Evaluation of Electrospun Self-Supporting Paper-Like Fibrous Membranes as Oil Sorbents
Abstract
:1. Introduction
2. Materials and Methods
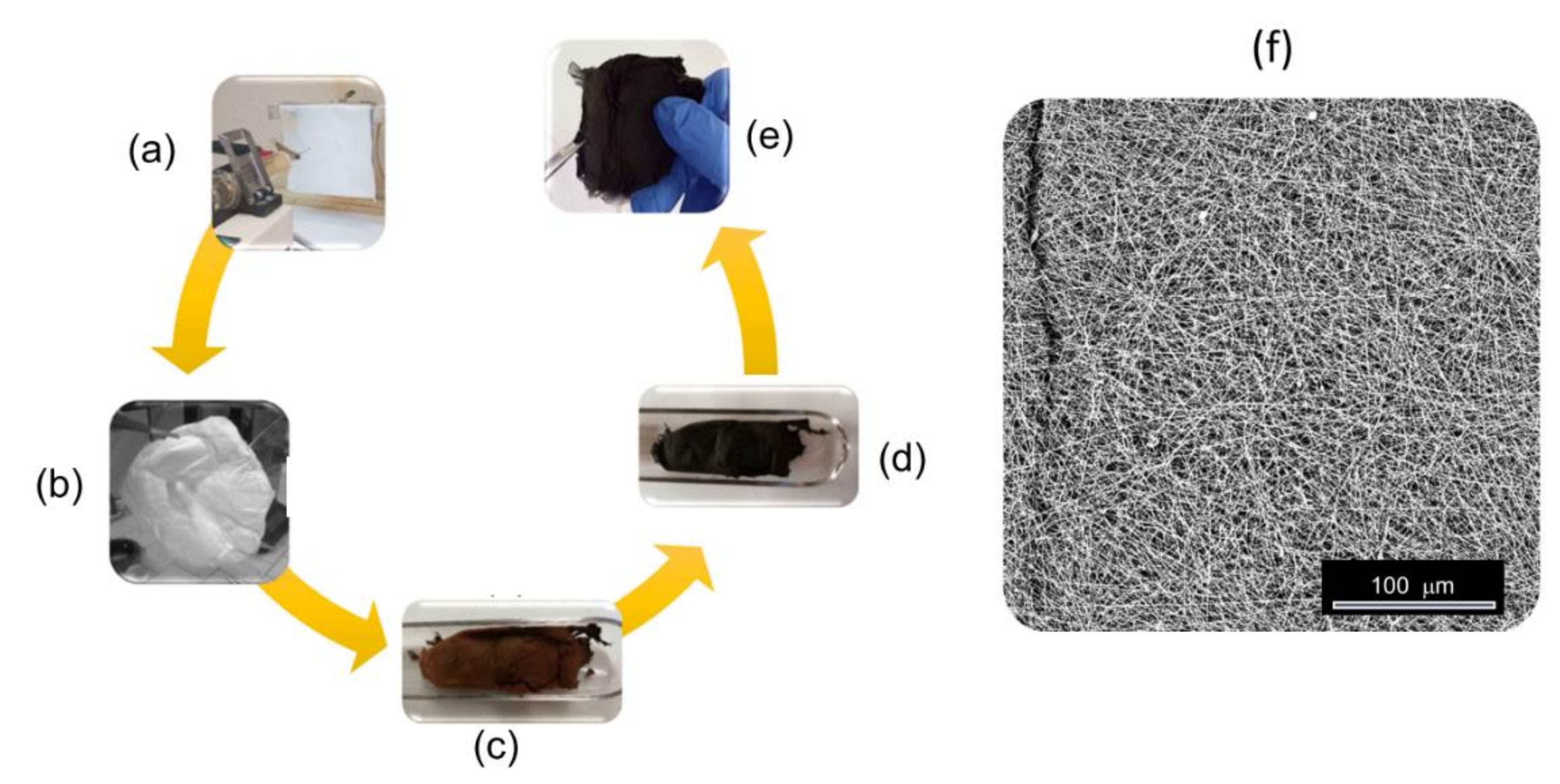
2.1. Preparation of the Self-Supporting Paper-Like Fibrous Membranes
2.2. Characterisation of the Produced Membranes
2.3. Contact Angle Measurements
2.4. Liquid Adsorption Tests
2.4.1. Immersion Tests
2.4.2. Spill Tests
3. Results and Discussion
3.1. Effect of the Thermal Treatments
3.2. Physico-Chemical Properties of the Sorbent Membranes
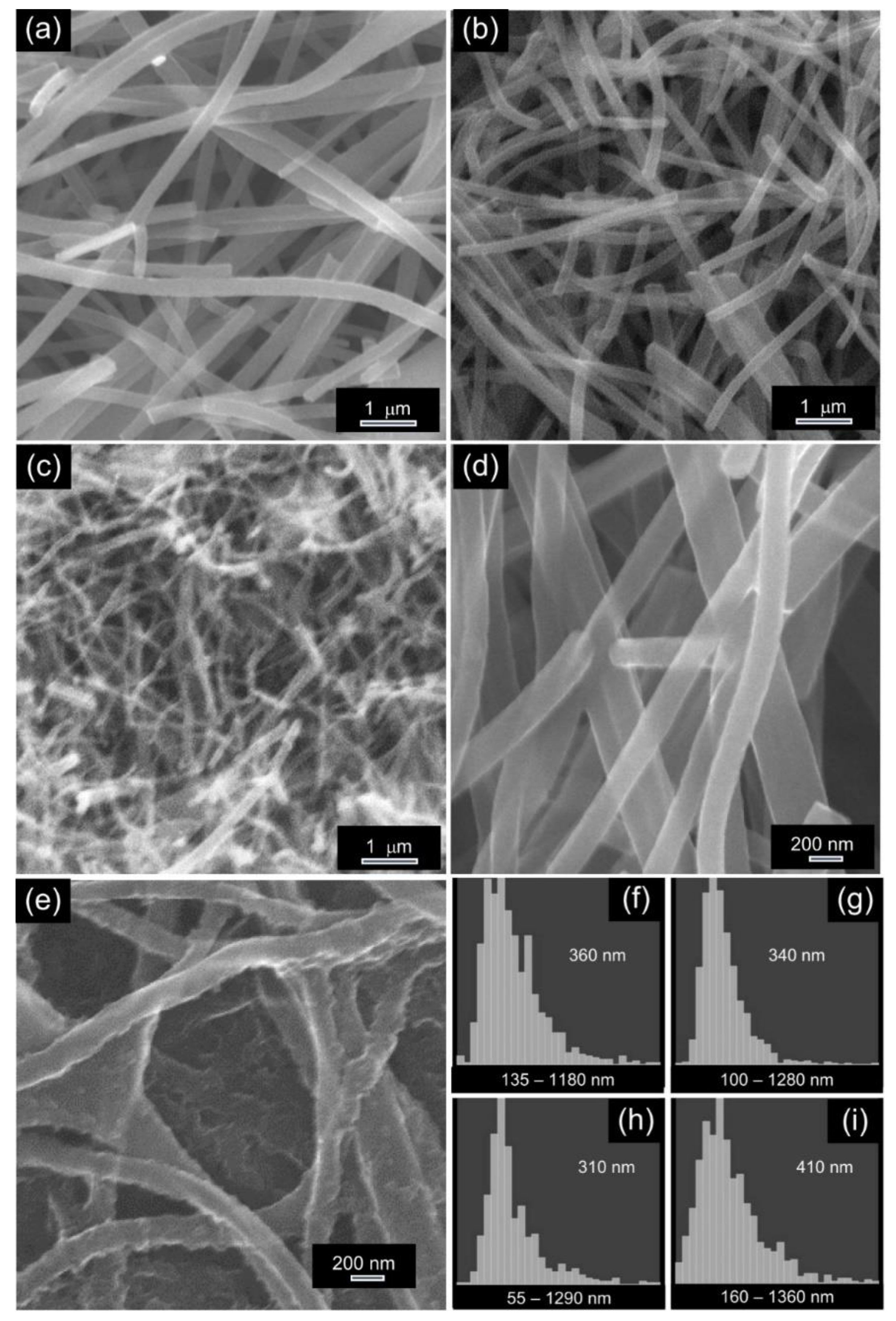
3.2.1. Morphology and Texture of the NFs
3.2.2. Porosity
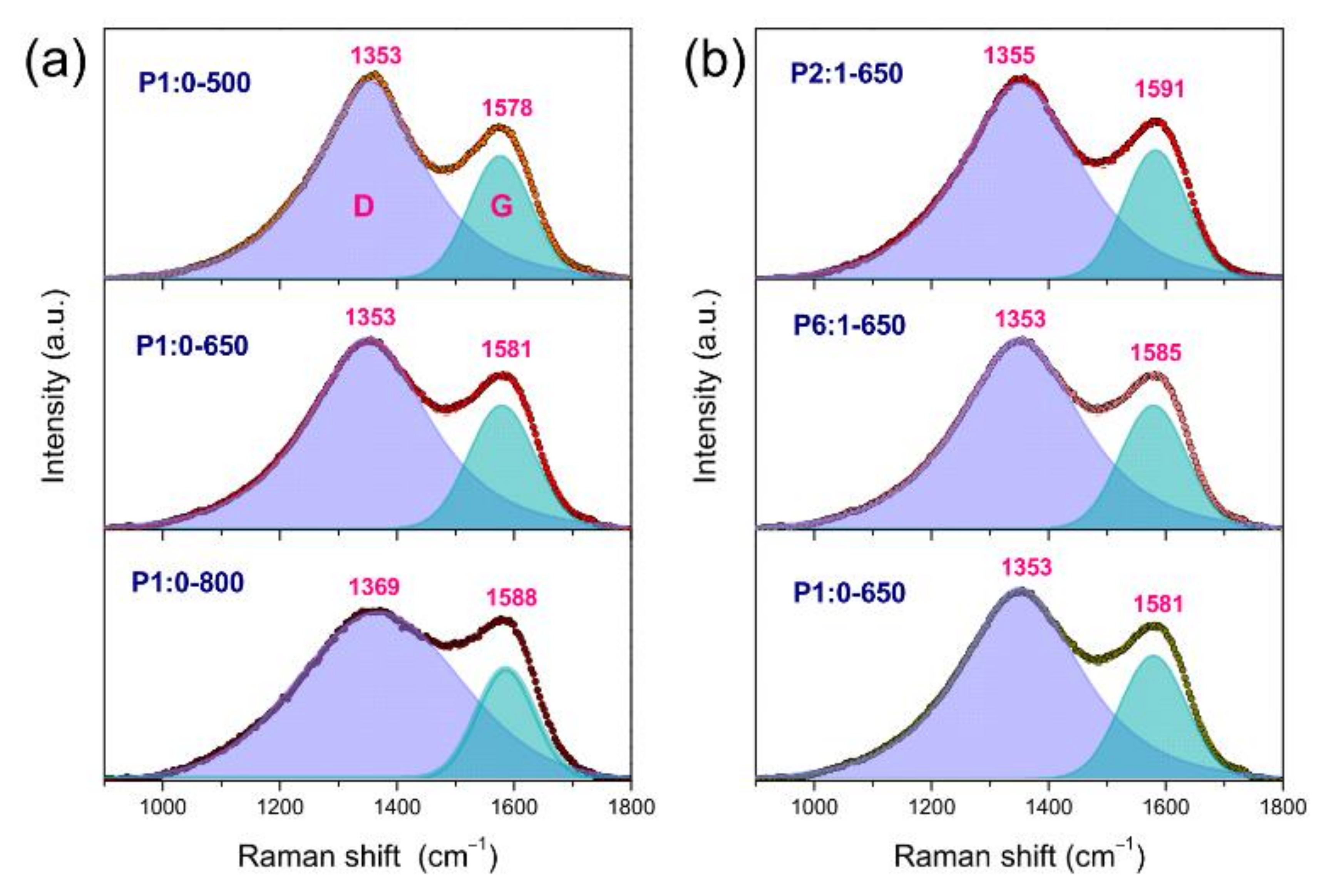
3.2.3. Amorphous Nature of the NFs
3.3. Sorption Performance of the Membranes
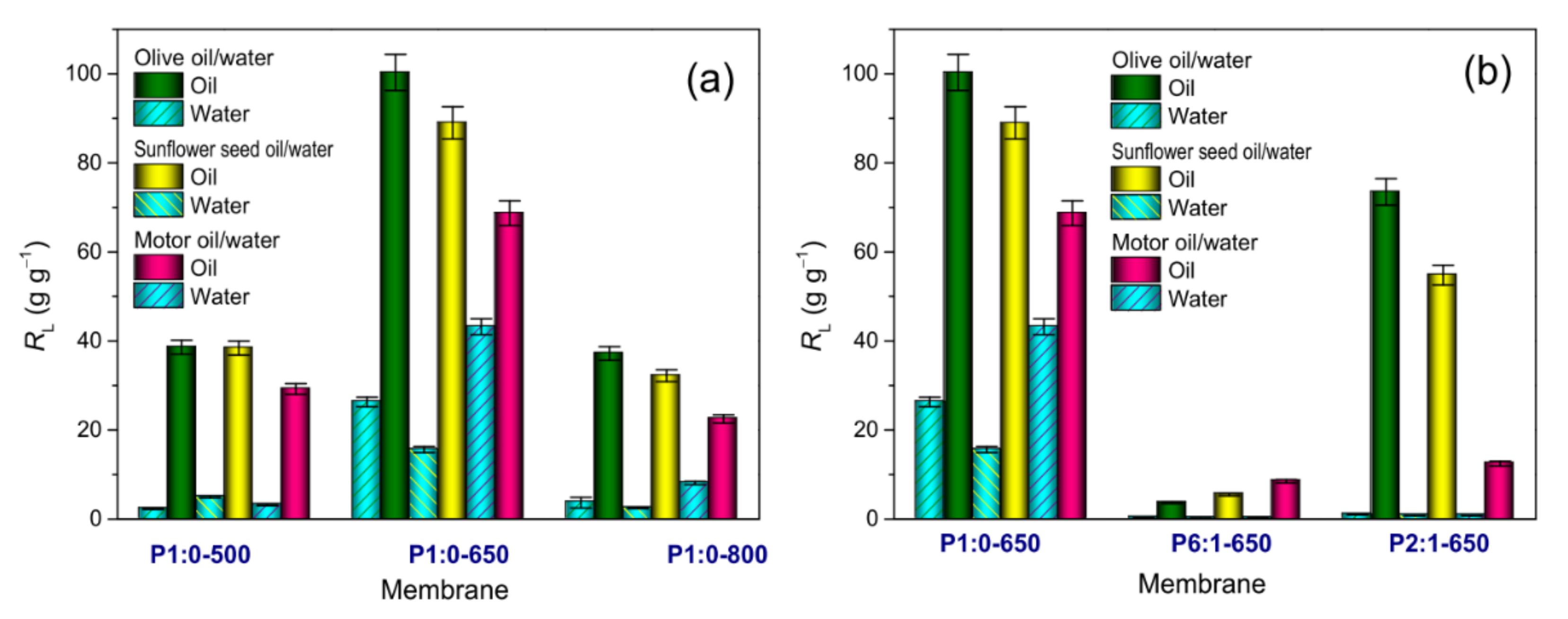
3.3.1. Water Immersion Tests
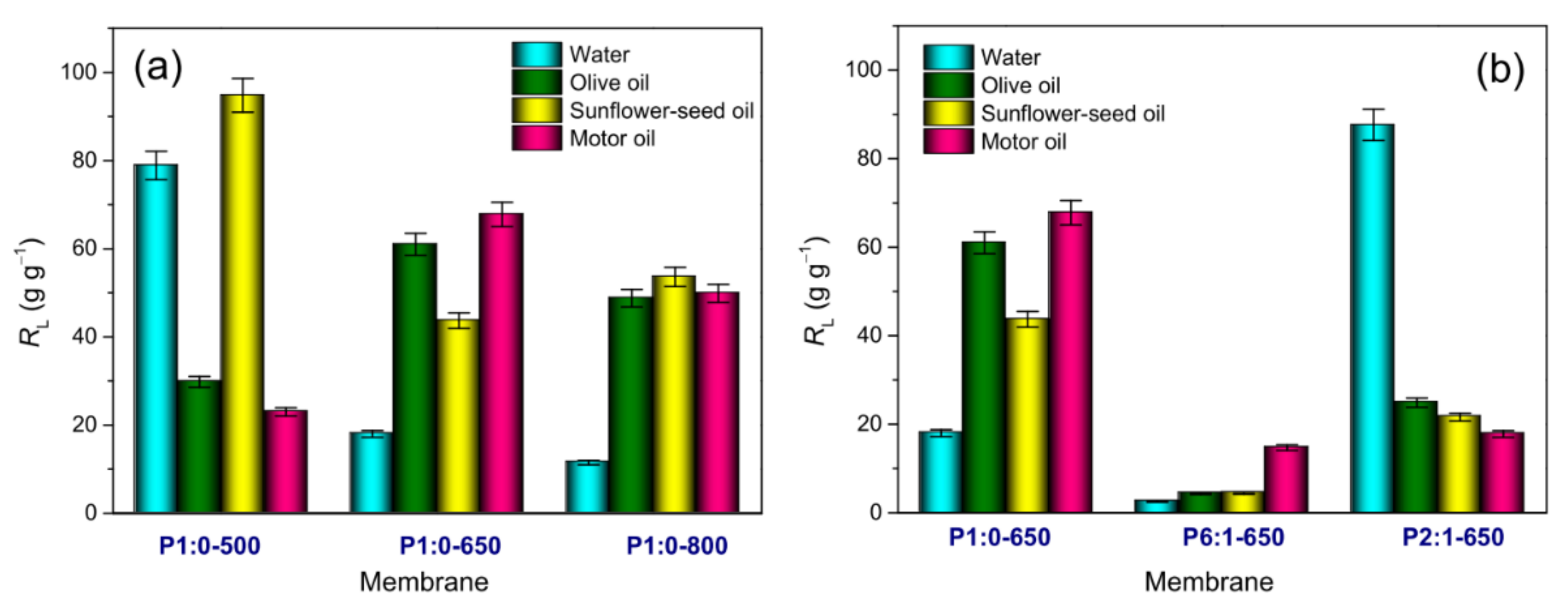
3.3.2. Oil Immersion Tests
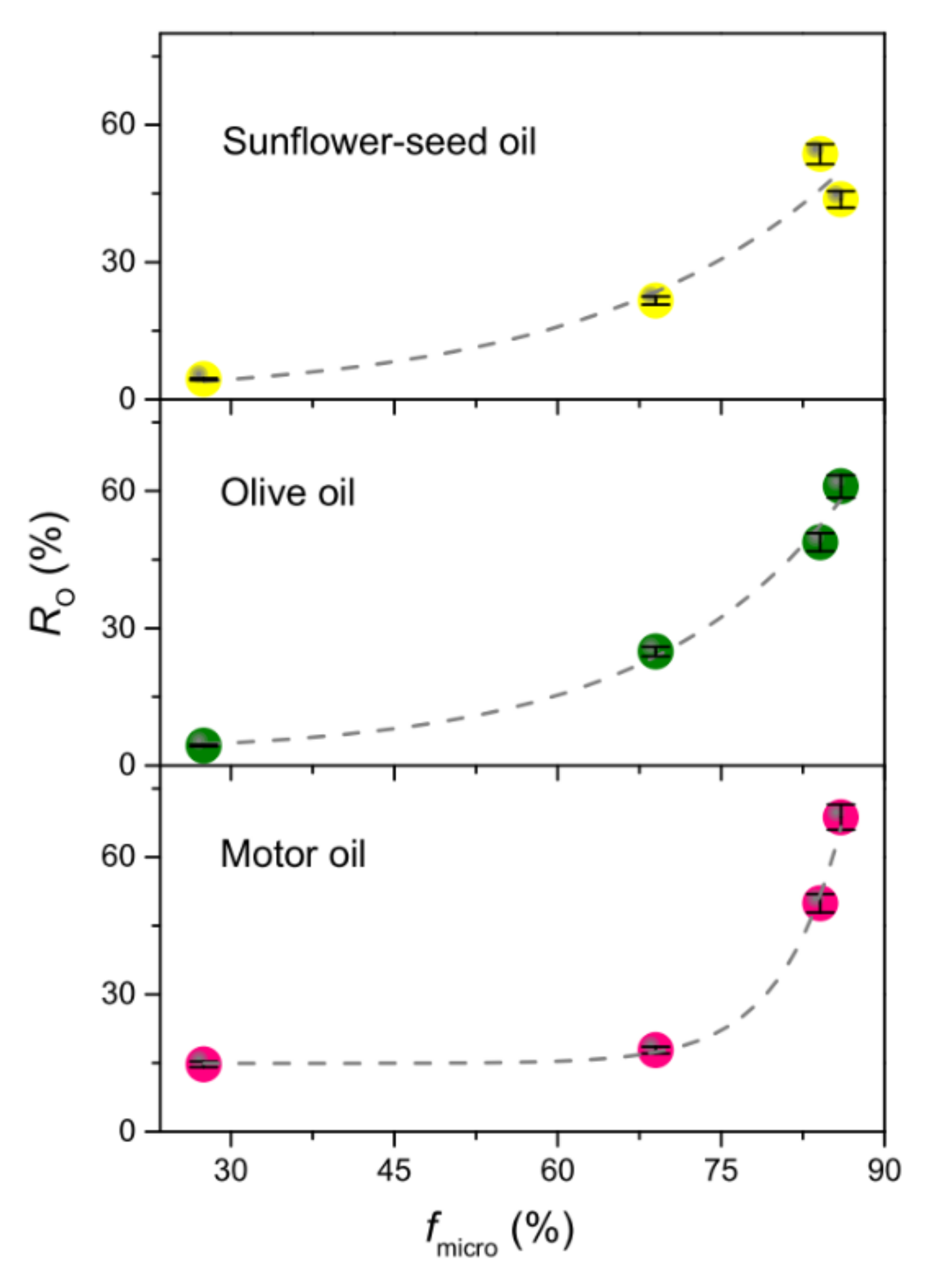
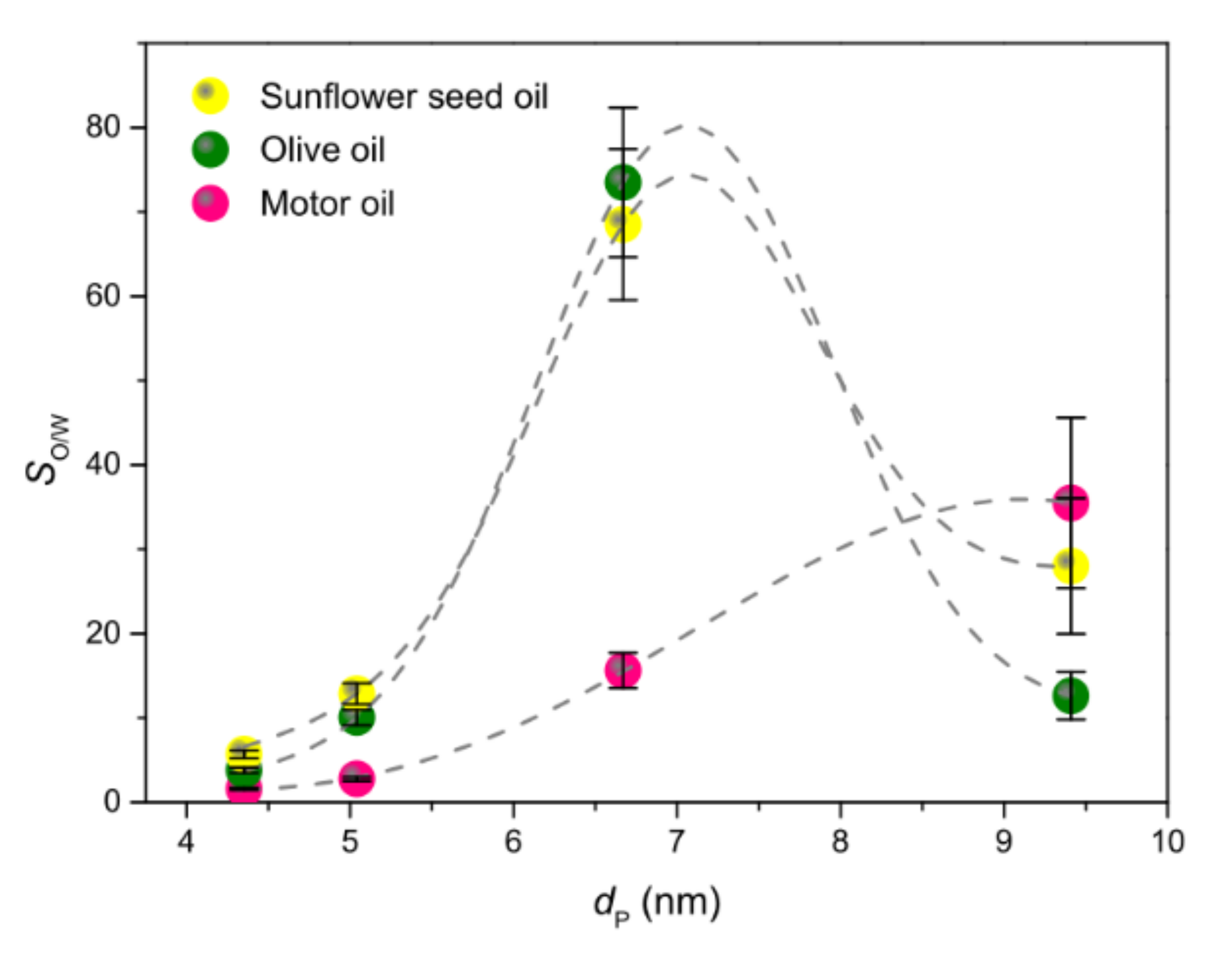
3.3.3. Spill Tests and Oil/Water Selectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Wang, C.; Zou, S.; Wei, Z.; Tong, Z. Simple, reversible emulsion system switched by pH on the basis of chitosan without any hydrophobic modification. Langmuir 2012, 28, 11017–11024. [Google Scholar] [CrossRef]
- Reddy, C.M.; Eglinton, T.I.; Hounshell, A.; White, H.K.; Xu, L.; Gaines, R.B.; Frysinger, G.S. The West Falmouth oil spill after thirty years: The persistence of petroleum hydrocarbons in marsh sediments. Environ. Sci. Technol. 2002, 36, 4754–4760. [Google Scholar] [CrossRef] [PubMed]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.H.; Tan, B.Y.L.; Juay, J.; Sun, D.D.; Leckie, J.O. A self-assembled superhydrophobic electrospun carbon-silica nanofiber sponge for selective removal and recovery of oils and organic solvents. Chem. Eur. J. 2015, 21, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-M.; Moreau, J.P. Oil sorption behavior of various sorbents studied by sorption capacity measurement and environmental scanning electron microscopy. Microsc. Res. Tech. 1993, 25, 447–455. [Google Scholar] [CrossRef]
- Deschamps, G.; Caruel, H.; Borredon, M.E.; Bonnin, C.; Vignoles, C. Oil removal from water by selective sorption on hydrophobic cotton fibers. 1. Study of sorption properties and comparison with other cotton fiber-based sorbents. Environ. Sci. Technol. 2003, 37, 1013–1015. [Google Scholar] [CrossRef]
- Zahid, M.A.; Halligan, J.E.; Johnson, R.F. Oil slick removal using matrices of polypropylene filaments. Ind. Eng. Chem. Process Des. Dev. 1972, 11, 550–555. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of olive mill wastewater into high-added value products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Pre-treatment of olive mill wastewaters at laboratory and mill scale and subsequent use in agriculture: Legislative framework and proposed soil quality indicators. Resour. Conserv. Recycl. 2012, 69, 82–89. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Ding, B.; Yang, J.; Yu, J.; Al-Deyab, S.S. Nanoporous polystyrene fibers for oil spill cleanup. Mar. Pollut. Bull. 2012, 64, 347–352. [Google Scholar] [CrossRef]
- Cerisuelo, A.; Castelló, L.; Moset, V.; Martínez, M.; Hernández, P.; Piquer, O.; Gómez, E.; Gasa, J.; Lainez, M. The inclusion of ensiled citrus pulp in diets for growing pigs: Effects on voluntary intake, growth performance, gut microbiology and meat quality. Livest. Sci. 2010, 134, 180–182. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the tolerance to polyphenols of the anaerobic digestion of olive wastewater through microbial adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Gómez, A.; Zubizarreta, J.; Rodrigues, M.; Dopazo, C.; Fueyo, N. An estimation of the energy potential of agro-industrial residues in Spain. Resour. Conserv. Recycl. 2010, 54, 972–984. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-enabled membranes for water purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Ceylan, D.; Dogu, S.; Karacik, B.; Yakan, S.D.; Okay, O.S.; Okay, O. Evaluation of butyl rubber as sorbent material for the removal of oil and polycyclic aromatic hydrocarbons from seawater. Environ. Sci. Technol. 2009, 43, 3846–3852. [Google Scholar] [CrossRef] [PubMed]
- Alassod, A.; Abedalwafa, M.A.; Xu, G. Evaluation of polypropylene melt blown nonwoven as the interceptor for oil. Environ. Technol. 2020, 2784–2796. [Google Scholar] [CrossRef]
- Hyung-Mln, C.; Cloud, R.M. Natural sorbents in oil spill cleanup. Environ. Sci. Technol. 1992, 26, 772–776. [Google Scholar]
- Radetić, M.M.; Jocić, D.M.; Jovančić, P.M.; Petrović, Z.L.; Thomas, H.F. Recycled wool-based nonwoven material as an oil sorbent. Environ. Sci. Technol. 2003, 37, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qiu, S.; Jiang, W.; Wu, D.; Zhang, C. Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup. Environ. Sci. Technol. 2011, 45, 4527–4531. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2016, 19, 403–414. [Google Scholar] [CrossRef]
- Jiang, Z.; Tijing, L.D.; Amarjargal, A.; Park, C.H.; An, K.J.; Shon, H.K.; Kim, C.S. Removal of oil from water using magnetic bicomponent composite nanofibers fabricated by electrospinning. Compos. Part B Eng. 2015, 77, 311–318. [Google Scholar] [CrossRef]
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced sorbents for oil-spill cleanup: Recent advances and future perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef]
- Sarbatly, R.; Krishnaiah, D.; Kamin, Z. A review of polymer nanofibres by electrospinning and their application in oil–water separation for cleaning up marine oil spills. Mar. Pollut. Bull. 2016, 106, 8–16. [Google Scholar] [CrossRef]
- Ding, B.; Yu, J. Electrospun Nanofibers for Energy and Environmental Applications; Ding, B., Jianyong, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-54159-9. [Google Scholar]
- Bhardwaj, N.; Bhaskarwar, A.N. A review on sorbent devices for oil-spill control. Environ. Pollut. 2018, 243, 1758–1771. [Google Scholar] [CrossRef]
- Hadji, E.M.; Fu, B.; Abebe, A.; Bilal, H.M.; Wang, J. Sponge-based materials for oil spill cleanups: A review. Front. Chem. Sci. Eng. 2020, 14, 749–762. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, C.; He, X.; Guo, Y.; Qiao, M.; Gu, J.; Zhang, Q. Novel reusable porous polyimide fibers for hot-oil adsorption. J. Hazard. Mater. 2017, 340, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Song, J.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Electrospun flexible nanofibrous membranes for oil/water separation. J. Mater. Chem. A 2019, 7, 20075–20102. [Google Scholar] [CrossRef]
- Liu, H.; Cao, C.-Y.; Wei, F.-F.; Huang, P.-P.; Sun, Y.-B.; Jiang, L.; Song, W.-G. Flexible macroporous carbon nanofiber film with high oil adsorption capacity. J. Mater. Chem. A 2014, 2, 3557–3562. [Google Scholar] [CrossRef]
- Brettmann, B.K.; Tsang, S.; Forward, K.M.; Rutledge, G.C.; Myerson, A.S.; Trout, B.L. Free surface electrospinning of fibers containing microparticles. Langmuir 2012, 28, 9714–9721. [Google Scholar] [CrossRef]
- Santangelo, S. Electrospun nanomaterials for energy applications: Recent advances. Appl. Sci. 2019, 9, 1049. [Google Scholar] [CrossRef] [Green Version]
- Qi, D.; Kang, X.; Chen, L.; Zhang, Y.; Wei, H.; Gu, Z. Electrospun polymer nanofibers as a solid-phase extraction sorbent for the determination of trace pollutants in environmental water. Anal. Bioanal. Chem. 2008, 390, 929–938. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, H.Y.; La, Y.M.; Lee, D.R.; Sung, N.H. Influence of a mixing solvent with tetrahydrofuran and N,N-dimethylformamide on electrospun poly(vinyl chloride) nonwoven mats. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2259–2268. [Google Scholar] [CrossRef]
- Zhang, M.; Uchaker, E.; Hu, S.; Zhang, Q.; Wang, T.; Cao, G.; Li, J. CoO-carbon nanofiber networks prepared by electrospinning as binder-free anode materials for lithium-ion batteries with enhanced properties. Nanoscale 2013, 5, 12342–12349. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, T.X.; Branford-White, C.J.; Zhu, L.M. Electrospun shikonin-loaded PCL/PTMC composite fiber mats with potential biomedical applications. Int. J. Pharm. 2009, 382, 215–221. [Google Scholar] [CrossRef]
- McCann, J.T.; Li, D.; Xia, Y. Electrospinning of nanofibers with core-sheath, hollow, or porous structures. J. Mater. Chem. 2005, 15, 735–738. [Google Scholar] [CrossRef]
- Zussman, E.; Yarin, A.L.; Bazilevsky, A.V.; Avrahami, R.; Feldman, M. Electrospun polyacrylonitrile/poly(methyl methacrylate)-derived turbostratic carbon micro-/nanotubes. Adv. Mater. 2006, 18, 348–353. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Karabulut, F. Melt-Blown Fibres vs Electrospun Nanofibres as Filtration Media. Available online: https://www.innovationintextiles.com/uploads/12280/MB-vs.-NF-White-Paper.pdf (accessed on 22 June 2021).
- Hu, L.; Zhang, S.; Zhang, Y.; Li, B. A flexible nanofiber-based membrane with superhydrophobic pinning properties. J. Colloid Interface Sci. 2016, 472, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Proverbio, E.; Milone, C. Sustainable reuse of char waste for oil spill recovery foams. Water Air Soil Pollut. 2020, 231, 293. [Google Scholar] [CrossRef]
- Mantripragada, S.; Gbewonyo, S.; Deng, D.; Zhang, L. Oil absorption capability of electrospun carbon nanofibrous membranes having porous and hollow nanostructures. Mater. Lett. 2020, 262, 127069. [Google Scholar] [CrossRef]
- Karki, H.P.; Kafle, L.; Ojha, D.P.; Song, J.H.; Kim, H.J. Three-dimensional nanoporous polyacrylonitrile-based carbon scaffold for effective separation of oil from oil/water emulsion. Polymer 2018, 153, 597–606. [Google Scholar] [CrossRef]
- Yuan, J.; Gao, R.; Wang, Y.; Cao, W.; Yun, Y.; Dong, B.; Dou, J. A novel hydrophobic adsorbent of electrospun SiO2@MUF/PAN nanofibrous membrane and its adsorption behaviour for oil and organic solvents. J. Mater. Sci. 2018, 53, 16357–16370. [Google Scholar] [CrossRef]
- Liu, C.K.; Feng, Y.; He, H.J.; Zhang, J.; Sun, R.J.; Chen, M.Y. Effect of carbonization temperature on properties of aligned electrospun polyacrylonitrile carbon nanofibers. Mater. Des. 2015, 85, 483–486. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, X.; Yang, C.; Lian, Y.; Razzaq, A.A.; Shah, R.; Guo, J.; Zhao, X.; Peng, Y.; Deng, Z. γ-Fe2O3 nanoparticles embedded in porous carbon fibers as binder-free anodes for high-performance lithium and sodium ion batteries. J. Alloys Compd. 2019, 777, 127–134. [Google Scholar] [CrossRef]
- Yarova, S.; Jones, D.; Jaouen, F.; Cavaliere, S. Strategies to hierarchical porosity in carbon nanofiber webs for electrochemical applications. Surfaces 2019, 2, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Belaustegui, Y.; Zorita, S.; Fernández-Carretero, F.; García-Luis, A.; Pantò, F.; Stelitano, S.; Frontera, P.; Antonucci, P.; Santangelo, S. Electro-spun graphene-enriched carbon fibres with high nitrogen-contents for electrochemical water desalination. Desalination 2018, 428, 40–49. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Electrospun porous structure fibrous film with high oil adsorption capacity. ACS Appl. Mater. Interfaces 2012, 4, 3207–3212. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Schierholz, R.; Kröger, D.; Kröger, K.; Weinrich, H.; Gehring, M.; Tempel, H.; Kungl, H.; Cde, J.M.; Eichel, R.U.-A. The carbonization of polyacrylonitrile-derived electrospun carbon nanofibers studied by in situ transmission electron microscopy. RSC Adv. 2019, 9, 6267–6277. [Google Scholar] [CrossRef] [Green Version]
- Janus, R.; Natkański, P.; Wach, A.; Drozdek, M.; Piwowarska, Z.; Cool, P.; Kuśtrowski, P. Thermal transformation of polyacrylonitrile deposited on SBA-15 type silica: Effect on adsorption capacity of methyl-ethyl ketone vapor. J. Therm. Anal. Calorim. 2012, 110, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J.L. Thermal degradation of poly(methyl methacrylate) (PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stab. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, T.; Khan, A.U.; Liu, G. Block copolymer–based porous carbon fibers. Sci. Adv. 2019, 5, eaau6852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Matei Ghimbeu, C.; Górka, J.; Simone, V.; Simonin, L.; Martinet, S.; Vix-Guterl, C. Insights on the Na+ ion storage mechanism in hard carbon: Discrimination between the porosity, surface functional groups and defects. Nano Energy 2018, 44, 327–335. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E. Powder Surface Area and Porosity (Vol. 2), 3rd ed.; Chapman & Hall Ltd., Ed.; Springer Science & Business Media: London, UK, 2013. [Google Scholar]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Serrano, S.; Santiago-Avilés, J.J. Raman characterization of carbon nanofibers prepared using electrospinning. Synth. Met. 2003, 138, 423–427. [Google Scholar] [CrossRef]
- Malara, A.; Leonardi, S.G.; Bonavita, A.; Fazio, E.; Stelitano, S.; Neri, G.; Neri, F.; Santangelo, S. Origin of the different behavior of some platinum decorated nanocarbons towards the electrochemical oxidation of hydrogen peroxide. Mater. Chem. Phys. 2016, 184, 269–278. [Google Scholar] [CrossRef]
- Santangelo, S. Controlled surface functionalization of carbon nanotubes by nitric acid vapors generated from sub-azeotropic solution. Surf. Interface Anal. 2016, 48, 17–25. [Google Scholar] [CrossRef]
- Tagliaferro, A.; Rovere, M.; Padovano, E.; Bartoli, M.; Giorcelli, M. Introducing the novel mixed gaussian-lorentzian lineshape in the analysis of the raman signal of biochar. Nanomaterials 2020, 10, 1748. [Google Scholar] [CrossRef] [PubMed]
- Pantò, F.; Fan, Y.; Stelitano, S.; Fazio, E.; Patanè, S.; Frontera, P.; Antonucci, P.; Pinna, N.; Santangelo, S. Are electrospun fibrous membranes relevant electrode materials for Li-ion batteries? The case of the C/Ge/GeO2 composite fibers. Adv. Funct. Mater. 2018, 28, 1800938. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, H.; Lyu, Z.; Jiang, Y.; Xie, K.; Wang, X.; Wu, Q.; Yang, L.; Jin, Z.; Ma, Y.; et al. Hydrophilic hierarchical nitrogen-doped carbon nanocages for ultrahigh supercapacitive performance. Adv. Mater. 2015, 27, 3541–3545. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, L.; Liu, Y.; Lu, T.; Sun, Z. Enhanced capacitive deionization performance of graphene by nitrogen doping. J. Colloid Interface Sci. 2015, 445, 143–150. [Google Scholar] [CrossRef]
- Kumar, K.V.; Preuss, K.; Guo, Z.X.; Titirici, M.M. Understanding the hydrophilicity and water adsorption behavior of nanoporous nitrogen-doped carbons. J. Phys. Chem. C 2016, 120, 18167–18179. [Google Scholar] [CrossRef]
- Vazquez-Duhalt, R. Environmental impact of used motor oil. Sci. Total Environ. 1989, 79, 1–23. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.S.; Chemat, F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Białecka-Florjańczyk, E. Factors influencing synthesis of extracellular lipases by Yarrowia lipolytica in medium containing vegetable oils. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Aluyor, E.O.; Ozigagu, C.E.; Oboh, O.I.; Aluyor, P. Chromatographic analysis of vegetable oils: A review. Sci. Res. Essay 2009, 4, 191–197. [Google Scholar]
- Chen, B.; Mcclements, D.J.; Decker, E.A. Minor components in food oils: A critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit. Rev. Food Sci. Nutr. 2011, 51, 901–916. [Google Scholar] [CrossRef]
- Purcaro, G.; Barp, L.; Beccaria, M.; Conte, L.S. Characterisation of minor components in vegetable oil by comprehensive gas chromatography with dual detection. Food Chem. 2016, 212, 730–738. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Minor components of fats and oils. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Shin, C.; Chase, G.G. Water-in-oil coalescence in micro-nanofiber composite filters. AIChE J. 2004, 50, 343–350. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured Fibers via Electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Esteban, B.; Riba, J.R.; Baquero, G.; Puig, R.; Rius, A. Characterization of the surface tension of vegetable oils to be used as fuel in diesel engines. Fuel 2012, 102, 231–238. [Google Scholar] [CrossRef]
- Diamante, L.M.; Lan, T. Absolute Viscosities of Vegetable Oils at Different Temperatures and Shear Rate Range of 64.5 to 4835 s−1. J. Food Process. 2014, 2014, 234583. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Zhao, L.; Li, P.; Sun, H.; Li, S. Electrospun polystyrene/polyacrylonitrile fiber with high oil sorption capacity. J. Reinf. Plast. Compos. 2014, 33, 1849–1858. [Google Scholar] [CrossRef]

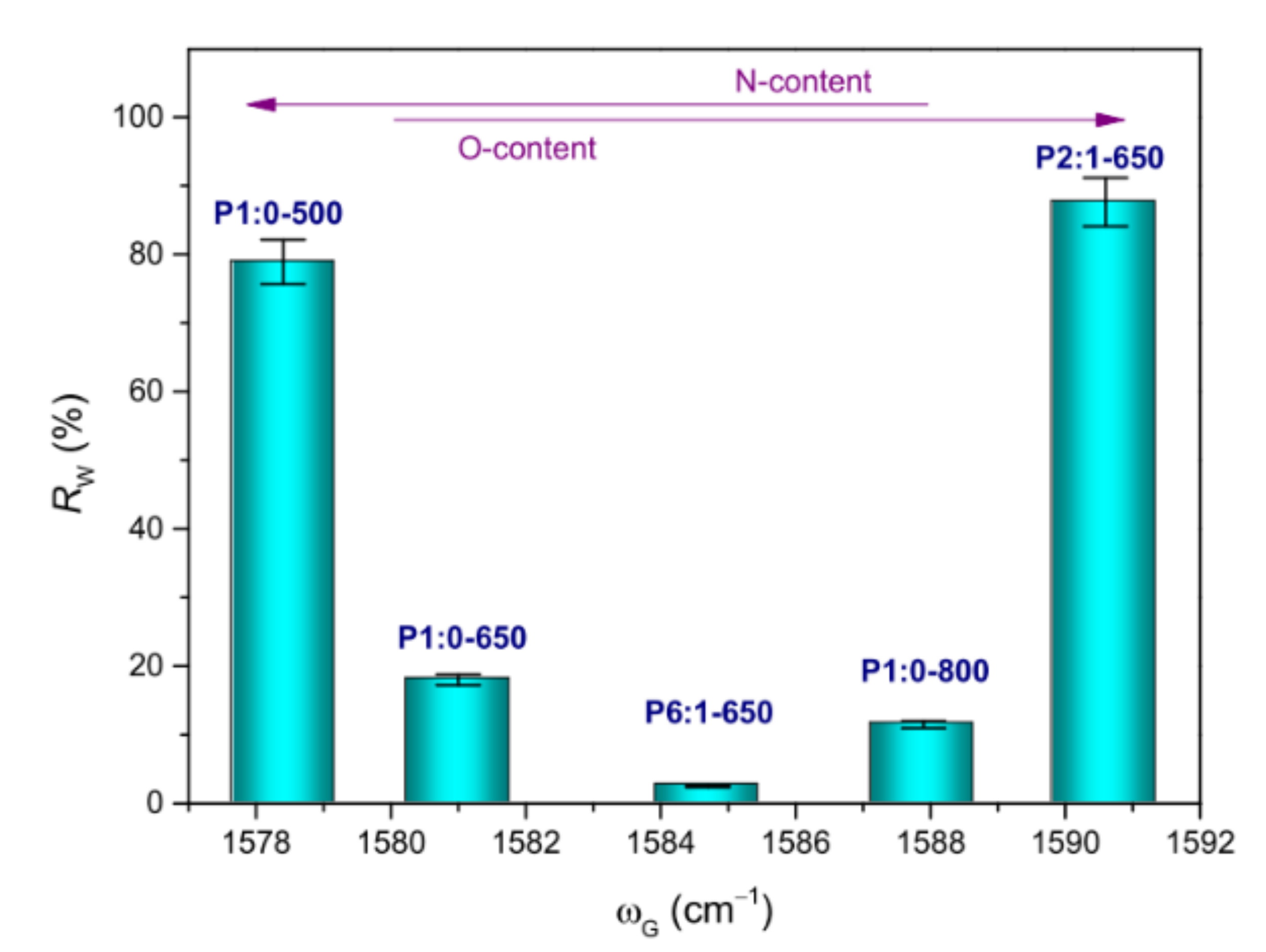

| Sample Code | Polymer(s) in the Spinnable Solution | Post-Electrospinning Heat Treatments | ||
|---|---|---|---|---|
| PAN:PMMA | PAN + PMMA (wt%) | TS (°C)/tS (h) | TC (°C)/tC (h) | |
| P1:0-500 | 1:0 | 6.5 | 280/3 | 500/3 |
| P1:0-650 | 650/3 | |||
| P1:0-800 | 800/3 | |||
| P6:1-650 | 6:1 | 650/3 | ||
| P2:1-650 | 2:1 | |||
| Sample Code | ST (m2 g−1) | Smicro (m2 g−1) | Vmicro (mm3 g−1) | fmicro (%) | fmeso (%) | fmacro (%) | dP (nm) |
|---|---|---|---|---|---|---|---|
| P1:0-500 | 20.3 ± 0.1 | 2.9 | 2.57 | 14.4 | 51.5 | 34.1 | 4.37 |
| P1:0-650 | 139.7 ± 0.9 | 120.1 | 46.41 | 86.0 | 13.9 | 0.1 | 4.35 |
| P1:0-800 | 128.8 ± 0.6 | 108.3 | 42.13 | 84.1 | 15.8 | 0.1 | 5.04 |
| P6:1-650 | 17.5 ± 0.1 | 4.8 | 2.08 | 27.5 | 70.2 | 2.3 | 9.41 |
| P2:1-650 | 53.3 ± 0.2 | 36.8 | 14.05 | 69.0 | 30.5 | 0.5 | 6.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folino, A.; Triolo, C.; Petrovičová, B.; Pantò, F.; Zema, D.A.; Santangelo, S. Evaluation of Electrospun Self-Supporting Paper-Like Fibrous Membranes as Oil Sorbents. Membranes 2021, 11, 515. https://doi.org/10.3390/membranes11070515
Folino A, Triolo C, Petrovičová B, Pantò F, Zema DA, Santangelo S. Evaluation of Electrospun Self-Supporting Paper-Like Fibrous Membranes as Oil Sorbents. Membranes. 2021; 11(7):515. https://doi.org/10.3390/membranes11070515
Chicago/Turabian StyleFolino, Adele, Claudia Triolo, Beatrix Petrovičová, Fabiolo Pantò, Demetrio A. Zema, and Saveria Santangelo. 2021. "Evaluation of Electrospun Self-Supporting Paper-Like Fibrous Membranes as Oil Sorbents" Membranes 11, no. 7: 515. https://doi.org/10.3390/membranes11070515
APA StyleFolino, A., Triolo, C., Petrovičová, B., Pantò, F., Zema, D. A., & Santangelo, S. (2021). Evaluation of Electrospun Self-Supporting Paper-Like Fibrous Membranes as Oil Sorbents. Membranes, 11(7), 515. https://doi.org/10.3390/membranes11070515










