Preliminary Study on Fish Scale Collagen Lamellar Matrix as Artificial Cornea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Scale Structure and Composition Analysis
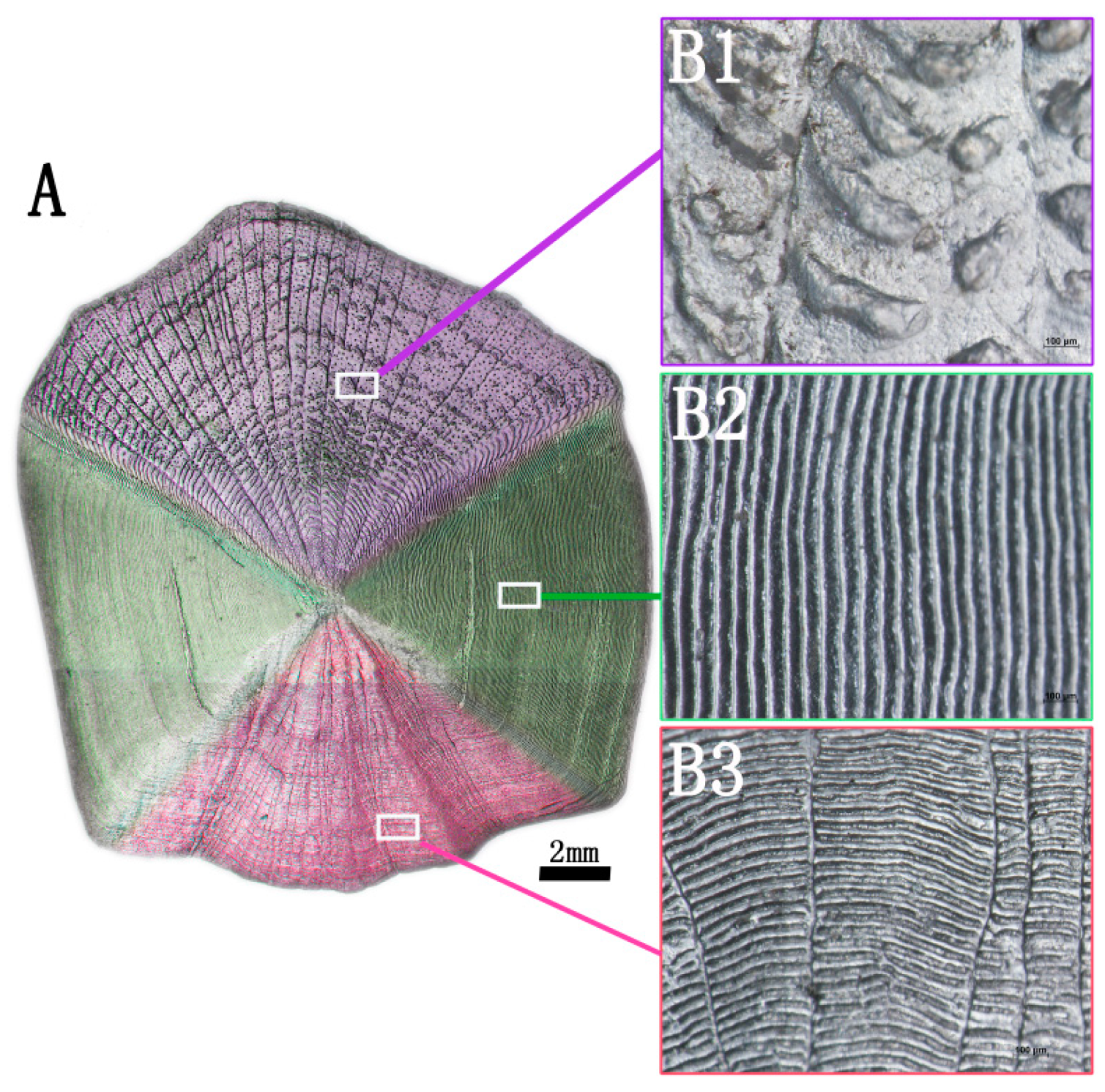
2.1.1. Surface Structure Analysis
2.1.2. Analysis of Calcium Content
2.1.3. Analysis of Decalcification Rate
2.2. Preparation of Fish Scale Collagen Lamellar Matrix
2.3. Detection for Physical and Chemical Properties of Fish Scale Collagen Lamellar Matrix
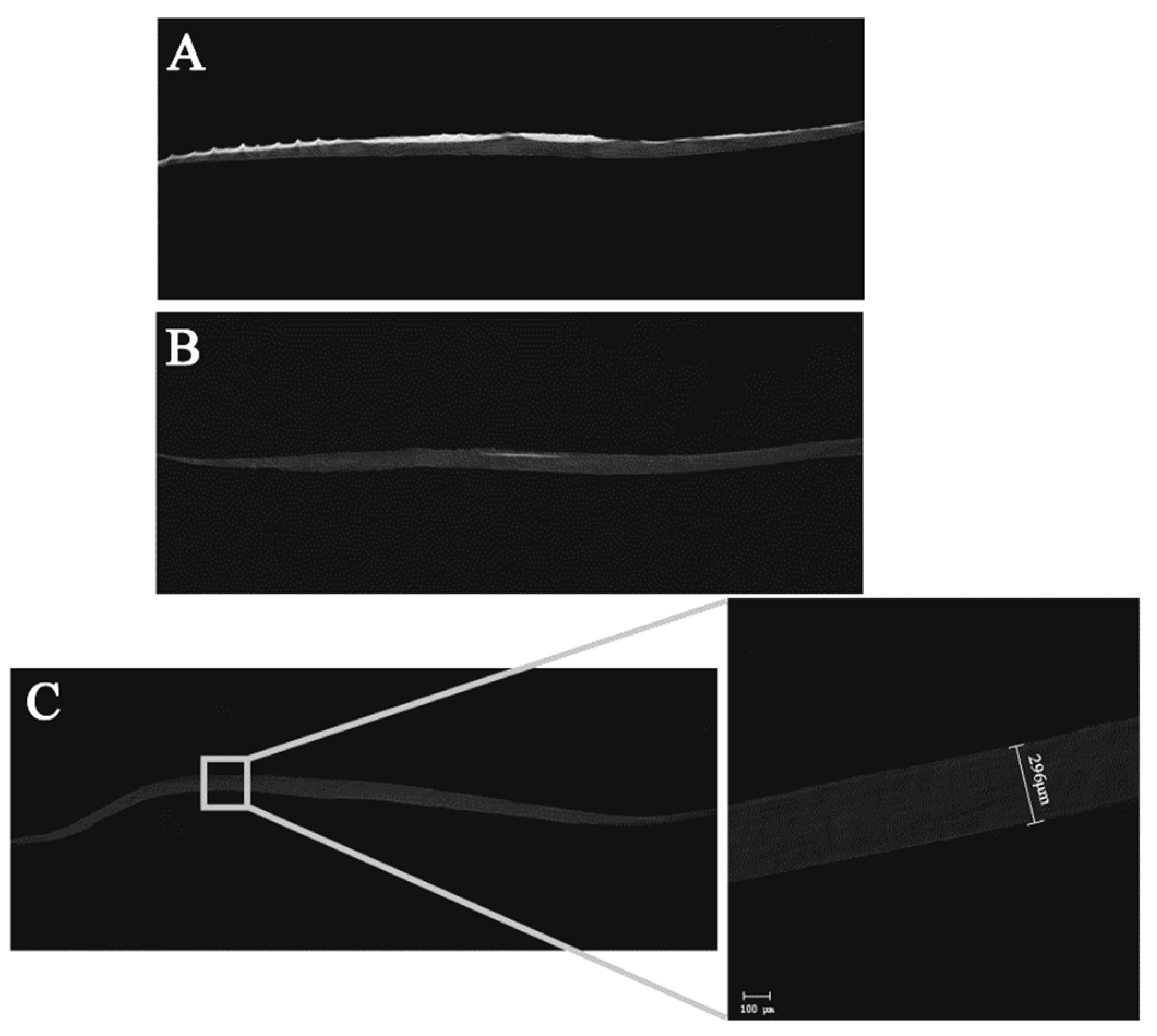
2.3.1. Thickness Characterization
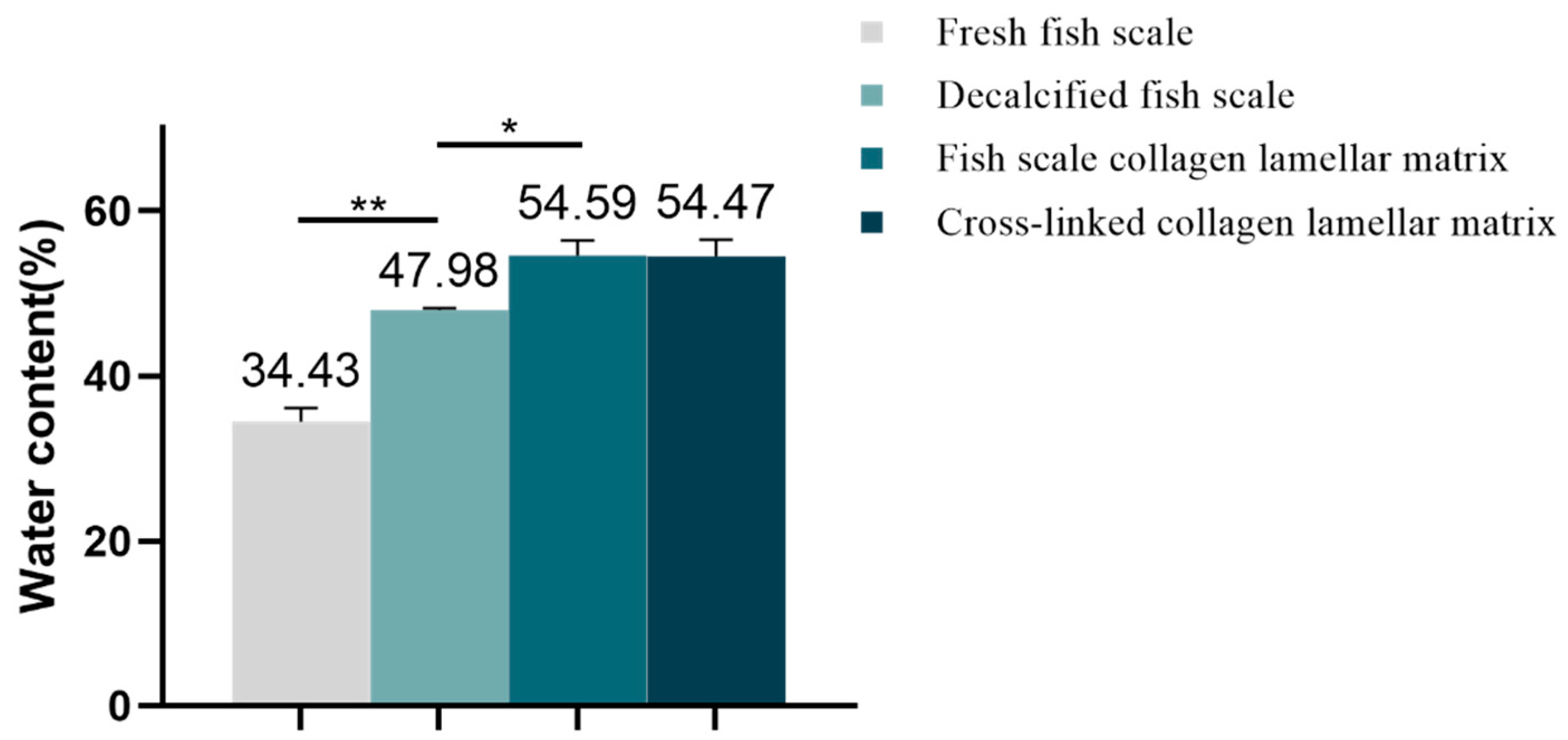
2.3.2. Water Content Analysis
2.3.3. In Vitro Atomization Analysis
2.3.4. Optical Property Detection
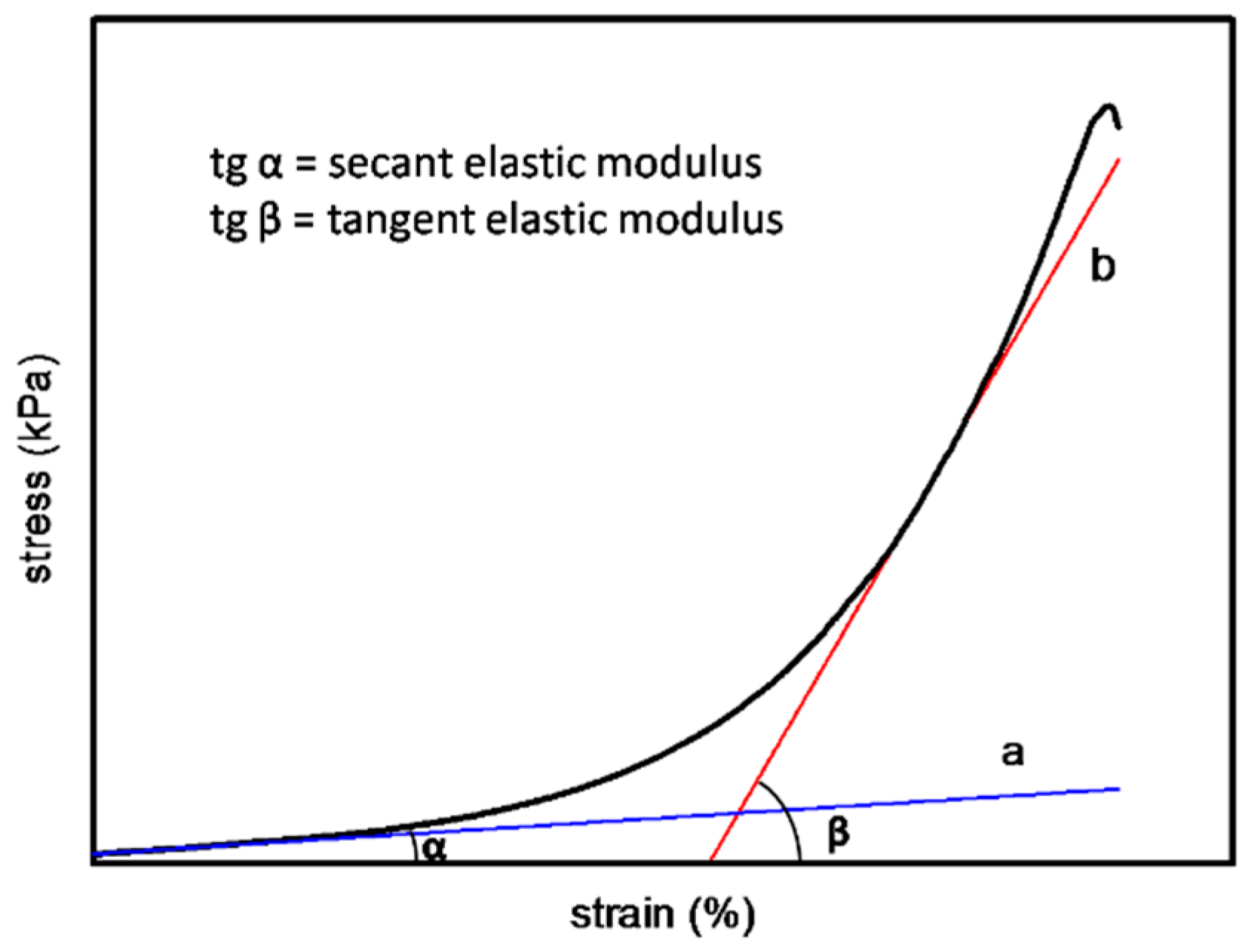
2.3.5. Mechanical Property Detection
2.4. Cellular Compatibility Detection of Fish Scale Collagen Lamellar Matrix
2.4.1. Primary Culture of Rat Bone Marrow Mesenchymal Stem Cells (BMSCs)
2.4.2. BMSCs Adhesion
2.4.3. BMSCs Proliferation
2.5. Statistical Analysis
3. Results
3.1. The Result of Fish Scale Structure and Composition Analysis
3.1.1. Surface Structure Analysis of Fish Scale
3.1.2. Analysis of Calcium Content in Fish Scale
3.1.3. Analysis of Decalcification Rate
3.2. Physical and Chemical Properties of Fish Scale Collagen Lamellar Matrix
3.2.1. Thickness Characterization
3.2.2. Water Content Analysis
3.2.3. In Vitro Atomization Analysis
3.2.4. Light Transmittance Analysis
3.2.5. Refractive Index Analysis
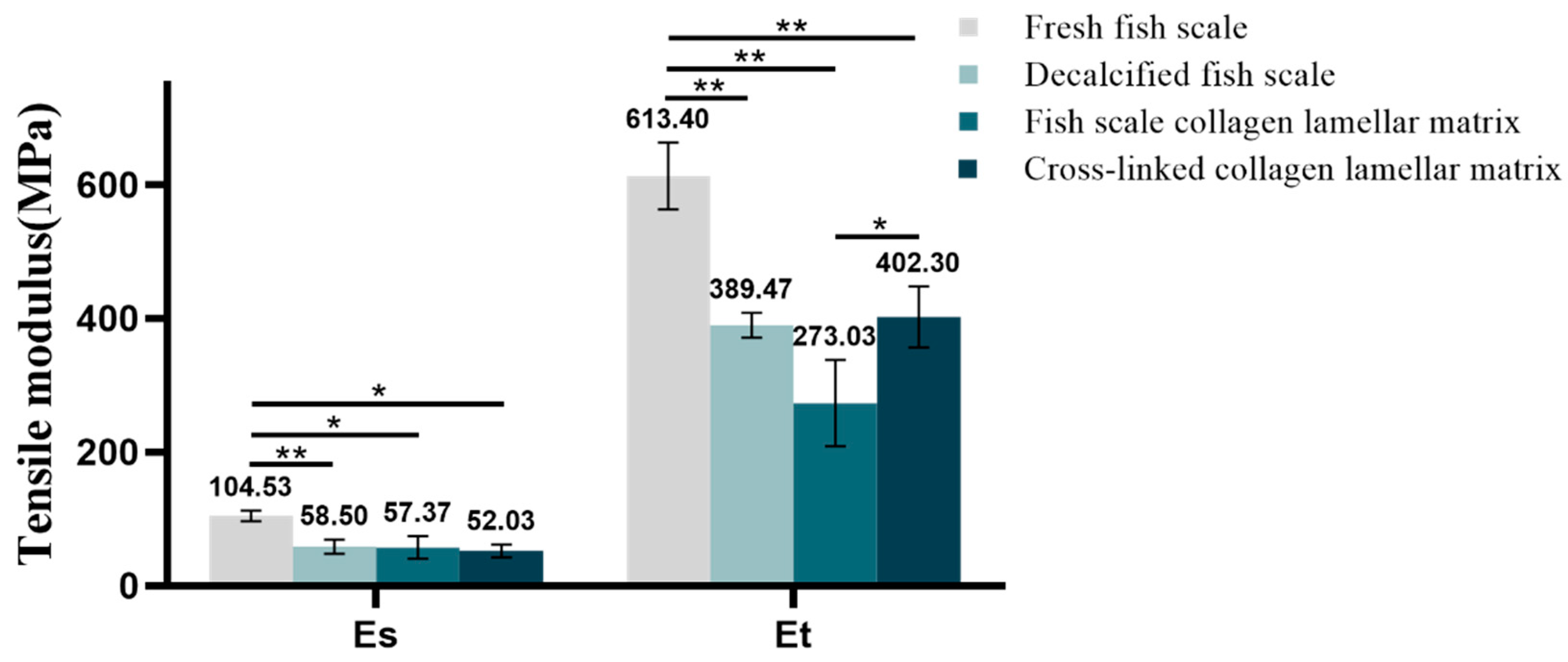
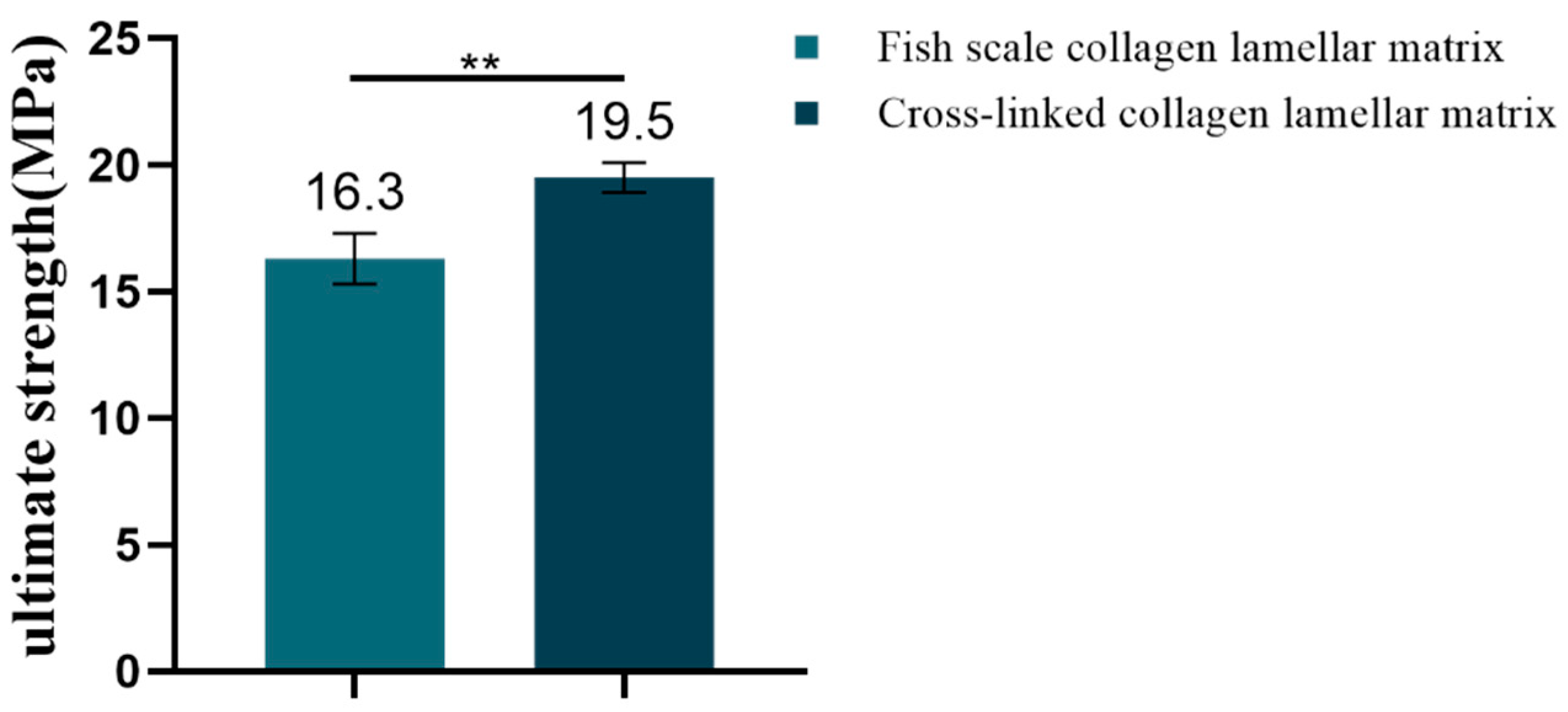
3.2.6. Mechanical Property Detection
3.3. Cellular Compatibility Test of Fish Scale Collagen Lamellar Matrix
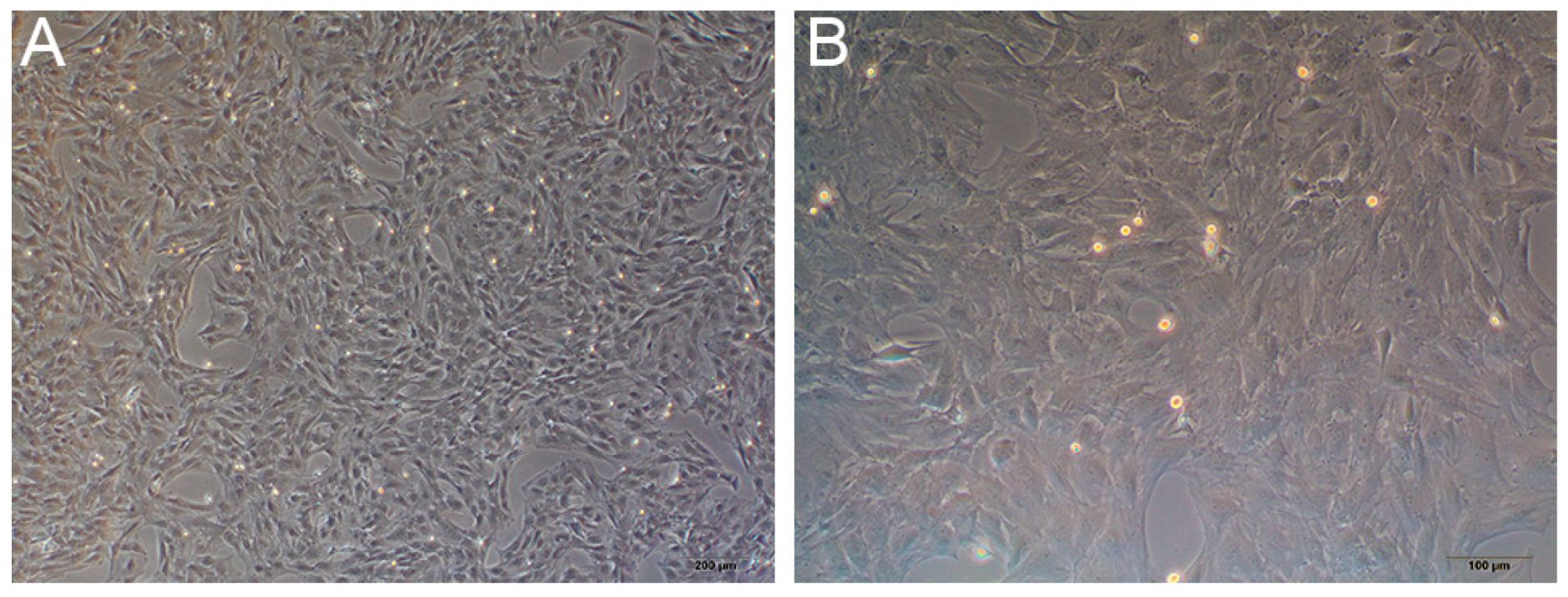
3.3.1. Primary Culture of Rat BMSCs
3.3.2. Cell Adhesion
3.3.3. Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beuerman, R.W.; Pedroza, L. Ultrastructure of the human cornea. Microsc. Res. Tech. 2015, 33, 320–335. [Google Scholar] [CrossRef]
- Sayers, Z.; Koch, M.H.J.; Whitburn, S.B.; Meek, K.M.; Elliott, G.F.; Harmsen, A. Synchrotron X-ray diffraction study of corneal stroma. J. Mol. Biol. 1982, 160, 593–607. [Google Scholar] [CrossRef]
- Myung, D.D.P.; Cochran, J.R.; Noolandi, J.; Ta, C.N.; Frank, C.W. Development of hydrogel-based keratoprostheses: A materials perspective. Biotechnol. Prog. 2008, 24, 735–741. [Google Scholar] [CrossRef] [Green Version]
- van Essen, T.H.; Lin, C.C.; Hussain, A.K.; Maas, S.; Lai, H.J.; Linnartz, H.; van den Berg, T.J.; Salvatori, D.C.; Luyten, G.P.; Jager, M.J. A fish scale-derived collagen matrix as artificial cornea in rats: Properties and potential. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3224–3233. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Xue, Q.; Li, J.; Ma, L.; Yao, Y.; Ye, H.; Cui, Z.; Yang, H. 3D bioprinting for artificial cornea: Challenges and perspectives. Med. Eng. Phys. 2019, 71, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Matthyssen, S.; Van den Bogerd, B.; Dhubhghaill, S.N.; Koppen, C.; Zakaria, N. Corneal regeneration: A review of stromal replacements. Acta Biomater. 2018, 69, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ghezzi, C.E.; Gomes, R.; Pollard, R.E.; Funderburgh, J.L.; Kaplan, D.L. In vitro 3D corneal tissue model with epithelium, stroma, and innervation. Biomaterials 2017, 112, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wray, L.S.; Orwin, E.J. Recreating the microenvironment of the native cornea for tissue engineering applications. Tissue Eng. Part A 2009, 15, 1463. [Google Scholar] [CrossRef]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, Å.; Ruggiero, F.; Hulmes, D.J.S. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- van Essen, T.H.; van Zijl, L.; Possemiers, T.; Mulder, A.A.; Zwart, S.J.; Chou, C.H.; Lin, C.C.; Lai, H.J.; Luyten, G.P.M.; Tassignon, M.J.; et al. Biocompatibility of a fish scale-derived artificial cornea: Cytotoxicity, cellular adhesion and phenotype, and in vivo immunogenicity. Biomaterials 2016, 81, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, D.; Wang, Y.; Qin, W. A comparative study of the properties and self-aggregation behavior of collagens from the scales and skin of grass carp (Ctenopharyngodon idella). Int. J. Biol. Macromol. 2018, 106, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Yeo, K.P.; Chun, Y.Y.; Tan, T.T.Y.; Tan, N.S.; Angeli, V.; Choong, C. Fish scale-derived collagen patch promotes growth of blood and lymphatic vessels in vivo. Acta Biomater. 2017, 63, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Huanhuan, F.; Xia, L.; Xiaoming, D.; Xiaolei, L.; Jitong, G.; Ke, M.; Bo, J. The lamellar structure and biomimetic properties of a fish scale matrix. RSC Adv. 2020, 10, 875–885. [Google Scholar]
- Tadashi, K.; Hiroaki, T. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar]
- Esmaeili, H.R.; Ansari, T.H.; Teimory, A. Scale structure of a cyprinid fish, capoeta damascina (valenciennes in cuvier and valenciennes, 1842) using scanning electron microscope (sem). Iran. J. Sci. Technol. Trans. A Sci. 2007, 31, 255–262. [Google Scholar]
- Arola, D.; Murcia, S.; Stossel, M.; Pahuja, R.; Linley, T.; Devaraj, A.; Ramulu, M.; Ossa, E.A.; Wang, J. The limiting layer of fish scales: Structure and properties. Acta Biomater. 2018, 67, 319–330. [Google Scholar] [CrossRef]
- Kashef, V. Development of a pericardial acellular matrix biomaterial: Biochemical and mechanical effects of cell extraction. J. Biomed. Mater. Res. Part A 1994, 28, 655. [Google Scholar]
- Yao, Q.; Zheng, Y.; Lan, Q.; Kou, L.; Xu, H.; Zhao, Y. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C 2019, 104, 109942. [Google Scholar] [CrossRef]
- Gamze Torun, K.S.; Feza, K.; Aykut, O.; Yasemin, S.; Taner, O.; Cemil, Y.; Vasif, H. Tissue engineered cartilage on collagen and PHBV matrices. Biomaterials 2005, 26, 5187–5197. [Google Scholar]
- Zhao, X.; Liu, Y.; Li, W.; Long, K.; Wang, L.; Liu, S.; Wang, Y.; Ren, L. Collagen based film with well epithelial and stromal regeneration as corneal repair materials: Improving mechanical property by crosslinking with citric acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Beems, E.M.; Van Best, J.A. Light transmission of the cornea in whole human eyes. Exp. Eye Res. 1990, 50, 393–395. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Z.; Ge, J.; Wan, P.; Li, N.; Xiang, P.; Gao, Q.; Wang, Z. Development and Characterization of Acellular Porcine Corneal Matrix Using Sodium Dodecylsulfate. Cornea 2011, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Schönbörner, A.A.; Boivin, G.; Baud, C.A. The mineralization processes in Teleost fish scales. Cell Tissue Res. 1979, 202, 203. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Watabe, N. Studies on fish scale formation and resorption. I. Fine structure and calcification of the scales in Fundulus heteroclitus (Atheriniformes: Cypnnodontidae). J. Morphol. 1979, 159, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Onozato, H.; Watabe, N. Studies on fish scale formation and resorption. III. Fine structure and calcification of the fibrillary plates of the scales in Carassius auratus (Cypriniformes: Cyprinidae). Cell Tissue Res. 1979, 201, 409–422. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2015, 55, 203–216. [Google Scholar] [CrossRef]
- He, Y.; Chen, D.; Yang, L.; Hou, Q.; Ma, H.; Xu, X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res. Ther. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.K.; Kim, J.I.; Hwang, T.I.; Sim, B.R.; Khang, G. Bioengineered Osteoinductive Broussonetia kazinoki/Silk Fibroin Composite Scaffolds for Bone Tissue Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Gierloff, M.; Nitsche, T.; Adam-Klages, S.; Liebs, K.; Hedderich, J.; Gassling, V.; Wiltfang, J.; Kabelitz, D.; Açil, Y. In vitro comparison of different carrier materials with rat bone marrow MSCs. Clin. Oral Investig. 2014, 18, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ng, S.M.; Akpek, E.K.; Ahmad, S. Artificial corneas versus donor corneas for repeat corneal transplants. Cochrane Database Syst. Rev. 2020, 5, CD009561. [Google Scholar] [CrossRef] [PubMed]

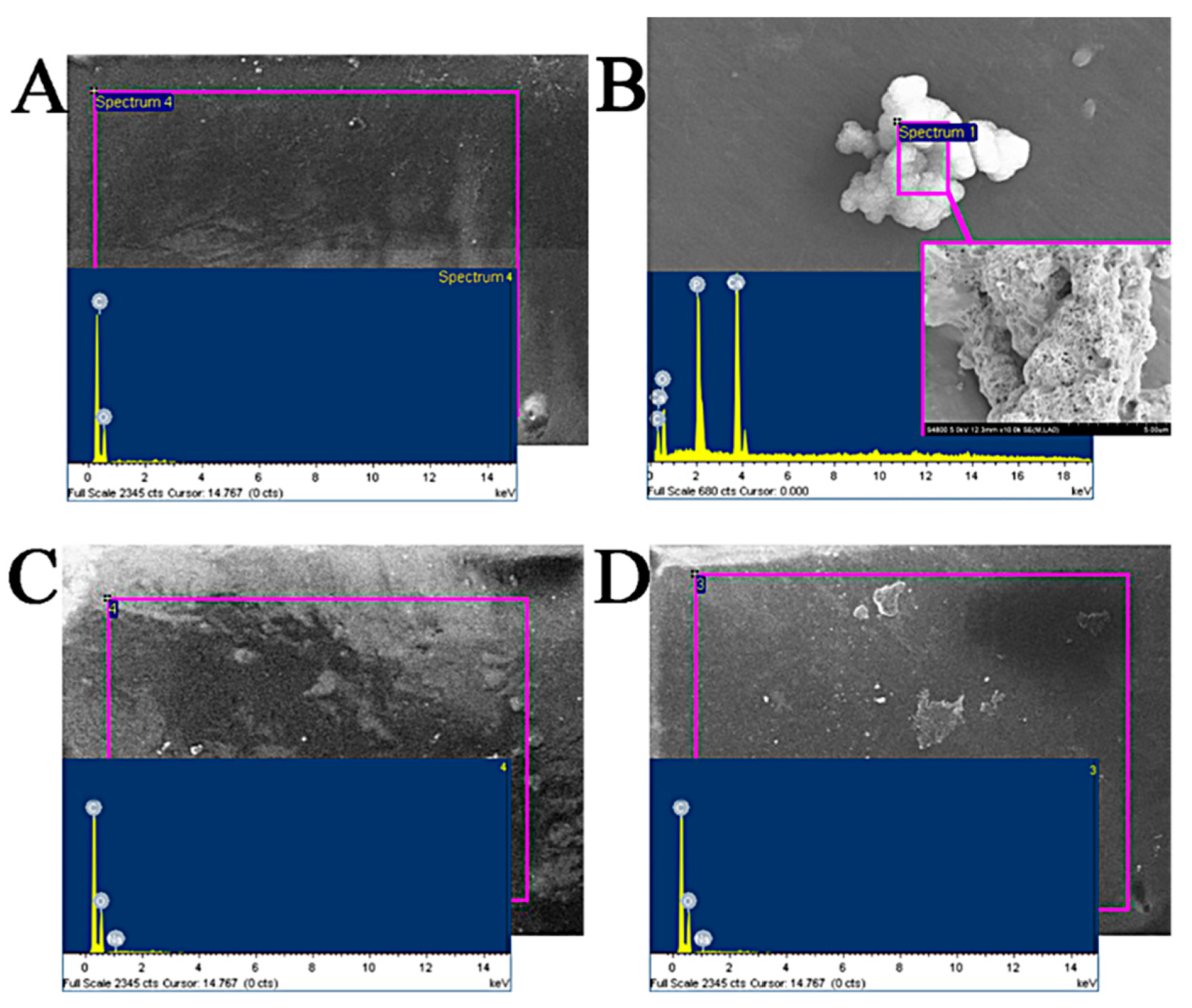





| EDTA Consumption Volume (ML) | Mass Concentration of Calcium Ion in Decalcification Solution (mg/mL) | Calcium Content in Fish Scale (%) (Mean) | |
|---|---|---|---|
| Sample 1 | 9.2 | 0.206 | 10.6 |
| Sample 2 | 9.8 | 0.219 | |
| Sample 3 | 9.6 | 0.215 |
| Volume of Standard Zinc Consumed in Titration of Original Decalcification Solution V0 (mL) | Standard Zinc Volume Consumed by Decalcification Solution after Titration and Decalcification V1 (mL) | Decalcification Rate (%) (Mean) | |
|---|---|---|---|
| Sample 1 | 5.0 | 2.9 | 99 |
| Sample 2 | 5.1 | 2.9 | |
| Sample 3 | 5.0 | 3.0 |
| Element | C | O | Na | Ca | P | |
|---|---|---|---|---|---|---|
| Percentage of atomic content (%) | Deionized water group | 67.59 | 32.41 | -- | -- | -- |
| SBF group | 35.77 | 45.62 | -- | 7.49 | 11.12 | |
| STF group | 66.8 | 32.99 | 0.21 | -- | -- | |
| Artificial ear fluid group | 61.96 | 37.3 | 0.74 | -- | -- | |
| Refractive Index | ||||
|---|---|---|---|---|
| Sample | Fresh Fish Scale | Decalcified Fish Scale | Fish Scale Collagen Lamellar Matrix | Cross-Linked Collagen Lamellar Matrix |
| 1 | 1.6566 | 1.5944 | 1.3314 | 1.3285 |
| 2 | 1.6449 | 1.5835 | 1.3421 | 1.3386 |
| 3 | 1.4646 | 1.5890 | 1.3353 | 1.339 |
| Mean (SD) | 1.5887 (0.1076) | 1.5890 (0.0055) | 1.3363 (0.0054) | 1.3354 (0.0060) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Chen, L.; Feng, H.; Jiang, B.; Ding, Y. Preliminary Study on Fish Scale Collagen Lamellar Matrix as Artificial Cornea. Membranes 2021, 11, 737. https://doi.org/10.3390/membranes11100737
Cheng G, Chen L, Feng H, Jiang B, Ding Y. Preliminary Study on Fish Scale Collagen Lamellar Matrix as Artificial Cornea. Membranes. 2021; 11(10):737. https://doi.org/10.3390/membranes11100737
Chicago/Turabian StyleCheng, Guoping, Liang Chen, Huanhuan Feng, Bo Jiang, and Yi Ding. 2021. "Preliminary Study on Fish Scale Collagen Lamellar Matrix as Artificial Cornea" Membranes 11, no. 10: 737. https://doi.org/10.3390/membranes11100737
APA StyleCheng, G., Chen, L., Feng, H., Jiang, B., & Ding, Y. (2021). Preliminary Study on Fish Scale Collagen Lamellar Matrix as Artificial Cornea. Membranes, 11(10), 737. https://doi.org/10.3390/membranes11100737






