Novel Poly(Vinylidene Fluoride)/Montmorillonite Polymer Inclusion Membrane: Application to Cr(VI) Extraction from Polluted Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Physicochemical Characterization
2.3.1. Tensile Tests
2.3.2. Water Contact Angle Measurements
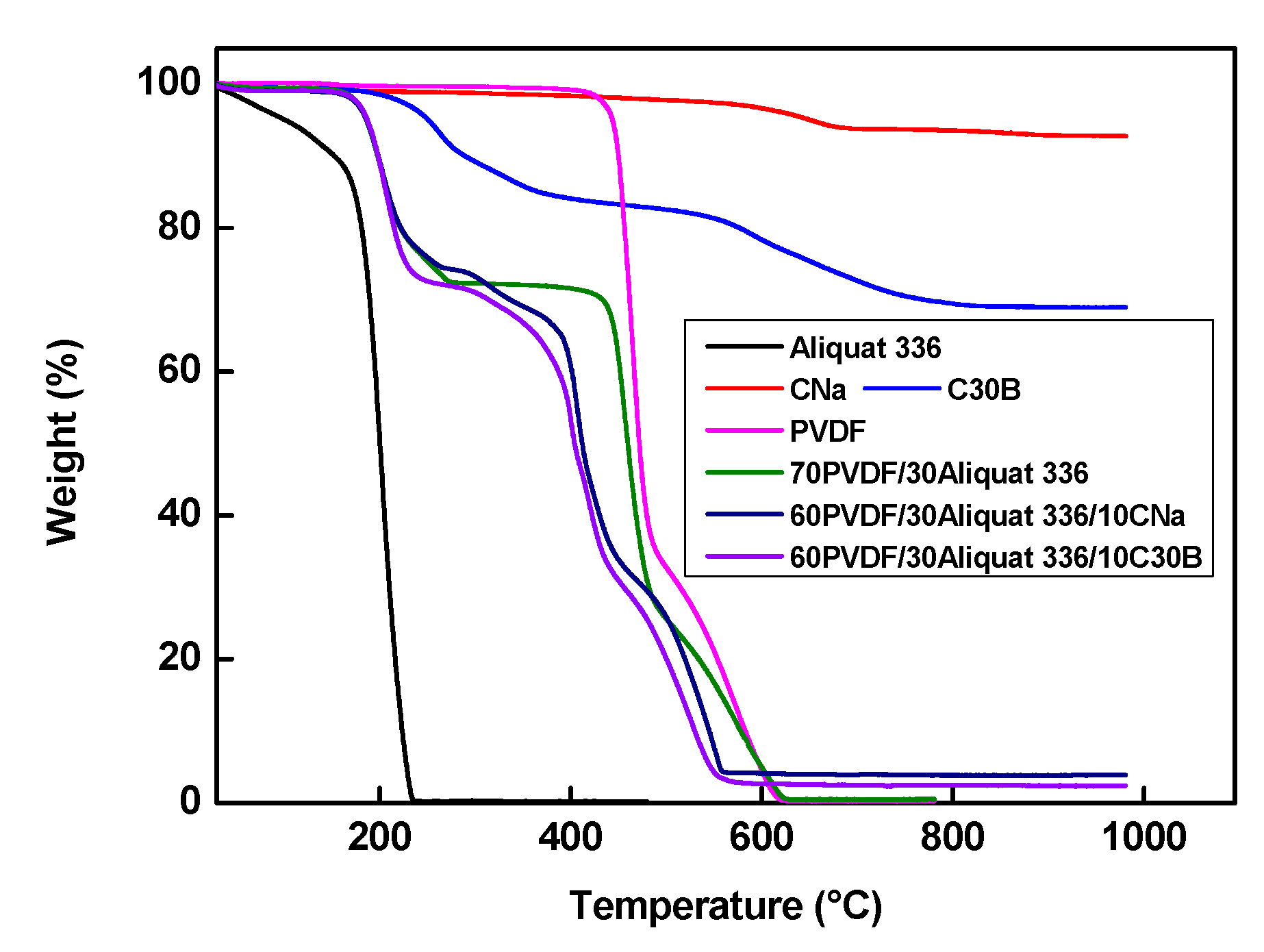
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Scanning Electron Microscopy (SEM) Analysis
2.3.6. Particle Size Distribution
2.4. Transport Experiments
3. Results and Discussion
3.1. Particle Size Distribution
3.2. Membrane Morphology
3.3. Hydrophobic/Hydrophilic Balance
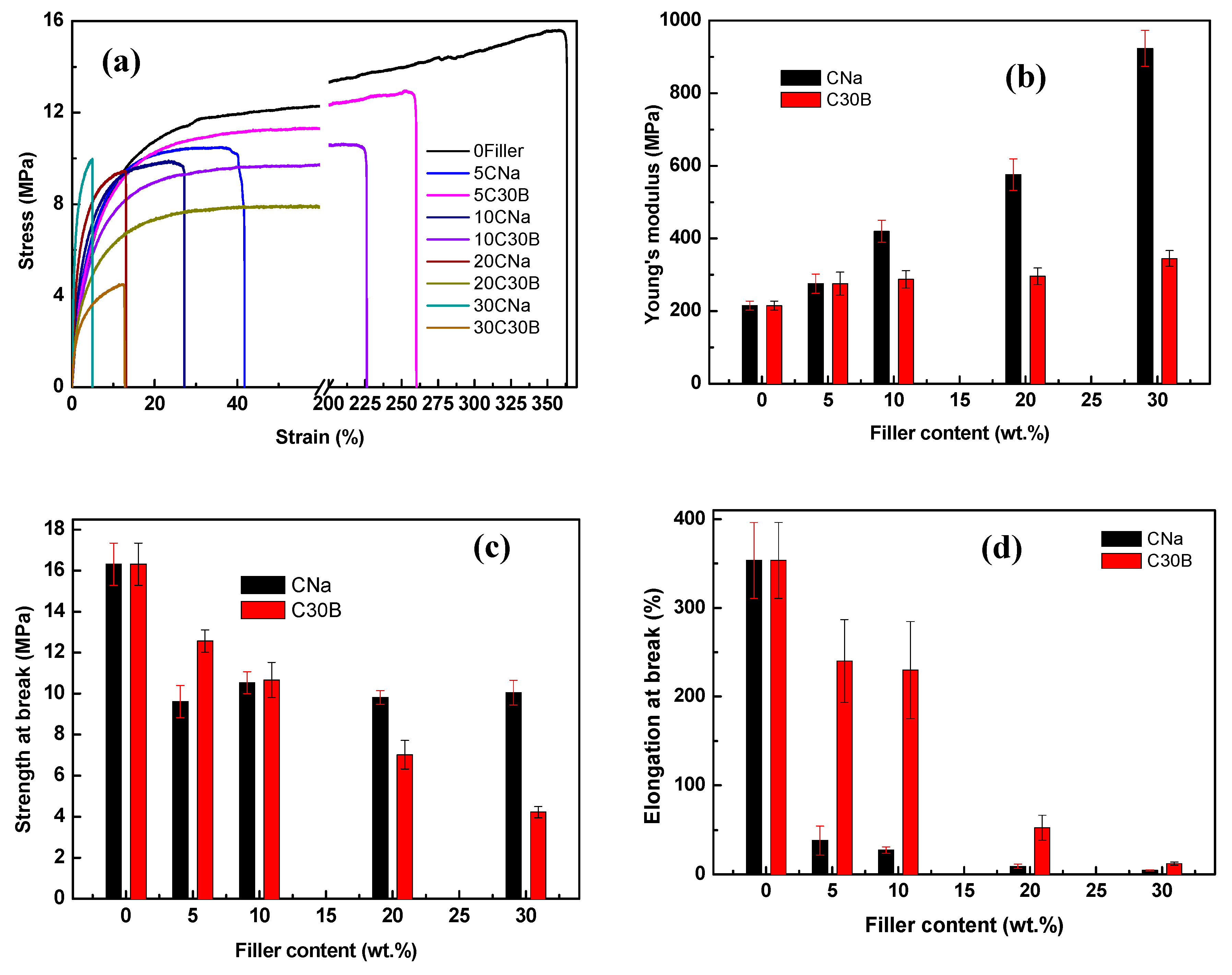
3.4. Membrane Mechanical Performance
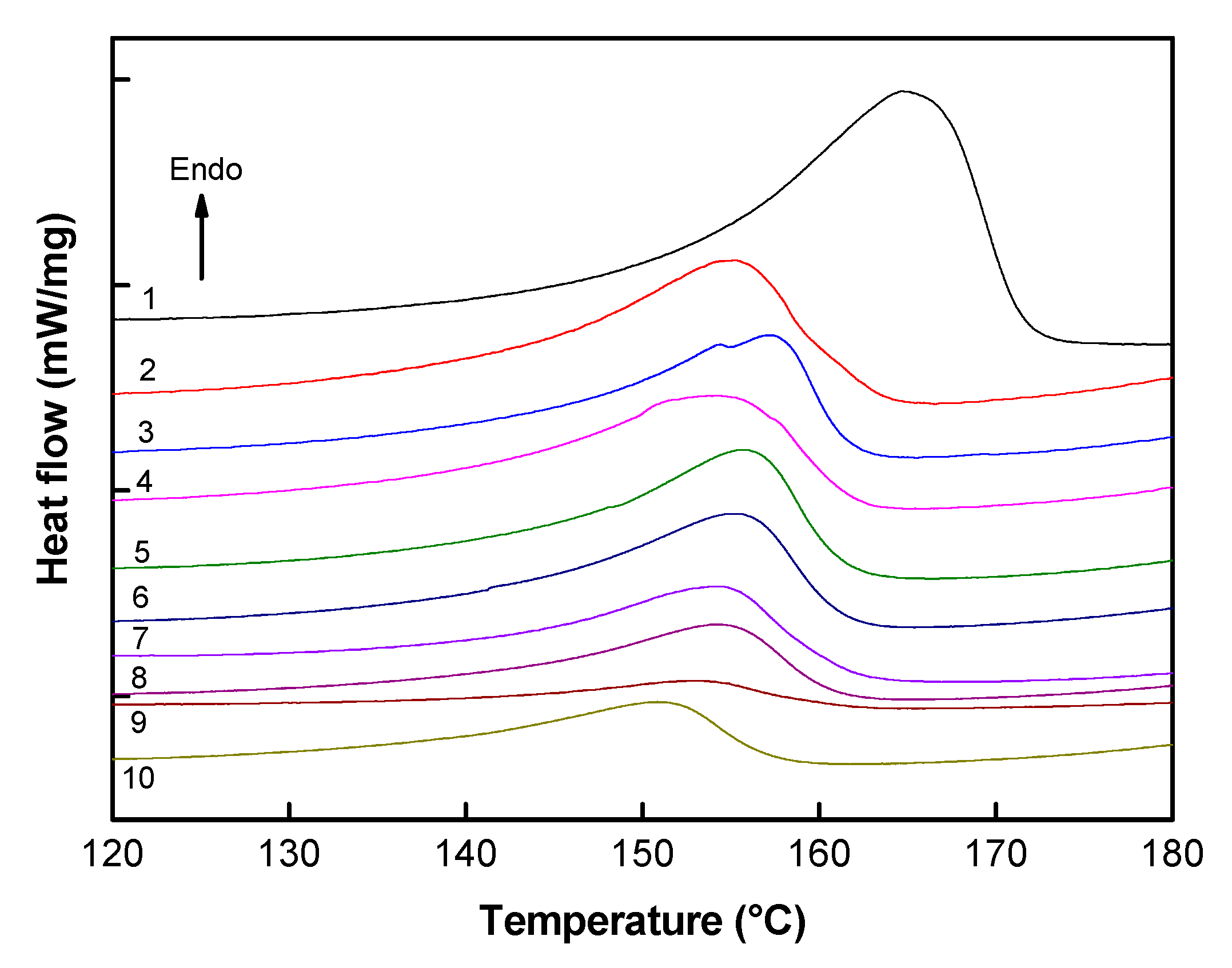
3.5. Membrane Thermal Characterization
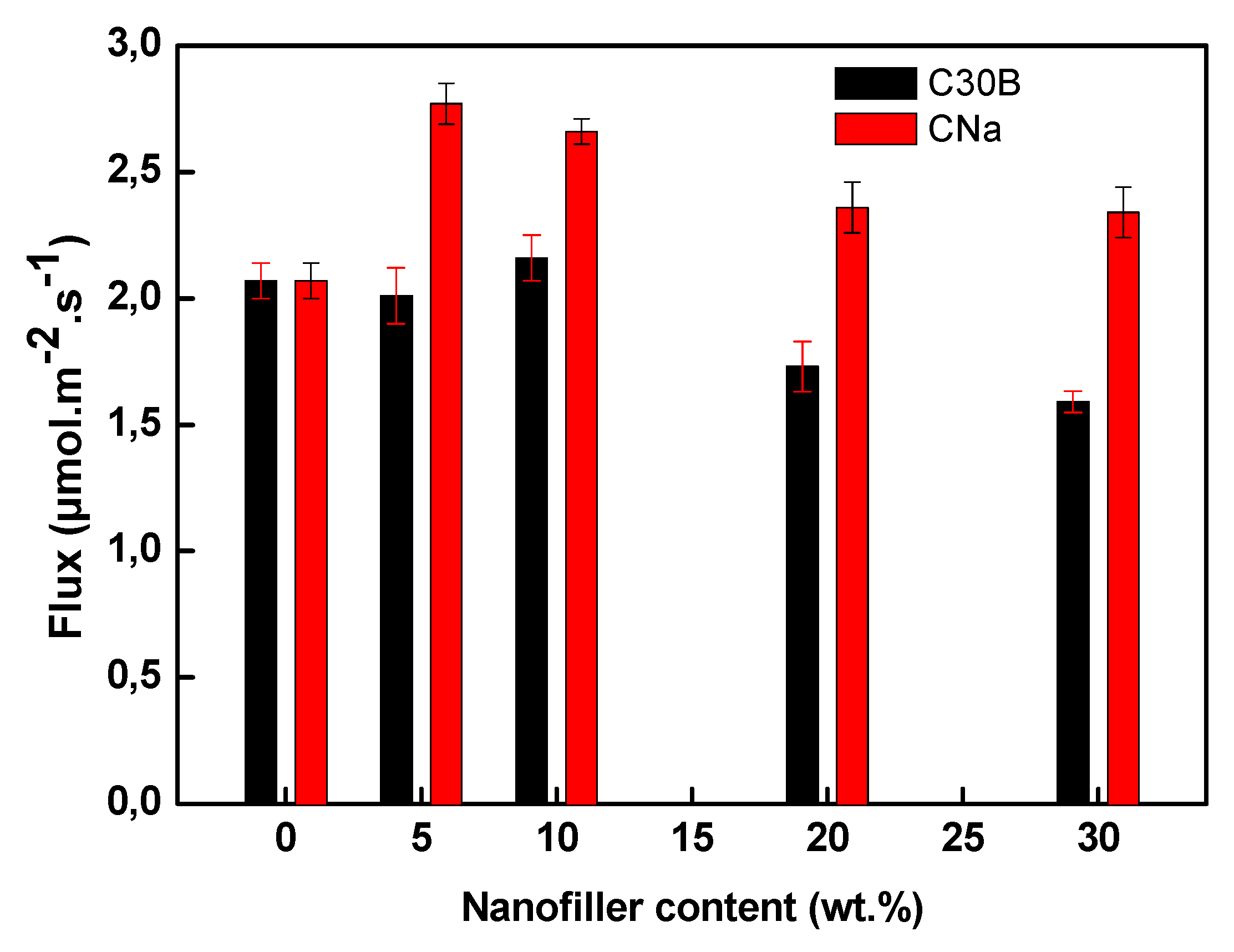
3.6. Membrane Transport Properties
3.6.1. Influence of the Filler Nature and Its Content
3.6.2. Membrane Stability and Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Premkumar, M.P.; Thiruvengadaravi, K.V.; Kumar, P.S.; Nandagopal, J.; Sivanesan, S. Eco-friendly treatment strategies for wastewater containing dyes and heavy metals. In Environmental Contaminants: Measurement, Modelling and Control; Gupta, T., Agarwal, A.K., Agarwal, R.A., Labhsetwar, N.K., Eds.; Springer: Singapore, 2018; pp. 317–360. [Google Scholar]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Couvrat, N.; Fatyeyeva, K. Polymer inclusion membranes based on CTA/PBAT blend containing Aliquat 336 as extractant for removal of Cr(VI): Efficiency, stability and selectivity. React. Funct. Polym. 2019, 139, 120–132. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Bourceanu, G.; Olariu, R.-I.; Arsene, C. A novel polymer inclusion membrane applied in chro-mium (VI) separation from aqueous solutions. J. Hazard. Mater. 2011, 197, 244–253. [Google Scholar] [CrossRef]
- El-Hefny, N.E. Comparison of liquid–liquid extraction of Cr(VI) from acidic and alkaline solutions by two dif-ferent amine extractants. Sep. Purif. Technol. 2009, 67, 44–49. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Golder, A.K.; Chanda, A.K.; Samanta, A.N.; Ray, S. Removal of hexavalent chromium by electrochemical reduc-tion–precipitation: Investigation of process performance and reaction stoichiometry. Sep. Purif. Technol. 2011, 76, 345–350. [Google Scholar] [CrossRef]
- Sapari, N.; Idris, A.; Hamid, N.H.A. Total removal of heavy metal from mixed plating rinse wastewater. Desalination 1996, 106, 419–422. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Bharagava, R.N. Emerging Eco-Friendly Green Technologies for Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H.; Giorno, L.; Drioli, E. Introduction to Membrane Science and Technology; Wiley-VCH Weinheim: Hoboken, NJ, USA, 2011. [Google Scholar]
- Nghiem, L.; Mornane, P.; Potter, I.; Perera, J.; Cattrall, R.; Kolev, S. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Román, M.F.S.; Bringas, E.; Ibañez, R.; Ortiz, I. Liquid membrane technology: Fundamentals and review of its ap-plications. J. Chem. Technol. Biotechnol. 2010, 85, 2–10. [Google Scholar] [CrossRef]
- Yang, X.J.; Fane, A.G.; Bi, J.; Griesser, H.J. Stabilization of supported liquid membranes by plasma polymerization surface coating. J. Membr. Sci. 2000, 168, 29–37. [Google Scholar] [CrossRef]
- Maiphetlho, K.; Chimuka, L.; Tutu, H.; Richards, H. Technical design and optimization of polymer inclusion membranes (PIMs) for sample pretreatment and passive sampling—A review. Sci. Total Environ. 2021, 799, 149483. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Kikkawa, M.; Urita, S. Effect of Plasticizer on Carrier-Mediated Transport of Zinc Ion through Cellulose Triacetate Membranes. Sep. Sci. Technol. 1987, 22, 2263–2268. [Google Scholar] [CrossRef]
- Khaldoun, I.A.; Mitiche, L.; Sahmoune, A.; Fontàs, C. An efficient polymer inclusion membrane-based device for Cd monitoring in seawater. Membranes 2018, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Ulewicz, M. Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier. Membranes 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Colasse, L.; Fatyeyeva, K. Enhanced removal of Cr(VI) by polymer in-clusion membrane based on poly(vinylidene fluoride) and Aliquat 336. Sep. Purif. Technol. 2020, 248, 117038. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using poly-mer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Yoshida, W.; Baba, Y.; Kubota, F.; Kolev, S.D.; Goto, M. Selective transport of scandium(III) across polymer inclu-sion membranes with improved stability which contain an amic acid carrier. J. Membr. Sci. 2019, 572, 291–299. [Google Scholar] [CrossRef]
- O’Bryan, Y.; Cattrall, R.W.; Truong, Y.B.; Kyratzis, I.L.; Kolev, S.D. The use of poly(vinylidenefluoride-co-hexafluoropropylene) for the preparation of polymer inclusion membranes. Application to the extraction of thiocyanate. J. Membr. Sci. 2016, 510, 481–488. [Google Scholar] [CrossRef]
- O’Bryan, Y.; Truong, Y.B.; Cattrall, R.W.; Kyratzis, I.L.; Kolev, S.D. A new generation of highly stable and permeable polymer inclusion membranes (PIMs) with their carrier immobilized in a crosslinked semi-interpenetrating polymer network. Application to the transport of thiocyanate. J. Membr. Sci. 2017, 529, 55–62. [Google Scholar] [CrossRef]
- Cho, Y.; Xu, C.; Cattrall, R.W.; Kolev, S.D. A polymer inclusion membrane for extracting thiocyanate from weakly alkaline solutions. J. Membr. Sci. 2011, 367, 85–90. [Google Scholar] [CrossRef]
- Bahrami, S.; Yaftian, M.R.; Najvak, P.; Dolatyari, L.; Shayani-Jam, H.; Kolev, S.D. PVDF-HFP based polymer inclu-sion membranes containing Cyphos® IL 101 and Aliquat® 336 for the removal of Cr(VI) from sulfate solutions. Sep. Purif. Technol. 2020, 250, 117251. [Google Scholar] [CrossRef]
- Kagaya, S.; Ryokan, Y.; Cattrall, R.W.; Kolev, S. Stability studies of poly(vinyl chloride)-based polymer inclusion membranes containing Aliquat 336 as a carrier. Sep. Purif. Technol. 2012, 101, 69–75. [Google Scholar] [CrossRef]
- Zhang, L.L.; Cattrall, R.W.; Ashokkumar, M.; Kolev, S.D. On-line extractive separation in flow injection analysis based on polymer inclusion membranes: A study on membrane stability and approaches for improving mem-brane permeability. Talanta 2012, 97, 382–387. [Google Scholar] [CrossRef]
- Kaya, A.; Onac, C.; Alpoğuz, H.; Agarwal, S.; Gupta, V.K.; Atar, N.; Yilmaz, A. Reduced graphene oxide based a novel polymer inclusion membrane: Transport studies of Cr(VI). J. Mol. Liq. 2016, 219, 1124–1130. [Google Scholar] [CrossRef]
- Anticó, E.; Vera, R.; Vázquez, F.; Fontàs, C.; Lu, C.; Ros, J. Preparation and characterization of nanoparticle-doped polymer inclusion membranes. Application to the removal of arsenate and phosphate from waters. Materials 2021, 14, 878. [Google Scholar] [CrossRef]
- Cui, Y.; Kumar, S.; Kona, B.R.; Van Houcke, D. Gas barrier properties of polymer/clay nanocomposites. RSC Adv. 2015, 5, 63669–63690. [Google Scholar] [CrossRef]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of Wyoming Montmorillonite Surfaces Using a Cationic Surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef]
- Thomas, S.; Joseph, K.; Malhotra, S.K.; Goda, K.; Sreekala, M.S. Polymer Composites, Macro- and Microcomposites; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Dong, Z.; Zhang, Q.; Yu, C.; Peng, J.; Ma, J.; Ju, X.; Zhai, M. Effect of ionic liquid on the properties of poly(vinylidene fluoride)-based gel polymer electrolytes. Ionics 2013, 19, 1587–1593. [Google Scholar] [CrossRef]
- Vollenberg, P.H.T.; Heikens, D. Particle size dependence of the Young’s modulus of filled polymers: 1 Prelimi-nary experiments. Polymer 1989, 30, 1656–1662. [Google Scholar] [CrossRef]
- Tantekin-Ersolmaz, Ş.B.; Atalay-Oral, Ç.; Tatlıer, M.; Erdem-Şenatalar, A.; Schoeman, B.; Sterte, J. Effect of zeolite particle size on the performance of polymer–zeolite mixed matrix membranes. J. Membr. Sci. 2000, 175, 285–288. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A review of the water barrier properties of polymer/clay and polymer/graphene nano-composites. J. Membr. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.; Robeson, L. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-J.; Jeong, S.-K.; Jo, N.-J. PVDF-Based Nanocomposite Solid Polymer Electrolytes; the Effect of Affinity between PVDF and Filler on Ionic Conductivity. Compos. Interfaces 2009, 16, 347–358. [Google Scholar] [CrossRef]
- Song, Y.M.; Zhao, Z.D.; Yu, W.X.; Li, B.; Chen, X.F. Morphological structures of poly(vonylidene fluo-ride)/Montmorillonite nanocomposites. Sci. China Ser. B Chem. 2007, 50, 790–796. [Google Scholar] [CrossRef]
- Layek, R.K.; Das, A.K.; Park, J.; Kim, N.H.; Lee, J.H. Enhancement of physical, mechanical, and gas barrier proper-ties in noncovalently functionalized graphene oxide/poly(vinylidene fluoride) composites. Carbon 2015, 81, 329–338. [Google Scholar] [CrossRef]
- Manfredi, L.B.; Puglia, D.; Tomasucci, A.; Kenny, J.M.; Vázquez, A. Influence of Clay Modification on the Properties of Resol Nanocomposites. Macromol. Mater. Eng. 2008, 293, 878–886. [Google Scholar] [CrossRef]
- Fina, A.; Cuttica, F.; Camino, G. Ignition of polypropylene/montmorillonite nanocomposites. Polym. Degrad. Stab. 2012, 97, 2619–2626. [Google Scholar] [CrossRef]
- Morawiec, J.; Pawlak, A.; Slouf, M.; Galeski, A.; Piorkowska, E.; Krasnikowa, N. Preparation and properties of compatibilized LDPE/organo-modified montmorillonite nanocomposites. Eur. Polym. J. 2005, 41, 1115–1122. [Google Scholar] [CrossRef]
- Arroyo, M.; López-Manchado, M.; Herrero, B. Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 2003, 44, 2447–2453. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J. Asymmetric Membrane Containing Ionic Liquid [A336][P507] for the Preconcentration and Separation of Heavy Rare Earth Lutetium. ACS Sustain. Chem. Eng. 2016, 4, 2644–2650. [Google Scholar] [CrossRef]
- Braga, F.J.C.; Rogero, S.O.; Couto, A.A.; Marques, R.F.C.; Ribeiro, A.A.; Campos, J.S.D.C. Characterization of PVDF/HAP composites for medical applications. Mater. Res. 2007, 10, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Nasser, I.I.; Amor, F.I.E.H.; Donato, L.; Algieri, C.; Garofalo, A.; Drioli, E.; Ahmed, C. Removal and recovery of Ag(CN)2- from synthetic electroplating baths by polymer inclusion membrane containing Aliquat 336 as a carrier. Chem. Eng. J. 2016, 295, 207–217. [Google Scholar] [CrossRef]
- Maiphetlho, K.; Shumbula, N.; Motsoane, N.; Chimuka, L.; Richards, H. Evaluation of silver nanocomposite pol-ymer inclusion membranes (PIMs) for trace metal transports: Selectivity and stability studies. J. Water Proc. Eng. 2020, 37, 101527. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- El Achaby, M.; Arrakhiz, F.-E.; Vaudreuil, S.; Essassi, E.M.; Qaiss, A.; Bousmina, M. Nanocomposite films of poly(vinylidene fluoride) filled with polyvinylpyrrolidone-coated multiwalled carbon nanotubes: Enhancement of β-polymorph formation and tensile properties. Polym. Eng. Sci. 2013, 53, 34–43. [Google Scholar] [CrossRef]
- Ferreira, J.; Monteiro, T.; Lopes, A.C.; Costa, C.M.; Silva, M.M.; Machado, A.; Lanceros-Mendez, S. Variation of the physicochemical and morphological characteristics of solvent casted poly(vinylidene fluoride) along its binary phase diagram with dimethylformamide. J. Non-Cryst. Solids 2015, 412, 16–23. [Google Scholar] [CrossRef]
- Mejri, R.; Dias, J.C.; Lopes, A.C.; Hentati, S.B.; Silva, M.M.; Botelho, G.; de Ferro, A.M.; Esperança, J.M.S.S.; Maceiras, A.; Laza, J.M.; et al. Effect of ionic liquid anion and cation on the physico-chemical properties of poly(vinylidene fluoride)/ionic liquid blends. Eur. Polym. J. 2015, 71, 304–313. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Abtahi, M.; Wong, S.-C.; Liu, Y.; Li, Q. Microstructure development in electrospun carbon nanotube reinforced polyvinylidene fluoride fibers and its influence on tensile strength and dielectric permittiv-ity. Compos. Sci. Technol. 2013, 88, 1–8. [Google Scholar] [CrossRef]
- Patro, T.U.; Mhalgi, M.V.; Khakhar, D.V.; Misra, A. Studies on poly(vinylidene fluoride)–clay nanocomposites: Effect of different clay modifiers. Polymer 2008, 49, 3486–3499. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Gharayebi, Y.; Salit, M.S.; Hussein, M.Z.; Shameli, K. Comparison of in situ polymerization and so-lution-dispersion techniques in the preparation of polyimide/Montmorillonite (MMT) nanocomposites. Int. J. Mol. Sci. 2011, 12, 6040–6050. [Google Scholar] [CrossRef] [Green Version]
- Kazemzadeh, H.; Karimi-Sabet, J.; Darian, J.T.; Adhami, A. Evaluation of polymer inclusion membrane ef-ficiency in selective separation of lithium ion from aqueous solution. Sep. Purif. Technol. 2020, 251, 117298. [Google Scholar] [CrossRef]
- Heidarbeigi, M.; Saraji, M.; Jafari, M.T. Mg-Al-CO3 layered double hydroxide reinforced polymer inclusion mem-brane as an extractant phase for thin-film microextraction of cyanide from environmental water samples. Environ. Sci. Pollut. Res. 2019, 26, 27854–27861. [Google Scholar] [CrossRef]
- Turgut, H.I.; Eyupoglu, V.; Kumbasar, R.A.; Şişman, I. Alkyl chain length dependent Cr(VI) transport by polymer inclusion membrane using room temperature ionic liquids as carrier and PVDF-co-HFP as polymer matrix. Sep. Purif. Technol. 2017, 175, 406–417. [Google Scholar] [CrossRef]
- Alexandre, B.; Colasse, L.; Langevin, D.; Médéric, P.; Aubry, T.; Chappey, C.; Marais, S. Transport mechanisms of small molecules through polyamide 12/Montmorillonite nanocomposites. J. Phys. Chem. Part B 2010, 114, 8827–8837. [Google Scholar] [CrossRef] [PubMed]
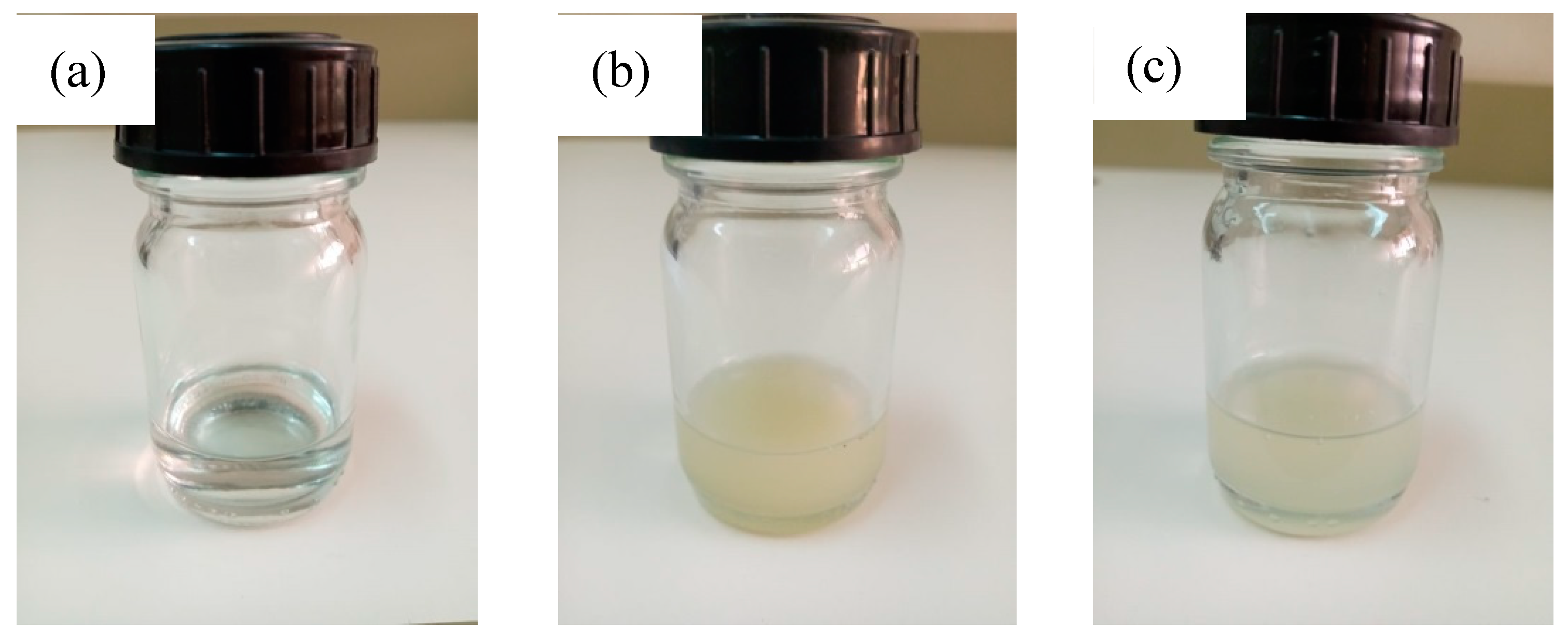
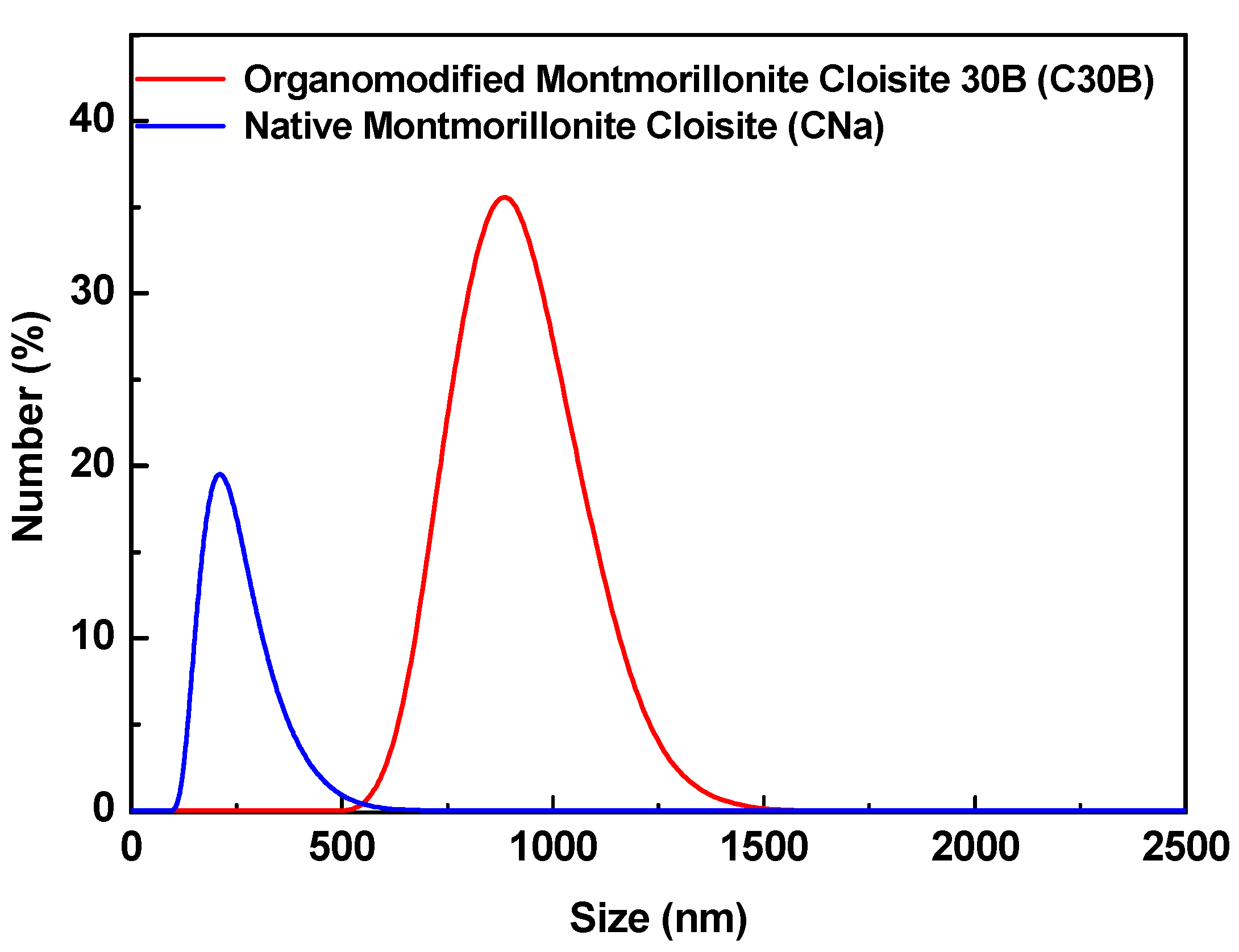
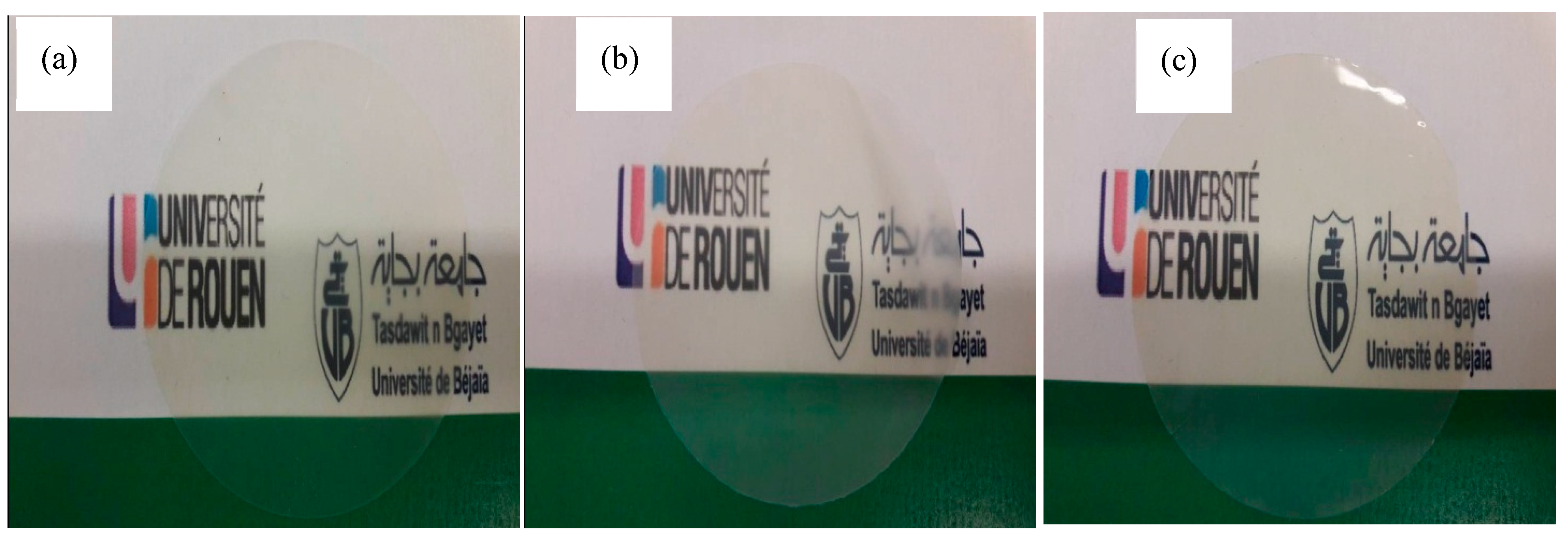
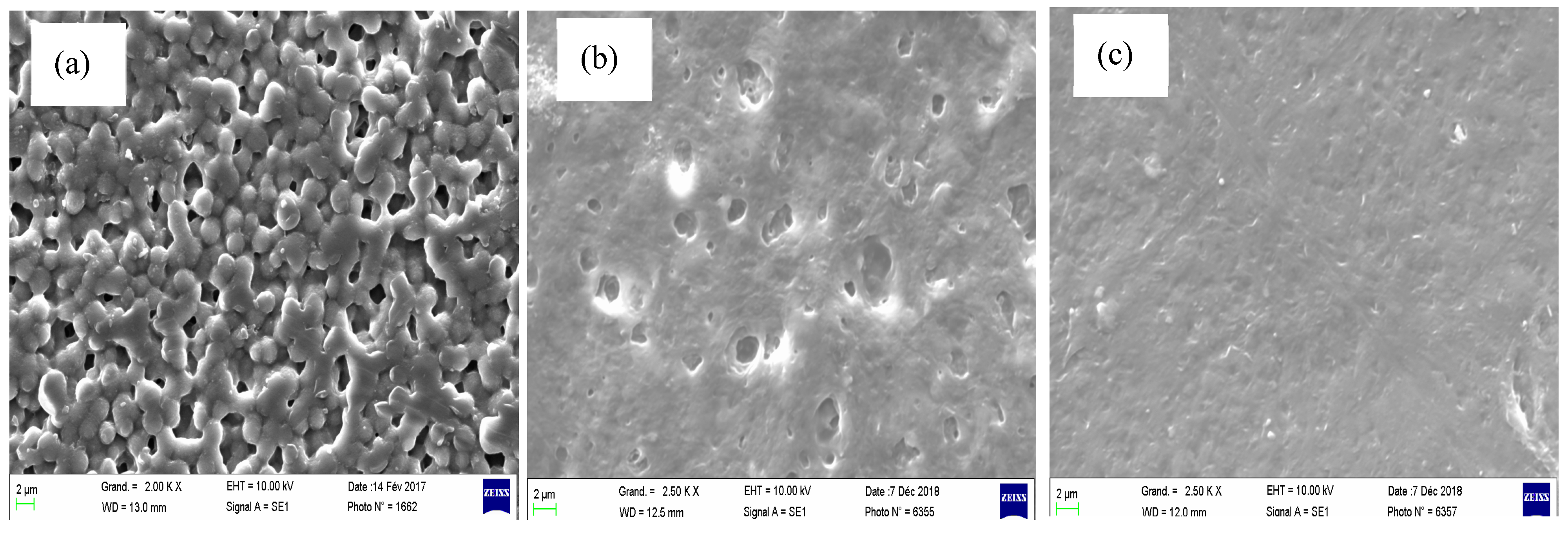

| Type of MMT | Average Diameter (nm ± SD) | Polydispersity Index |
|---|---|---|
| CNa | 273 ± 12 | 0.348 ± 0.008 |
| C30B | 851 ± 35 | 0.840 ± 0.010 |
| Membrane | Melting Temperature (°C) | Melting Enthalpy ΔHm (mW/mg) | Crystallinity Degree Xc(PVDF) (%) |
|---|---|---|---|
| PVDF | 166.9 | 51.9 | 50 |
| 70PVDF/30Aliquat 336 | 155.2 | 28.1 | 38 |
| 65PVDF/30Aliquat 336/5CNa | 154.2 | 23.7 | 34 |
| 65PVDF/30Aliquat 336/5C30B | 157.1 | 22.9 | 34 |
| 60PVDF/30Aliquat 336/10CNa | 155.3 | 21.6 | 34 |
| 60PVDF/30Aliquat 336/10C30B | 155.7 | 23.2 | 37 |
| 50PVDF/30Aliquat 336/20CNa | 154.2 | 14.1 | 27 |
| 50PVDF/30Aliquat 336/20C30B | 154.5 | 17.1 | 33 |
| 40PVDF/30Aliquat 336/30CNa | 150.8 | 11.0 | 26 |
| 40PVDF/30Aliquat 336/30C30B | 153.1 | 5.0 | 12 |
| Membrane | Flux (µmol/(m2·s)) |
|---|---|
| 70PVDF/30Aliquat 336 | 2.07 ± 0.07 |
| 65PVDF/30Aliquat 336/5CNa | 2.77 ± 0.08 |
| 65PVDF/30Aliquat 336/5C30B | 2.01 ± 0.11 |
| 60PVDF/30Aliquat 336/10CNa | 2.66 ± 0.05 |
| 60PVDF/30Aliquat 336/10C30B | 2.16 ± 0.09 |
| 50PVDF/30Aliquat 336/20CNa | 2.36 ± 0.10 |
| 50PVDF/30Aliquat 336/20C30B | 1.73 ± 0.10 |
| 40PVDF/30Aliquat 336/30CNa | 2.34 ± 0.10 |
| 40PVDF/30Aliquat 336/30C30B | 1.59 ± 0.04 |
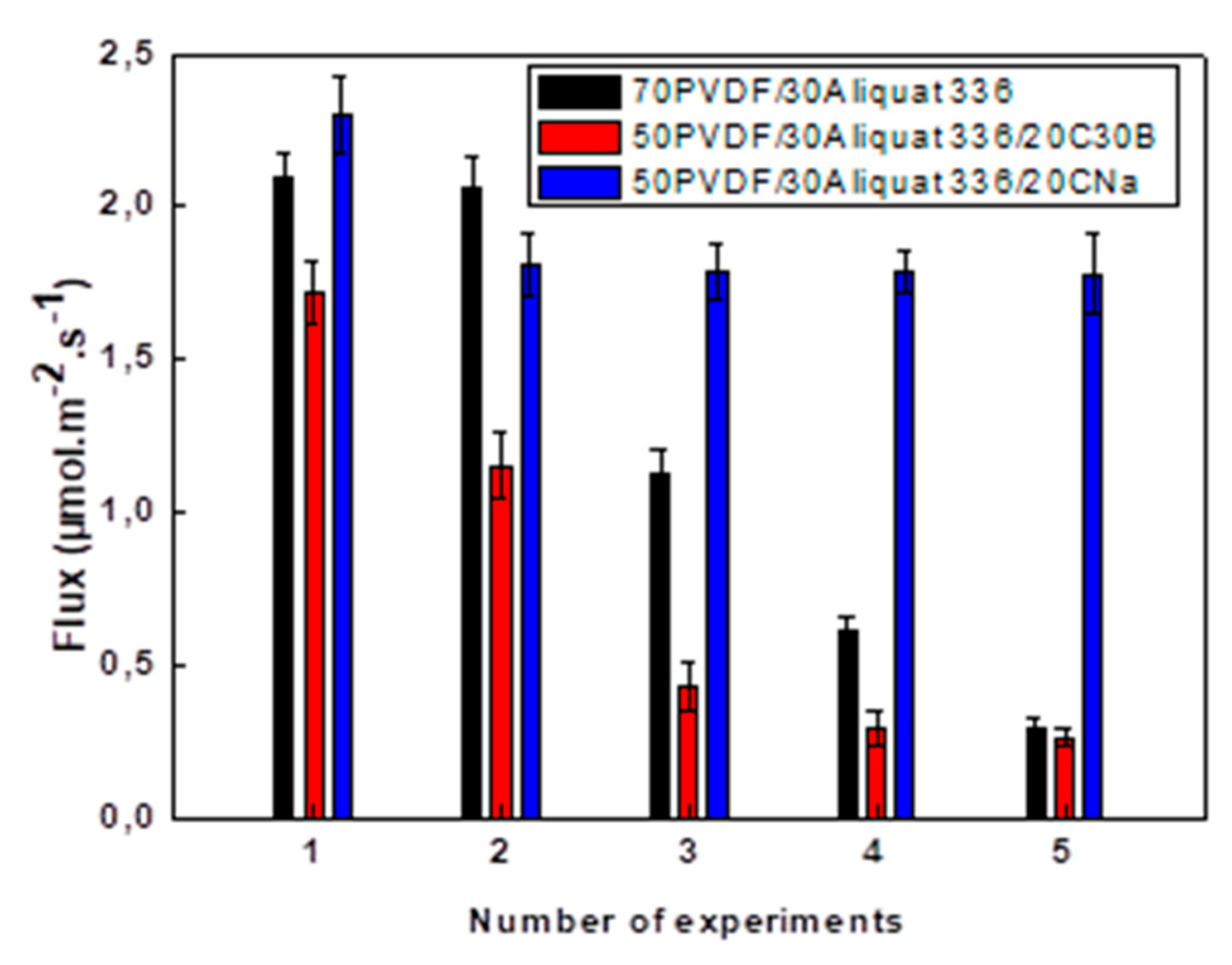
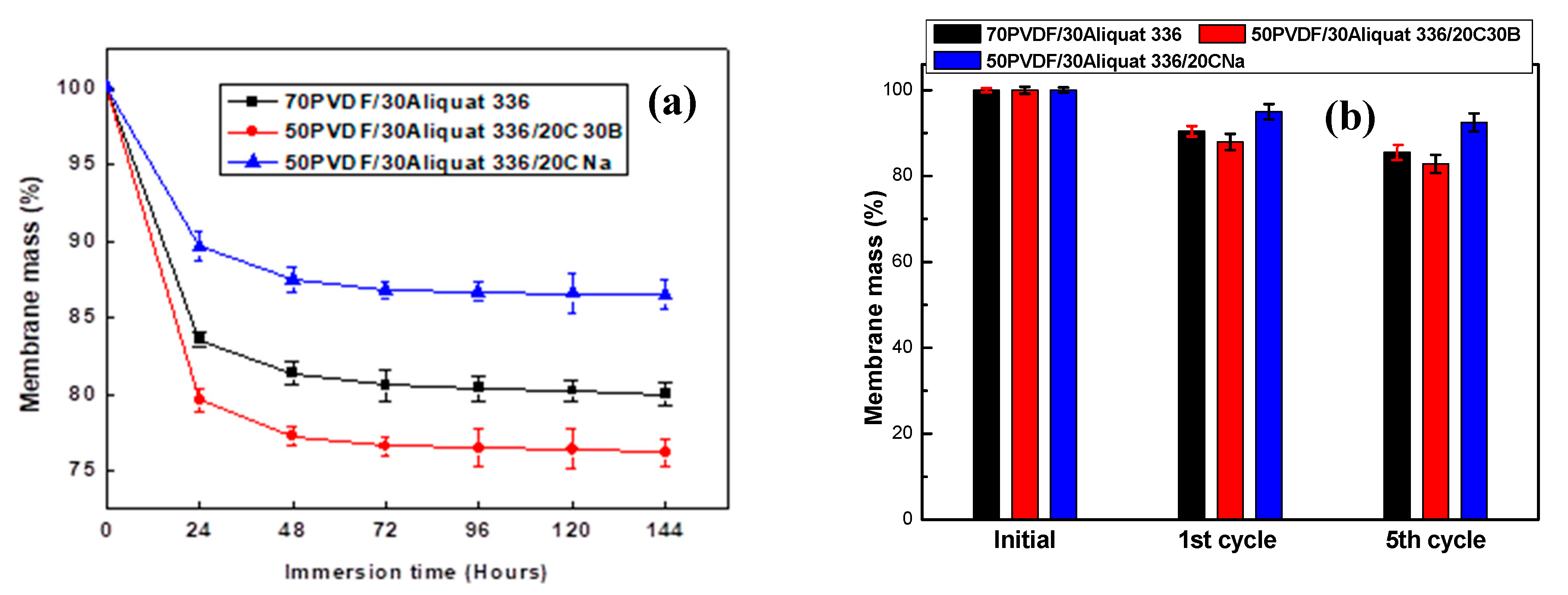
| Number of Cycles | Flux (µmol/(m2·s)) | ||
|---|---|---|---|
| 70PVDF/30Aliquat 336 | 50PVDF/30Aliquat 336/20CNa | 50PVDF/30Aliquat 336/20C30B | |
| 1 | 2.09 ± 0.09 | 2.30 ± 0.13 | 1.72 ± 0.10 |
| 2 | 2.06 ± 0.10 | 1.81 ± 0.10 | 1.15 ± 0.11 |
| 3 | 1.13 ± 0.07 | 1.79 ± 0.09 | 0.43 ± 0.08 |
| 4 | 0.62 ± 0.04 | 1.79 ± 0.07 | 0.29 ± 0.06 |
| 5 | 0.29 ± 0.06 | 1.78 ± 0.13 | 0.26 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Lanel, C.; Fatyeyeva, K. Novel Poly(Vinylidene Fluoride)/Montmorillonite Polymer Inclusion Membrane: Application to Cr(VI) Extraction from Polluted Water. Membranes 2021, 11, 682. https://doi.org/10.3390/membranes11090682
Sellami F, Kebiche-Senhadji O, Marais S, Lanel C, Fatyeyeva K. Novel Poly(Vinylidene Fluoride)/Montmorillonite Polymer Inclusion Membrane: Application to Cr(VI) Extraction from Polluted Water. Membranes. 2021; 11(9):682. https://doi.org/10.3390/membranes11090682
Chicago/Turabian StyleSellami, Ferhat, Ounissa Kebiche-Senhadji, Stéphane Marais, Charles Lanel, and Kateryna Fatyeyeva. 2021. "Novel Poly(Vinylidene Fluoride)/Montmorillonite Polymer Inclusion Membrane: Application to Cr(VI) Extraction from Polluted Water" Membranes 11, no. 9: 682. https://doi.org/10.3390/membranes11090682
APA StyleSellami, F., Kebiche-Senhadji, O., Marais, S., Lanel, C., & Fatyeyeva, K. (2021). Novel Poly(Vinylidene Fluoride)/Montmorillonite Polymer Inclusion Membrane: Application to Cr(VI) Extraction from Polluted Water. Membranes, 11(9), 682. https://doi.org/10.3390/membranes11090682







