Free Standing, Large-Area Silicon Nitride Membranes for High Toxin Clearance in Blood Surrogate for Small-Format Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. NPN Membrane Fabrication
2.2. Single-Pass Benchtop Dialysis with Chip-Based Devices
2.3. Benchtop Dialysis Studies with NPN Sheet Membranes
3. Results
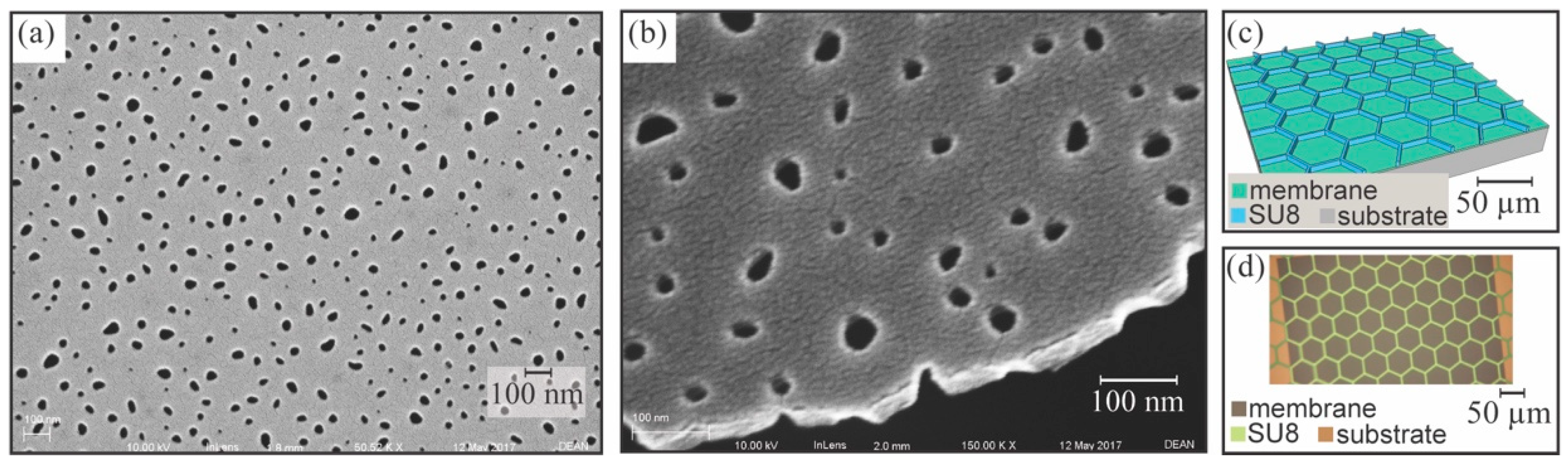
3.1. Sheet Membrane Fabrication
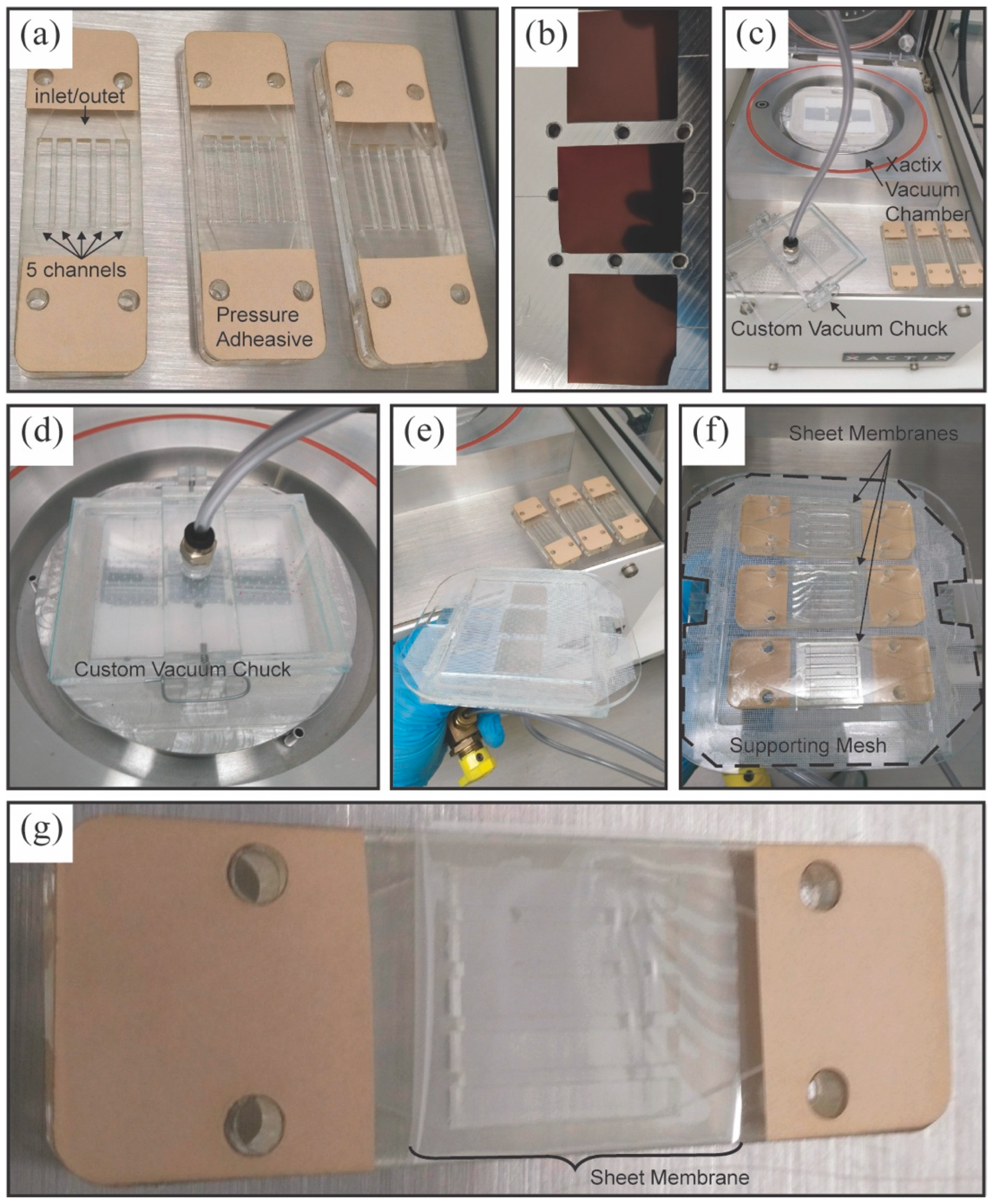
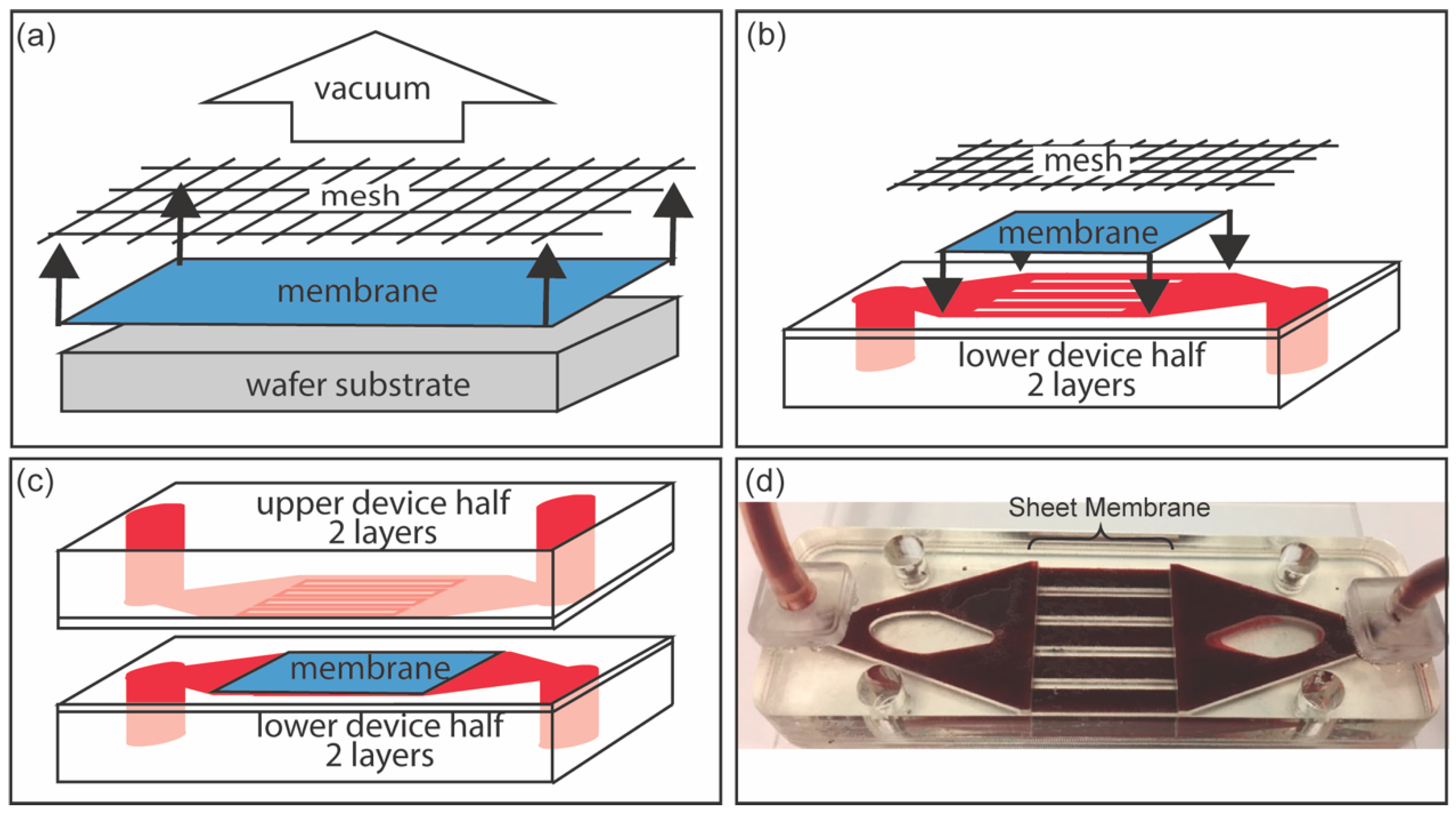
3.2. Fluidic Device Assembly for NPN Sheet Membranes
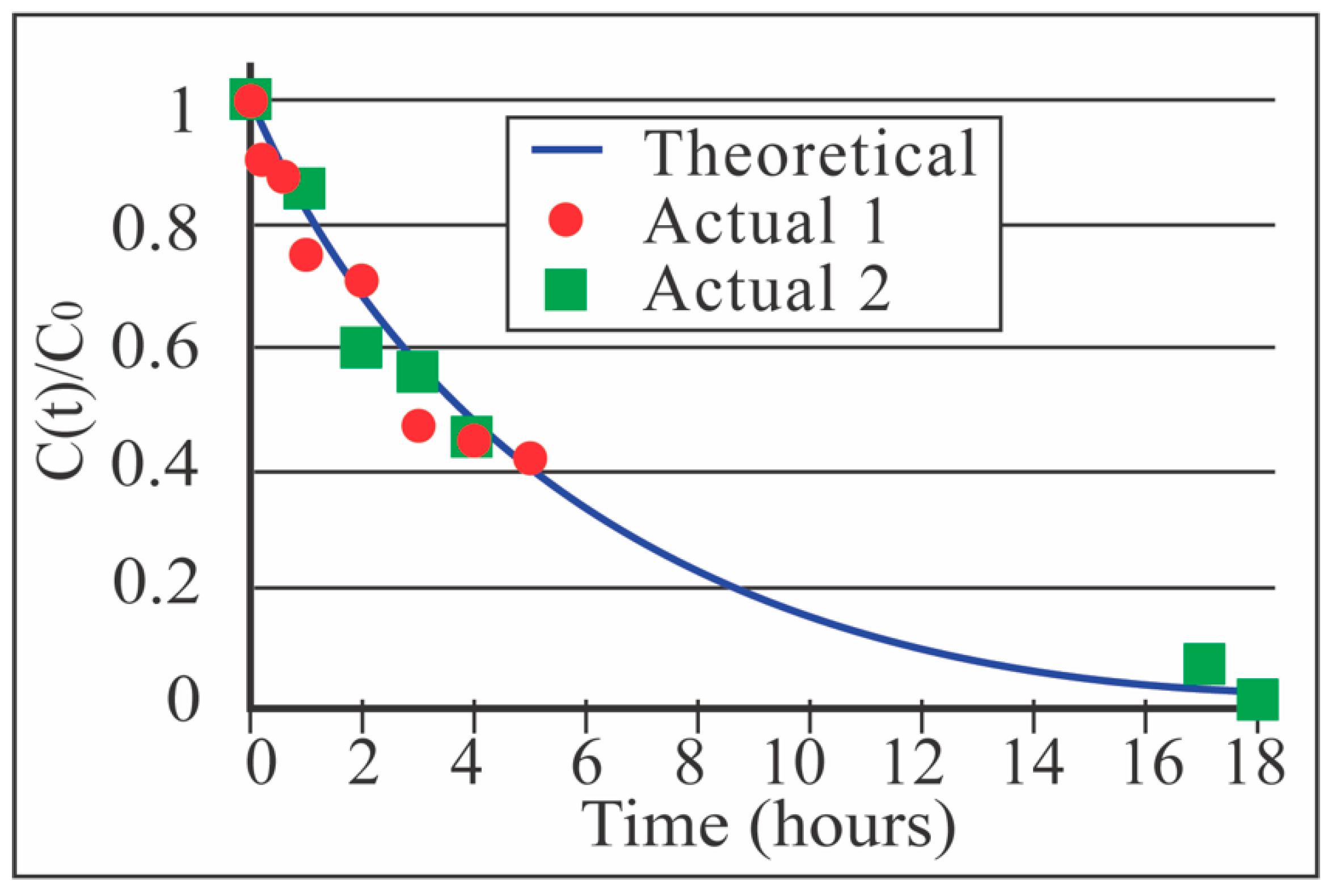
3.3. Benchtop Dialysis Studies with NPN Sheet Membranes
3.4. Analytical Model Relating Single-Pass Clearance, k, and Multi-Pass Clearance, K
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- USRDS. United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2019.
- Chien, C.-W.; Huang, C.-J.; Chao, Z.-H.; Huang, S.-K.; Chen, P.-E.; Tung, T.-H. Hemodialysis interval and its association with emergency care and mortality. Medicine 2019, 98, e14816. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Gilbertson, D.T.; Murray, T.; Collins, A.J. Long Interdialytic Interval and Mortality among Patients Receiving Hemodialysis. N. Engl. J. Med. 2011, 365, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Loutradis, C.; Sarafidis, P.A.; Papadopoulos, C.E.; Papagianni, A.; Zoccali, C. The Ebb and Flow of Echocardiographic Cardiac Function Parameters in Relationship to Hemodialysis Treatment in Patients with ESRD. J. Am. Soc. Nephrol. 2018, 29, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.; Walker, S.N.; Salminen, A.; Chung, H.L.; Li, X.; Ezzat, B.; Miller, J.J.; Desormeaux, J.S.; Zhang, J.; Hayden, A.; et al. Second Generation Nanoporous Silicon Nitride Membranes for High Toxin Clearance and Small Format Hemodialysis. Adv. Health Mater. 2020, 9, e1900750. [Google Scholar] [CrossRef]
- Kolff, W.J. The beginning of the artificial kidney. Artif. Organs 1993, 17, 293–299. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Ikizler, T.A. Hemodialysis. N. Engl. J. Med. 2010, 363, 1833–1845. [Google Scholar] [CrossRef]
- Ronco, C.; Davenport, A.; Gura, V. A wearable artificial kidney: Dream or reality? Nat. Clin. Pract. Nephrol. 2008, 4, 604–605. [Google Scholar] [CrossRef]
- USRDS. United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018.
- Striemer, C.C.; Gaborski, T.R.; McGrath, J.L.; Fauchet, P.M. Charge- and size-based separation of macromolecules using ultrathin silicon membranes. Nature 2007, 445, 749–753. [Google Scholar] [CrossRef]
- Snyder, J.; Clark, A.; Fang, D.; Gaborski, T.; Striemer, C.; Fauchet, P.; McGrath, J.L. An experimental and theoretical analysis of molecular separations by diffusion through ultrathin nanoporous membranes. J. Membr. Sci. 2011, 369, 119–129. [Google Scholar] [CrossRef]
- Gaborski, T.R.; Snyder, J.L.; Striemer, C.C.; Fang, D.Z.; Hoffman, M.; Fauchet, P.M.; McGrath, J.L. High-Performance Separation of Nanoparticles with Ultrathin Porous Nanocrystalline Silicon Membranes. ACS Nano 2010, 4, 6973–6981. [Google Scholar] [CrossRef]
- Desormeaux, J.P.S.; Winans, J.D.; Wayson, S.E.; Gaborski, T.R.; Khire, T.S.; Striemer, C.C.; McGrath, J.L. Nanoporous silicon nitride membranes fabricated from porous nanocrystalline silicon templates. Nanoscale 2014, 6, 10798–10805. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.P.; Winans, J.; McGrath, J.L. Ultrathin Membrane Fouling Mechanism Transitions in Dead-End Filtration of Protein. In Proceedings of the ASME 2016 14th International Conference on Nanochannels, Microchannels, and Minichannels, Washington, DC, USA, 10–14 July 2016. [Google Scholar]
- Johnson, D.G.; Khire, T.S.; Lyubarskaya, Y.L.; Smith, K.J.; Desormeaux, J.-P.S.; Taylor, J.G.; Gaborski, T.R.; Shestopalov, A.A.; Striemer, C.C.; McGrath, J.L. Ultrathin Silicon Membranes for Wearable Dialysis. Adv. Chronic Kidney Dis. 2013, 20, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Mossu, A.; Rosito, M.; Khire, T.; Chung, H.L.; Nishihara, H.; Gruber, I.; Luke, E.; Dehouck, L.; Sallusto, F.; Gosselet, F.; et al. A silicon nanomembrane platform for the visualization of immune cell trafficking across the human blood–brain barrier under flow. Br. J. Pharmacol. 2018, 39, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Carter, R.N.; McNabb, K.B.; Desormeaux, J.-P.S.; Striemer, C.C.; Winans, J.D.; Gaborski, T.R. Lift-off of large-scale ultrathin nanomembranes. J. Micromech. Microeng. 2014, 25, 15011. [Google Scholar] [CrossRef]
- Fang, D.Z.; Striemer, C.C.; Gaborski, T.R.; McGrath, J.L.; Fauchet, P.M. Methods for controlling the pore properties of ultra-thin nanocrystalline silicon membranes. J. Phys. Condens. Matter 2010, 22, 454134. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Striemer, C.C.; Gaborski, T.R.; McGrath, J.L.; Fauchet, P.M. Highly Porous Silicon Membranes Fabricated from Silicon Nitride/Silicon Stacks. Small 2014, 10, 2946–2953. [Google Scholar] [CrossRef]
- Zawada, R.J.; Kwan, P.; Olszewski, K.L.; Llinás, M.; Huang, S.-G. Quantitative determination of urea concentrations in cell culture medium. Biochem. Cell Biol. 2009, 87, 541–544. [Google Scholar] [CrossRef]
- Gillmer, S.R.; Fang, D.Z.; Wayson, S.E.; Winans, J.D.; Abdolrahim, N.; Desormeaux, J.-P.S.; Getpreecharsawas, J.; Ellis, J.; Fauchet, P.M.; McGrath, J.L. Predicting the failure of ultrathin porous membranes in bulge tests. Thin Solid Films 2017, 631, 152–160. [Google Scholar] [CrossRef]
- Ouseph, R.; Ward, R.A. Increasing Dialysate Flow Rate Increases Dialyzer Urea Mass Transfer-Area Coefficients During Clinical Use. Am. J. Kidney Dis. 2001, 37, 316–320. [Google Scholar] [CrossRef]
- Leypoldt, J.K.; Cheung, A.K.; Agodoa, L.Y.; Daugirdas, J.T.; Greene, T.; Keshaviah, P.R.; Beck, G.J. Hemodialyzer mass transfer-area coefficients for urea increase at high dialysate flow rates. Kidney Int. 1997, 51, 2013–2017. [Google Scholar] [CrossRef]
- Hootkins, R. Lessons in dialysis, dialyzers, and dialysate. Dial. Transplant. 2011, 40, 392–396. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, J.J.; Carter, J.A.; Hill, K.; DesOrmeaux, J.-P.S.; Carter, R.N.; Gaborski, T.R.; Roussie, J.A.; McGrath, J.L.; Johnson, D.G. Free Standing, Large-Area Silicon Nitride Membranes for High Toxin Clearance in Blood Surrogate for Small-Format Hemodialysis. Membranes 2020, 10, 119. https://doi.org/10.3390/membranes10060119
Miller JJ, Carter JA, Hill K, DesOrmeaux J-PS, Carter RN, Gaborski TR, Roussie JA, McGrath JL, Johnson DG. Free Standing, Large-Area Silicon Nitride Membranes for High Toxin Clearance in Blood Surrogate for Small-Format Hemodialysis. Membranes. 2020; 10(6):119. https://doi.org/10.3390/membranes10060119
Chicago/Turabian StyleMiller, Joshua J., Jared A. Carter, Kayli Hill, Jon-Paul S. DesOrmeaux, Robert N. Carter, Thomas R. Gaborski, James A. Roussie, James L. McGrath, and Dean G. Johnson. 2020. "Free Standing, Large-Area Silicon Nitride Membranes for High Toxin Clearance in Blood Surrogate for Small-Format Hemodialysis" Membranes 10, no. 6: 119. https://doi.org/10.3390/membranes10060119
APA StyleMiller, J. J., Carter, J. A., Hill, K., DesOrmeaux, J.-P. S., Carter, R. N., Gaborski, T. R., Roussie, J. A., McGrath, J. L., & Johnson, D. G. (2020). Free Standing, Large-Area Silicon Nitride Membranes for High Toxin Clearance in Blood Surrogate for Small-Format Hemodialysis. Membranes, 10(6), 119. https://doi.org/10.3390/membranes10060119






