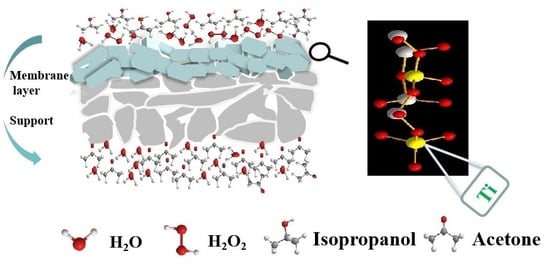
Effects of Seed Crystals on the Growth and Catalytic Performance of TS-1 Zeolite Membranes
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of TS-1 Zeolite Membrane
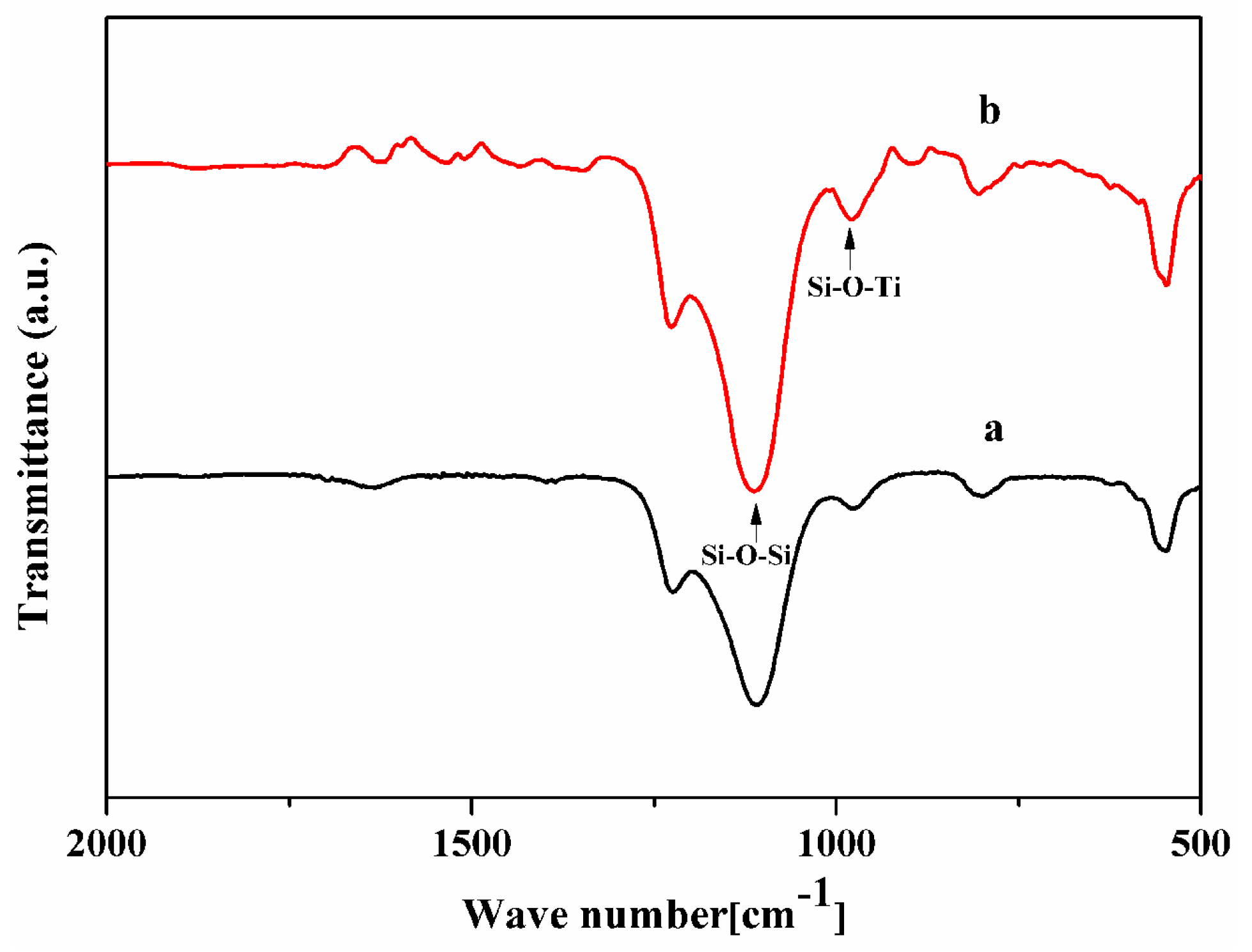
2.3. Characterizations

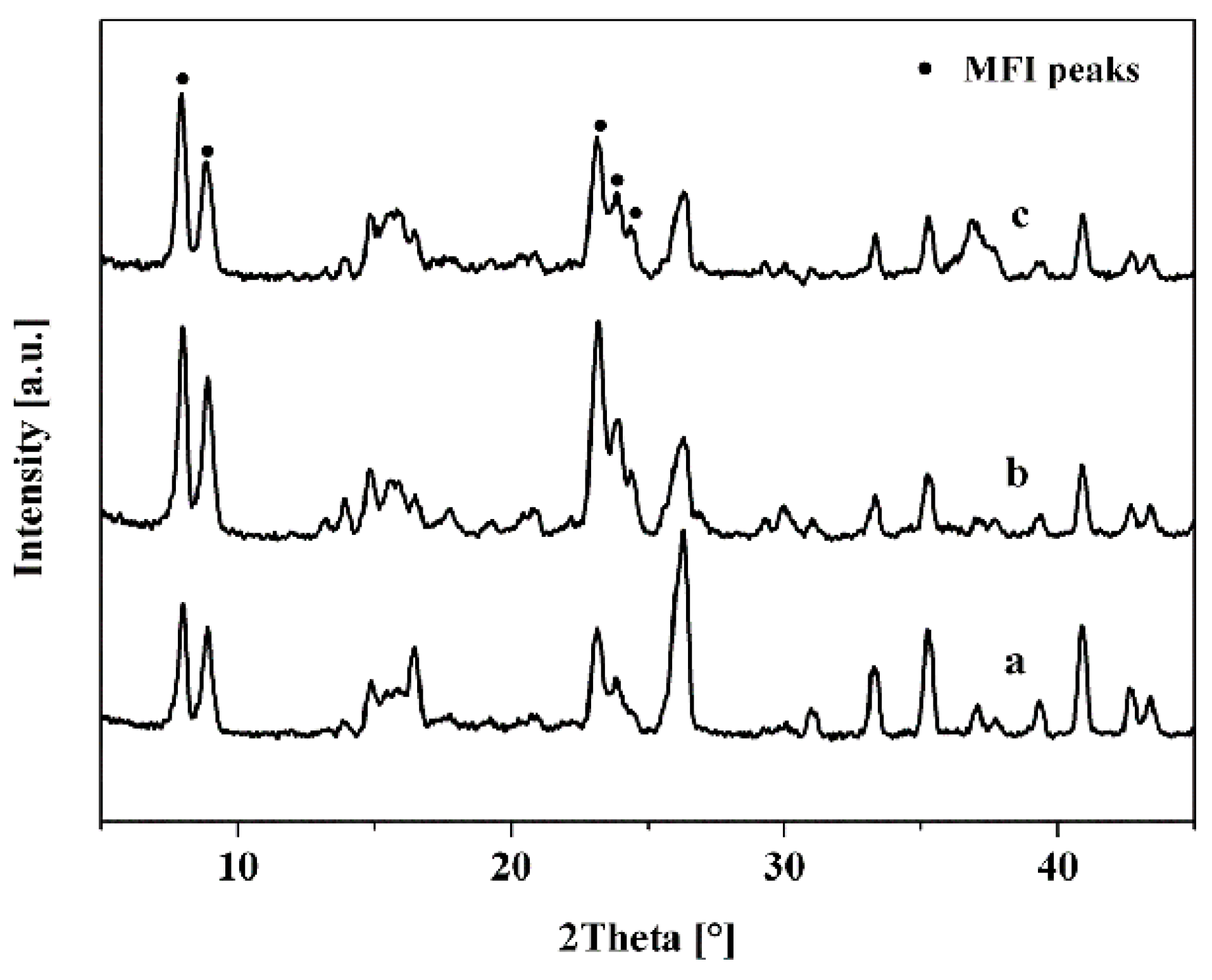
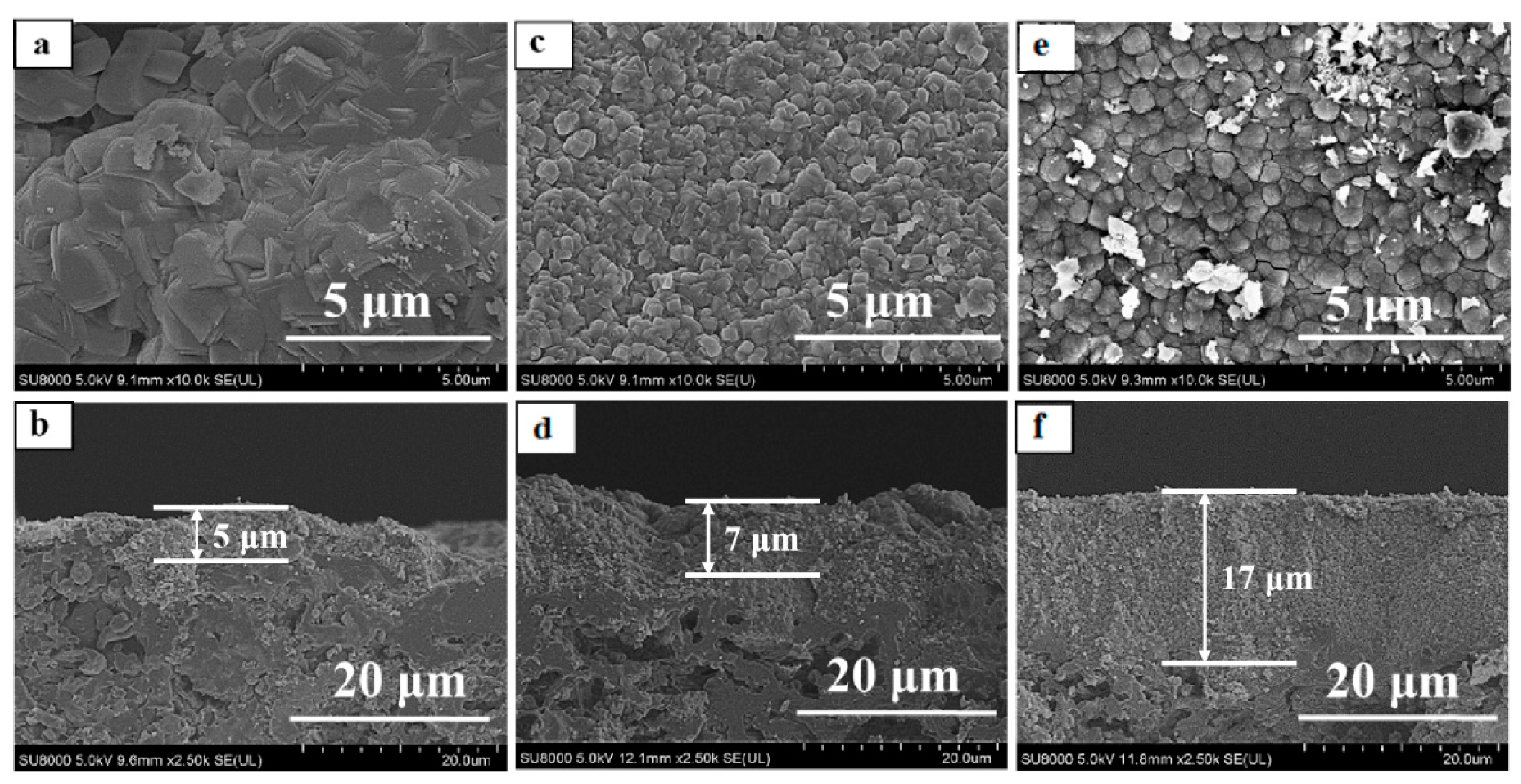
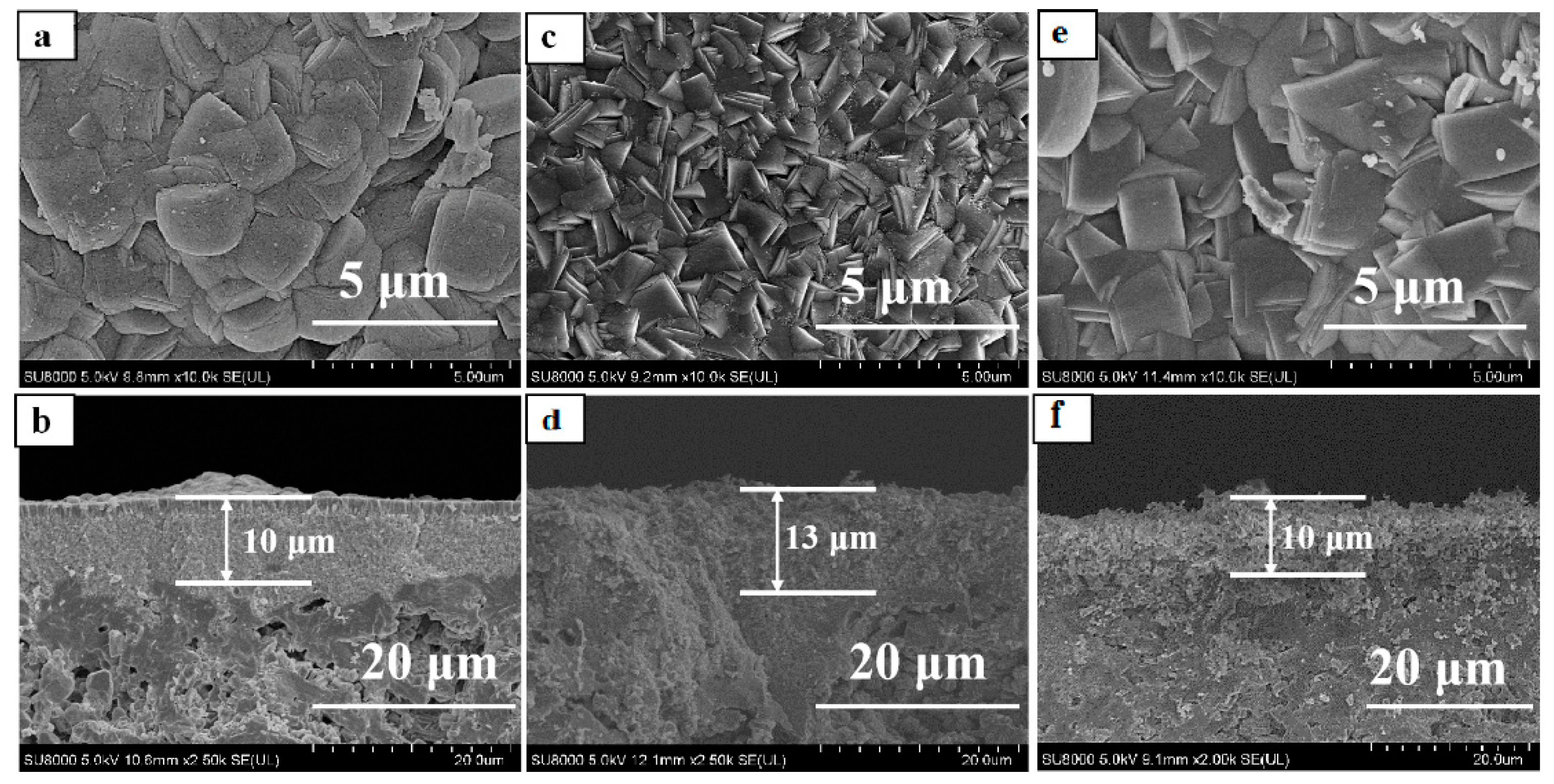
3. Results and Discussion
3.1. Effect of Seed Crystals Ti/Si Ratio
3.2. Effect of Seed Crystals Morphology
3.3. Effect of Seed Crystals Suspension Concentration
3.4. Comparison of Bi-Layer and Mono-Layer TS-1 Zeolite Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taramasso, M.; Perego, G.; Notari, B. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. US4410501. 1983. Available online: https://patents.glgoo.top/patent/US4410501A/en (accessed on 10 March 2020).
- Thangaraj, A.; Sivasanker, S.; Ratnasamy, P. Catalytic properties of crystalline titanium silicalites III. Ammoximation of cyclohexanone. J. Catal. 1991, 131, 394–400. [Google Scholar] [CrossRef]
- Murugavel, R.; Roesky, H.W. Titanosilicates: Recent Developments in Synthesis and Use as Oxidation Catalysts. Angew. Chem. Int. Ed. Eng. 1997, 36, 477–479. [Google Scholar] [CrossRef]
- Perego, C.; Carati, A.; Ingallina, P.; Mantegazza, M.A.; Bellussi, G. Production of titanium containing molecular sieves and their application in catalysis. Appl. Catal. A Gen. 2001, 221, 63–72. [Google Scholar] [CrossRef]
- Shin, S.B.; Chadwick, D. Kinetics of Heterogeneous Catalytic Epoxidation of Propene with Hydrogen Peroxide over Titanium Silicalite (TS-1). Ind. Eng. Chem. Res. 2010, 49, 8125–8134. [Google Scholar] [CrossRef]
- Wang, W.; Fu, Y.; Guo, Y.; Guo, Y.; Gong, X.; Lu, G.J. Preparation of lamellar-stacked TS-1 and its catalytic performance for the ammoximation of butanone with H2O2. Mater. Sci. 2017, 53, 4034–4045. [Google Scholar] [CrossRef]
- Lu, T.; Zou, J.; Zhan, Y.; Yang, X.; Wen, Y.; Wang, X.; Zhou, L.; Xu, J. Highly Efficient Oxidation of Ethyl Lactate to Ethyl Pyruvate Catalyzed by TS-1 Under Mild Conditions. ACS Catal. 2018, 8, 1287–1296. [Google Scholar] [CrossRef]
- Lovallo, M.C.; Gouzinis, A.; Tsapatsis, M. Synthesis and characterization of oriented MFI membranes prepared by secondary growth. AIChE J. 1998, 44, 1903–1913. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P.; Schäfer, R. Zeolite membranes—State of their development and perspective. Microporous Mesoporous Mater. 2000, 38, 3–24. [Google Scholar] [CrossRef]
- Zhu, B.; Kim, J.H.; Na, Y.; Moon, I.S.; Connor, G.; Morris, G.; Gray, S.; Duke, M. Temperature and Pressure Effects of Desalination Using a MFI-Type Zeolite Membrane. Membranes 2013, 3, 155–168. [Google Scholar] [CrossRef]
- Capel-Sanchez, M.C.; Campos-Martin, J.M.; Fierro, J.L.G. Removal of refractory organosulfur compoundsviaoxidation with hydrogen peroxide on amorphous Ti/SiO2catalysts. Energy. Environ. Sci. 2010, 3, 328–333. [Google Scholar] [CrossRef]
- Zhang, F.; Shang, H.; Jin, D.; Chen, R.; Xing, W. High efficient synthesis of methyl ethyl ketone oxime from ammoximation of methyl ethyl ketone over TS-1 in a ceramic membrane reactor. Chem. Eng. Process. 2017, 116, 1–8. [Google Scholar] [CrossRef]
- Warzywoda, J.; Edelman, R.D.; Thompson, R.W. Crystallization of high-silica ZSM-5 in the presence of seeds. Zeolites 1991, 11, 318–324. [Google Scholar] [CrossRef]
- Gora, L.; Thompson, R.W. Controlled addition of aged mother liquor to zeolite NaA synthesis solutions. Zeolites 1997, 18, 132–141. [Google Scholar] [CrossRef]
- Xu, X.; Yang, W.; Liu, J.; Lin, L. Synthesis and perfection evaluation of NaA zeolite membrane. Sep. Purif. Technol. 2001, 25, 475–485. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Huang, W. Synthesis of b-oriented TS-1 zeolite membranes with high performance in the oxyfunctionalization of n-hexane. Thin Solid Films 2013, 534, 40–44. [Google Scholar] [CrossRef]
- Zhu, M.H.; Liu, Y.S.; Yao, Y.; Jiang, J.; Zhang, F.; Yang, Z.; Lu, Z.; Kumakiri, I.; Chen, X.; Kita, H. Preparation and catalytic performance of Ti-MWW zeolite membrane for phenol hydroxylation. Microporous Mesoporous Mater. 2018, 268, 84–87. [Google Scholar] [CrossRef]
- Liu, X.; Ge, P.; Zhang, Y.; Zhang, B.; Yao, Z.; Li, X.; Hu, M. Highly Oriented Thin Membrane Fabrication with Hierarchically Porous Zeolite Seed. Cryst. Growth. Des. 2018, 18, 4544–4554. [Google Scholar] [CrossRef]
- Wang, X.; Meng, B.; Zhang, X.; Tan, X.; Liu, S. Synthesis of stable Ti-containing mesoporous tubular membrane using silicalite-1 nanoparticles as seeds. Chem. Eng. J. 2014, 255, 344–355. [Google Scholar] [CrossRef]
- Zhu, M.; Li, L.; Chen, L.; Liao, Y.; Ding, W.; Feng, Z.; Wan, Z.; Hu, N.; Chen, X.; Kita, H. Preparation of TS-1 zeolite membrane from dilute precursor synthesis solution. Microporous Mesoporous Mater. 2019, 273, 212–218. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Zhang, K.; Peng, H.; Wu, H.; Jiang, J.; Liu, Y.; Wu, P. Multilayer structured MFI-type titanosilicate: Synthesis and catalytic properties in selective epoxidation of bulky molecules. J. Catal. 2012, 288, 16–23. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, M.; Hong, L.; Wu, M.; Zhang, T.; Ma, M.; Song, C.; Guo, X. Role of Supports in the Tetrapropylammonium Hydroxide Treated Titanium Silicalite-1 Extrudates. Ind. Eng. Chem. Res. 2015, 54, 1513–1519. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, M.; Zhang, T.; Hong, L.; Guo, X.; Song, C.; Chen, Y.; Zhu, P.; Jaye, C.; Fischer, D. Role of pentahedrally coordinated titanium in titanium silicalite-1 in propene epoxidation. RSC. Adv. 2015, 5, 17897–17904. [Google Scholar] [CrossRef]
- Thangaraj, A.; Eapen, M.J.; Sivasanker, S.; Ratnasamy, P. Studies on the synthesis of titanium silicalite, TS-1. Zeolites 1992, 12, 943–950. [Google Scholar] [CrossRef]
- Wong, W.C.; Au, L.T.Y.; Lau, P.P.S.; Ariso, C.T.; Yeung, K.L. Effects of synthesis parameters on the zeolite membrane morphology. J. Membr. Sci. 2001, 193, 141–161. [Google Scholar] [CrossRef]
- Huang, A.; Lin, Y.S.; Yang, W. Synthesis and properties of A-type zeolite membranes by secondary growth method with vacuum seeding. J. Membr. Sci. 2004, 245, 41–51. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Liu, H.; Yeung, K.; Wang, J. Preparation of titanium silicalite-1 catalytic films and application as catalytic membrane reactors. Chem. Eng. J. 2010, 156, 562–570. [Google Scholar] [CrossRef]
- Wu, P.; Tatsumi, T. A novel titanosilicate with MWW structure III. Highly efficient and selective production of glycidol through epoxidation of allyl alcohol with H2O2. J. Catal. 2003, 214, 317–326. [Google Scholar] [CrossRef]
- Campanella, A.; Baltanás, M.A.; Capel-Sánchez, M.C.; Campos-Martín, J.M.; Fierro, J.L.G. Soybean oil epoxidation with hydrogen peroxide using an amorphous Ti/SiO2catalyst. Green Chem. 2004, 6, 330–334. [Google Scholar] [CrossRef]
- Chen, C.; Cai, L.; Li, L.; Bao, L.; Lin, Z.; Wu, G. Heterogeneous and non-acid process for production of epoxidized soybean oil from soybean oil using hydrogen peroxide as clean oxidant over TS-1 catalysts. Microporous Mesoporous Mater. 2019, 276, 89–97. [Google Scholar] [CrossRef]

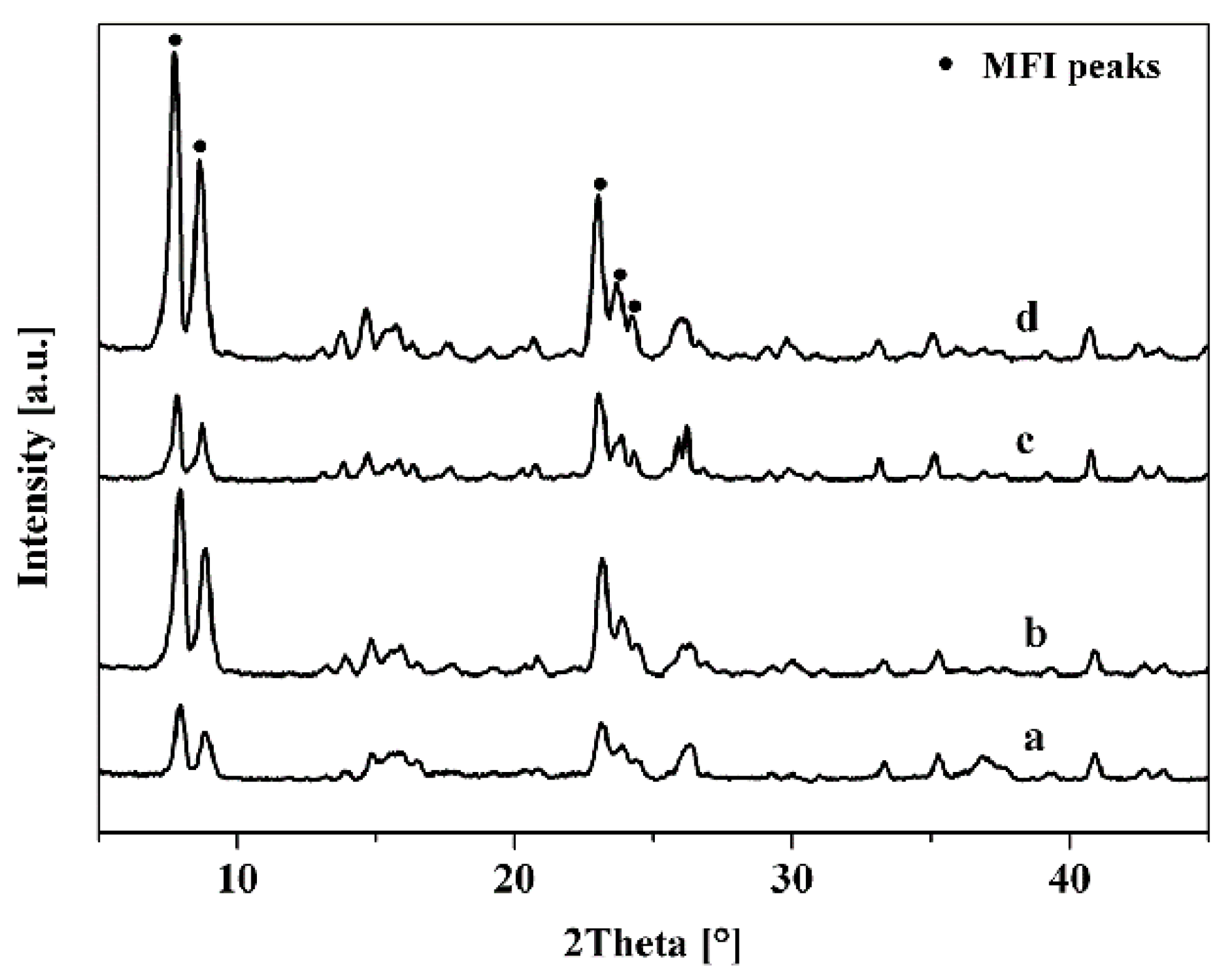
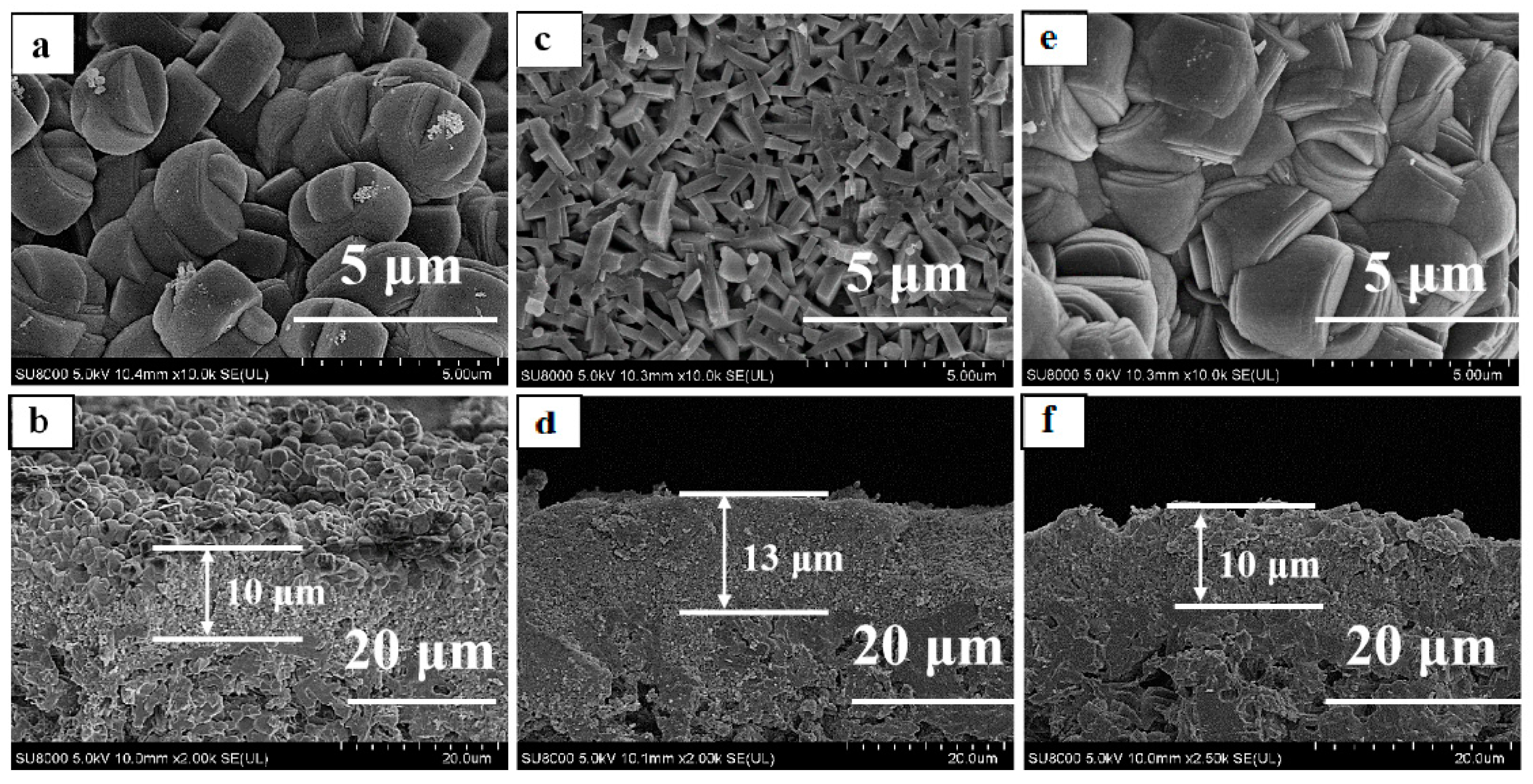
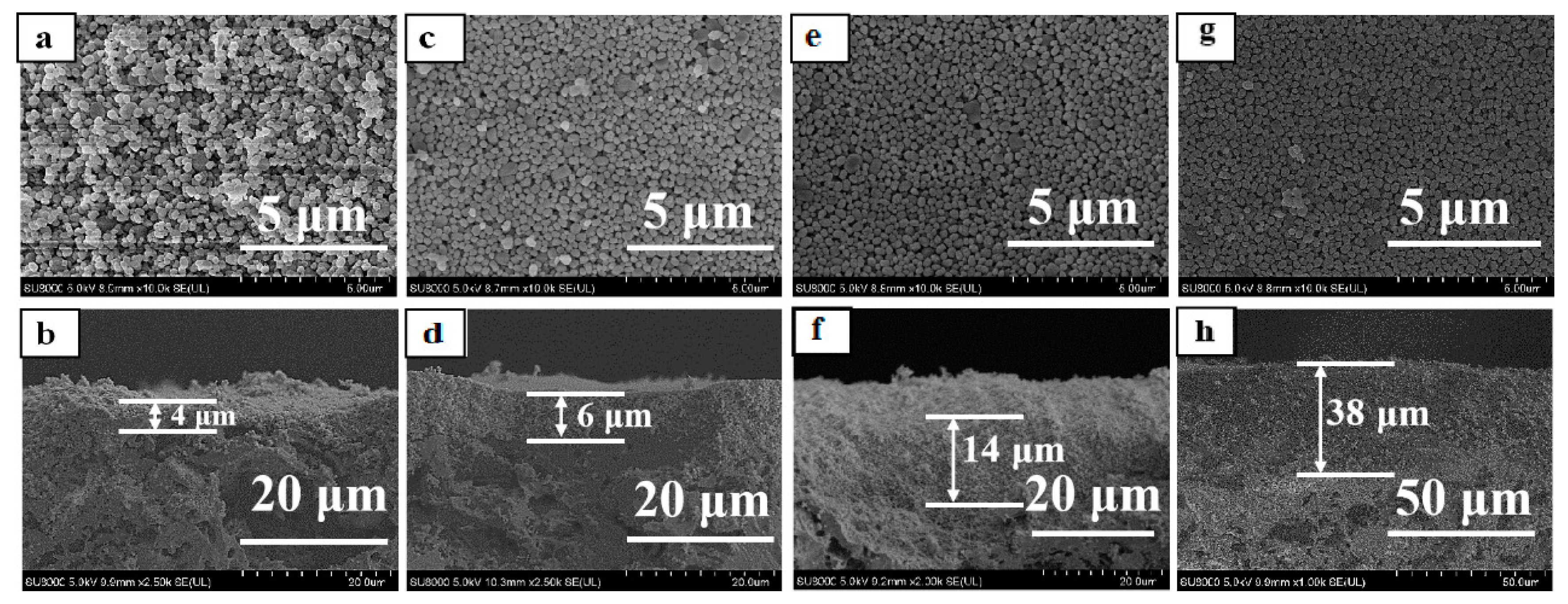
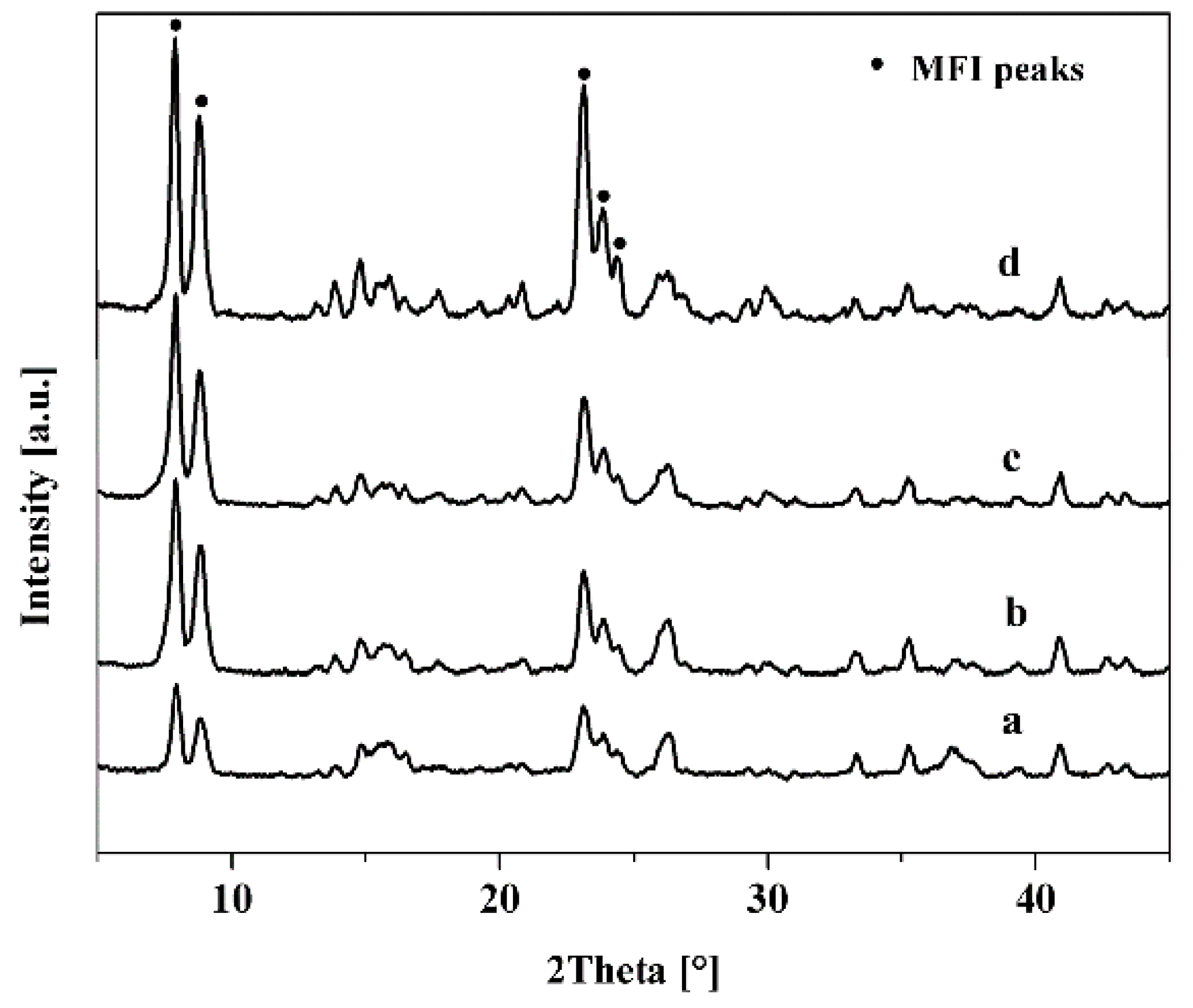

| No. | Morphology | Size 2 (nm) | Ti/Si 1 Ratio |
|---|---|---|---|
| Z-1 | ellipsoid | 230 | 0 |
| Z-2 | ellipsoid | 300 | 0.022 |
| Z-3 | ellipsoid | 300 | 0.030 |
| Z-4 | agglomerate | 540 | 0.030 |
| Z-5 | petals | 540 | 0.030 |
| Z-6 | reunion | 750 | 0.030 |
| Membrane No. | Seed Suspension | Ti/Si Ratio | Q [kg·m−2·h−1] | CIPA [%] | |
|---|---|---|---|---|---|
| Seed No. | Seed Concentration (wt%) | ||||
| M-1 | Z-1 | 5% | 0.024 | 1.79 | 37.78 |
| M-2 | Z-2 | 5% | 0.036 | 1.90 | 81.88 |
| M-3 | Z-3 | 5% | 0.055 | 2.58 | 98.18 |
| M-4 | Z-4 | 5% | 0.027 | 1.84 | 69.02 |
| M-5 | Z-5 | 5% | 0.0319 | 1.97 | 86.70 |
| M-6 | Z-6 | 5% | 0.024 | 2.80 | 64.89 |
| M-7 | Z-3 | 2% | 0.025 | 1.06 | 68.72 |
| M-8 | Z-3 | 3% | 0.035 | 1.29 | 82.56 |
| M-9 | Z-3 | 7% | 0.030 | Leak | Leak |
| M-10 * | Z-3 | 5% | 0.051 | 1.98 | 93.27 |
| Support (g) | Seeded Support (g) | Amount of Seed (g) | Membrane (g) | Amount of Zeolite (g) |
|---|---|---|---|---|
| 9.44 | 10.06 | 0.62 | 10.19 | 0.75 |
| No. | Q (kg·m−2·h−1) | C (%) |
|---|---|---|
| M-3 | 2.58 | 98.18 |
| M-11 | 2.63 | 97.34 |
| M-12 | 2.30 | 93.87 |
| M-13 | 2.48 | 96.64 |
| M-14 | 2.18 | 97.29 |
| Running Times | M-3 | M-10 | ||
|---|---|---|---|---|
| Q (kg·m−2·h−1) | C (%) | Q (kg·m−2·h−1) | C (%) | |
| 1 | 2.58 | 98.23 | 1.98 | 93.27 |
| 2 | 2.45 | 98.18 | 1.82 | 93.04 |
| 3 | 2.34 | 97.91 | 1.62 | 93.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Xiang, S.; Ye, F.; Gui, T.; Li, Y.; Zhang, F.; Hu, N.; Zhu, M.; Chen, X. Effects of Seed Crystals on the Growth and Catalytic Performance of TS-1 Zeolite Membranes. Membranes 2020, 10, 41. https://doi.org/10.3390/membranes10030041
Ding W, Xiang S, Ye F, Gui T, Li Y, Zhang F, Hu N, Zhu M, Chen X. Effects of Seed Crystals on the Growth and Catalytic Performance of TS-1 Zeolite Membranes. Membranes. 2020; 10(3):41. https://doi.org/10.3390/membranes10030041
Chicago/Turabian StyleDing, Wenjuan, Sitong Xiang, Fei Ye, Tian Gui, Yuqin Li, Fei Zhang, Na Hu, Meihua Zhu, and Xiangshu Chen. 2020. "Effects of Seed Crystals on the Growth and Catalytic Performance of TS-1 Zeolite Membranes" Membranes 10, no. 3: 41. https://doi.org/10.3390/membranes10030041
APA StyleDing, W., Xiang, S., Ye, F., Gui, T., Li, Y., Zhang, F., Hu, N., Zhu, M., & Chen, X. (2020). Effects of Seed Crystals on the Growth and Catalytic Performance of TS-1 Zeolite Membranes. Membranes, 10(3), 41. https://doi.org/10.3390/membranes10030041