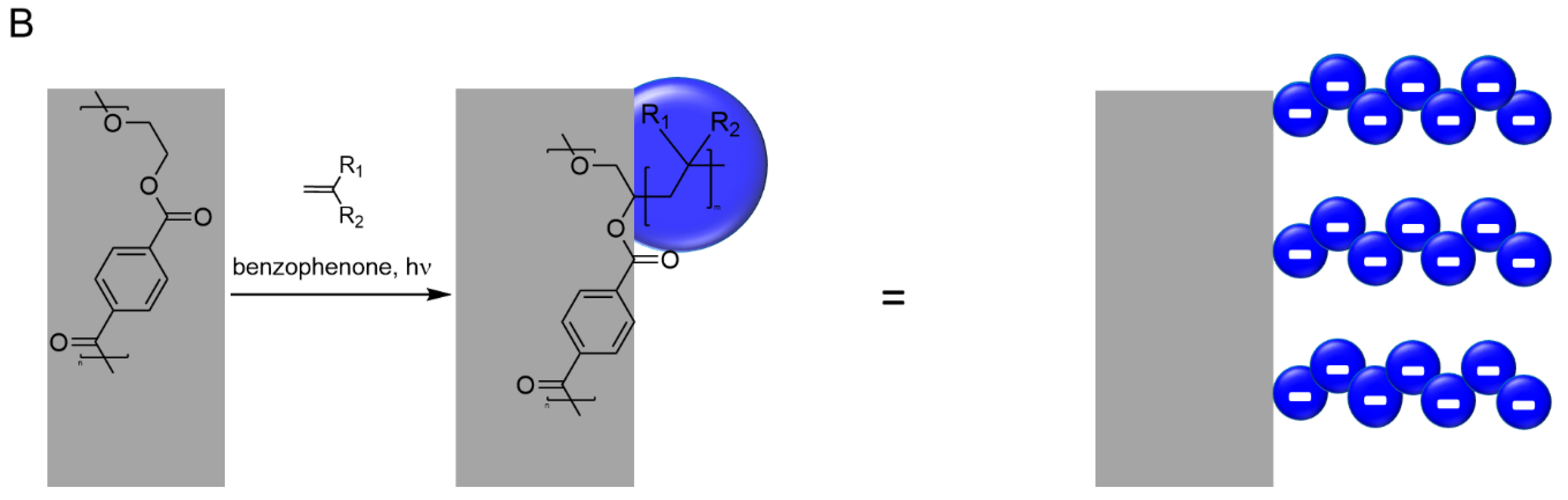
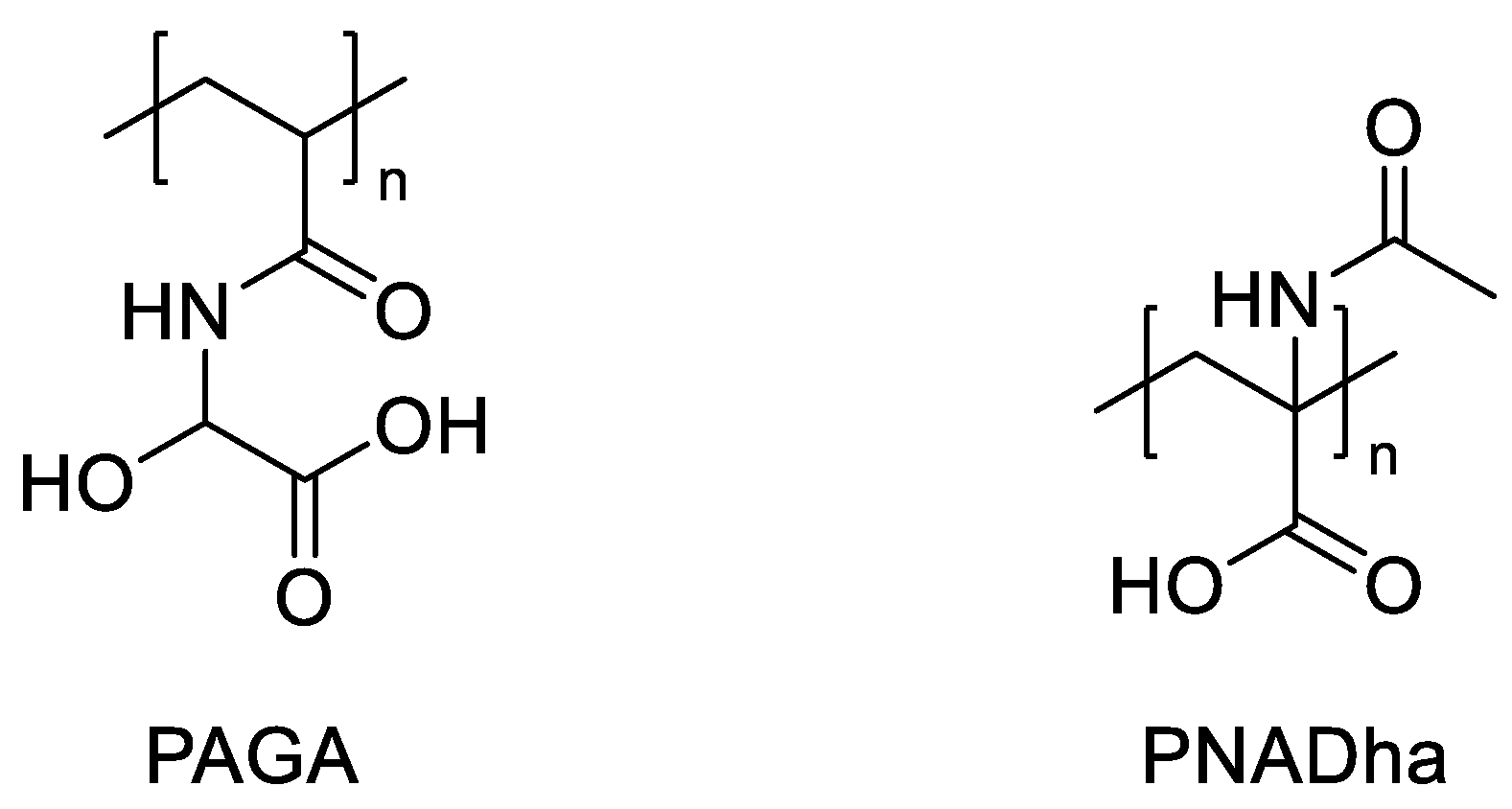3.2. Photo-Initiated Graft Copolymerization of Polyelectrolytes to Porous Track-Etched Membranes
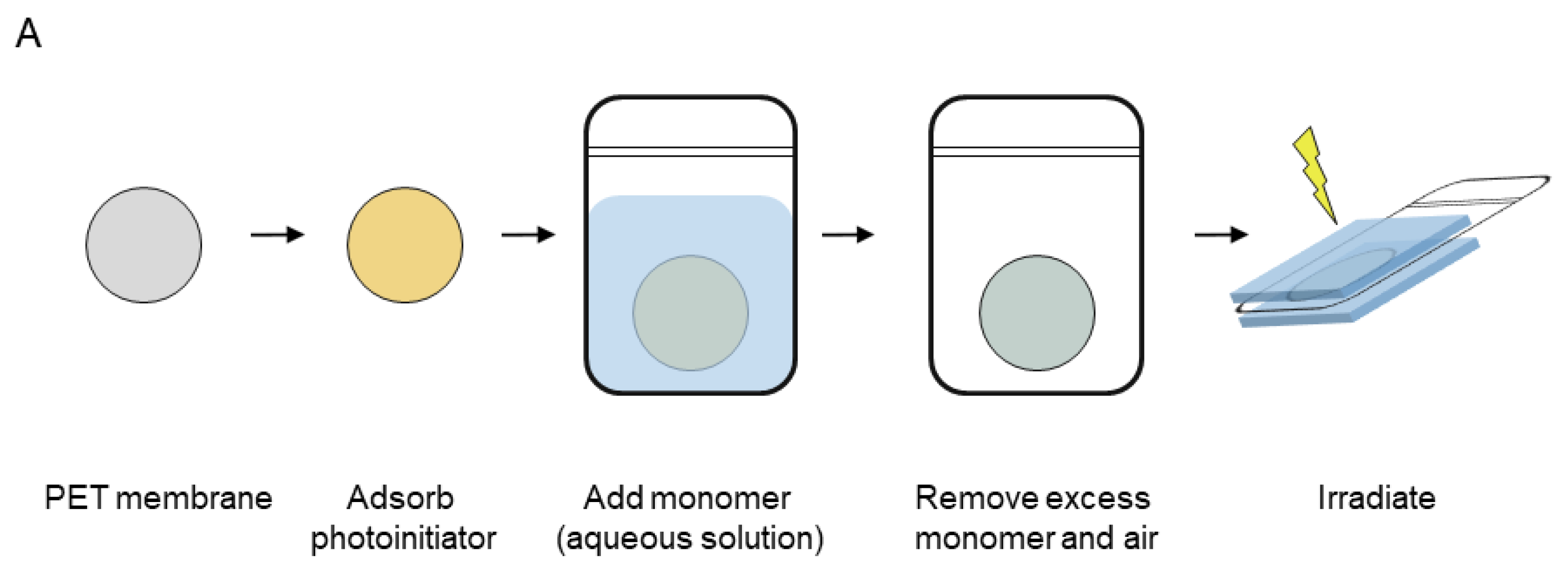
Membranes were functionalised with the polyelectrolytes via heterogeneous grafting onto the surface of track-etched membranes. A protocol (cf. Materials and Methods) established in the Ulbricht group and depicted in
Scheme 2. was used for the polymerization [
6]. The grafting technique exploits differences in hydrophilicity between the aqueous (highly hydrophilic) monomer solution, the moderately hydrophilic membrane material (PET) and the highly hydrophobic PE-bags used as the reaction vessel. In this way, an optimal coverage of the membrane and pore surface by the monomer solution was promoted. The use of the bag further protects the reaction mixture from exposure to atmospheric oxygen during the equilibration and irradiation steps. It is also transparent to UVA irradiation so that the photoinitiator can be activated efficiently.
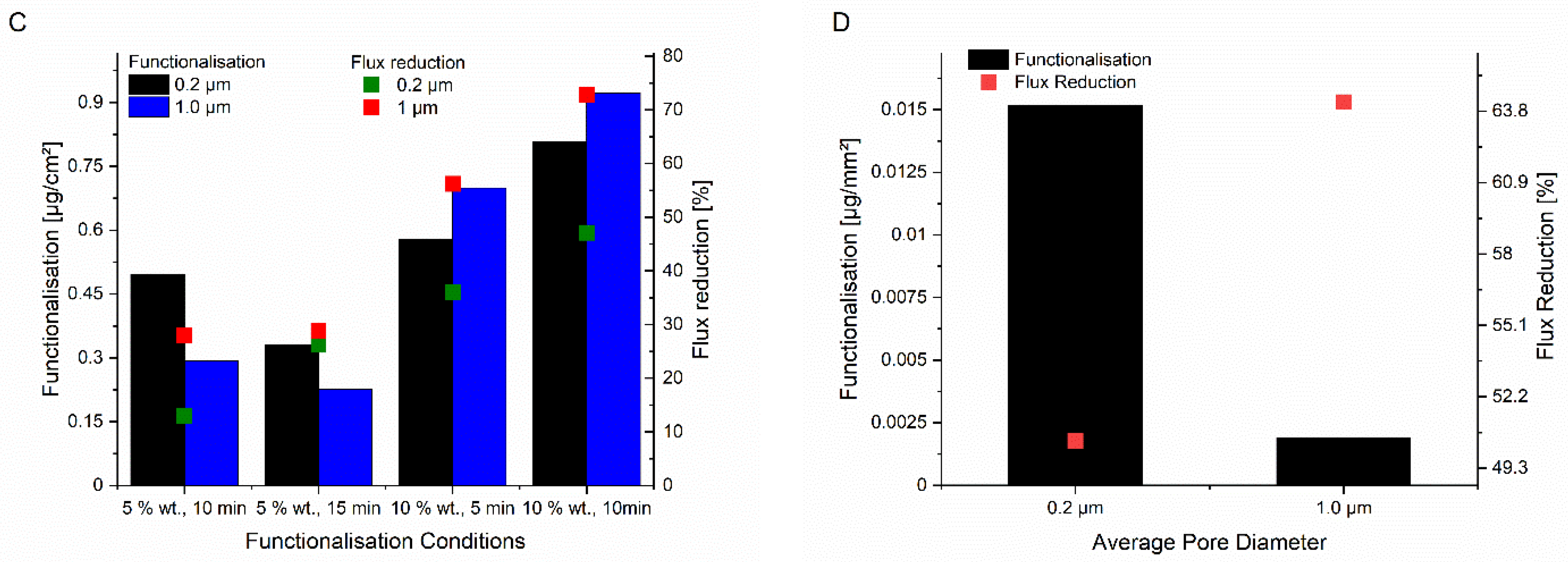
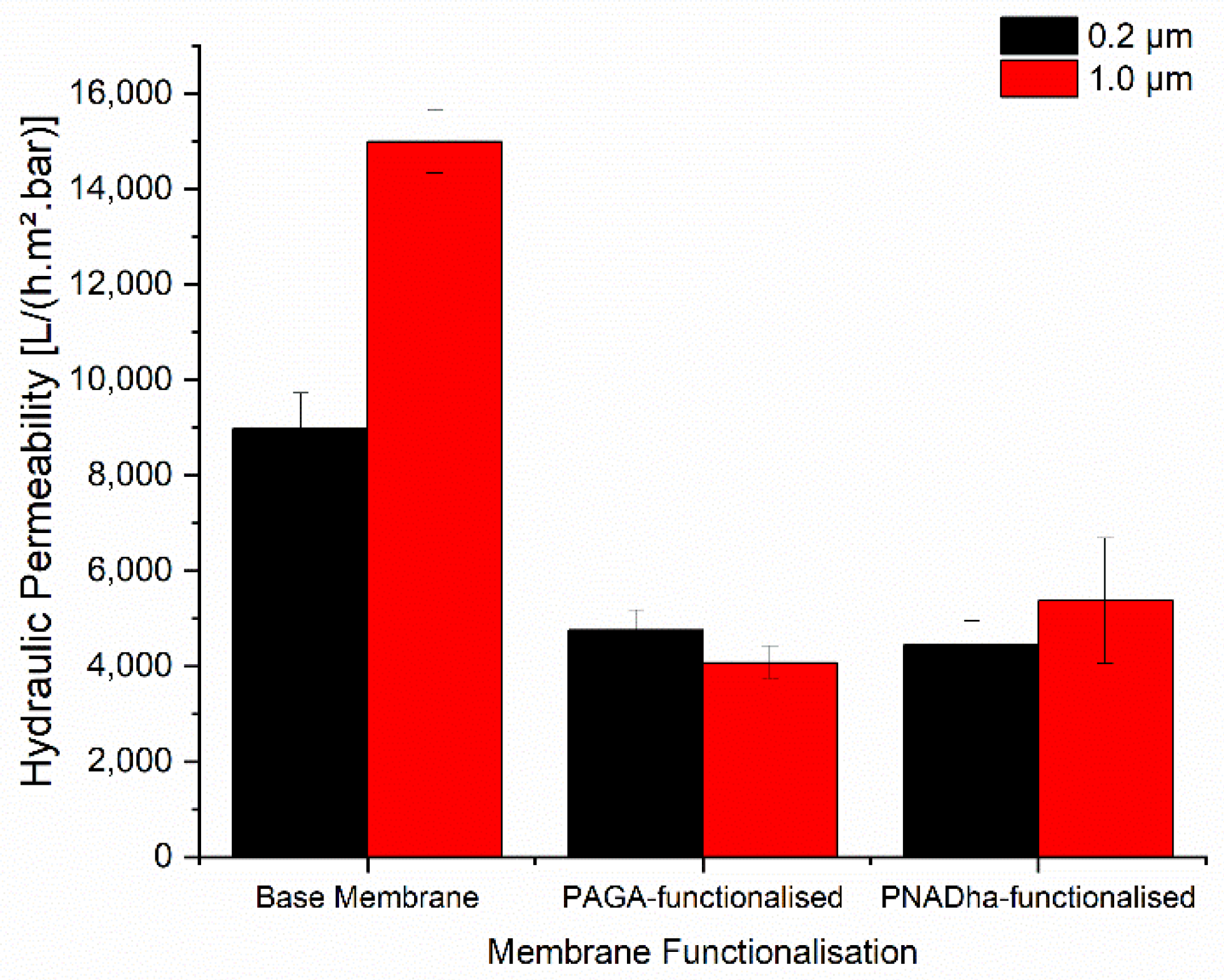
Initially, the general feasibility of grafting PAGA to the surface of track-etched membranes via UV-initiated surface polymerisation was investigated. A monomer concentration of 10 wt.% and an irradiation time of 10 min were chosen. Using these conditions, membranes with nominal pore sizes of 0.2, 1, and 5 µm could be readily functionalised. The membranes with a 5 µm pore size showed the highest degree of functionalization, with an average increase in weight of 6.7% of total membrane mass, yet the permeability was only reduced by 5% (from 179,000 L·(h·m2·bar)−1 to 170,000 L·(h·m2·bar)−1). Both effects can be attributed to the large pore size. While the pores are easily accessible for reagents permitting efficient functionalisation, a thick layer of polyelectrolyte is needed to significantly decrease the pore size and, thus, the membrane permeability. The low effect of grafted polyelectrolyte layers on effective membrane pore sizes for base membranes with a 5 µm pore diameter led to the decision to not perform further evaluations of these membranes.
The results for the membranes with smaller pore sizes were more promising. For the membrane with a pore size of 1 µm, an average functionalisation of 1.2 wt.% PAGA could be achieved, resulting in a 73% decrease in permeability from 14,990 L·(h·m
2·bar)
−1 to 4100 L·(h·m
2·bar)
−1. In the case of the membrane with a pore size of 0.2 µm, the average PAGA-functionalisation that could be achieved was 4.4 wt.%, even higher than for the membranes with 1 µm pore size. On the other hand, the permeability decreased less strongly, by 47% from 8990 L·(h·m
2·bar)
−1 to 4750 L·(h·m
2·bar)
−1. The extent of reduction in permeability as well as functionalisation for the different membranes are shown in
Figure 1.
After establishing the general feasibility of surface-grafting of PAGA onto PET track-etched membranes, the influence of monomer concentration and irradiation time was studied (
Figure 1C). Owing to solubility, the maximum monomer concentration used was 10 wt.% Additionally, the monomer concentration was reduced to 5 wt.% and the irradiation time was varied. Based on the strongly reduced functionalisation and change in permeability for the samples with a monomer concentration of 5 wt.% and an irradiation time of 10 min, the respective experiment with an irradiation time of 5 min was not performed. Increasing the irradiation time to 15 min still could not mitigate the effects of the decreased monomer concentration. Owing to the satisfactory functionalisation and permeability reduction obtained for a monomer concentration of 10 wt.% with an irradiation time of 10 min, the irradiation time was not further increased. A decrease of the reaction time to 5 min caused decreasing functionalisation and permeability reduction, as expected. Based on these results, PAGA-functionalised membranes for the following experiments were prepared using monomer solutions with a concentration of 10 wt.% and an irradiation time of 10 min.
Using the same strategy, the track-etched membranes were also functionalised with PNADha. Here, the irradiation time was increased to 15 min to compensate for the lower reactivity. Independent of the membrane pore size, the sample weight increased, and the hydraulic permeability decreased upon functionalisation with PNADha (
Figure 1D). For the degree of functionalisation, the effect was more pronounced for the membrane with a pore size of 0.2 µm. Here, an average increase of 90 µg or 0.8% of total membrane mass could be observed, while for the membrane with a pore size of 1 µm the increase in mass was only 0.03% on average. Concerning the reduction in permeability, the strength of the effects was different. Here, the decrease in permeability was 64% for the 1 µm membrane (from 14,990 L·(h·m
2·bar)
−1 to 5370 L·(h·m
2·bar)
−1) upon functionalisation with PNADha, while for the membrane with 0.2 µm pore size PNADha functionalisation reduced the permeability by 50% from 8990 L·(h·m
2·bar)
−1 to 4460 L·(h·m
2·bar)
−1. The fact that PNADha-functionalisation seems less effective but leads to a stronger reduction in permeability compared to the results obtained with PAGA will be discussed below.
In addition to membrane functionalisation with PNADha, deprotection to yield PDha was attempted via acidic hydrolysis [
30]. Although the cleavage of the acetyl group could be achieved, the PET membranes were not stable under the reaction conditions. Therefore, no PDha functionalised membranes could be obtained via this pathway and the experiments were discontinued. Instead, the work was focused on PNADha-grafted membranes, as the protected precursor polymer also has the ability to interact with heavy metal ions [
20,
21].
The influence of functionalisation on the wettability of the membranes was also investigated (
Table 1,
Figure S2). Upon functionalisation of a membrane with a polyelectrolyte, two distinct effects are expected. On the one hand, the polyelectrolyte should increase the hydrophilicity of the surface (thus decreasing the contact angle). On the other hand, any surface functionalisation itself affects surface composition and structure as well as pore size. This has, in turn, effects on the contact angle. For example, trapped air can cause a high contact angle even with a highly hydrophilic surface [
31,
32].
For membranes grafted with PNADha, the contact angle increased for both used pore sizes. The increase was stronger for the membrane with the larger pore size of 1 µm. This indicates a small difference in hydrophilicity between PET and PNADha, as well as a larger influence of surface texture. In the case of PAGA-grafting the effect of functionalisation on the contact angle differs with pore size. For the membrane with a pore size of 1 µm, the contact angle increases upon PAGA functionalisation, which we attribute to changes in the surface roughness. For membranes with a pore size of 0.2 µm, the grafting of the polyelectrolyte caused a significant decrease in the contact angle. This is attributed to an increase in surface hydrophilicity as the dominant effect. The reasons for these differences between PAGA and PNADha functionalization will be discussed below.
Moreover, the functionalised membranes were analysed using scanning electron microscopy (SEM), although no significant differences were visible after functionalisation. The micrographs were assessed visually and with the program ImageJ. As shown in
Figures S3 and S4, all membranes were between 21 and 23 µm in thickness, which was not significantly influenced by grafting a polyelectrolyte onto the membranes. In addition to the membrane thickness, the pore size distributions were analysed. Here, samples with 60–600 pores were evaluated automatically using the particle analyser function of the software ImageJ. No significant difference could be found after polyelectrolyte grafting (
Table 1). This lack of difference in pore size and appearance is attributed to the fact that samples are analysed in the dry state and after metal sputtering, so that the thin grafted layers cannot be visualized.
The changes in membrane weight, hydraulic permeability, and contact angle in association with the surface grafting confirmed a successful functionalisation. The extent of functionalisation and the pK
a values of the carboxylic groups differ between the two polyelectrolytes. The difference in acidity causes differences in the degree of protonation and, thus, the total charge of the polyelectrolyte chains, which translates into variations in chain extension. Owing to the higher degree of functionalisation achieved with PAGA and the higher acidity of this polyelectrolyte, the decrease in permeability is significantly stronger in the case of membranes with a pore size of 1 µm (
Figure 2). The unexpectedly large reduction in permeability found after PNADha-functionalisation suggests additional effects, besides simple chain extension influencing the permeability of polyelectrolyte grafted membranes. Possible explanations are differences in grafting density and chain length or different locations of the grafted polyelectrolytes (i.e., on the top and bottom surfaces vs. inside the pores).
In fact, that PNADha functionalisation is less effective but leads to a stronger reduction in permeability compared to the results obtained with PAGA suggests a different location of the polymer chains. PAGA seems to be located on the top and bottom surfaces as well as inside the pores, while PNADha might be solely located on the inner pore walls. Thus, the effect of a certain degree of functionalisation is more pronounced for PNADha. This consideration also agrees with the changes induced on the contact angle. Functionalisation with PNADha inside the pores leads to a reduced pore diameter and, thus, surface roughness, increasing the contact angle. For functionalisation with PAGA, the strong decrease of the contact angle of the membrane with a pore size of 0.2 µm agrees with the supposed functionalisation on the top and bottom surface, increasing the surface hydrophilicity. In the case of PAGA functionalisation of the membranes with a pore size of 1 µm the higher accessibility of the pores promotes the functionalisation inside the pores over the functionalisation on the top and bottom surfaces, and the effect of the decreased surface roughness is more dominant, thus increasing the contact angle. A possible explanation for the different location of the polyelectrolytes is their different reactivity. While AGA polymerises quickly and readily all over the available membrane surface, the polymerisation of NADha is rather inhibited and might need an acceleration of the reaction rate via the cage effect (i.e., confinement in the pore space).
3.3. Stimuli-Responsive Permeability of Polyelectrolyte Grafted Membranes
A different location (i.e., on the outer membrane surface and within the pores, as illustrated in
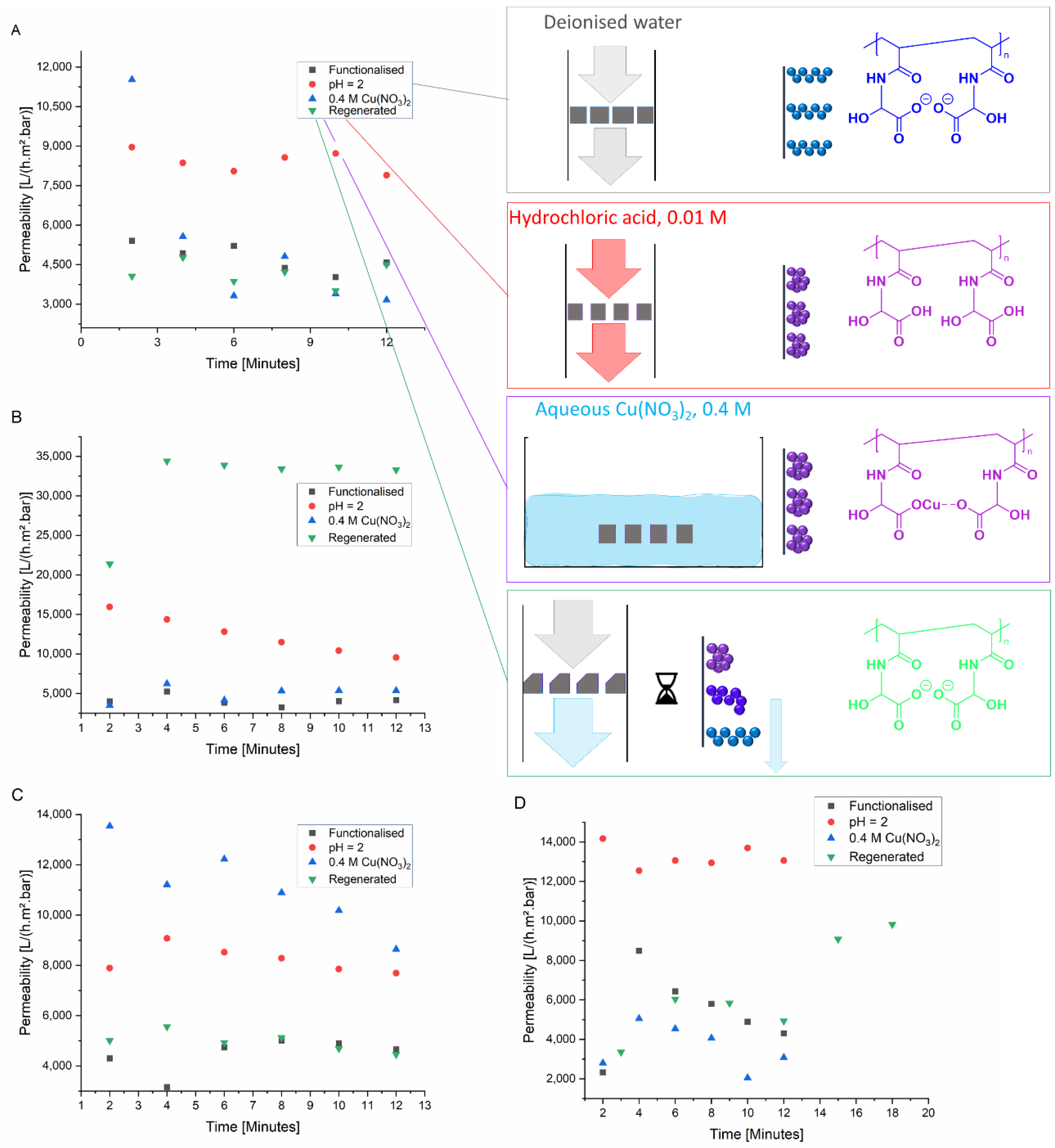
Figure 3 will have additional implications for the response of permeability to stimuli by solution conditions. Even though both arrangements can occur simultaneously, they will be discussed separately to improve clarity. When the polyelectrolyte chains extend into the pores, they decrease the hydraulic permeability of the membrane. When the chains are charged alike, the repulsive forces cause the chains to be extended and block larger parts of the pore volume, thereby decreasing the membrane permeability. When the charges are neutralised, there is no electrostatic repulsion. The polyelectrolyte chains collapse, and a larger proportion of the pore volume becomes accessible allowing an increased permeability. In case the chains are located on the top and bottom surfaces of the membranes, the effects of charge neutralisation are opposite to what is expected for a functionalisation within the pores. When the net charge is not zero, the polyelectrolyte chains are extended due to electrostatic repulsion. However, the chains do not block the interior of the pores. They are only present in dilute polymer state on the PET surface or at the entrance of the pore. When the charges are neutralised, the chains collapse and block access to the pores. Thus, the permeability will decrease upon charge neutralisation. As illustrated by the very high permeability of the membrane in
Figure 4A the effect of chain collapse seems to be more pronounced in the case of copper as bivalent cations. In case of the extended chains, there are only few crosslinks possible by the counterions. Yet, previously published results suggest no significant differences between protons and sodium ions [
26].
The effect of the stimuli Cu
2+ ions and pH on the hydraulic permeability of the membranes was assessed. The stimuli induced changes in the membrane permeability, regardless of pore size and type of polyelectrolyte used. The results of the stimuli response tests are summarised in
Figure 4.
In case of the membranes with PAGA-functionalisation and a pore size of 0.2 µm (
Figure 4A), the permeability was significantly increased in the presence of acid. After the exposure to Cu
2+, the permeability initially increased strongly, and afterwards quickly decreased to values close to the initial state before the application of any stimulus. This difference can be explained, as in the case of Cu
2+, wherein the exposure was limited to a single event prior to the measurement. Thus, the cations are partially washed out of the membrane during the measurement, whereas the addition of acid leads to a permanent drop in pH value and, with that, an increased permeability. Yet, the leaking of the Cu
2+ ions as well as the stability of the permeability after washing the membrane in an acidic solution to regenerate indicated a recyclability of the membranes. The stimuli-responsive behaviour is consistent with a functionalisation within the pores.
For membranes with PAGA functionalisation and a pore size of 1 µm (
Figure 4B), a similar but stronger response was observed in the presence of acid. Both PAGA-functionalised membranes showed a comparable initial permeability regardless of the pore size. This indicates the most pronounced chain extension of PAGA, leading to lower permeability. The presence of acid then induced the collapse of the polyelectrolyte chains, allowing a much higher permeability under acidic conditions. Exposure to Cu
2+ ions seemed to have no effect. Yet, the washing-out of the metal ions, as was observed for the PAGA-functionalised membrane with a 0.2 µm pore size, could be occurring in this case as well. For the membrane with the larger pore size, a higher water permeability is possible with collapsed pores possibly accelerating the leeching of ions. The reverting of the permeability to values observed before the application of any stimuli demonstrates the recyclability of the system. Attempts to regenerate the membrane often resulted in strongly increased values for permeability, possibly indicating some mechanical damage to the membranes caused by the handling.
The effects of exposure to Cu
2+ and acid on the permeability of PNADha-functionalised membranes with a pore size of 0.2 µm are comparable to those for PAGA-functionalised membranes. Both stimuli led to an increase in membrane permeability, while the membranes can be regenerated to values found before the application of any stimulus (
Figure 4C). This behavior is again consistent with a functionalisation inside the pores. The strength of the increase of permeability differs between PAGA and PNADha functionalised membranes. Moreover, the permeability remained increased after exposure to Cu
2+. These differences could be correlated to differences in pK
a; Cu
2+ binding was stronger and therefore leaching was less extensive in case of PNADha.
In response to acid, PNADha-functionalised membranes with a pore size of 1 µm showed an increase in permeability, as did the other functionalised membranes (
Figure 4D). Against what was observed for the other membranes, the exposure to Cu
2+ led to a decrease in permeability. This could possibly be due to the higher Cu
2+: polymer ratio in this scenario, owing to the lower extent of PNADha functionalisation. The membranes can, however, be regenerated to show a permeability in the range observed before the application of any stimuli.
Generally, the membrane responses are consistent between the polyelectrolytes and the different pore sizes. The main differences were found in the strength of the effects. These differences were attributed to differences in pKa values. The largest exception was found for the response of PNADha functionalised membranes to the presence of Cu2+ ions. In this case, the change in permeability was opposite to what was expected and observed for the other membranes. Here, a congestion of the pores is assumed. The responses to the stimuli applied occurred fast, with changes being visible within minutes. In case of the membranes’ reaction towards an acidic environment, the deswelling took place during the change of the filtration feed, even before the first measurement was taken. Apart from these observations, the kinetics of swelling and deswelling were not investigated further. Thus the deswelling of the membrane in response to an exposure to Cu2+ ions cannot be accurately timed to values of less than an hour. Additionally, minor differences were observed concerning the regeneration. Except for samples that had been damaged due to handling, the permeability reverted to values in the range observed before stimuli were applied.
3.4. Adsorption of Cationic Solutes
In addition to the changes of permeability in response to certain stimuli, the absorption of charged species was investigated. In a first step, the membranes were exposed to Cu
2+ ions and changes in the contact angle were monitored. In some cases, the contact angle increased after exposure to Cu
2+. This indicates a decrease in hydrophilicity and/or a decrease in surface roughness upon formation of the polyelectrolyte-Cu
2+ complexes (
Figure S5). In the other cases, a decreasing contact angle was observed, which we tentatively attributed to changes in the surface roughness. The largest difference was found for the membranes with a pore size of 1.0 µm and PNADha functionalisation. Interestingly, small increases could also be observed for the non-functionalised base membrane, which can possibly be assigned to surface hydroxyl groups from the hydrophilization process interacting with the Cu
2+ ions.
The adsorption of the model dye methylene blue was studied. In a first step, the interaction between the homopolymers and solutions of methylene blue in ethanol was assessed via UV–Vis spectroscopy. For PNADha, adsorption could only be shown for the higher concentrated solution (
Figure S6). The results are strongly influenced by scattering which was probably caused by polymer left in dispersion after filtration. In general, both polyelectrolytes exhibited a comparable adsorption of methylene blue from non-aqueous solutions. In aqueous solutions, the adsorption behaviour could additionally be influenced by the degree of protonation of the polyelectrolyte.
Subsequently, the adsorption of methylene blue from aqueous solution to the polyelectrolyte-grafted membranes was investigated. As expected based on the pK
a values, the highest adsorption was observed for the PAGA-grafted membranes (pK
a(PAGA) = 4.32) [
26]. The absorption on base membranes and PNADha-functionalised membranes is much lower and little differences are found between these types of membranes (pK
a(PNADha) = 4.95) [
20] (
Table 2). This agrees with the lower degree of functionalisation of the PNADha-grafted membranes, and could indicate a low degree of ionisation of the PNADha chains in water at neutral pH. Although this seems to contradict the results for copper adsorption, the adsorption of metal ions is not solely influenced by charge. Complex formation between metal ions and polymers can also be influenced by the nature and geometry of the ligand polymer. For example, a typical binding mode for Cu
2+ ions is the bridging of two carboxylic acid groups of the same or different macromolecule chains.
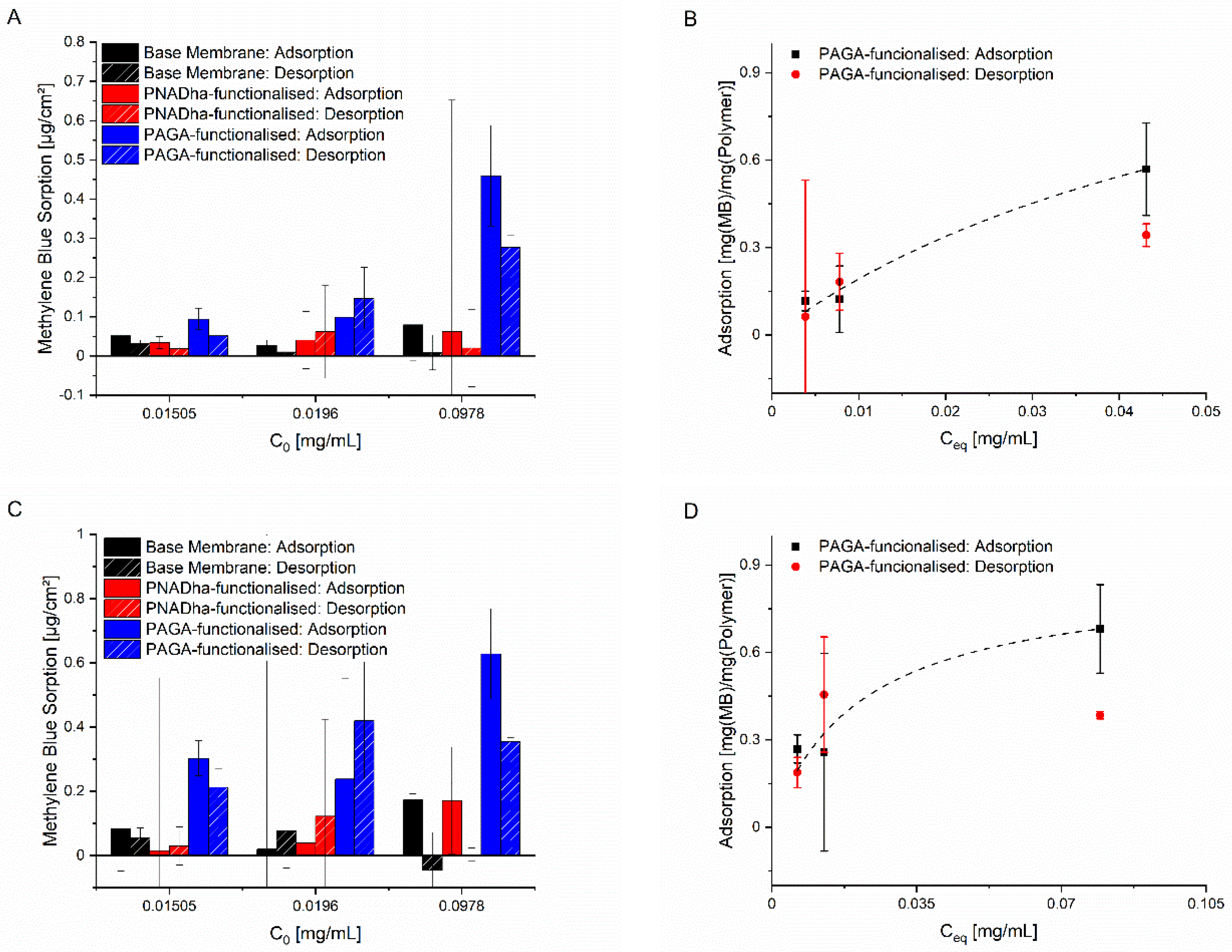
In the case of the PAGA-grafted membranes, plotting of the membrane loading against the equilibrium concentration of methylene blue revealed an adsorption behaviour following the Langmuir model (
Figure 5B,D). In the case of the other membranes, the extent of adsorption was much lower and did not follow any clear trend (
Figure 5A,C). The PNADha-functionalised membrane with a pore size of 0.2 µm showed a slight increase in the amount of methylene blue adsorbed and desorbed with the increasing dye-concentration. In case of the PNADha-functionalised membrane with a pore size 1 µm, the adsorption increased as expected. However, with increasing dye concentration, the desorption decreased. As expected, the changes observed for the base membrane are very small and fluctuate around zero for both adsorption and desorption.
Generally, the influence of the initial dye concentration is pronounced only for the PAGA-grafted membranes. Here, the dye adsorption also takes place to the largest extent. The membranes grafted with PNADha also seem to be effective in adsorbing a low amount of dye. However, the low degree of functionalization, in combination with a low degree of ionization, does not enable the adsorption of larger amounts. Compared to the adsorption to and desorption from the non-functionalised base membranes, the PNADha-functionalised membranes perform within the margin of error (
Figure 5A,C).
Fitting the adsorption data based on a Langmuir model revealed comparable parameters for both PAGA-grafted membranes, as expected for surfaces grafted with the same substance. In case of the membrane with a pore size of 0.2 µm a maximum monolayer adsorption of qmono, max = 1.40 ± 0.74 and a Langmuir coefficient of K = 15.95 ± 14.03 mL·mg−1 were found, while for the membrane with the same functionalisation and a pore size of 1 µm values of qmono, max = 0.86 ± 0.10 and K = 47.20 ± 25.52 mL·mg−1 were found. Owing to the large confidence intervals, there is an overlap even though the numbers seem to be very different at first glance.