Stability of Ar/O2 Plasma-Treated Polypropylene Membranes Applied for Membrane Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Treatment
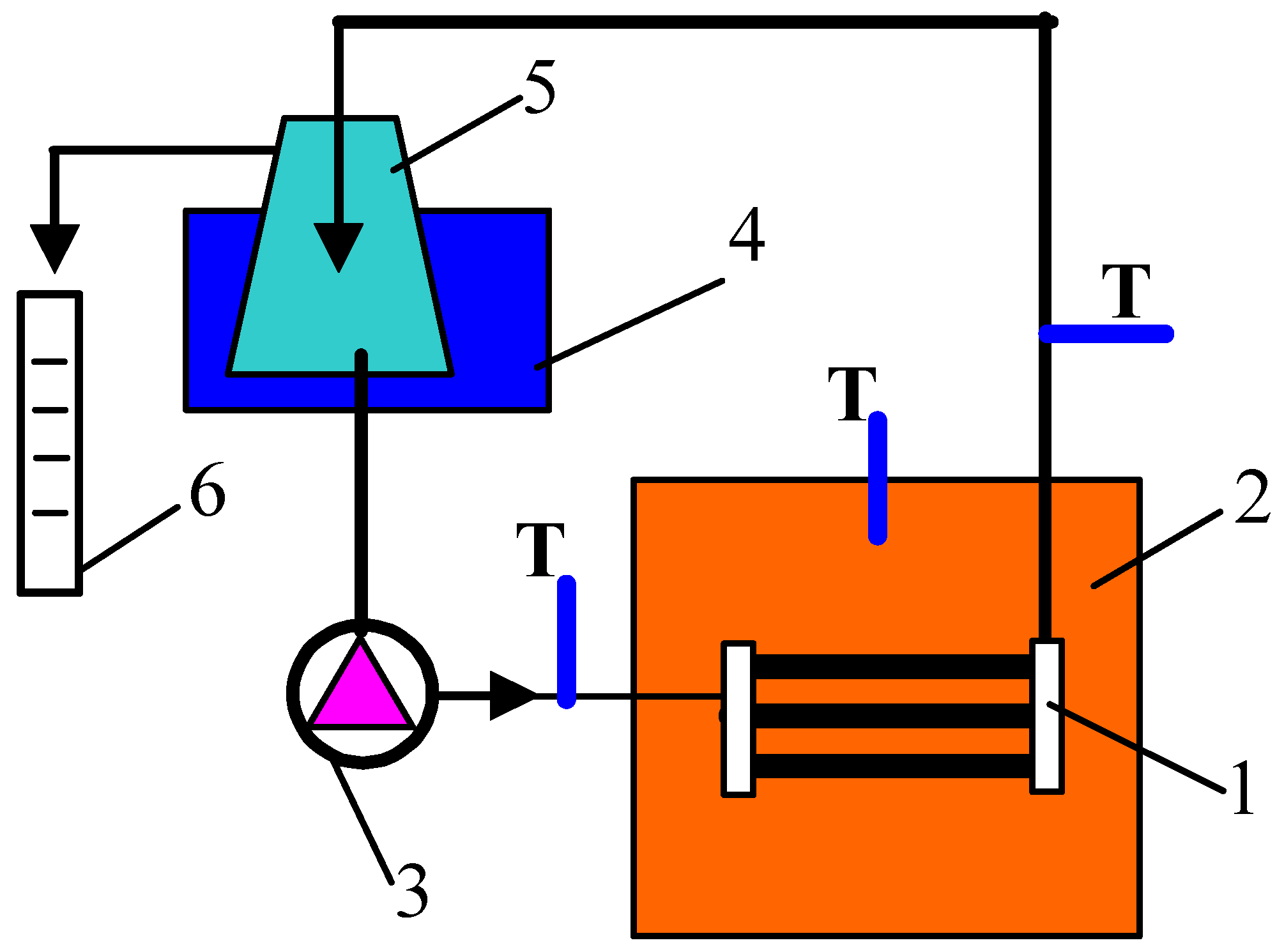
2.2. Membrane Distillation
2.3. Analytical Methods
3. Results and Discussion
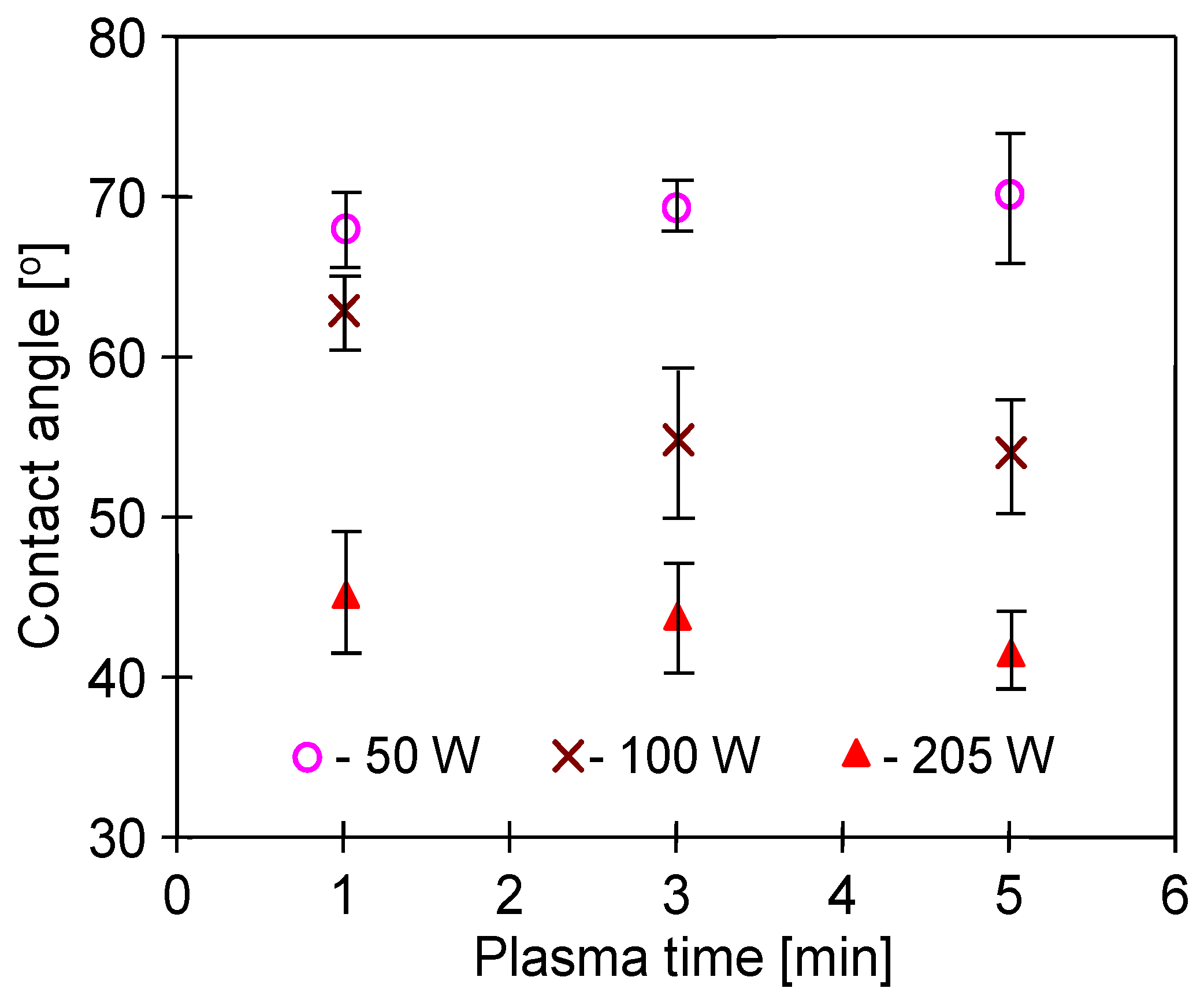
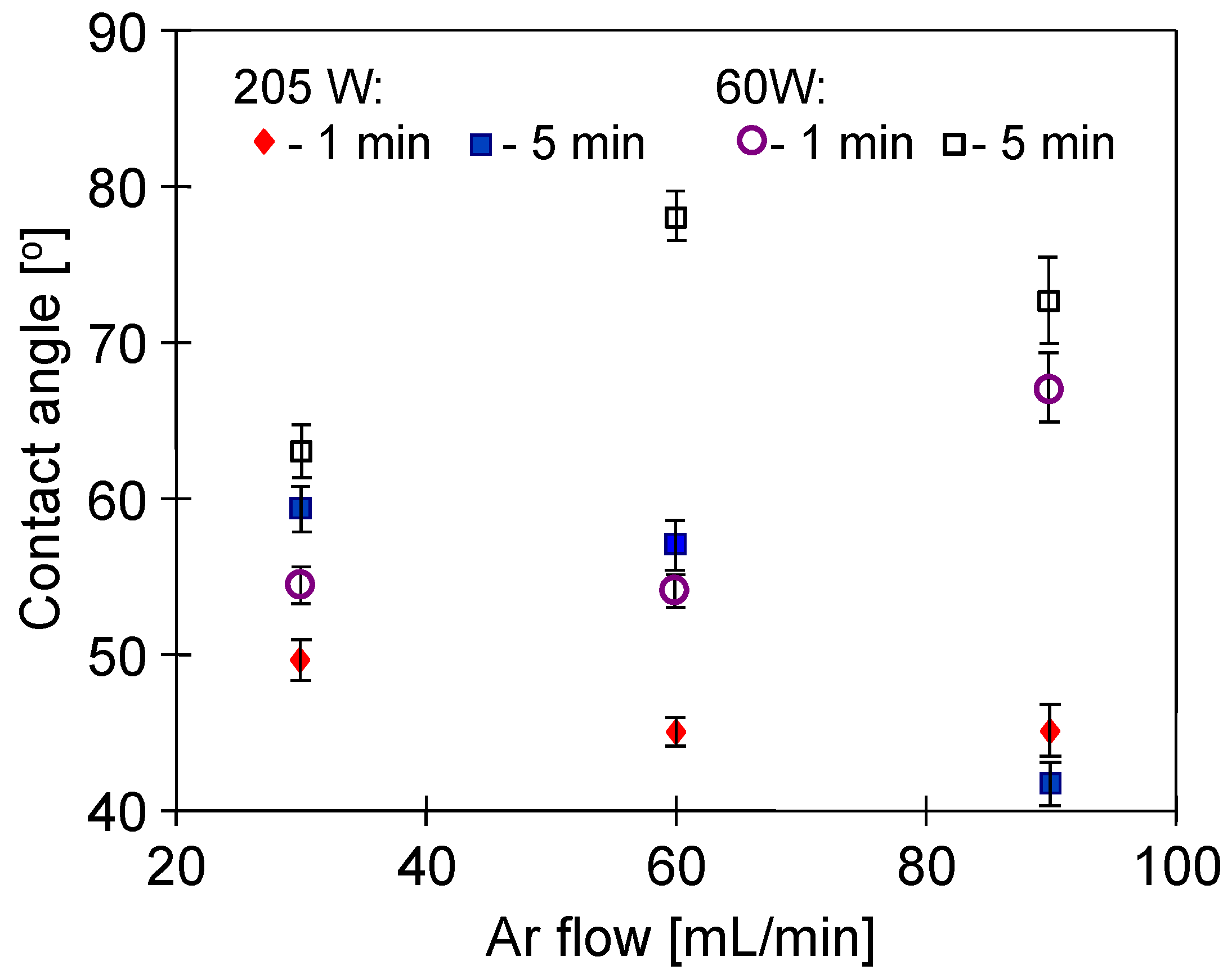
3.1. Conditions of Plasma Treatment
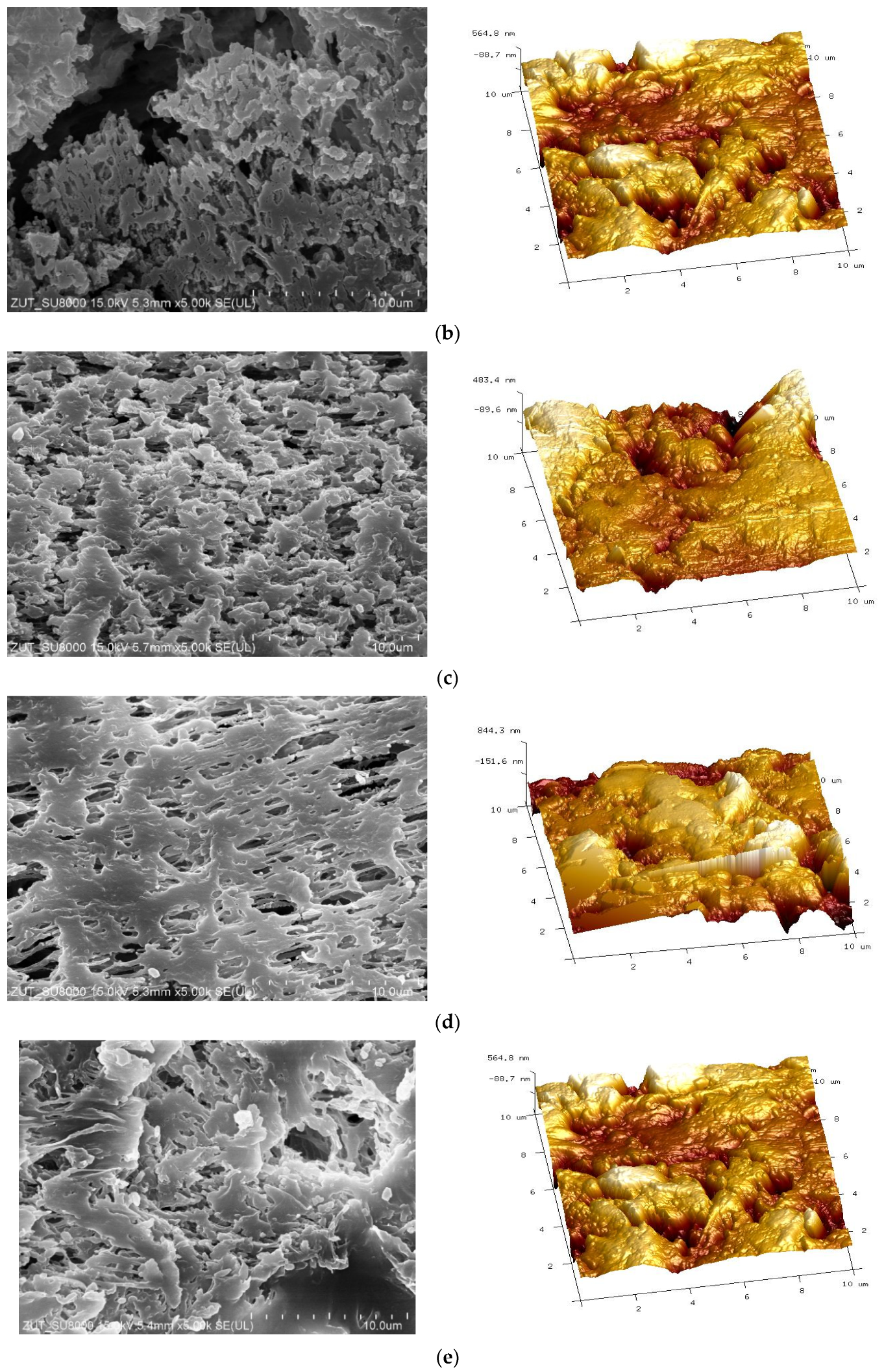
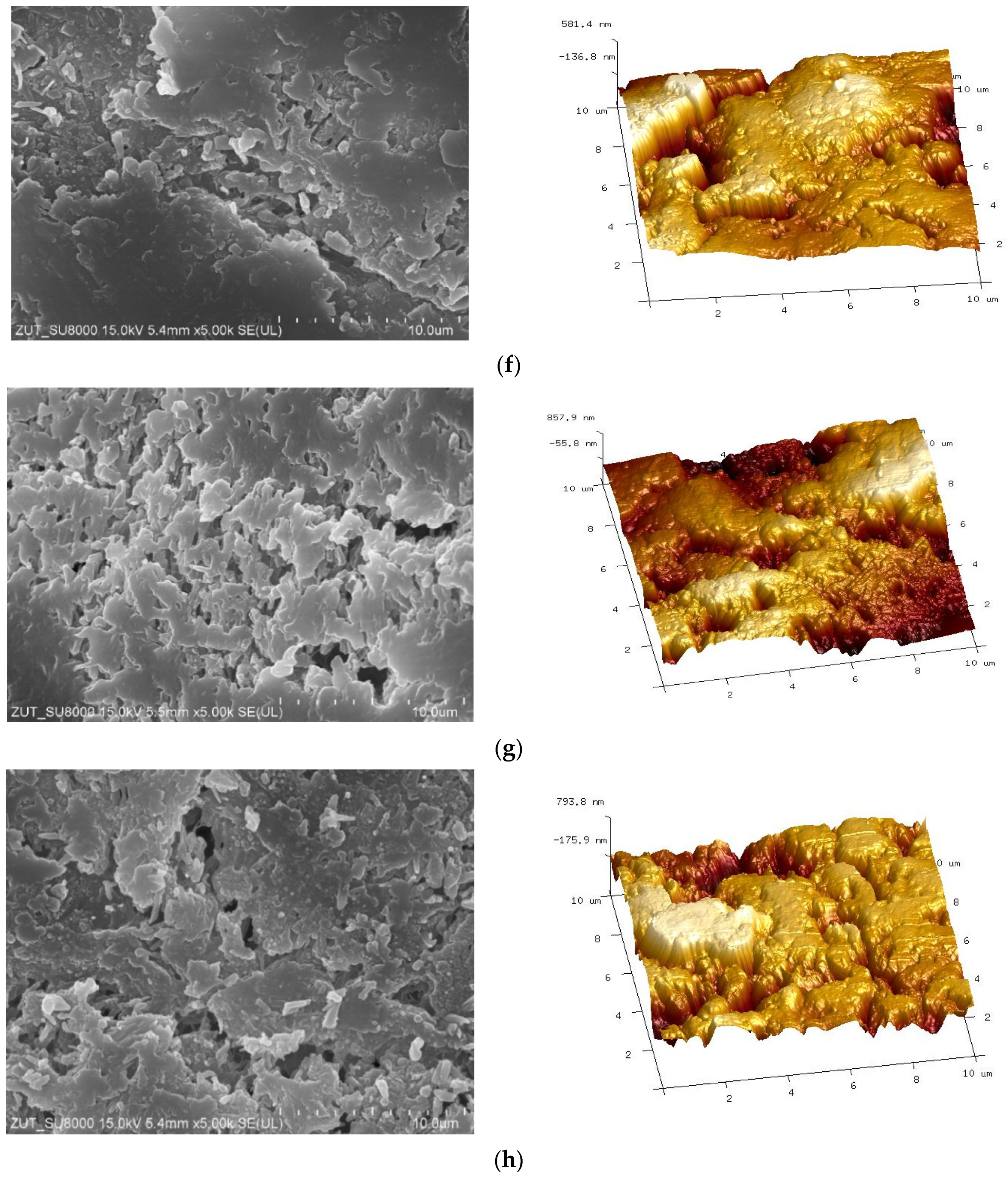
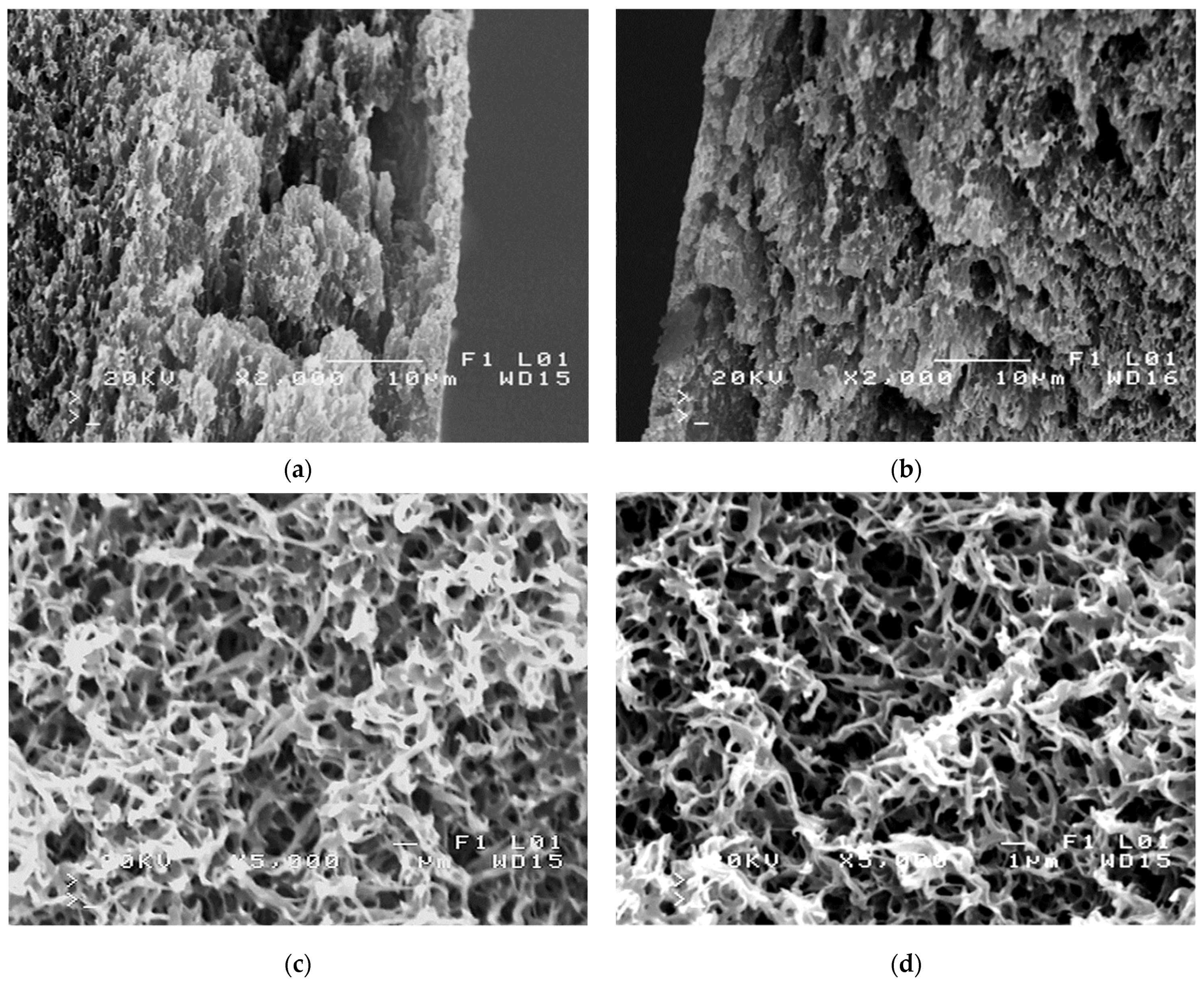
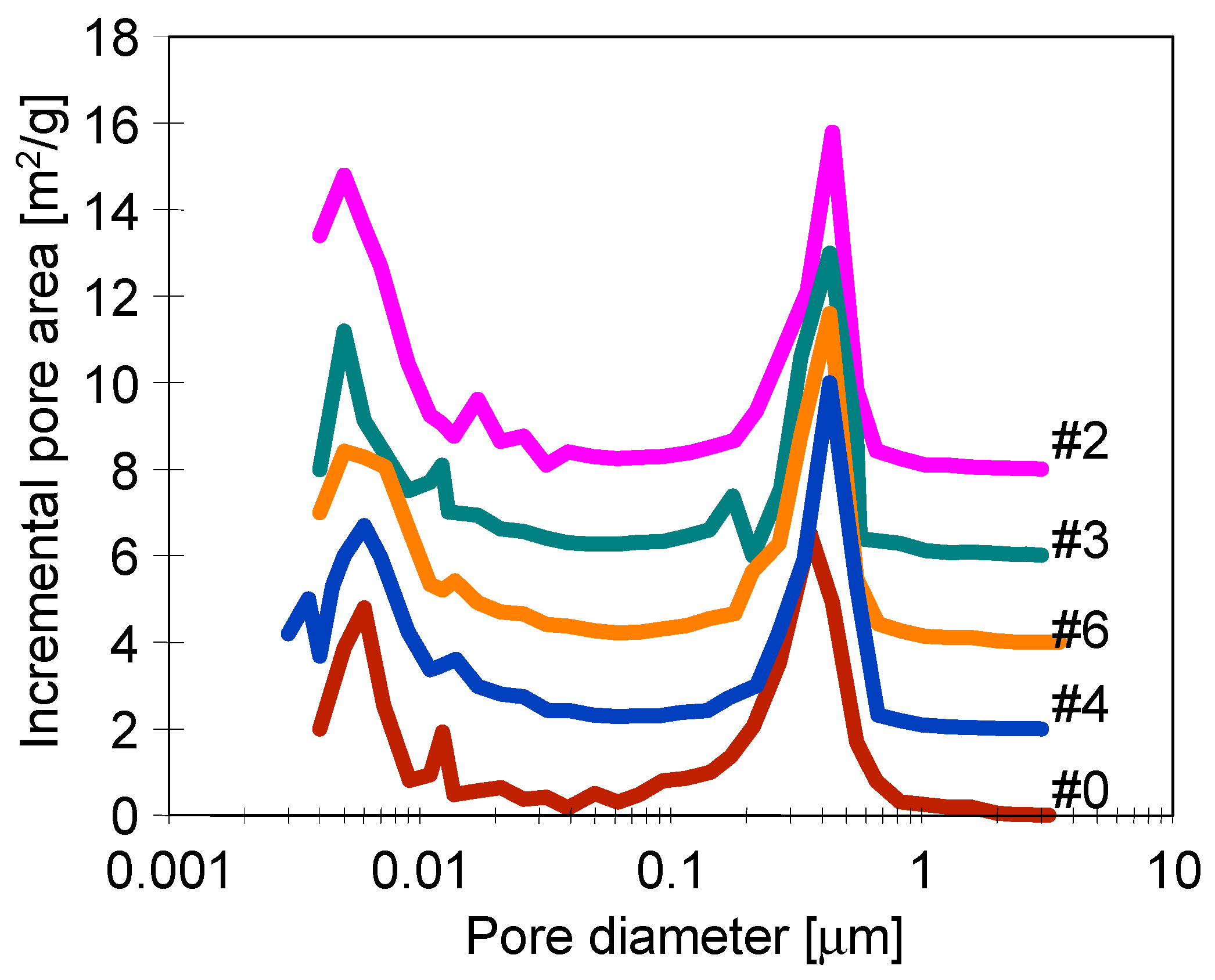
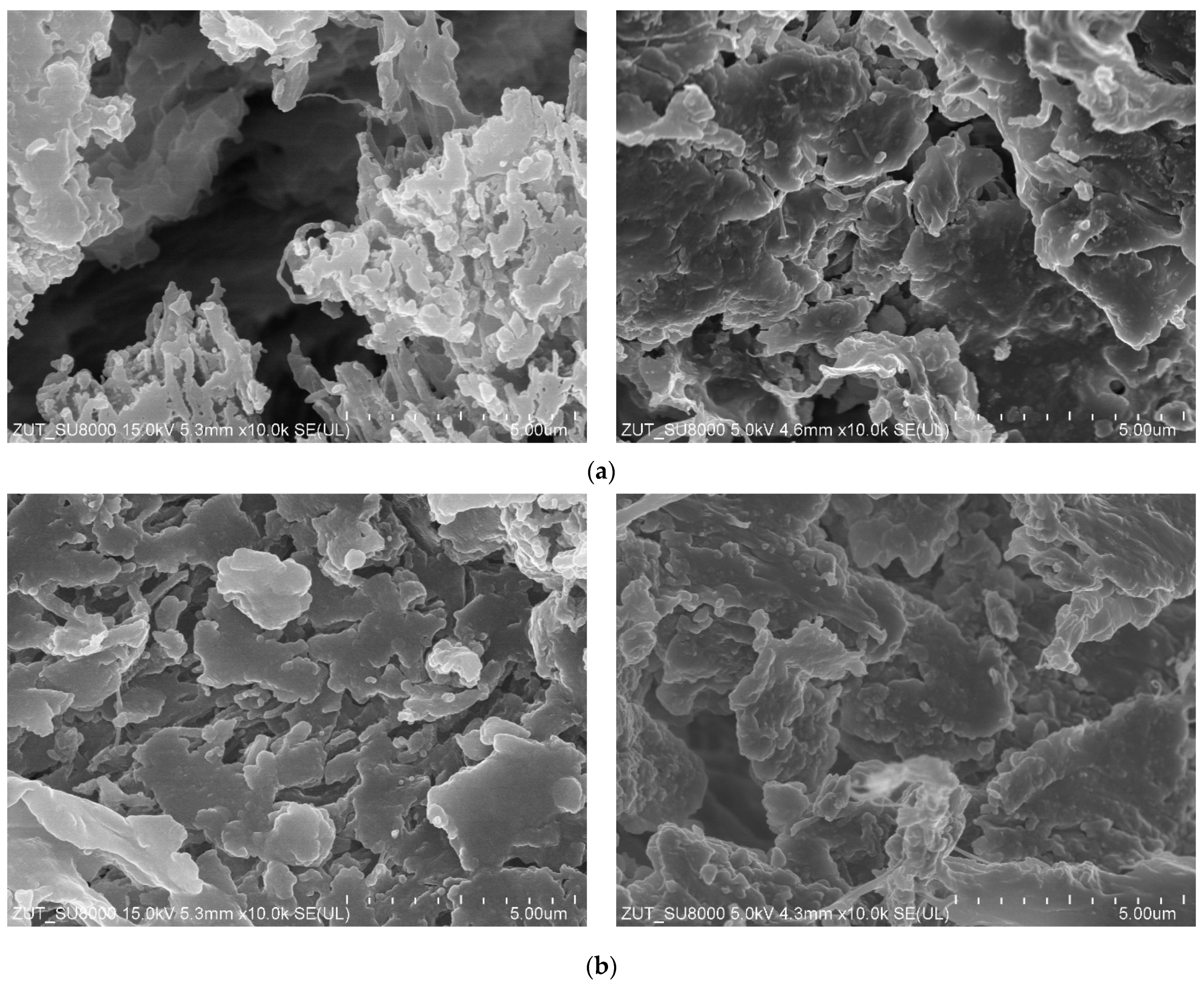
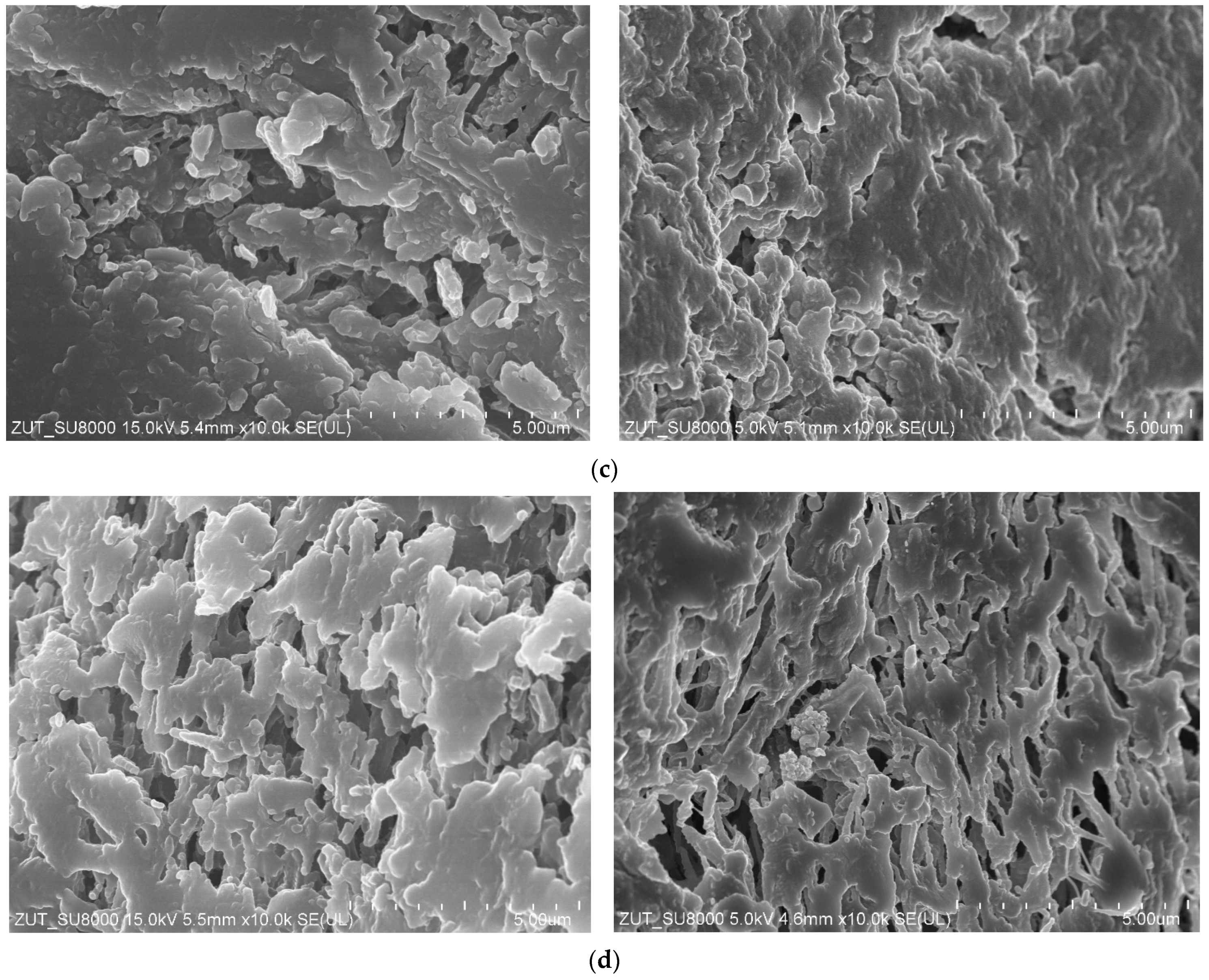
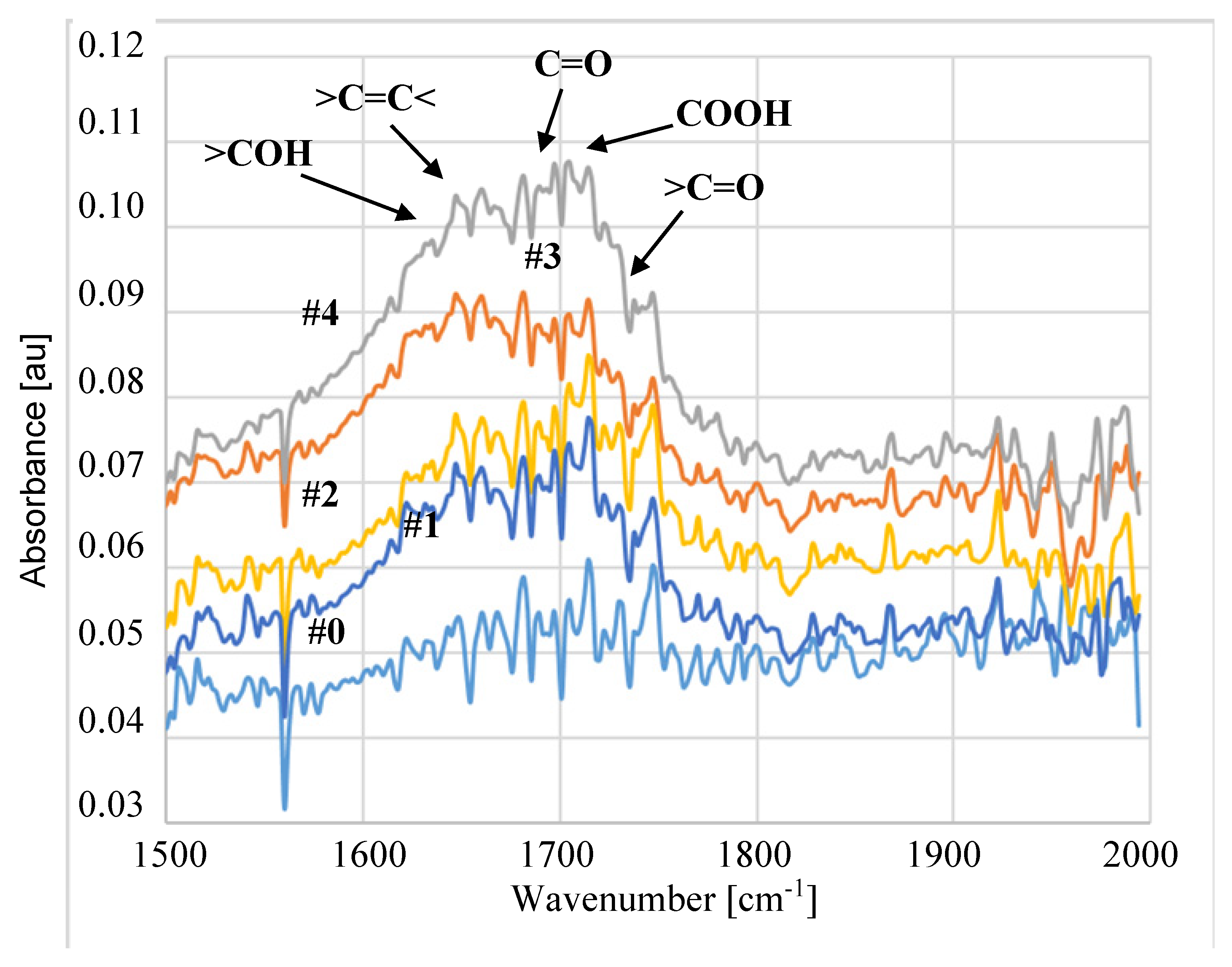
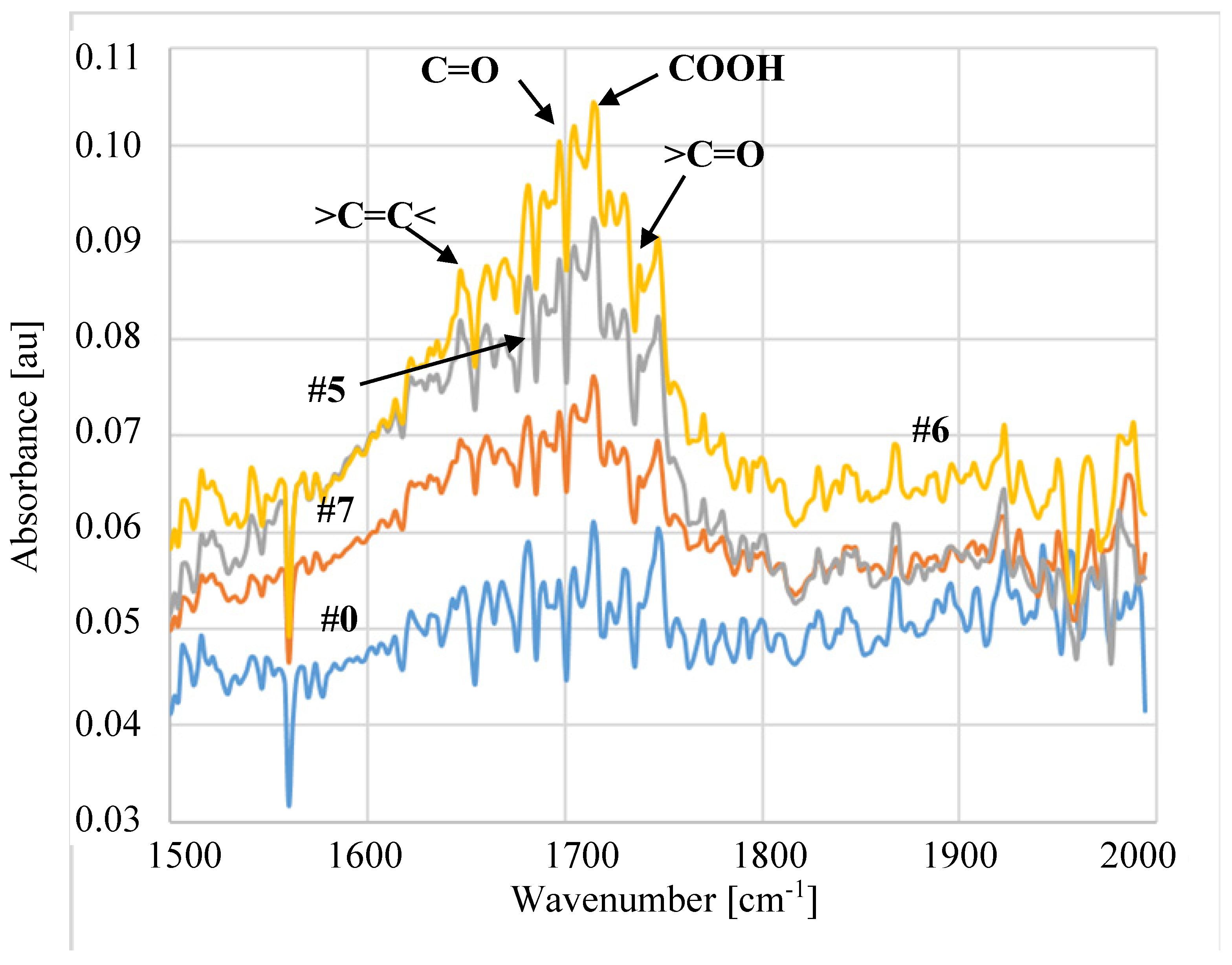
3.2. Parameters of Membrane Modification
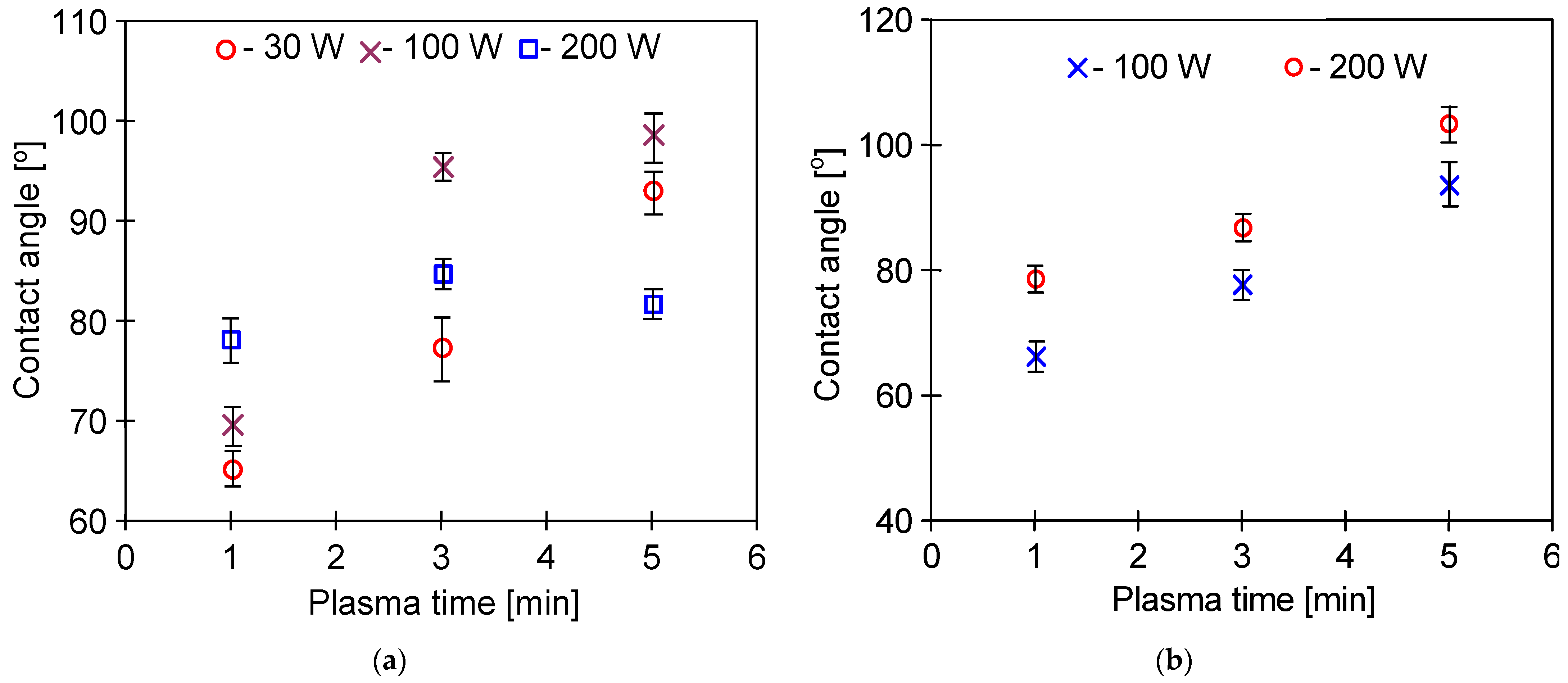
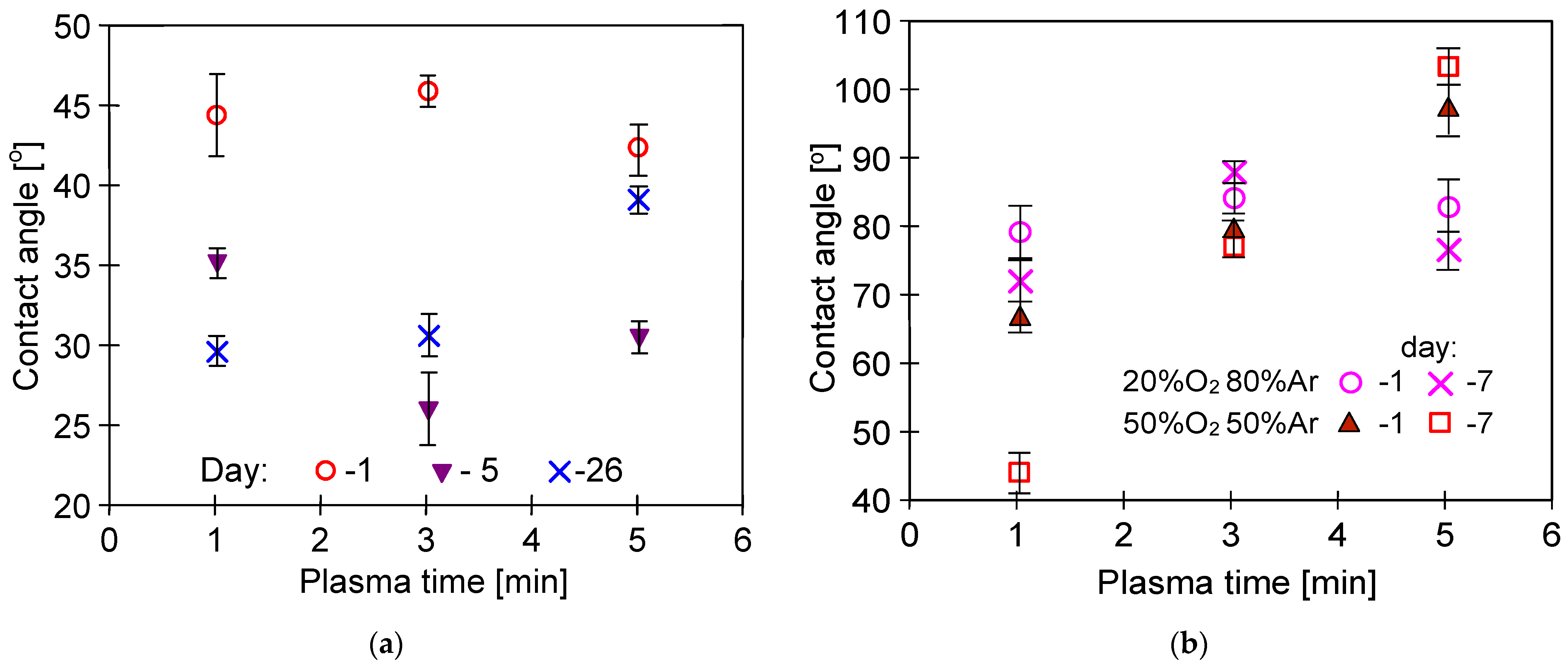
3.3. Membrane Wettability
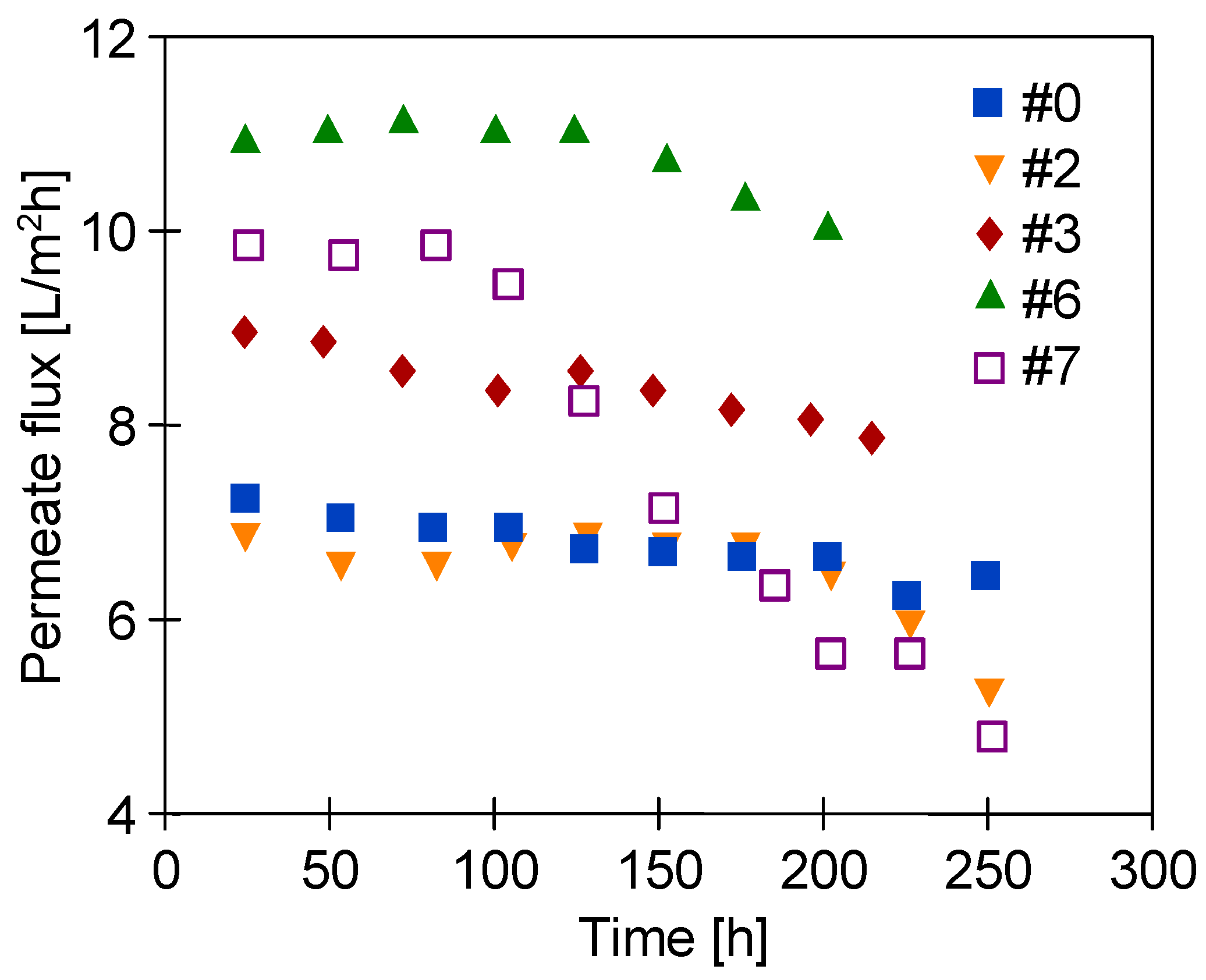
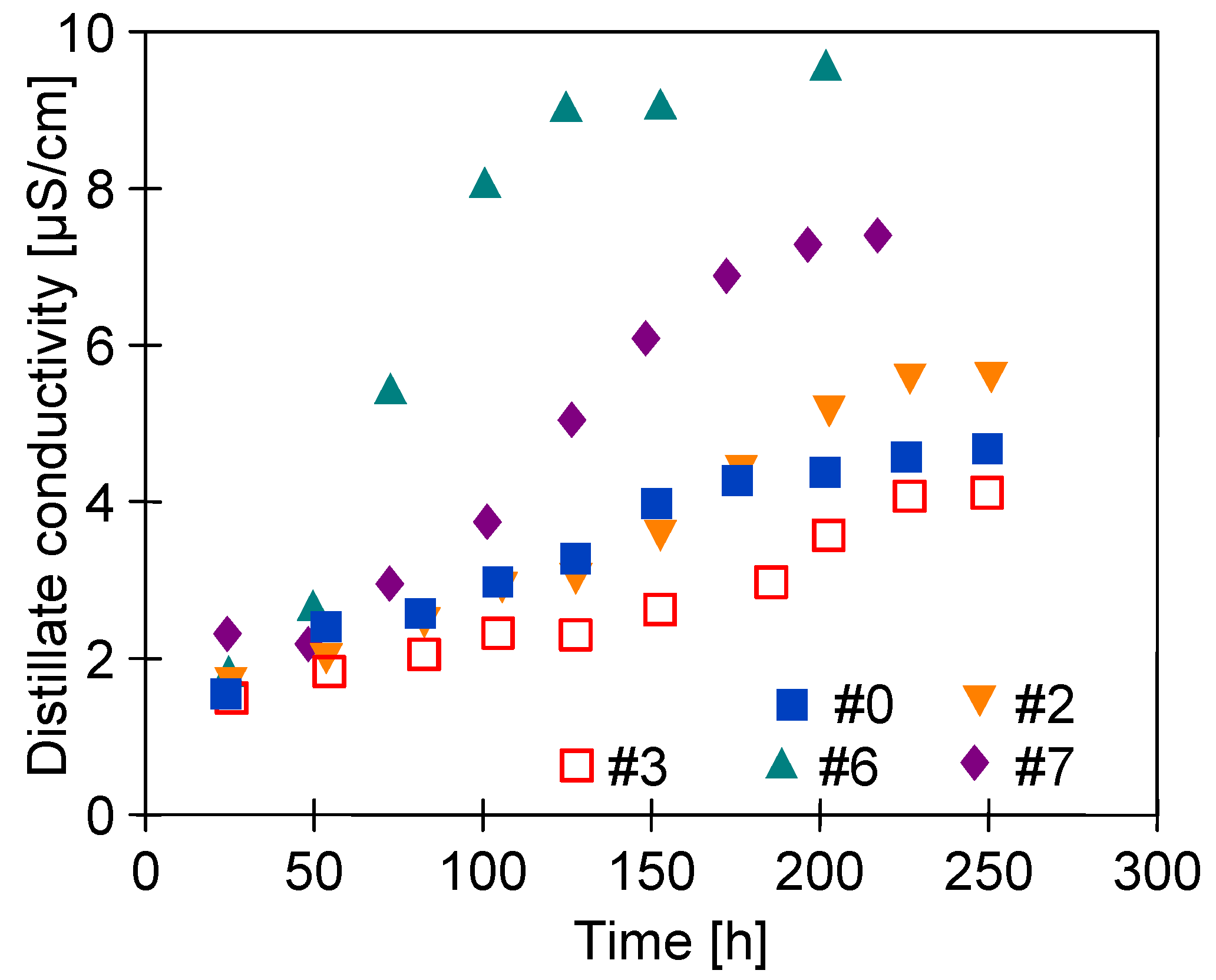
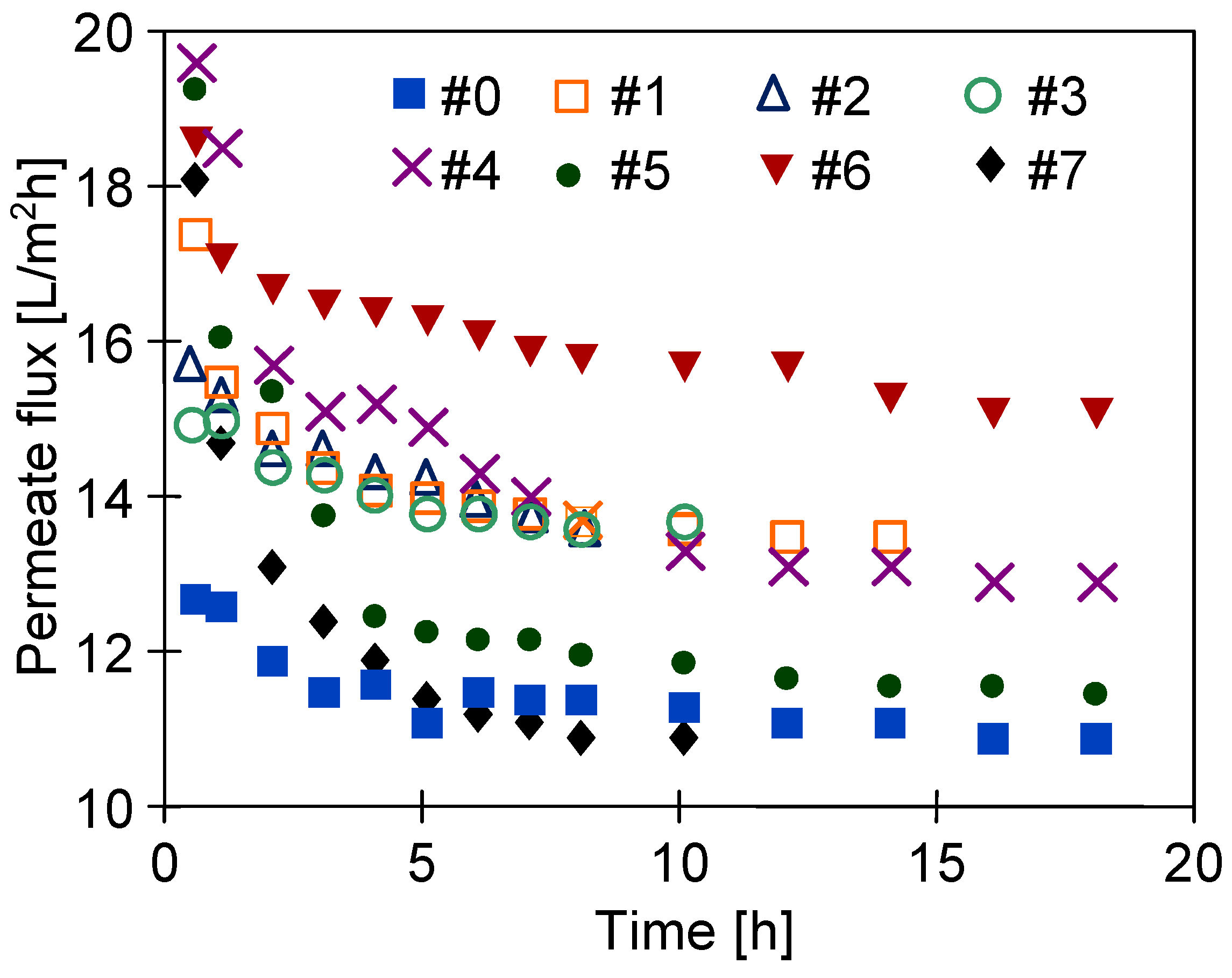
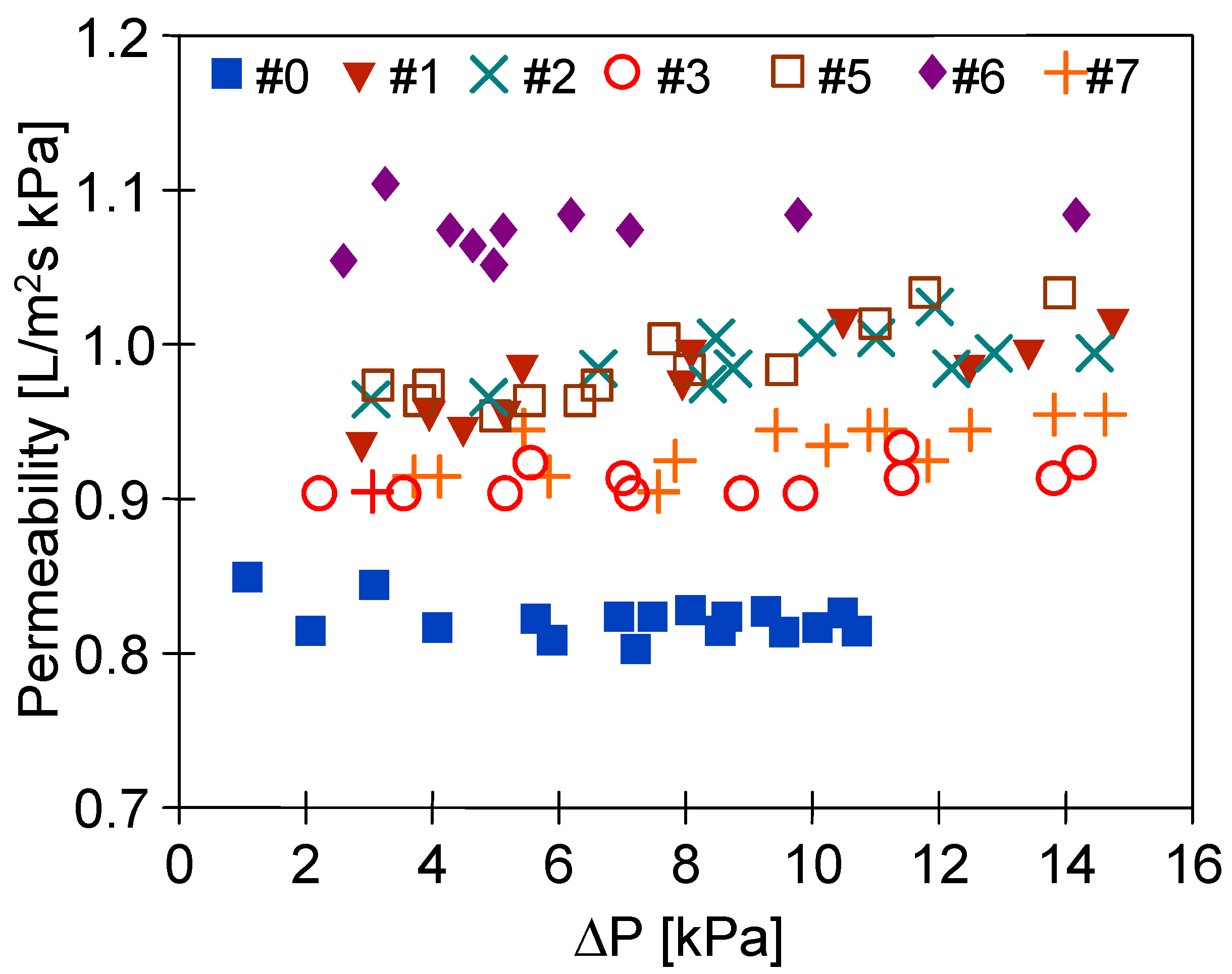
3.4. Long-Term Stabilization of Membranes
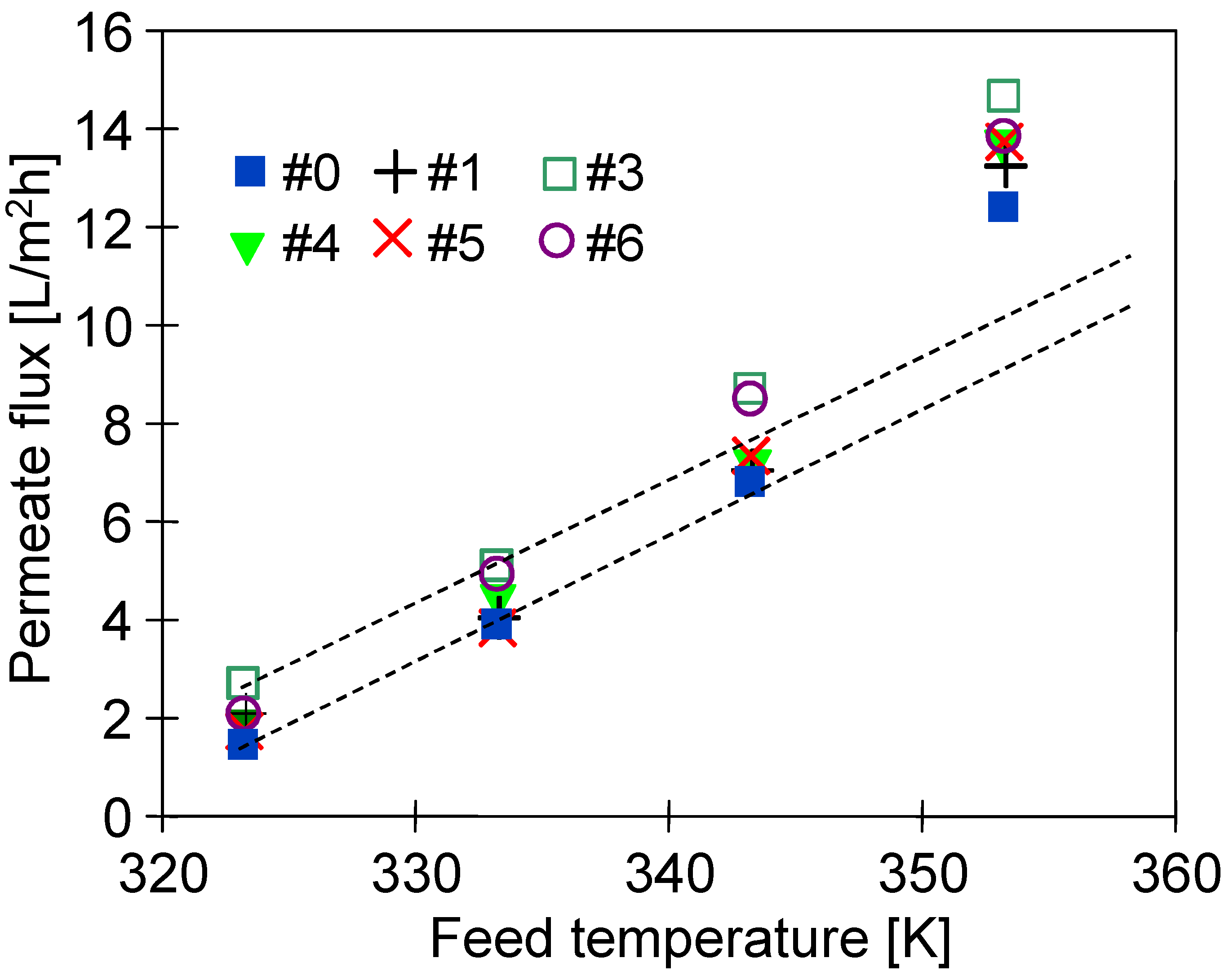
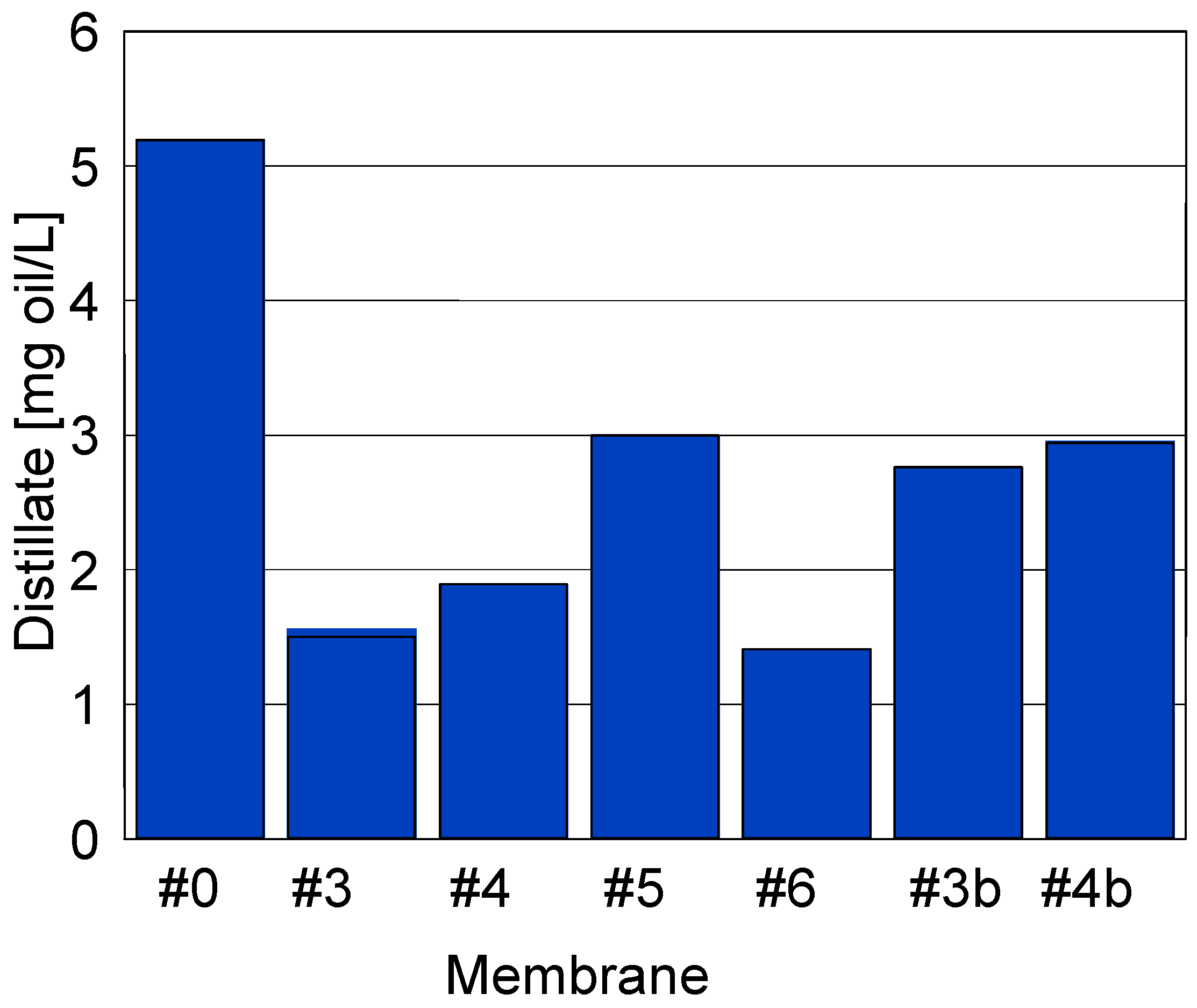
3.5. Desalination of Oily Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Yang, B.; Xiao, K.; Zhao, H. Separation, Anti-Fouling, and Chlorine Resistance of the Polyamide Reverse Osmosis Membrane: From Mechanisms to Mitigation Strategies. Water Res. 2021, 195, 116976. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Ravi, J.; Othman, M.H.D.; Matsuura, T.; Bilad, M.R.I.; El-badawy, T.H.; Aziz, F.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Polymeric Membranes for Desalination Using Membrane Distillation: A Review. Desalination 2020, 490, 114530. [Google Scholar] [CrossRef]
- Tibi, F.; Charfi, A.; Cho, J.; Kim, J. Fabrication of Polymeric Membranes for Membrane Distillation Process and Application for Wastewater Treatment: Critical Review. Process Saf. Environ. Prot. 2020, 141, 190–201. [Google Scholar] [CrossRef]
- Anvari, A.; Yancheshme, A.A.; Kekre, K.M.; Ronen, A. State-of-the-Art Methods for Overcoming Temperature Polarization in Membrane Distillation Process: A Review. J. Membr. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Ibrahim, S.S.; Khaleel, S.R. Performance of Vacuum Poly(Propylene) Membrane Distillation (VMD) for Saline Water Desalination. Chem. Eng. Process. Process Intensif. 2017, 120, 68–80. [Google Scholar] [CrossRef]
- Floros, I.N.; Kouvelos, E.P.; Pilatos, G.I.; Hadjigeorgiou, E.P.; Gotzias, A.D.; Favvas, E.P.; Sapalidis, A.A. Enhancement of Flux Performance in PTFE Membranes for Direct Contact Membrane Distillation. Polymers 2020, 12, 345. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Chamani, H.; Matsuura, T.; Rana, D.; Lan, C. Effect of Temperature and Salt on PVDF Membrane Wetting Properties. J. Constr. Mater. 2021. [Google Scholar] [CrossRef]
- Kiss, A.A.; Readi, O.M.K. An Industrial Perspective on Membrane Distillation Processes: Membrane Distillation Processes. J. Chem. Technol. Biotechnol. 2018, 93, 2047–2055. [Google Scholar] [CrossRef]
- Grossi, L.B.; Alvim, C.B.; Alvares, C.M.S.; Martins, M.F.; Amaral, M.C.S. Purifying Surface Water Contaminated with Industrial Failure Using Direct Contact Membrane Distillation. Sep. Purif. Technol. 2020, 233, 116052. [Google Scholar] [CrossRef]
- Gontarek-Castro, E.; Castro-Muñoz, R.; Lieder, M. New Insights of Nanomaterials Usage toward Superhydrophobic Membranes for Water Desalination via Membrane Distillation: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 1–46. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Liu, Y.; Li, X.; Yin, H.; Volkov, A.; He, T. Understanding the Fouling/Scaling Resistance of Superhydrophobic/Omniphobic Membranes in Membrane Distillation. Desalination 2021, 499, 114864. [Google Scholar] [CrossRef]
- Ray, S.S.; Bakshi, H.S.; Dangayach, R.; Singh, R.; Deb, C.K.; Ganesapillai, M.; Chen, S.-S.; Purkait, M.K. Recent Developments in Nanomaterials-Modified Membranes for Improved Membrane Distillation Performance. Membranes 2020, 10, 140. [Google Scholar] [CrossRef]
- Alsebaeai, M.K.; Ahmad, A.L. Membrane Distillation: Progress in the Improvement of Dedicated Membranes for Enhanced Hydrophobicity and Desalination Performance. J. Ind. Eng. Chem. 2020, 86, 13–34. [Google Scholar] [CrossRef]
- Madalosso, H.B.; Machado, R.; Hotza, D.; Marangoni, C. Membrane Surface Modification by Electrospinning, Coating, and Plasma for Membrane Distillation Applications: A State-of-the-Art Review. Adv. Eng. Mater. 2021, 23, 2001456. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and Perspectives in PTFE Membrane: Preparation, Modification, and Applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Reis, R.; Chen, Z.; Milne, N.; Winther-Jensen, B.; Kong, L.; Dumée, L. Plasma Modification and Synthesis of Membrane Materials—A Mechanistic Review. Membranes 2018, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.S.; Dangayach, R.; Kwon, Y.-N. Surface Engineering for Anti-Wetting and Antibacterial Membrane for Enhanced and Fouling Resistant Membrane Distillation Performance. Chem. Eng. J. 2021, 405, 126702. [Google Scholar] [CrossRef]
- Rabiei, S.; Mousavi, S.M.; Paterson, A.H.J. Development of Hierarchical Surface Roughness on Porous Poly (Vinylidene Fluoride) Membrane for Membrane Distillation Process. Polym. Eng. Sci. 2020, 60, 1686–1698. [Google Scholar] [CrossRef]
- Xiao, Z.; Guo, H.; He, H.; Liu, Y.; Li, X.; Zhang, Y.; Yin, H.; Volkov, A.V.; He, T. Unprecedented Scaling/Fouling Resistance of Omniphobic Polyvinylidene Fluoride Membrane with Silica Nanoparticle Coated Micropillars in Direct Contact Membrane Distillation. J. Membr. Sci. 2020, 599, 117819. [Google Scholar] [CrossRef]
- Thomas, N.; Kumar, M.; Palmisano, G.; Al-Rub, R.K.A.; Alnuaimi, R.Y.; Alhseinat, E.; Rowshan, R.; Arafat, H.A. Antiscaling 3D Printed Feed Spacers via Facile Nanoparticle Coating for Membrane Distillation. Water Res. 2021, 189, 116649. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, Y.; Fan, X.; Liu, Z.; Song, Y.; Wang, Y.; Tao, P.; Song, C.; Shao, M. In-Situ Silica Nanoparticle Assembly Technique to Develop an Omniphobic Membrane for Durable Membrane Distillation. Desalination 2021, 499, 114832. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Wan, L.-S.; Xu, Z.-K. Surface Engineering of Microporous Polypropylene Membrane for Antifouling: A Mini-Review. J. Adhes. Sci. Technol. 2011, 25, 245–260. [Google Scholar] [CrossRef]
- Gryta, M. Surface Modification of Polypropylene Membrane by Helium Plasma Treatment for Membrane Distillation. J. Membr. Sci. 2021, 628, 119265. [Google Scholar] [CrossRef]
- Pedram, S.; Mortaheb, H.R.; Arefi-Khonsari, F. Plasma Treatment of Polyethersulfone Membrane for Benzene Removal from Water by Air Gap Membrane Distillation. Environ. Technol. 2018, 39, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Shin, B.; Jo, W.; Kim, H.-Y.; Moon, M.-W.; Lee, S. Nanostructured PVDF Membrane for MD Application by an O2 and CF4 Plasma Treatment. Desalination 2016, 399, 178–184. [Google Scholar] [CrossRef]
- Woo, Y.C.; Chen, Y.; Tijing, L.D.; Phuntsho, S.; He, T.; Choi, J.-S.; Kim, S.-H.; Kyong Shon, H. CF4 Plasma-Modified Omniphobic Electrospun Nanofiber Membrane for Produced Water Brine Treatment by Membrane Distillation. J. Membr. Sci. 2017, 529, 234–242. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, B.; Li, X.-M.; Wang, Z.; He, B.-Q.; He, T.; Jiang, B. CF4 Plasma Surface Modification of Asymmetric Hydrophilic Polyethersulfone Membranes for Direct Contact Membrane Distillation. J. Membr. Sci. 2012, 407, 164–175. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.-M.; Gilron, J.; Kong, D.; Yin, Y.; Oren, Y.; Linder, C.; He, T. CF4 Plasma-Modified Superhydrophobic PVDF Membranes for Direct Contact Membrane Distillation. J. Membr. Sci. 2014, 456, 155–161. [Google Scholar] [CrossRef]
- Lai, C.-L.; Liou, R.-M.; Chen, S.-H.; Huang, G.-W.; Lee, K.-R. Preparation and Characterization of Plasma-Modified PTFE Membrane and Its Application in Direct Contact Membrane Distillation. Desalination 2011, 267, 184–192. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic Modification of Polyethersulfone Membranes by Low Temperature Plasma-Induced Graft Polymerization. J. Membr. Sci. 2002, 209, 255–269. [Google Scholar] [CrossRef]
- Liu, L.; Shen, F.; Chen, X.; Luo, J.; Su, Y.; Wu, H.; Wan, Y. A Novel Plasma-Induced Surface Hydrophobization Strategy for Membrane Distillation: Etching, Dipping and Grafting. J. Membr. Sci. 2016, 499, 544–554. [Google Scholar] [CrossRef]
- Kang, M.S.; Chun, B.; Kim, S.S. Surface Modification of Polypropylene Membrane by Low-Temperature Plasma Treatment. J. Appl. Polym. Sci. 2001, 81, 1555–1566. [Google Scholar] [CrossRef]
- Liang, C.-H.; Li, C.; Chen, T.-H.; Cheng, C.-Y.; Huang, C. Surface Evaluation of Reactive Plasma-Modified Microporous Polypropylene Membrane by Static Contact Angle Analysis. Polym. Degrad. Stab. 2019, 160, 89–95. [Google Scholar] [CrossRef]
- Kawakami, R.; Yoshitani, Y.; Mitani, K.; Niibe, M.; Nakano, Y.; Azuma, C.; Mukai, T. Effects of Air-Based Nonequilibrium Atmospheric Pressure Plasma Jet Treatment on Characteristics of Polypropylene Film Surfaces. Appl. Surf. Sci. 2020, 509, 144910. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Liu, G.; Ren, Y.; Lv, J.; Gao, D.; Lu, Z. Smart Adhesion by Surface Treatment Experimental and Theoretical Insights. Therm. Sci. 2019, 23, 2355–2363. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C.; Gengembre, L.; Payen, E. Treatment of Polymer Films with a Dielectric Barrier Discharge in Air, Helium and Argon at Medium Pressure. Coat. Technol. 2007, 201, 7066–7075. [Google Scholar] [CrossRef]
- Raveh, A.; Brewer, J.; Irene, E.A. Nitridation of Thermal SiO2 FIlms by Radio-Frequency Plasma Assisted Electron Cyclotron Resonance: Layer Structure and Composition. J. Vac. Sci. Technol. A 2001, 19, 17–24. [Google Scholar]
- Khaing, T.-H.; Li, J.; Li, Y.; Wai, N.; Wong, F.S. Feasibility Study on Petrochemical Wastewater Treatment and Reuse Using a Novel Submerged Membrane Distillation Bioreactor. Sep. purif. Technol. 2010, 74, 138–143. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Tang, Z.-Q.; Huang, L.; Cheng, G.; Li, W.; Zhou, J.; Yan, M.-G.; Gu, J.-S.; Wei, X.-W. Surface Modification of Polypropylene Macroporous Membrane to Improve Its Antifouling Characteristics in a Submerged Membrane-Bioreactor: H2O Plasma Treatment. Water Res. 2008, 42, 4341–4347. [Google Scholar] [CrossRef] [PubMed]
- Nishime, T.M.C.; Wagner, R.; Kostov, K.G. Study of Modified Area of Polymer Samples Exposed to a He Atmospheric Pressure Plasma Jet Using Different Treatment Conditions. Polymers 2020, 12, 1028. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Hein, L.R.O.; Toth, A. Study of Polypropylene Surface Modification by Air Dielectric Barrier Discharge Operated at Two Different Frequencies. Surf. Coat. Technol. 2013, 234, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.P.; Lei, M.K. Nanotexturing and Wettability Ageing of Polypropylene Surfaces Modified by Oxygen Capacitively Coupled Radio Frequency Plasma. J. Mater. Sci. Technol. 2014, 30, 965–972. [Google Scholar] [CrossRef]
- Reis, R.; Dumée, L.F.; Merenda, A.; Orbell, J.D.; Schütz, J.A.; Duke, M.C. Plasma-Induced Physicochemical Effects on a Poly(Amide) Thin-Film Composite Membrane. Desalination 2017, 403, 3–11. [Google Scholar] [CrossRef]
- Dumée, L.F.; Alglave, H.; Chaffraix, T.; Lin, B.; Magniez, K.; Schütz, J. Morphology-Properties Relationship of Gas Plasma Treated Hydrophobic Meso-Porous Membranes and Their Improved Performance for Desalination by Membrane Distillation. Appl. Surf. Sci. 2016, 363, 273–285. [Google Scholar] [CrossRef]
- Wade, W.L.; Mammone, R.J.; Binder, M. Surface Properties of Commercial Polymer Films Following Various Gas Plasma Treatments. J. Appl. Polym. Sci. 1991, 43, 1589–1591. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Rho, G.-H.; Lee, J.Y.; Kim, S.J.; Kim, H.-Y.; Jang, Y.-J.; Moon, M.-W.; Lee, K.-R. Nano-Embossed Structure on Polypropylene Induced by Low Energy Ar Ion Beam Irradiation. Surf. Coat. Technol. 2010, 205, S104–S108. [Google Scholar] [CrossRef]
- Barbeş, L.; Rădulescu, C.; Stihi, C. ATR-FTIR spectrometry characterisation of polymeric materials. Rom. Rep. Phys. 2014, 66, 765–777. [Google Scholar]
- Fontanella, S.; Bonhomme, S.; Brusson, J.M.; Pitteri, S.; Samuel, G.; Pichon, G.; Lacoste, J.; Fromageot, D.; Lemaire, J.; Delort, A.M. Comparison of Biodegradability of Various Polypropylene Films Containing Pro-Oxidant Additives Based on Mn, Mn/Fe or Co. Polym. Degrad. Stab. 2013, 98, 875–884. [Google Scholar] [CrossRef]





| Inner Diameter (mm) | Wall Thickness (mm) | Porosity (%) | Average Pore Diameters (µm) |
|---|---|---|---|
| 1.8 | 0.4 | 72 | 0.2 |
| Membrane | Power (W) | Bias (V) | Time (min) | Plasma | Water Contact Angle (°) | RA (nm) |
|---|---|---|---|---|---|---|
| #0 | - | - | - | - | 102.1 | 150 ± 29 |
| #1 | 205 | 180 | 5 | Ar | 65.1 ± 3.7 | 286 ± 71 |
| #2 | 205 | 270 | 3 | Ar | 57.8 ± 2.5 | 236 ± 63 |
| #3 | 205 | 272 | 1 | Ar | 61.9 | 275 ± 68 |
| #4 | 101 | 100 | 5 | Ar | 62.2 ± 2.9 | 200 ± 54 |
| #5 | 205 | 274 | 5 | Ar/O2 | 71.6 | 182 ± 80 |
| #6 | 205 | 215 | 5 | Ar/O2 | 55.6 ± 2.7 | 188 ± 55 |
| #7 | 101 | 139 | 5 | Ar/O2 | 62.4 ± 2.1 | 197 ± 20 |
| Membrane | Plasma | After 1 Day | After 4 Years | After MD |
|---|---|---|---|---|
| #0 | - | 103.9 ± 1.6° | 104.7 ± 1.9° | 90.1 ± 4.9° |
| #1 | Ar | 64.8 ± 2.9° | 98.4 ± 2.1° | 88.4 ± 3.7° |
| #2 | Ar | 62.8 ± 3.5° | 106.2 ± 3.4° | 100.1 ± 2.8° |
| #3 | Ar | 61.9 ± 1.4° | 95.8 ± 1.6° | 78.5 ± 1.1° |
| #4 | Ar | 62.1 ± 2.9° | 88.7 ± 2.8° | 65.8 ± 1.4° |
| #5 | Ar/O2 | 71.6 ± 1.5° | 92.9 ± 2.8° | 85.8 ± 3.1° |
| #6 | Ar/O2 | 69.6 ± 2.7° | 81.4 ± 5.7° | 72.4 ± 2.6° |
| #7 | Ar/O2 | 62.4 ± 2.1° | 106.1 ± 1.6° | 82.7 ± 5.7° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryta, M.; Tomczak, W. Stability of Ar/O2 Plasma-Treated Polypropylene Membranes Applied for Membrane Distillation. Membranes 2021, 11, 531. https://doi.org/10.3390/membranes11070531
Gryta M, Tomczak W. Stability of Ar/O2 Plasma-Treated Polypropylene Membranes Applied for Membrane Distillation. Membranes. 2021; 11(7):531. https://doi.org/10.3390/membranes11070531
Chicago/Turabian StyleGryta, Marek, and Wirginia Tomczak. 2021. "Stability of Ar/O2 Plasma-Treated Polypropylene Membranes Applied for Membrane Distillation" Membranes 11, no. 7: 531. https://doi.org/10.3390/membranes11070531
APA StyleGryta, M., & Tomczak, W. (2021). Stability of Ar/O2 Plasma-Treated Polypropylene Membranes Applied for Membrane Distillation. Membranes, 11(7), 531. https://doi.org/10.3390/membranes11070531







