sample annealed at 400 °C had the lowest values of 27.28, 4.84 and 4.29 mV, respectively. Mg doping incorporated with annealing at 400 °C had the most reliable response. Since the dangling bonds and traps might capture H+ or OH- ions in solutions, a membrane of better material quality might exhibit lower hysteresis voltages. Furthermore, to evaluate the capacitor’s long-term reliability, all of the tested samples were immersed in pH7 buffer solutions for 12 h, and the drift voltages were calculated. Similarly, the hysteresis voltages for the Ti-doped sample and the Mg-doped sample annealed at 400 °C had the smallest values of 3.01 and 1.99 mV/hr, respectively, as shown in Figure 8c,d. Since post-annealing at 400 °C incorporating Mg doping could effectively reduce vacancies and defects, the drift voltage of the Mg-doped sample annealed at 400 °C was greatly suppressed. These reliability tests were consistent with all of the other electrical measurements and material analyses [62].
Finally, to compare the sensing sensitivity of different ions on the EIS structure, the sensitivity and linearity of the K+ and Na+ ions of the undoped, the Ti-doped and Mg-doped samples were measured. As shown in Figure 9a–f, the sensitivity and linearity measurement of K+ ion for the samples reveal that the Mg-doped sample annealed at 400 °C had the optimized sensitivity of 17.3 mV/pK and linearity of 96.15%.
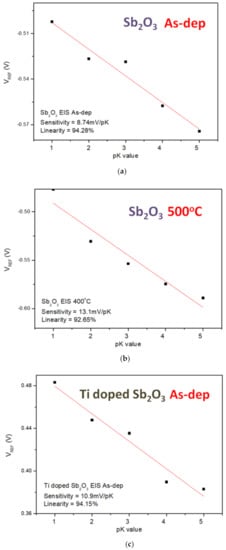
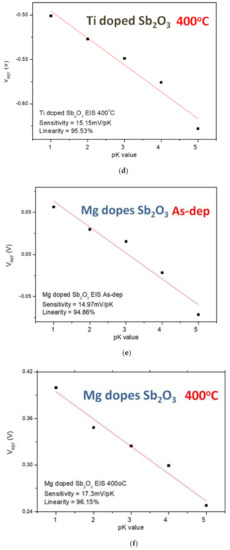
Figure 9.
The K+ sensing behaviors of (a) the as-deposited Sb2O3 sensing membrane, (b) the Sb2O3 sensing membrane annealed at 500 °C, (c) the as-deposited Ti-doped Sb2O3 sensing membrane, (d) the Ti-doped Sb2O3 sensing membrane annealed at 400 °C, (e) the as-deposited Mg-doped sensing membrane and (f) the Mg-doped O3 sensing membrane annealed at 400 °C.
Similarly, the sensitivity and linearity measurements of the Na+ ion for the samples reveal that the Mg-doped sample annealed at 400 °C had an optimized sensitivity of 21.01 mV/pK and a linearity of 96.15%, as shown in Figure 10a–f. Since the radius and mass of H+ ions are much smaller than the radius and mass of K+ and Na+, the sensitivity of the K+ and Na+ ions is smaller than that of the H+ ions.
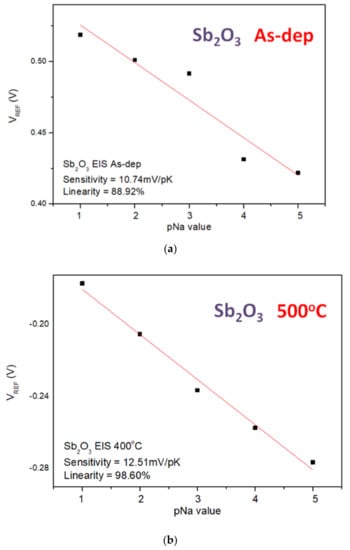
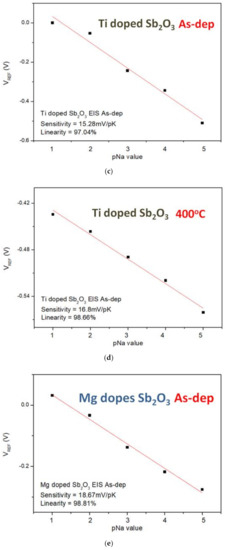
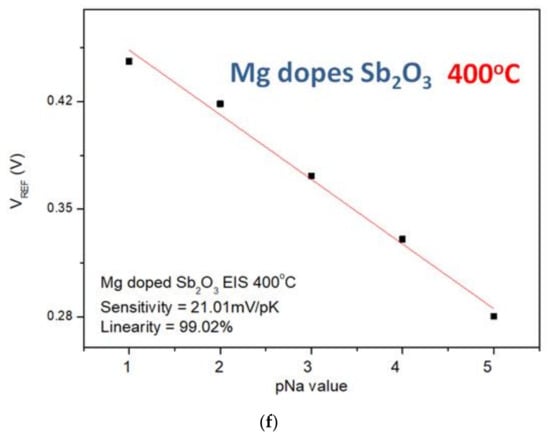
Figure 10.
The Na+ sensing behaviors of (a) the as-deposited Sb2O3 sensing membrane, (b) the Sb2O3 sensing membrane annealed at 500 °C, (c) the as-deposited Ti-doped Sb2O3 sensing membrane, (d) the Ti-doped Sb2O3 sensing membrane annealed at 400 °C, (e) the as-deposited Mg-doped sensing membrane and (f) the Mg-doped O3 sensing membrane annealed at 400 °C.
Finally, the sensitivity, linearity, hysteresis characteristics and drift characteristics of the appropriately annealed undoped, Ti-doped and Mg-doped Sb2O3 membranes were compared. The Mg-doped Sb2O3 membranes annealed at 400 °C still had the most preferable sensing characteristics compared with all of the other samples, as shown in Figure 11.
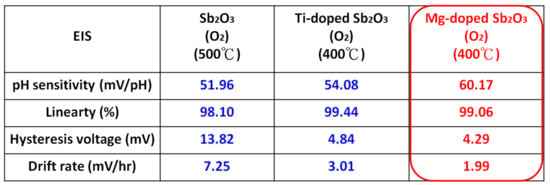
Figure 11.
Comparison of the sensing behaviors of the appropriately annealed, undoped, Ti-doped and Mg-doped Sb2O3 membranes.
4. Conclusions
In this study, undoped, Ti-doped and Mg-doped Sb2O3 sensing membranes with various annealing conditions were fabricated. Multiple material characterizations and sensing measurements were conducted to study the annealing and doping effects on the membranes. The results indicate that Mg doping incorporating annealing at an appropriate temperature of 400 °C could optimize the material quality and enhance the sensing behaviors due to the suppression of silicate, the enhancement of crystallization, the boosting of sensing factors and the removal of defects. Mg-doped Sb2O3-based membranes with appropriate annealing are promising for future industrial ion-sensing devices and for possible integration with Sb2O3-based devices.
Author Contributions
Conceptualization, C.-H.K., Y.-W.K., M.-L.L., L.J.-H.L. and H.C.; methodology, C.-H.K. and H.C.; data curation, K.-L.C.; writing—original draft preparation, J.-R.C., S.-M.C. and H.C.; writing—review and editing, H.C.; visualization, H.C.; supervision, C.-H.K. and H.C.; project administration, H.C.; funding acquisition, M.-L.L. and H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (MOST), Taiwan, grant number “110-2221-E-260-006-”, 110-2221-E-182-032, 110-2222-E-159 -002 -MY2 and the APC was funded by Chang Gung Medical Foundation grant CMRP program (Assistance Agreement CMRPD2J0092 and BMRPA00).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
This work was supported by the Ministry of Science and Technology, Taiwan, under the contract of MOST 107-2221-E-260-015-MY3.
Conflicts of Interest
There are no conflicts of interest to declare. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pan, T.-M.; Lin, J.-C.; Wu, M.-H.; Lai, C.-S. Structural properties and sensing performance of high-k Nd2TiO5 thin layer-based electrolyte–insulator–semiconductor for pH detection and urea biosensing. Biosens. Bioelectron. 2009, 24, 2864–2870. [Google Scholar] [CrossRef]
- Schöning, M.J. Playing around with field-effect sensors on the basis of EIS structures, LAPS and ISFETs. Sensors 2005, 5, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.-H.; Chen, H.; Kuo, L.-T.; Wang, J.-C.; Chen, Y.-T.; Chu, Y.-C.; Chen, C.-Y.; Lai, C.-S.; Chang, S.W.; Chang, C.W. Multi-analyte biosensors on a CF4 plasma treated Nb2O5-based membrane with an extended gate field effect transistor structure. Sens. Actuators B Chem. 2013, 194, 419–426. [Google Scholar] [CrossRef]
- Kao, C.H.; Chen, H.; Lee, M.L.; Liu, C.C.; Ueng, H.-Y.; Chu, Y.C.; Chen, C.B.; Chang, K.M. Effects of N2 and O2 annealing on the multianalyte biosensing characteristics of CeO2-based electrolyte–insulator–semiconductor structures. Sens. Actuators B Chem. 2014, 194, 503–510. [Google Scholar] [CrossRef]
- Tudorache, F.; Tigau, N.; Condurache-Bota, S. Humidity sensing characteristics of Sb2O3 thin films with transitional electrical behavior. Sens. Actuators A Phys. 2018, 285, 134–141. [Google Scholar] [CrossRef]
- Kwo, J.; Hong, M.; Kortan, A.R. Properties of high κ gate dielectrics Gd2O3 and Y2O3 for Si. J. Appl. Phys. 2001, 89, 3920–3927. [Google Scholar] [CrossRef] [Green Version]
- Ott, J.; Lorenz, A.; Harrer, M. The influence of Bi2O3 and Sb2O3 on the electrical properties of ZnO-based varistors. J. Electroceram. 2001, 6, 135–146. [Google Scholar] [CrossRef]
- Mestl, G.; Ruiz, P.; Delmon, B.; Knozinger, H. Sb2O3/Sb2O4 in reducing/oxidizing environments: An in situ Raman spectroscopy study. J. Phys. Chem. 1994, 98, 11276–11282. [Google Scholar] [CrossRef]
- Le, T.; Hai, L.C.; Hung, T.T.; Phuong, B. Multiwall carbon nanotube modified by antimony oxide (Sb2O3/MWCNTs) paste electrode for the simultaneous electrochemical detection of cadmium and lead ions. Microchem. J. 2020, 153, 104456. [Google Scholar]
- Gonçalves, R.A.; Baldan, M.R.; Ciapina, E.G.; Berengue, O.M. Nanostructured Pd/Sb2O3: A new and promising fuel cell electrocatalyst and non-enzymatic amperometric sensor for ethanol. Appl. Surf. Sci. 2019, 491, 9–15. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, H.; Wang, Y. Ex-situ XPS analysis of yolk-shell Sb2O3/WO3 for ultra-fast acetone resistive sensor. J. Hazard. Mater. 2021, 412, 125175. [Google Scholar] [CrossRef]
- Stojanović, Z.S.; Đurović, A.D.; Ashrafi, A.M.; Koudelková, Z.; Zítka, O.; Richtera, L. Highly sensitive simultaneous electrochemical determination of reduced and oxidized glutathione in urine samples using antimony trioxide modified carbon paste electrode. Sens. Actuators B Chem. 2020, 318, 128141. [Google Scholar] [CrossRef]
- Bai, H.; Guo, H.; Wang, J. Hydrogen gas sensor based on SnO2 nanospheres modified with Sb2O3 prepared by one-step solvothermal route. Sens. Actuators B Chem. 2021, 331, 129441. [Google Scholar] [CrossRef]
- Majidian, M.; Raoof, J.B.; Hosseini, S.R.; Fischer, J.; Barek, J. Determination of 8-hydroxy-7-iodo-5-quinoline sulfonic acid (HIQSA) at renewable electrode with Sb2O3/MWCNT-TiO2 nanohybrid. J. Electroanal. Chem. 2020, 858, 113775. [Google Scholar] [CrossRef]
- Li, Z.; Zong, L.; Liu, H.; Yao, Z.; Sun, Y.; Li, Z. A solid-state Sb/Sb2O3 biosensor for the in situ measurement of extracellular acidification associated with the multidrug resistance phenotype in breast cancer cells. Anal. Methods 2018, 10, 4445–4453. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M. Development of an efficient phenolic sensor based on facile Ag2O/Sb2O3nanoparticles for environmental safety. Nanoscale Adv. 2018, 1, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Kotchasak, N.; Inyawilert, K.; Wisitsorrat, A. Chemophysical acetylene-sensing mechanisms of Sb2O3/NaWO4-doped WO3 heterointerfaces. Phys. Chem. Chem. Phys. 2020, 22, 20482–20498. [Google Scholar] [CrossRef]
- Sukul, P.P.; Kumar, K. Afruitful demonstration in sensors based on upconversion luminescence of Yb3+/Er3+ codoped Sb2O3-WO3-Li2O (SWL) glass-ceramic. Mater. Res. Express 2016, 3, 076207. [Google Scholar] [CrossRef]
- Sen, S.; Nilabh, A.; Kundu, S. Room temperature acetone sensing performance of Pt/Sb2O3 impregnated Fe2O3 thin film: Noninvasive diabetes detection. Microchem. J. 2021, 165, 106111. [Google Scholar] [CrossRef]
- Tigau, N.; Ciupina, V.; Prodan, G. The effect of substrate temperature on the optical properties of polycrystalline Sb2O3 thin films. J. Cryst. Growth 2005, 277, 529–535. [Google Scholar] [CrossRef]
- Wang, L.; Gao, C.; Dai, L. Improvement of Al3+ ion conductivity by F doping of (Al0.2Zr0.8)4/3.8 NbP3O12 solid electrolyte for mixed potential NH3 sensors. Ceram. Int. 2018, 44, 8983–8991. [Google Scholar] [CrossRef]
- Palmer, M.; Masikini, M.; Jiang, L.W. Enhanced electrochemical glucose sensing performance of CuO: NiO mixed oxides thin film by plasma assisted nitrogen doping. J. Alloy. Compd. 2021, 853, 156900. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Y.-M.; Tasi, S.; Hsiung, T.-L.; Wang, H.P.; Jang, L. Conductivity enhancement and semiconductor–metal transition in Ti-doped ZnO films. Opt. Mater. 2007, 29, 1548–1552. [Google Scholar] [CrossRef]
- Lin, C.F.; Kao, C.H.; Lin, C.Y.; Liu, Y.W.; Wang, C.H. The electrical and physical characteristics of Mg-doped ZnO sensing membrane in EIS (electrolyte–insulator–semiconductor) for glucose sensing applications. Results Phys. 2020, 16, 102976. [Google Scholar] [CrossRef]
- Liston, E.M. Plasma Treatment for Improved Bonding: A Review. J. Adhes. 1989, 30, 199–218. [Google Scholar] [CrossRef]
- Tanaka, T.; Nagatomo, T.; Kawasaki, D.; Nishio, M.; Guo, Q.; Wakahara, A.; Yoshida, A.; Ogawa, H. Preparation of Cu2ZnSnS4 thin films by hybrid sputtering. J. Phys. Chem. Solids 2005, 66, 1978–1981. [Google Scholar] [CrossRef]
- Van Dover, R. Amorphous lanthanide-doped TiOx dielectric films. Appl. Phys. Lett. 1999, 74, 3041–3043. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, C.-W. Enhanced ferroelectricity in Ti-doped multiferroic BiFeO3 thin films. Appl. Phys. Lett. 2006, 89, 52903. [Google Scholar] [CrossRef]
- Pan, T.-M.; Lin, J.-C. A TiO2/Er2O3 stacked electrolyte/insulator/semiconductor film pH-sensor for the detection of urea. Sens. Actuators B Chem. 2009, 138, 474–479. [Google Scholar] [CrossRef]
- Chou, J.C.; Liao, L.P. Study of TiO2 thin films for ion sensitive field effect transistor application with rf sputtering deposition. Jpn. J. Appl. Phys. 2004, 43, 61. [Google Scholar] [CrossRef]
- Isabel, A.P.S.; Kao, C.H.; Mahanty, R.K.; Wu, Y.C.S.; Li, C.Y.; Lin, C.Y.; Lin, C.F. Sensing and structural properties of Ti-doped tin oxide (SnO2) membrane for bio-sensor applications. Ceram. Int. 2017, 43, 10386–10391. [Google Scholar] [CrossRef]
- Lee, M.L.; Wang, J.C.; Kao, C.H.; Chen, H.; Lin, C.Y.; Chang, C.W.; Mahanty, R.K.; Lin, C.F.; Chang, K.M. Comparison of ZnO and Ti-doped ZnO sensing membrane applied in electrolyte-insulator-semiconductor structure. Ceram. Int. 2018, 44, 6081–6088. [Google Scholar] [CrossRef]
- Kao, C.-H.; Su, Y.-L.; Liao, W.-J.; Li, M.-H.; Chan, W.-L.; Tsai, S.-C.; Chen, H. Effects of CF4 Plasma Treatment on Indium Gallium Oxide and Ti-doped Indium Gallium Oxide Sensing Membranes in Electrolyte–Insulator–Semiconductors. Crystals 2020, 10, 810. [Google Scholar] [CrossRef]
- Kao, C.-H.; Liu, C.S.; Xu, C.Y.; Lin, C.F.; Chen, H. Ti-doped indium gallium oxide electrolyte–insulator–semiconductor membranes for multiple ions and solutes detectors. J. Mater. Sci. Mater. Electron. 2019, 30, 20596–20604. [Google Scholar] [CrossRef]
- Kao, C.H.; Chang, C.W.; Chen, Y.T.; Su, W.M.; Lu, C.C. Influence of NH3 plasma and Ti doping on pH-sensitive CeO2 electrolyte-insulator-semiconductor biosensors. Sci. Rep. 2017, 7, 2405. [Google Scholar]
- Nakarmi, M.; Kim, K.; Khizar, M.; Fan, Z.; Lin, J.; Jiang, H. Electrical and optical properties of Mg-doped Al0.7Ga0.3 N alloys. Appl. Phys. Lett. 2005, 86, 092108. [Google Scholar] [CrossRef]
- Kılınç, N.; Arda, L.; Öztürk, S.; Öztürk, Z. Structure and electrical properties of Mg-doped ZnO nanoparticles. Cryst. Res. Technol. 2010, 45, 529–538. [Google Scholar] [CrossRef]
- Hautakangas, S.; Oila, J.; Alatalo, M.; Saarinen, K.; Liszkay, L.; Seghier, D.; Gislason, H.P. Vacancy Defects as Compensating Centers in Mg-Doped GaN. Phys. Rev. Lett. 2003, 90, 137402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohar, R.S.; Sugihartono, I.; Fauzia, V.; Umar, A.A. Dependence of optical properties of Mg-doped ZnO nanorods on Al dopant. Surf. Interfaces 2020, 19, 100518. [Google Scholar] [CrossRef]
- Kao, C.H.; Liu, C.S.; Lu, S.H.; Tsai, S.C.; Chan, W.L.; Lin, B.H.; Lin, C.F.; Chen, H.; Han, J. Multianalyte Mg-Doped InGaZnO Electrolyte-Insulator-Semiconductor Biosensors and Multiple Material Characterizations of Membrane Nanostructures. IEEE Sens. J. 2020, 20, 10653–10663. [Google Scholar] [CrossRef]
- Lin, C.F.; Kao, C.H.; Lin, C.Y.; Chen, K.L.; Lin, Y.H. NH3 Plasma-Treated Magnesium Doped Zinc Oxide in Biomedical Sensors with Electrolyte–Insulator–Semiconductor (EIS) Structure for Urea and Glucose Applications. Nanomaterials 2020, 10, 583. [Google Scholar] [CrossRef] [Green Version]
- Al-Khalqi, E.M.; Hamid, M.A.A.; Al-Hardan, N.H.; Keng, L.K. Highly Sensitive Magnesium-Doped ZnO Nanorod pH Sensors Based on Electrolyte–Insulator–Semiconductor (EIS) Sensors. Sensors 2021, 21, 2110. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst Limit in Dual-Gated Si-Nanowire FET Sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef]
- Harame, D.L.; Bousse, L.J.; Shott, J.D.; Meindl, D.J. Ion-sensing devices with silicon nitride and borosilicate glass insulators. IEEE Trans. Electron. Devices 1987, 34, 1700–1707. [Google Scholar] [CrossRef]
- Chang, L.-B.; Ko, H.-H.; Lee, Y.-L.; Lai, C.-S.; Wang, C.-Y. The Electrical and pH-Sensitive Characteristics of Thermal Gd2O3/SiO2-Stacked Oxide Capacitors. J. Electrochem. Soc. 2006, 153, G330. [Google Scholar] [CrossRef]
- Khan, M.I.; Mukherjee, K.; Shoukat, R.; Dong, H. A review on pH sensitive materials for sensors and detection methods. Microsyst. Technol. 2017, 23, 4391–4404. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, J.; Panda, S. Back-Channel Electrolyte-Gated a-IGZO Dual-Gate Thin-Film Transistor for Enhancement of pH Sensitivity Over Nernst Limit. IEEE Electron. Device Lett. 2016, 37, 500–503. [Google Scholar] [CrossRef]
- Poghossian, A. The super-Nernstian pH sensitivity of Ta2O5-gate ISFETs. Sens. Actuators B Chem. 1992, 7, 367–370. [Google Scholar] [CrossRef]
- Bousse, L.; de Rooij, N.F.; Bergveld, P. Operation of chemically sensitive field-effect sensors as a function of the insulator-electrolyte interface. IEEE Trans. Electron. Devices 1983, 30, 1263–1270. [Google Scholar] [CrossRef]
- Fung, C.D.; Cheung, P.W.; Ko, W.H. A generalized theory of an electrolyte-insulator-semiconductor field-effect transistor. IEEE Trans. Electron. Devices 1986, 33, 8–18. [Google Scholar] [CrossRef]
- Van Hal, R.; Eijkel, J.; Bergveld, P. A novel description of ISFET sensitivity with the buffer capacity and double-layer capacitance as key parameters. Sens. Actuators B Chem. 1995, 24, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Oldham, K.B. A Gouy–Chapman–Stern model of the double layer at a (metal)/(ionic liquid) interface. J. Electroanal. Chem. 2008, 613, 131–138. [Google Scholar] [CrossRef]
- Pruneanu, S.; Boughriet, A.; Henderson, A.; Malins, C.; Ali, Z.; Olenic, L. Impedimetric measurements for monitoring avidin-biotin interaction on self-assembled monolayer. Part. Sci. Technol. 2008, 26, 136–144. [Google Scholar] [CrossRef]
- Zeng, R.-H.; Li, W.-S.; Dong-Sheng, L.; Huang, Q.-M.; Zhao, L.-Z. Insertion/removal kinetics of lithium ion in spinel LiCuxMn2−xO4. Trans. Nonferrous Met. Soc. China 2007, 17, 1312–1318. [Google Scholar] [CrossRef]
- Divya, K.; Abraham, K. Ag nanoparticle decorated Sb2O3 thin film: Synthesis, characterizations and application. Nano Express 2020, 1, 020005. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Ma, F.; Wang, T.; Han, J.; Huang, Y.; Li, Q. Core@shell Sb@Sb2O3 nanoparticles anchored on 3D nitrogen-doped carbon nanosheets as advanced anode materials for Li-ion batteries. Nanoscale Adv. 2020, 2, 5578–5583. [Google Scholar] [CrossRef]
- Yule, Z.; Shouan, Z.; Tao, L. Drift characteristics of pH-ISFET output. Chin. J. Semiconduct. 1994, 12, 838–843. [Google Scholar]
- Tigau, N.; Ciupina, V.; Prodan, G. Structural, optical and electrical properties of Sb2O3 thin films with different thickness. J. Optoelectron. Adv. Mater. 2006, 8, 37. [Google Scholar]
- Chou, J.-C.; Weng, C.-Y. Sensitivity and hysteresis effect in Al2O3 gate pH-ISFET. Mater. Chem. Phys. 2001, 71, 120–124. [Google Scholar] [CrossRef]
- Bousse, L.; van den Vlekkert, H.; de Rooij, N. Hysteresis in al2o3-gate isfets. Sens. Actuators B Chem. 1990, 2, 103–110. [Google Scholar] [CrossRef]
- Mayergoyz, I. Mathematical models of hysteresis. IEEE Trans. Magn. 1986, 22, 603–608. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).