Permeation Properties of Ions through Inorganic Silica-Based Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Porous Ceramic Substrates
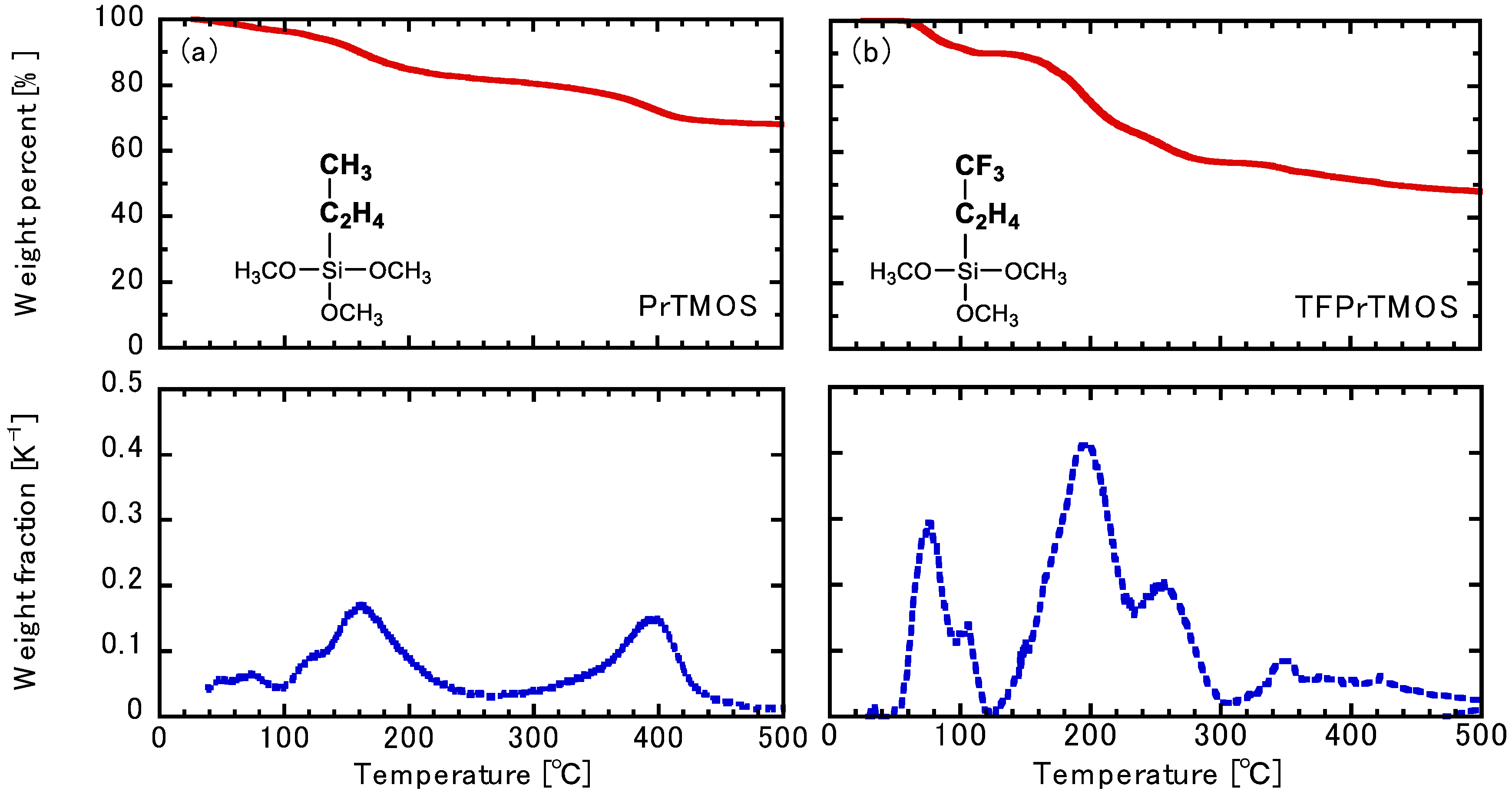
2.2. Thermogravimetric Analysis of the Hydrolysis Silica Powder
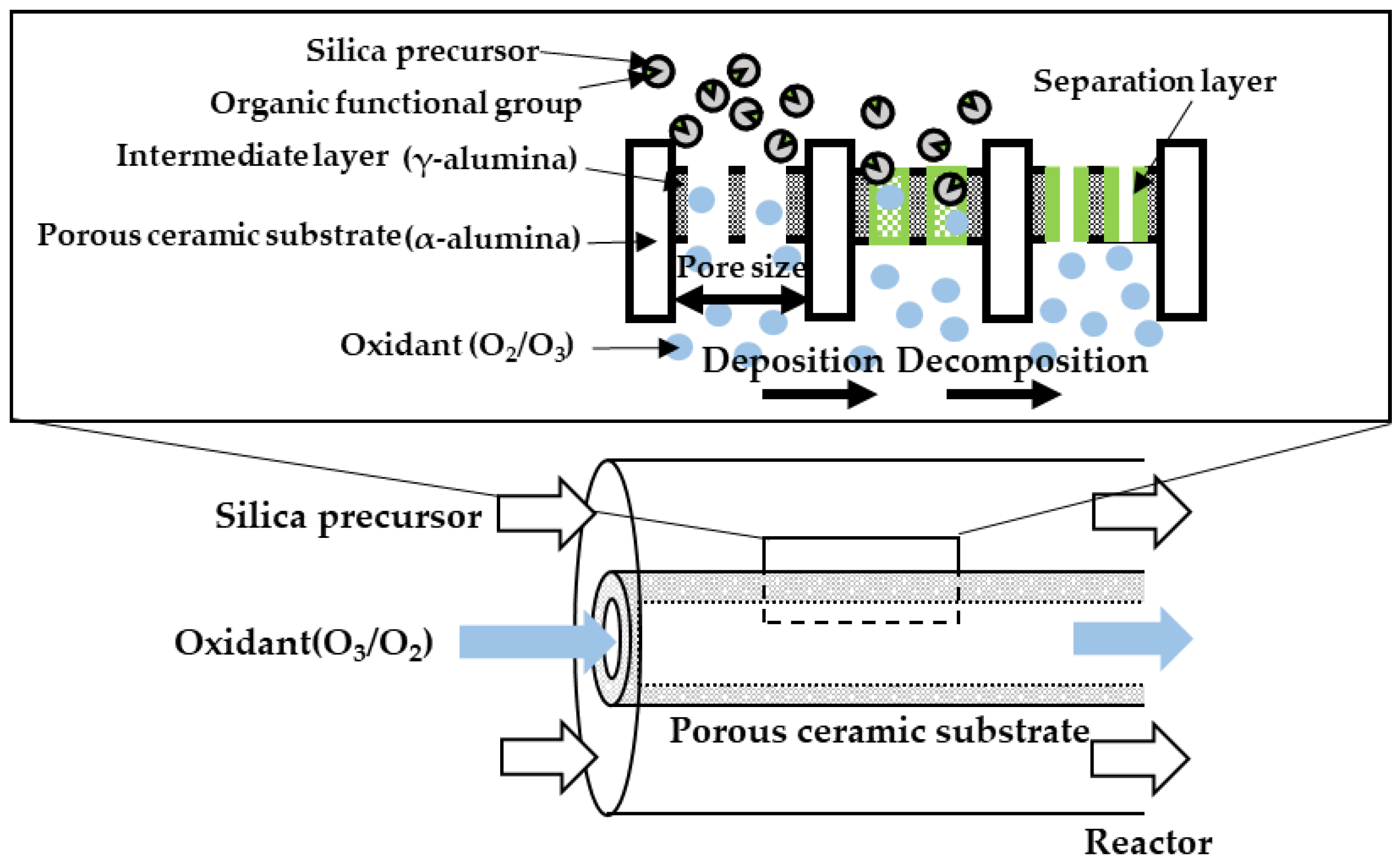
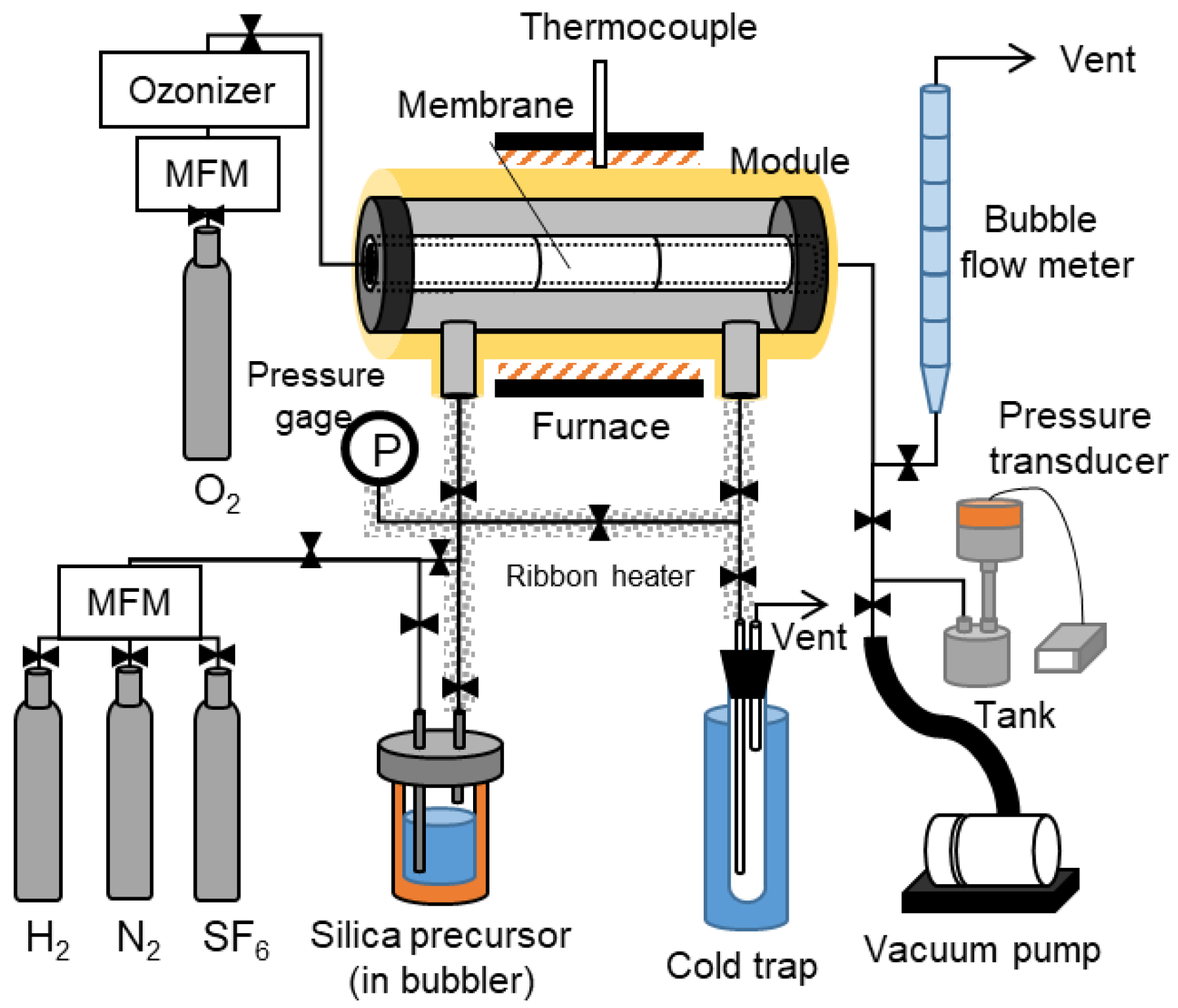
2.3. The Counter Diffusion CVD Method
2.4. Single Gas Permeation Tests
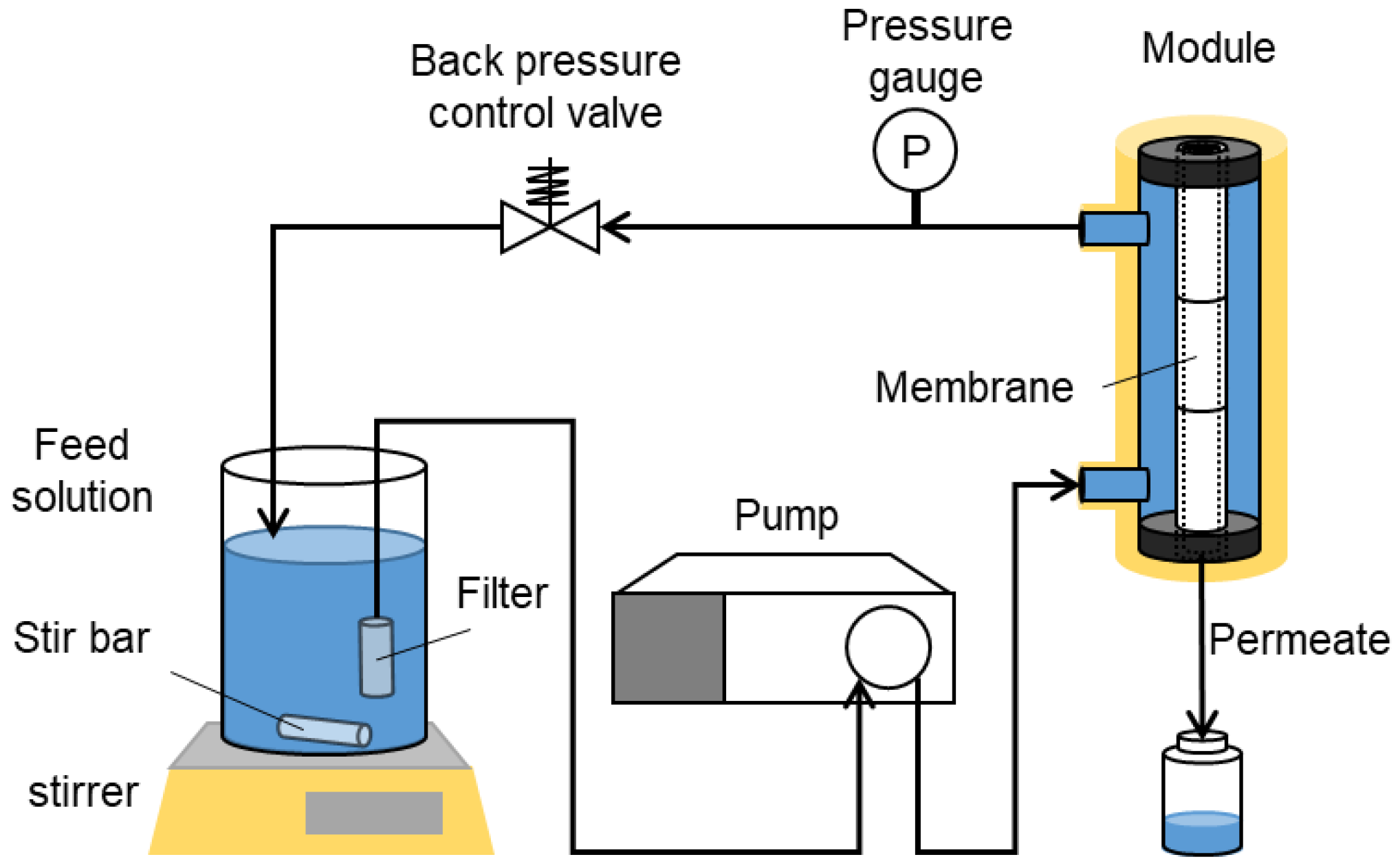
2.5. Liquid Permeation Tests
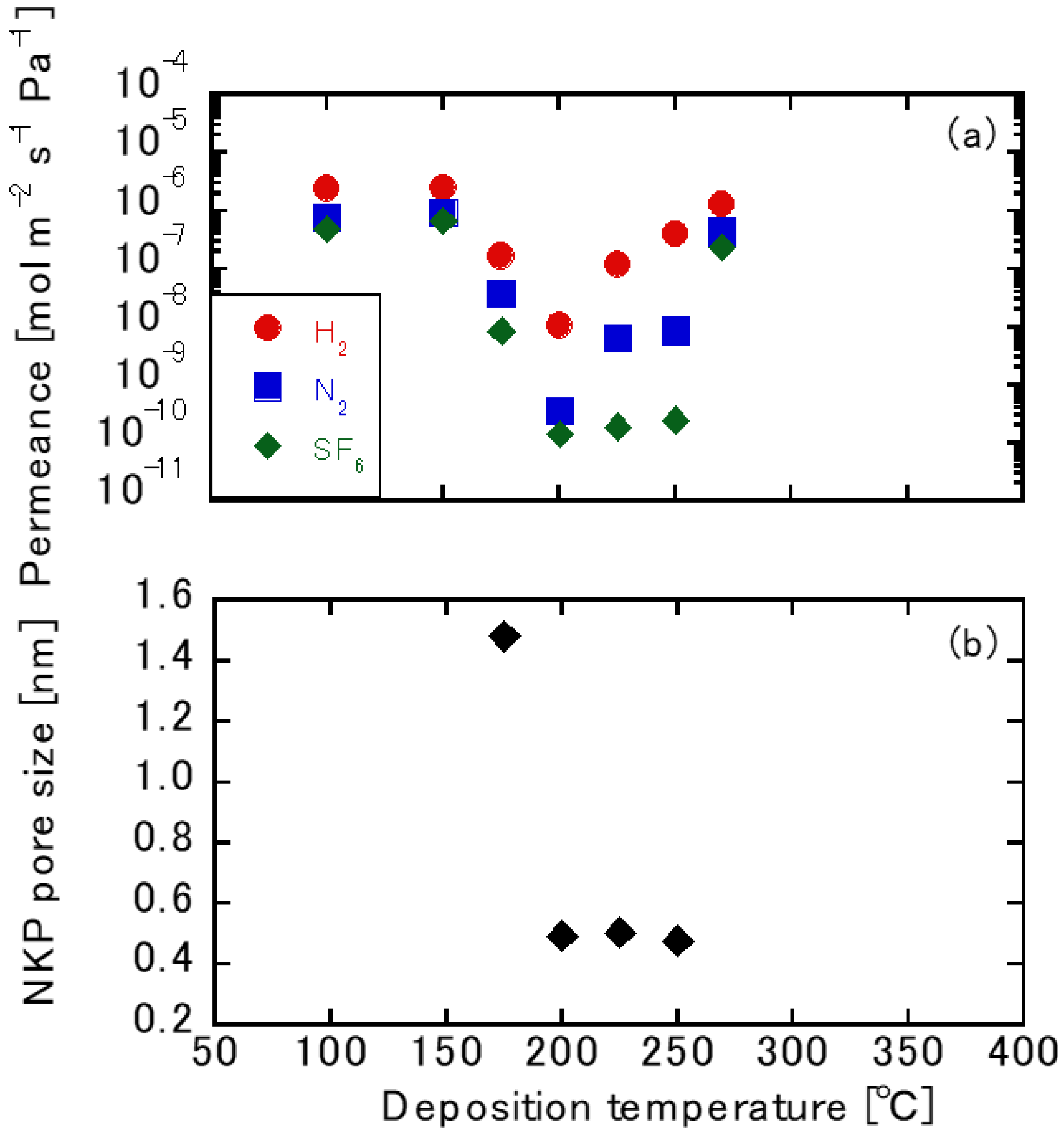
3. Results and Discussion
3.1. Thermogravimetric Analysis
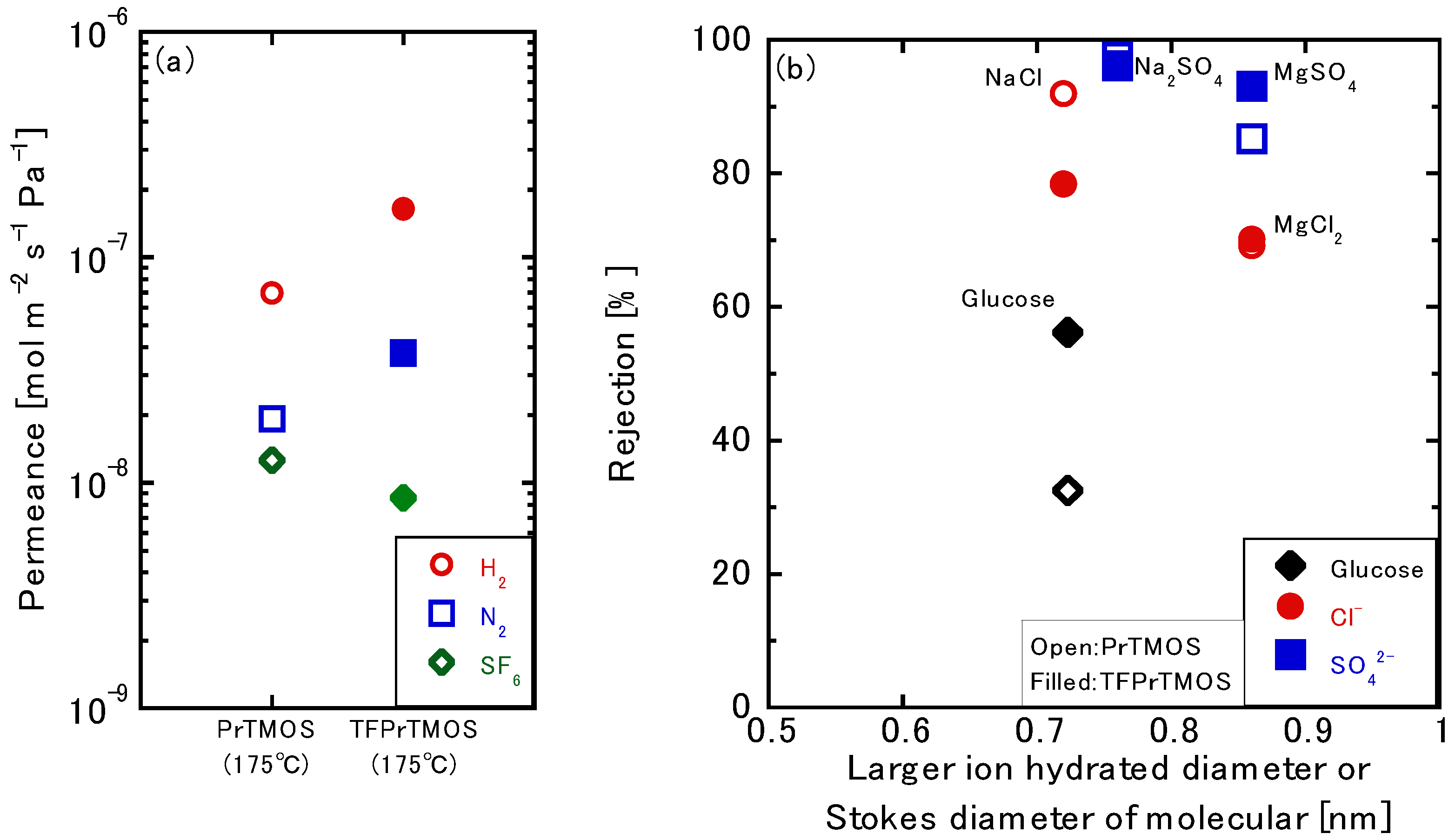
3.2. Gas Permeation Tests using TFPrTMOS-Derived Membranes
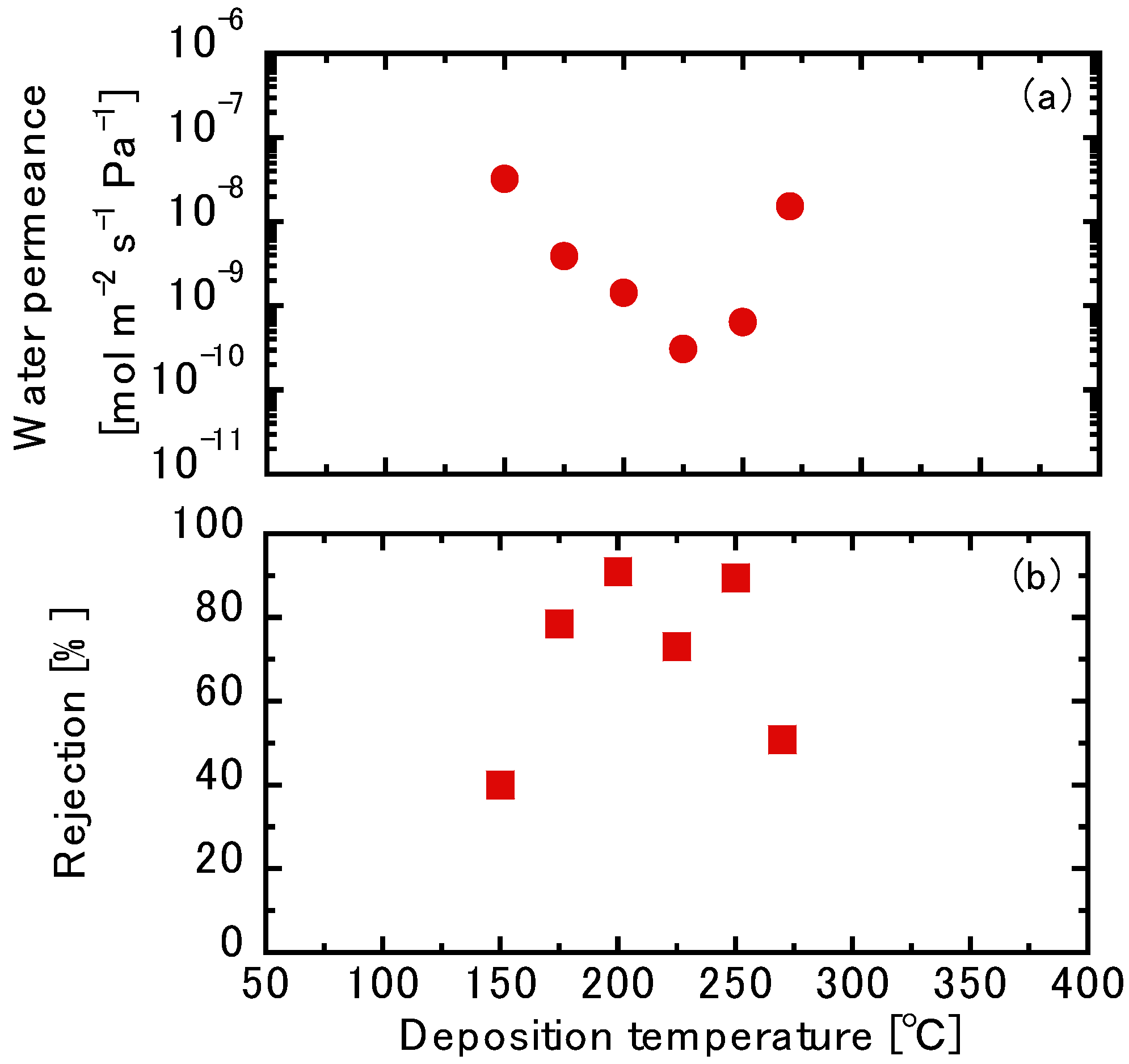
3.3. Liquid Permeation Tests using the TFPrTMOS-Derived Membranes
3.3.1. NaCl Permeation
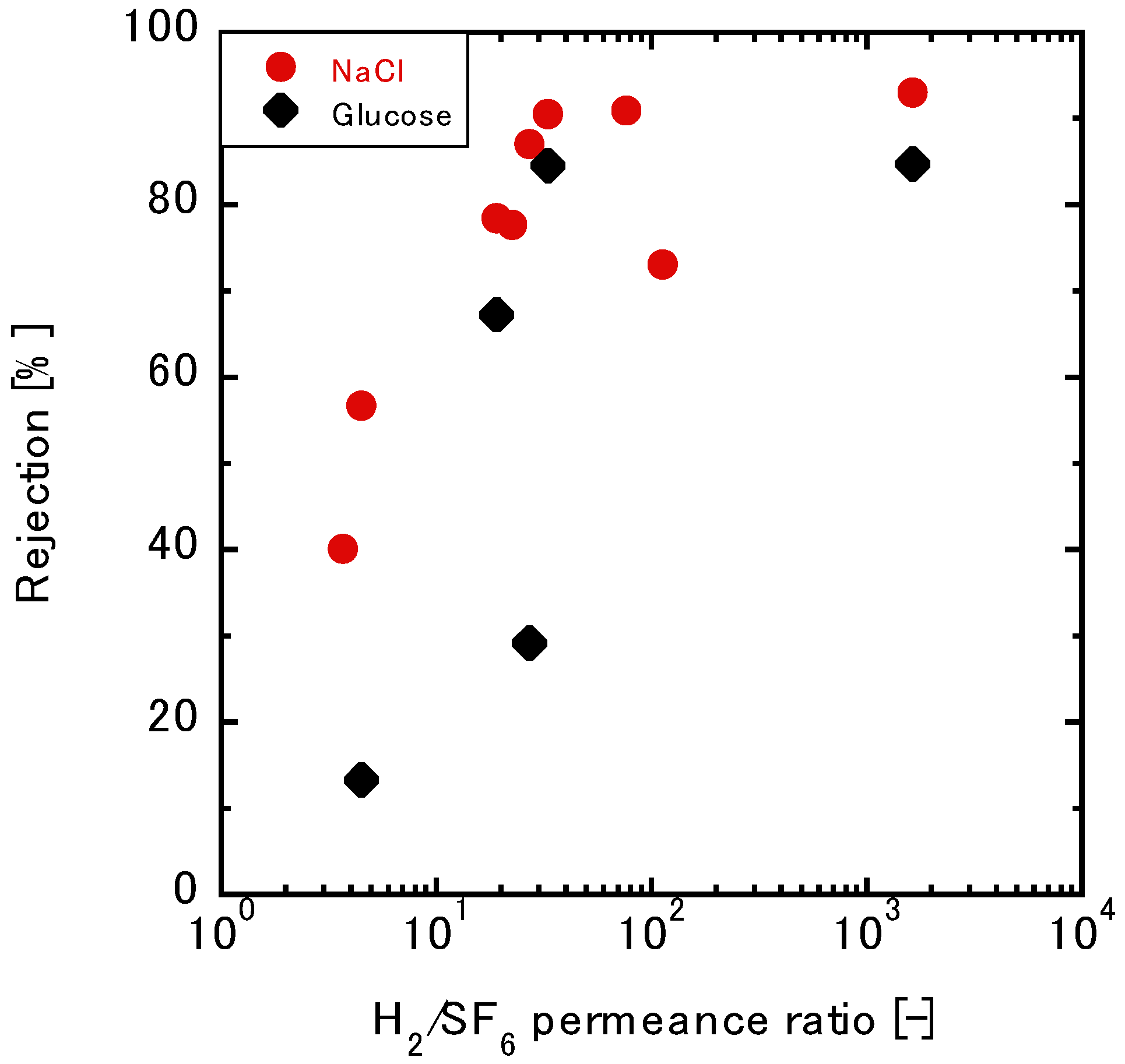
3.3.2. Relationship between the Gas and Liquid Permeation Performance
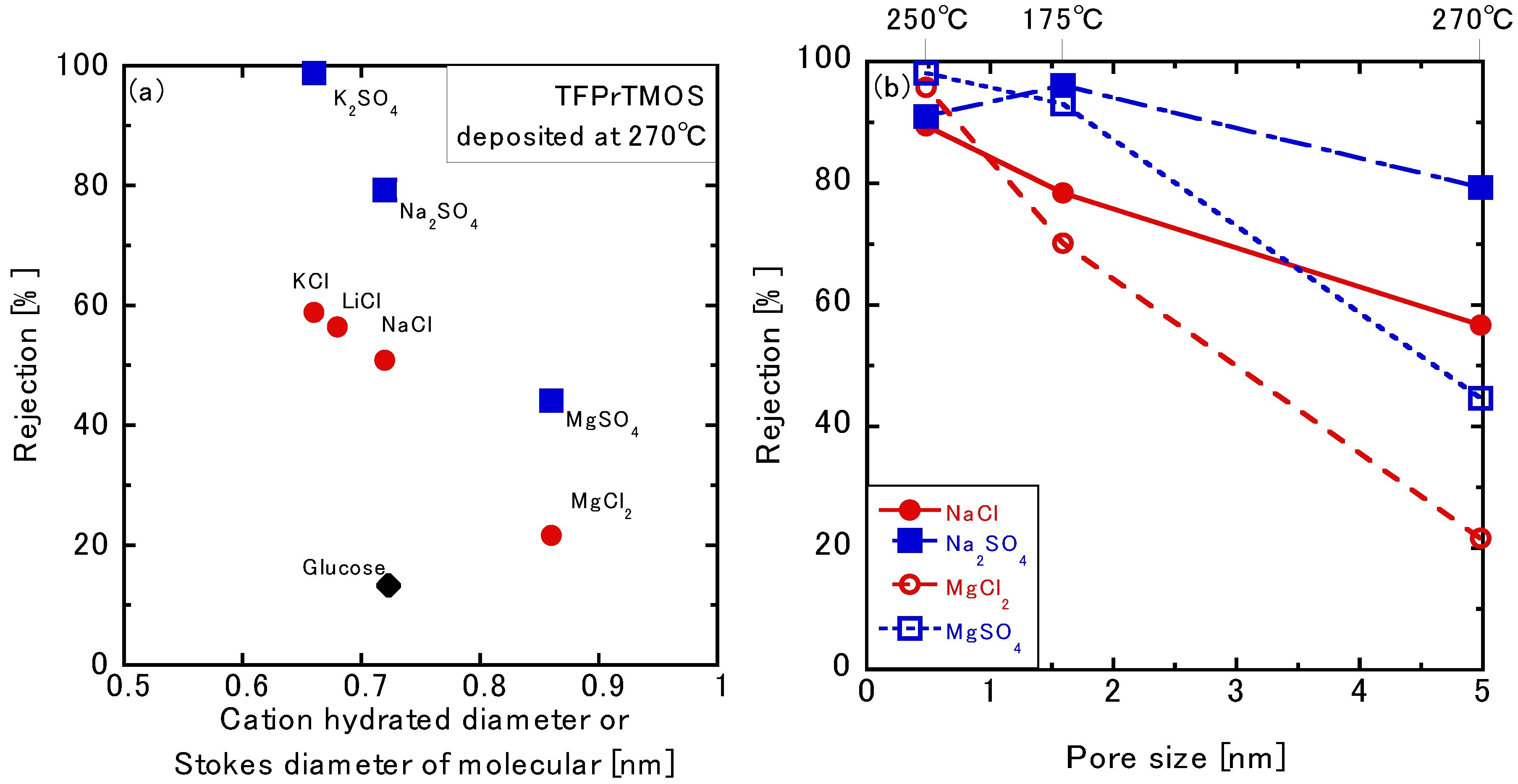
3.3.3. Permeation of the Multivalent Ions
3.3.4. Effects of the Silica Precursors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsuru, T. Inorganic porous membranes for liquid phase separation. Sep. Purif. Technol. 2001, 30, 191–220. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Yuan, B.; Li, P.; Wang, P.; Yang, H.; Sun, H.; Li, P.; Sun, H.; Niu, Q.J. Novel aliphatic polyamide membrane with high mono-/ divalent ion selectivity, excellent Ca2+, Mg2+ rejection, and improved antifouling properties. Sep. Purif. Technol. 2019, 224, 443–455. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Ikarugi, S.; Oura, K.; Ikeda, A.; Matsuyama, E.; Ono, R.; Nomura, M.; Tawarayama, H.; Saito, T.; Kuwahara, K. MFI Zeolite Membranes Prepared on Novel Silica Substrates. J. Chem. Eng. Jpn. 2015, 48, 891–896. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin Selective Ag-Exchanged X-Type Zeolite Membrane for Propylene/Propane and Ethylene/Ethane Separation. ACS Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, X.; Kita, H. Inexpensive synthesis of silicalite-1 membranes with high pervaporation performance. Chem. Lett. 2010, 39, 388–389. [Google Scholar] [CrossRef]
- Kato, H.; Lundin, B.S.; Ahn, S.; Takagaki, A.; Kikuchi, R.; Oyama, T. Gas Separation Silica Membranes Prepared by Chemical Vapor Deposition of Methyl-Substituted Silanes. Membranes 2019, 9, 144. [Google Scholar] [CrossRef]
- Nishida, R.; Tago, T.; Saitoh, T.; Seshimo, M.; Nalao, S. Development of CVD Silica Membranes Having High Hydrogen Permeance and Steam Durability and a Membrane Reactor for a Water Gas Shift Reaction. Membranes 2019, 9, 140. [Google Scholar] [CrossRef]
- Zhu, B.; Morris, G.; Moon, I.; Gray, S.; Duke, M. Diffusion behavior of multivalent ions at low pH through a MFI-type zeolite membrane. Desalination 2018, 440, 88–98. [Google Scholar] [CrossRef]
- Elma, M.; Yacou, C.; Costa, D.C.J.; Wang, K.D. Performance and Long Term Stability of Mesoporous Silica Membranes for Desalination. Membranes 2013, 3, 136–150. [Google Scholar] [CrossRef]
- Xu, R.; Kanezashi, M.; Yoshioka, T.; Okuda, T.; Ohshita, J.; Tsuru, T. Tailoring the affinity of organosilica membranes by introducing polarizable ethenylene bridges and aqueous ozone modification. ACS Appl. Mater. Interfaces 2013, 5, 6147–6154. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Ono, K.; Gopalakrishnan, S.; Sugawara, T.; Nakao, S. Preparation of a stable silica membrane by a counter diffusion chemical vapor deposition method. J. Membr. Sci. 2005, 251, 151–158. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Ikeda, A.; Tanaka, N.; Kubo, S.; Nomura, M. Preparation of an H2-permselective silica membrane for the separation of H2 from the hydrogen iodide decomposition reaction in the iodine-sulfur process. Int. J. Hydrogen Energy 2017, 42, 6012–6023. [Google Scholar] [CrossRef]
- Ishii, K.; Shibata, A.; Takeuchi, T.; Yoshiura, J.; Urabe, T.; Kameda, Y.; Nomura, M. Development of silica membranes to improve dehydration reactions. J. Jpn. Petrol. Inst. 2019, 62, 211–219. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Tanaka, N.; Nomura, M.; Kubo, S. Module design of silica membrane reactor for hydrogen production via thermochemical IS process. Int. J. Hydrogen Energy 2019, 44, 10207–10217. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Tanaka, N.; Nomura, M.; Kubo, S. Hydrogen production tests by hydrogen iodide decomposition membrane reactor equipped with silica-based ceramics membrane. Int. J. Hydrogen Energy 2017, 42, 29091–29100. [Google Scholar] [CrossRef]
- Ikeda, A.; Nomura, M. Preparation of amorphous silica based membranes for separation of hydrocarbons. J. Jpn. Petrol. Inst. 2016, 59, 259–265. [Google Scholar] [CrossRef][Green Version]
- Ikeda, A.; Ono, R.; Nomura, M. High hydrogen permeance silica membranes prepared by a chemical vapor deposition method. J. Membr. Sep. Tech. 2015, 4, 66–73. [Google Scholar]
- Matsuyama, E.; Ikeda, A.; Komatsuzaki, M.; Sasaki, M.; Nomura, M. High temperature propylene/propane separation through silica hybrid membranes. Sep. Purif. Technol. 2014, 128, 25–30. [Google Scholar] [CrossRef]
- Nomura, M.; Momma, K.; Negishi, Y.; Matsuyama, E.; Kimura, S. Preparation of silica hybrid membranes for high temperature gas separation. Desalin. Water Treat. 2010, 17, 288–293. [Google Scholar] [CrossRef]
- Nomura, M.; Nagayo, T.; Monma, K. Pore size control of a molecular sieve silica membrane prepared by a counter diffusion CVD method. J. Chem. Eng. Jpn. 2007, 40, 1235–1241. [Google Scholar] [CrossRef]
- Ikeda, A.; Matsuyama, E.; Komatsuzaki, M.; Sasaki, M.; Nomura, M. Development of inorganic silica reverse osmosis membranes by using a counter-diffusion chemical vapor deposition method. J. Chem. Eng. Jpn. 2014, 47, 574–578. [Google Scholar] [CrossRef]
- Ishii, K.; Ikeda, A.; Takeuchi, T.; Yoshiura, J.; Nomura, M. Silica-based RO membranes for separation of acidic solution. Membranes 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, E.R., Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Lee, H.R.; Kanezashi, M.; Shimomura, Y.; Yoshioka, T.; Tsuru, T. Evaluation and fabricatioin of pore-size-tuned silica membranes with tetraethoxydimethyl disiloxane for gas separation. AlChE J. 2011, 57, 2755–2765. [Google Scholar] [CrossRef]
- Tsuru, T. Ion Separation by Charged Membranes in Reverse Osmosis. Membrane 1995, 20, 423–429. [Google Scholar] [CrossRef]
| Solute | Manufacturer and Purity | MW [g mol−1] |
|---|---|---|
| D-Glucose | FUJIFILM Wako Pure Chemicals Co., Ltd. | 180.16 |
| NaCl | Sigma-Aldrich, 99.98% trace metals basis | 74.55 |
| Na2SO4 | Merck, ≥99.0% | 142.04 |
| MgCl2 | Merck, ≥99.0% | 95.21 |
| MgSO4 | Sigma-Aldrich, ACS reagent, ≥98% | 120.37 |
| Cation | Hydrated Diameter [nm] | Anion | Hydrated Diameter [nm] |
|---|---|---|---|
| Na+ | 0.72 | Cl− | 0.66 |
| Mg2+ | 0.86 | SO42− | 0.76 |
| Membranes | Deposition Temperature [°C] | The Order of ion Rejections | Glucose Rejection [%] | Pore Size [nm] |
|---|---|---|---|---|
| TFPrTMOS | 175 | Na2SO4 > MgSO4 > NaCl > MgCl2 | 67.2 | 1.48 |
| 250 | MgSO4 > MgCl2 > Na2SO4 > NaCl | 84.7 | 0.48 | |
| 270 | Na2SO4 > NaCl > MgSO4 > MgCl2 | 13.3 | 1.48< | |
| PrTMOS | 175 | Na2SO4 > NaCl > MgSO4 > MgCl2 | 35.9 | 1.48< |
| Polymeric membrane [26] | - | Na2SO4 > MgSO4 > NaCl > MgCl2 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshiura, J.; Ishii, K.; Saito, Y.; Nagataki, T.; Nagataki, Y.; Ikeda, A.; Nomura, M. Permeation Properties of Ions through Inorganic Silica-Based Membranes. Membranes 2020, 10, 27. https://doi.org/10.3390/membranes10020027
Yoshiura J, Ishii K, Saito Y, Nagataki T, Nagataki Y, Ikeda A, Nomura M. Permeation Properties of Ions through Inorganic Silica-Based Membranes. Membranes. 2020; 10(2):27. https://doi.org/10.3390/membranes10020027
Chicago/Turabian StyleYoshiura, Junko, Katsunori Ishii, Yuta Saito, Takaya Nagataki, Yuhei Nagataki, Ayumi Ikeda, and Mikihiro Nomura. 2020. "Permeation Properties of Ions through Inorganic Silica-Based Membranes" Membranes 10, no. 2: 27. https://doi.org/10.3390/membranes10020027
APA StyleYoshiura, J., Ishii, K., Saito, Y., Nagataki, T., Nagataki, Y., Ikeda, A., & Nomura, M. (2020). Permeation Properties of Ions through Inorganic Silica-Based Membranes. Membranes, 10(2), 27. https://doi.org/10.3390/membranes10020027





