Enhanced BDNF Actions Following Acute Hypoxia Facilitate HIF-1α-Dependent Upregulation of Cav3-T-Type Ca2+ Channels in Rat Cardiomyocytes
Abstract
1. Introduction
2. Results
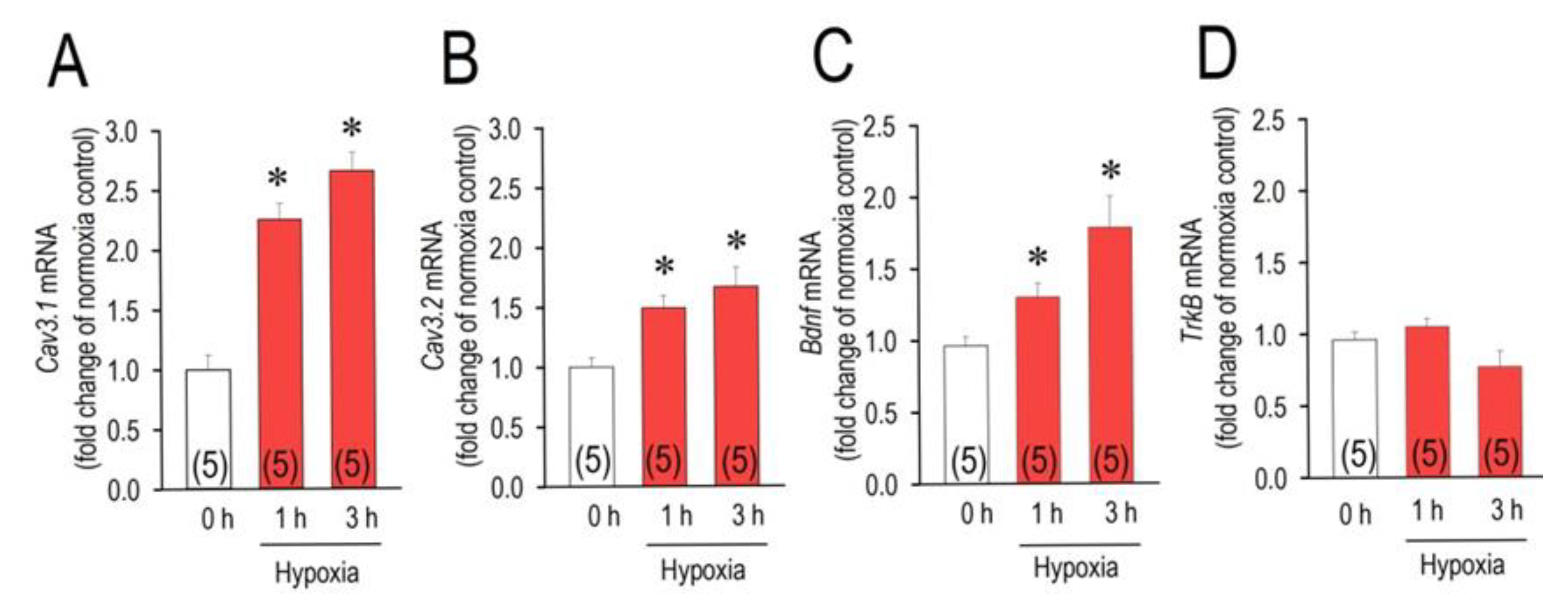
2.1. Acute Hypoxia Upregulates Cav3.1, Cav3.2 and Bdnf
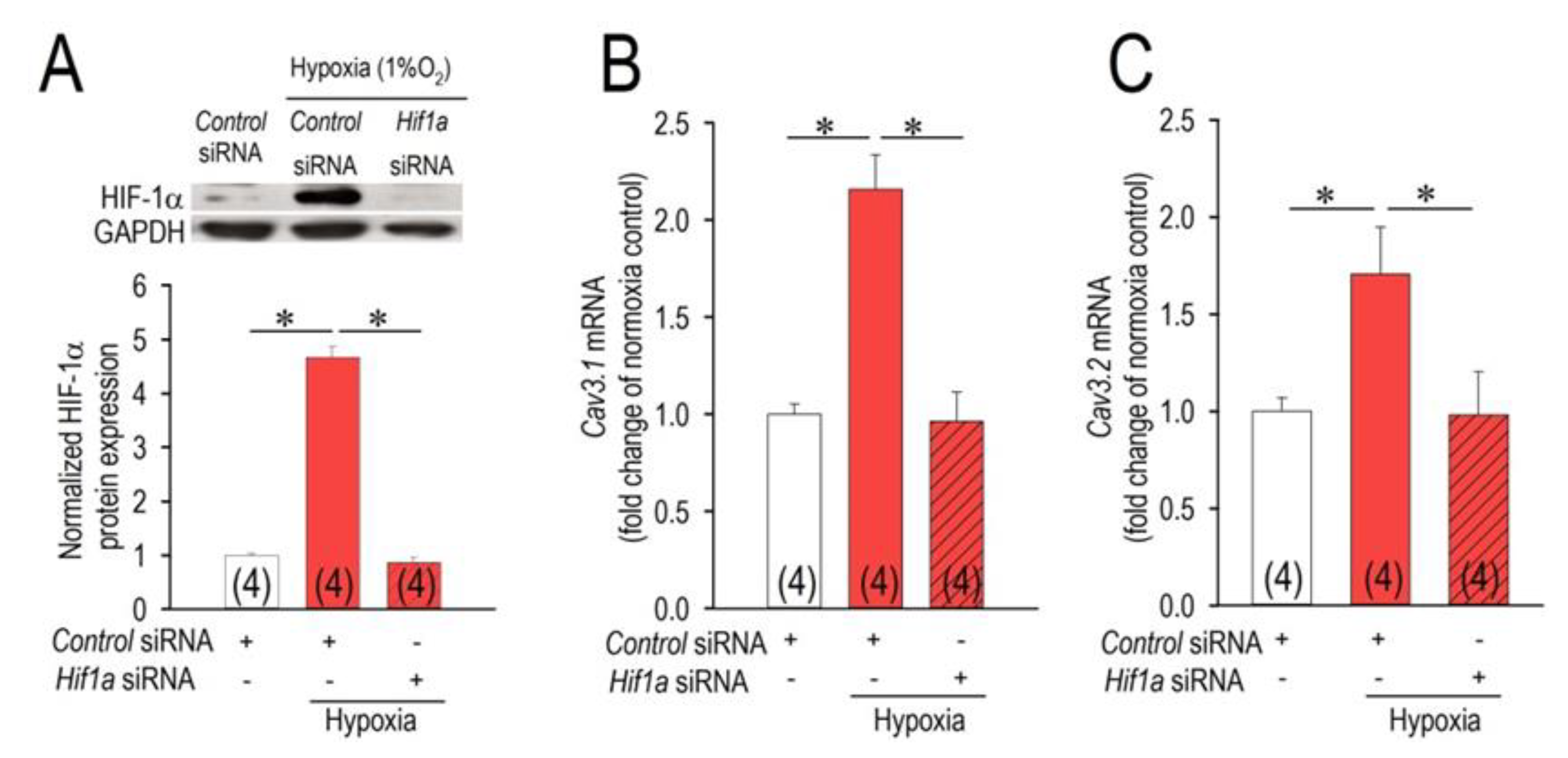
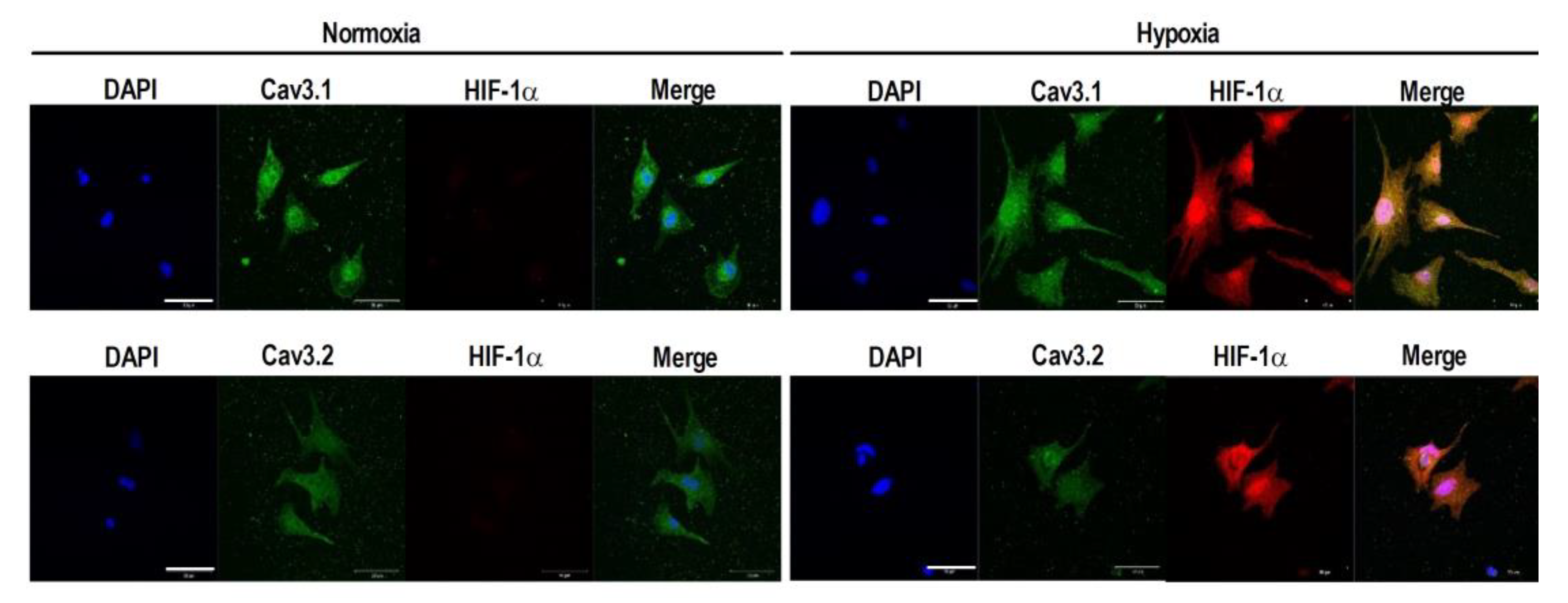
2.2. HIF-1α as a Modulator of the Cav3 Channels
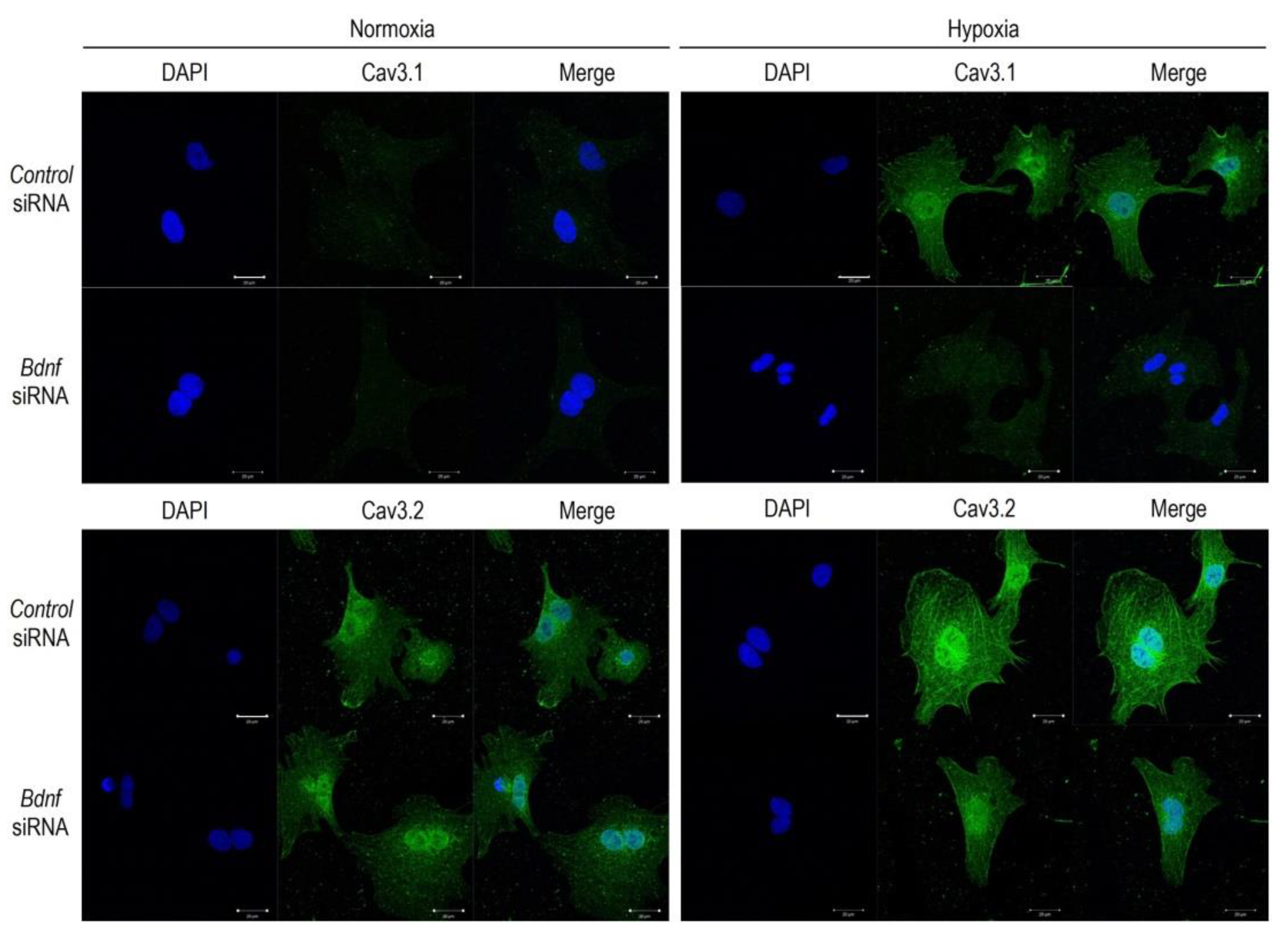
2.3. BDNF Upregulates the Cav3 Channels in Hypoxia
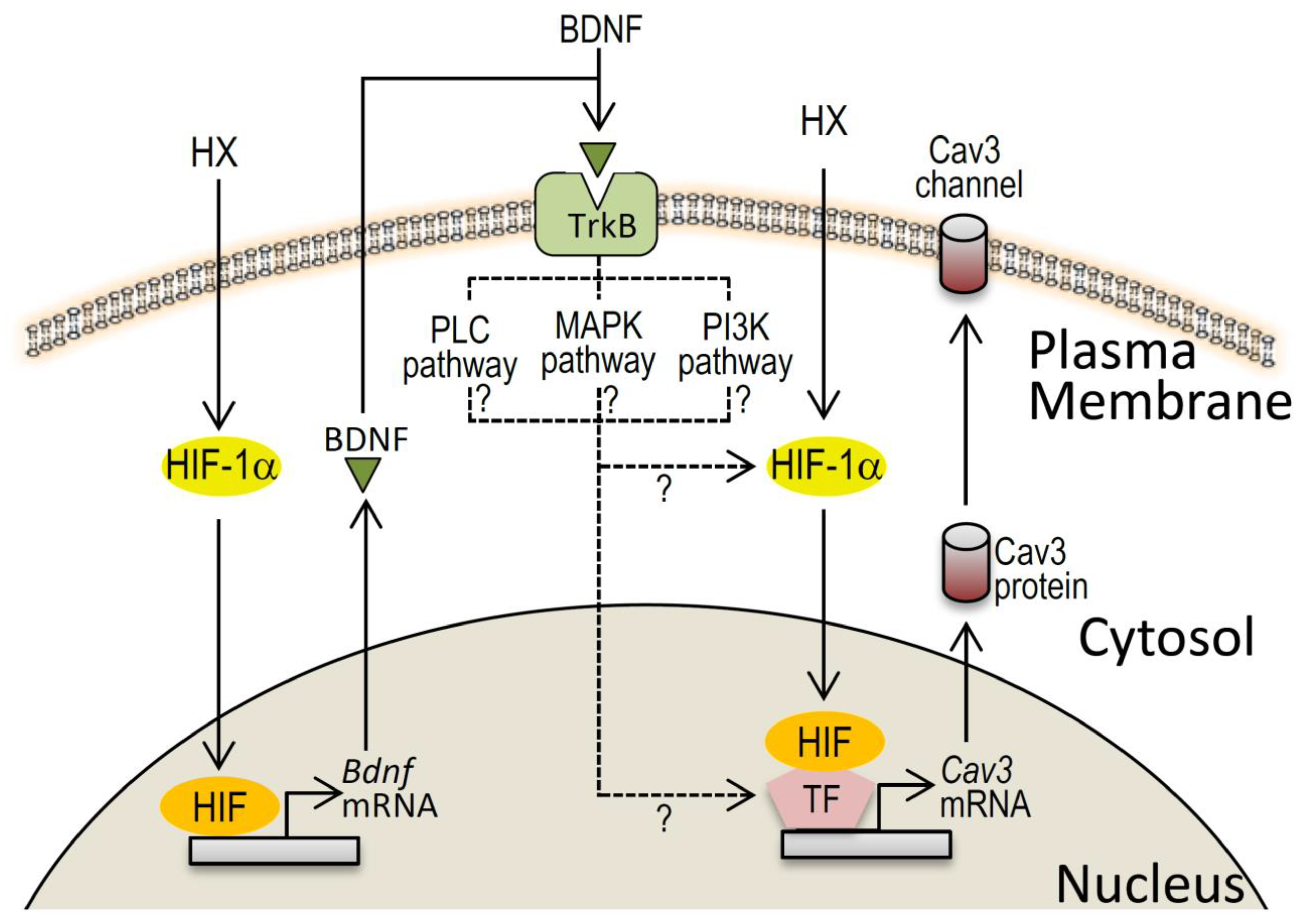
3. Discussion
3.1. Acute Hypoxia Causes Upregulation of Cav3 Channels
3.2. HIF-1α/BDNF Involvement in Cav3 Channel Upregulation
3.3. Pathophysiological Significance of BDNF in the Heart Rhythm
4. Materials and Methods
4.1. Regents
4.2. Preparation of Neonatal Rat Cardiomyocytes and Hypoxia Treatment
4.3. Transfection
4.4. Quantitative Real-Time PCR
4.5. Western Blot Analysis
4.6. Immunocytochemistry
4.7. Confocal Fluorescence Microscopy
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| HIF-1a | hypoxia-inducible factor 1a |
| TrkB | tropomyosin-related kinase receptor B |
| siRNA | small interfering RNA |
| DAPI | 4′,6-diamidino-2-phenylindole dihydrochloride |
| HX | hypoxia |
| PLC | phospholipase C |
| MAPK | mitogen-activated protein kinase |
| PI3K | phosphatidylinositol-3-kinase |
References
- Libby, P.; Braunwald, E. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Saunders/Elsevier: Philadelphia, PA, USA, 2008. [Google Scholar]
- Nattel, S.; Maguy, A.; Le Bouter, S.; Yeh, Y.H. Arrhythmogenic ion-channel remodeling in the heart: Heart failure, myocardial infarction, and atrial fibrillation. Physiol. Rev. 2007, 87, 425–456. [Google Scholar] [CrossRef]
- Takebayashi, S.; Li, Y.; Kaku, T.; Inagaki, S.; Hashimoto, Y.; Kimura, K.; Miyamoto, S.; Hadama, T.; Ono, K. Remodeling excitation-contraction coupling of hypertrophied ventricular myocytes is dependent on T-type calcium channels expression. Biochem. Biophys. Res. Commun. 2006, 345, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Ono, K.; Hadama, T.; Uchida, Y.; Arita, M. Roles of α1 and α1/β subunits derived from cardiac L-type Ca2+ channels on voltage-dependent facilitation mechanisms. Jpn. J. Physiol. 2001, 51, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Kaku, T.; Takebayashi, S.; Uchino, T.; Miyamoto, S.; Hadama, T.; Perez-Reyes, E.; Ono, K. Actions of mibefradil, efonidipine and nifedipine block of recombinant T- and L-type Ca2+ channels with distinct inhibitory mechanisms. Pharmacology 2006, 78, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, L.L.; Martin, B.L.; Schroder, E.A.; Keller, B.B.; Delisle, B.P.; Satin, J. Identification of the T-type calcium channel (Cav3.1d) in developing mouse heart. Circ. Res. 2001, 88, 403–407. [Google Scholar] [CrossRef]
- Hagiwara, H.; Irisawa, H.; Kameyama, M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 1988, 395, 233–253. [Google Scholar] [CrossRef]
- Carabelli, V.; Marcantoni, A.; Comunanza, V.; de Luca, A.; Díaz, J.; Borges, R.; Carbone, E. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J. Physiol. 2007, 584, 149–165. [Google Scholar] [CrossRef]
- Wan, J.; Yamamura, A.; Zimnicka, A.M.; Voiriot, G.; Smith, K.A.; Tang, H.; Ayon, R.J.; Choudhury, M.S.; Ko, E.A.; Wang, J.; et al. Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav1.2 and Cav3.2. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L154–L164. [Google Scholar] [CrossRef]
- Fearon, I.M.; Randall, A.D.; Perez-Reyes, E.; Peers, C. Modulation of recombinant T-type Ca2+ channels by hypoxia and glutathione. Pflug. Arch. 2000, 441, 181–188. [Google Scholar] [CrossRef]
- Makarenko, V.V.; Ahmmed, G.U.; Peng, Y.J.; Khan, S.A.; Nanduri, J.; Kumar, G.K.; Fox, A.P.; Prabhakar, N.R. Cav3.2 T-type Ca2+ channels mediate the augmented calcium influx in carotid body glomus cells by chronic intermittent hypoxia. J. Neurophysiol. 2016, 115, 345–354. [Google Scholar] [CrossRef]
- Pluteanu, F.; Cribbs, L.L. T-type calcium channels are regulated by hypoxia/reoxygenation in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1304–H1313. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; Falcón, D.; Castro, M.J.; Ureña, J.; López-Barneo, J.; Castellano, A. Hypoxic induction of T-type Ca2+ channels in rat cardiac myocytes: Role of HIF-1α and RhoA/ROCK signalling. J. Physiol. 2015, 593, 4729–4745. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Kermani, P.; Hempstead, B. BDNF actions in the cardiovascular system: Roles in development, adulthood and response to injury. Front. Physiol. 2019, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Hang, P.Z.; Zhu, H.; Li, P.F.; Liu, J.; Ge, F.Q.; Zhao, J.; Du, Z.M. The emerging role of BDNF/TrkB signaling in cardiovascular diseases. Life 2021, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, J.O.; Laurikainen, A.; Väkevä, A.; Meri, S.; Saarma, M. Nerve growth factor and brain-derived neurotrophic factor mRNAs are regulated in distinct cell populations of rat heart after ischaemia and reperfusion. J. Pathol. 2001, 194, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Yokoyama, M.; Toko, H.; Tateno, K.; Moriya, J.; Shimizu, I.; Nojima, A.; Ito, T.; Yoshida, Y.; Kobayashi, Y.; et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arter. Thromb. Vasc. Biol. 2012, 32, 1902–1909. [Google Scholar] [CrossRef]
- Fulgenzi, G.; Tomassoni-Ardori, F.; Babini, L.; Becker, J.; Barrick, C.; Puverel, S.; Tessarollo, L. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J. Cell Biol. 2015, 210, 1003–1012. [Google Scholar] [CrossRef]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- Ambrose, L.J.; Abd-Jamil, A.H.; Gomes, R.S.; Carter, E.E.; Carr, C.A.; Clarke, K.; Heather, L.C. Investigating mitochondrial metabolism in contracting HL-1 cardiomyocytes following hypoxia and pharmacological HIF activation identifies HIF-dependent and independent mechanisms of regulation. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 574–585. [Google Scholar] [CrossRef]
- Elgenaidi, I.S.; Spiers, J.P. Hypoxia modulates protein phosphatase 2A through HIF-1α dependent and independent mechanisms in human aortic smooth muscle cells and ventricular cardiomyocytes. Br. J. Pharmacol. 2019, 176, 1745–1763. [Google Scholar] [CrossRef] [PubMed]
- Huai, R.; Han, X.; Wang, B.; Li, C.; Niu, Y.; Li, R.; Qu, Z. Vasorelaxing and antihypertensive effects of 7,8-dihydroxyflavone. Am. J. Hypertens. 2014, 27, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Yan, X.Z.; Xu, S.F.; Pang, Z.Q.; Li, L.B.; Yang, Y.; Fan, Y.G.; Wang, Z.; Yu, X.; Guo, C.; et al. α-Lipoic Acid Maintains Brain Glucose Metabolism via BDNF/TrkB/HIF-1alpha Signaling Pathway in P301S Mice. Front. Aging Neurosci. 2020, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Martin, K.C.; Jackson, J.K.; Beppu, K.; Woo, C.W.; Thiele, C.J. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006, 66, 4249–4255. [Google Scholar] [CrossRef] [PubMed]
- Wurzelmann, M.; Romeika, J.; Sun, D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural. Regen. Res. 2017, 12, 7–12. [Google Scholar] [PubMed]
- The BDNF Study Group (Phase III). A controlled trial of recombinant methionyl human BDNF in ALS. Neurology 1999, 52, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, K.J.; Wan, R.; Brown, T.R.; Okun, E.; Camandola, S.; Mughal, M.R.; Phillips, T.M.; Mattson, M.P. Aberrant heart rate and brainstem brain-derived neurotrophic factor (BDNF) signaling in a mouse model of Huntington’s disease. Neurobiol. Aging 2012, 33, 1481.e1–e5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wan, R.; Weigand, L.A.; Bateman, R.; Griffioen, K.; Mendelowitz, D.; Mattson, M.P. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J. Neurochem. 2014, 129, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Slonimsky, J.D.; Birren, S.J. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat. Neurosci. 2002, 5, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Kaku, T.; Uchino, T.; Isomoto, S.; Yoshimatsu, H.; Ono, K. Short- and long-term amiodarone treatments regulate Cav3.2 low-voltage-activated T-type Ca2+ channel through distinct mechanisms. Mol. Pharmacol. 2006, 69, 1684–1691. [Google Scholar] [CrossRef]
- Wang, Y.; Morishima, M.; Zheng, M.; Uchino, T.; Mannen, K.; Takahashi, A.; Nakaya, Y.; Komuro, I.; Ono, K. Transcription factors Csx/Nkx2.5 and GATA4 distinctly regulate expression of Ca2+ channels in neonatal rat heart. J. Mol. Cell Cardiol. 2007, 42, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, M.; Sanyal, S.N.; Miyamoto, S.; Hadama, T.; Isomoto, S.; Ono, K. Three different bradycardic agents, zatebradine, diltiazem and propranolol, distinctly modify heart rate variability and QT-interval variability. Pharmacology 2007, 80, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, Y.; Pu, Y.; Jiang, D.; Jiang, X.; Zhang, Y.; Tao, J. Brain-derived neurotrophic factor stimulation of T-type Ca2+ channels in sensory neurons contributes to increased peripheral pain sensitivity. Sci. Signal. 2019, 12, eaaw2300. [Google Scholar] [CrossRef]
- Trimarchi, T.; Pachuau, J.; Shepherd, A.; Dey, D.; Martin-Caraballo, M. CNTF-evoked activation of JAK and ERK mediates the functional expression of T-type Ca2+ channels in chicken nodose neurons. J. Neurochem. 2009, 108, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Pachuau, J.; Martin-Caraballo, M. Extrinsic regulation of T-type Ca2+ channel expression in chick nodose ganglion neurons. Dev. Neurobiol. 2007, 67, 1915–1931. [Google Scholar] [CrossRef]
- Wang, Y.; Morishima, M.; Li, D.; Takahashi, N.; Saikawa, T.; Nattel, S.; Ono, K. Binge alcohol exposure triggers atrial fibrillation through T-type Ca2+ channel upregulation via protein kinase C (PKC)/glycogen synthesis kinase 3β (GSK3β)/nuclear factor of activated T-cells (NFAT) signaling—An experimental account of holiday heart syndrome. Circ. J. 2020, 84, 1931–1940. [Google Scholar] [PubMed]
- Morishima, M.; Tahara, S.; Wang, Y.; Ono, K. Oxytocin downregulates the Cav1.2 L-type Ca2+ Channel via Gi/cAMP/PKA/CREB signaling pathway in cardiomyocytes. Membranes 2021, 11, 234. [Google Scholar] [CrossRef]
- Masuda, K.; Takanari, H.; Morishima, M.; Ma, F.; Wang, Y.; Takahashi, N.; Ono, K. Testosterone-mediated upregulation of delayed rectifier potassium channel in cardiomyocytes causes abbreviation of QT intervals in rats. J. Physiol. Sci. 2018, 68, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, M.; Zhu, X.; Liu, Y.; Yoshimura, K.; Zheng, M.; Liu, G.; Kume, S.; Morishima, M.; Kurokawa, T.; et al. Nitric oxide down-regulates voltage-gated Na+ channel in cardiomyocytes possibly through S-nitrosylation-mediated signaling. Sci. Rep. 2021, 11, 11273. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morishima, M.; Fujita, T.; Osagawa, S.; Kubota, H.; Ono, K. Enhanced BDNF Actions Following Acute Hypoxia Facilitate HIF-1α-Dependent Upregulation of Cav3-T-Type Ca2+ Channels in Rat Cardiomyocytes. Membranes 2021, 11, 470. https://doi.org/10.3390/membranes11070470
Morishima M, Fujita T, Osagawa S, Kubota H, Ono K. Enhanced BDNF Actions Following Acute Hypoxia Facilitate HIF-1α-Dependent Upregulation of Cav3-T-Type Ca2+ Channels in Rat Cardiomyocytes. Membranes. 2021; 11(7):470. https://doi.org/10.3390/membranes11070470
Chicago/Turabian StyleMorishima, Masaki, Takafumi Fujita, Satoshi Osagawa, Hiroshi Kubota, and Katsushige Ono. 2021. "Enhanced BDNF Actions Following Acute Hypoxia Facilitate HIF-1α-Dependent Upregulation of Cav3-T-Type Ca2+ Channels in Rat Cardiomyocytes" Membranes 11, no. 7: 470. https://doi.org/10.3390/membranes11070470
APA StyleMorishima, M., Fujita, T., Osagawa, S., Kubota, H., & Ono, K. (2021). Enhanced BDNF Actions Following Acute Hypoxia Facilitate HIF-1α-Dependent Upregulation of Cav3-T-Type Ca2+ Channels in Rat Cardiomyocytes. Membranes, 11(7), 470. https://doi.org/10.3390/membranes11070470




