Correlation between the Porosity and Permeability of a Polymer Filter Fabricated via CO2-Assisted Polymer Compression
Abstract
1. Introduction
2. Materials and Methods
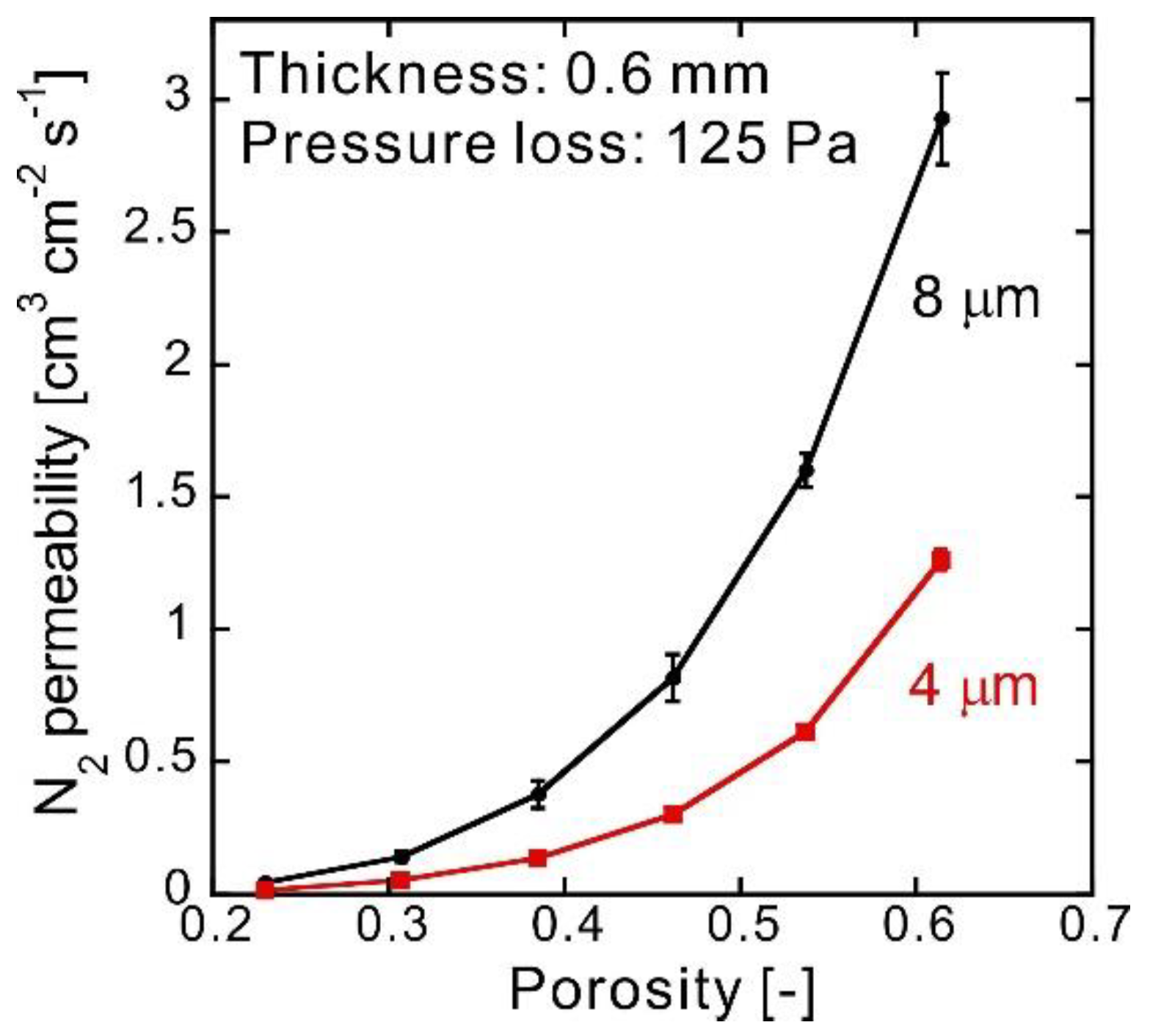
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Young, R.J.; Lovell, P.A. Introduction to Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-0849339295. [Google Scholar]
- Ishizaki, K.; Komarneni, S.; Nanko, M. Porous Materials—Process Technology and Applications; Springer Science + Business Media: Dordrecht, NL, USA, 2014; ISBN 978-1461376637. [Google Scholar]
- Russell, S.J. Handbook of Nonwovens; Woodhead Publishing: Cambridge, UK, 2007; ISBN 978-1855736030. [Google Scholar]
- Hutten, I.M. Handbook of Nonwoven Filter Media, 2nd ed.; Butterworth-Heinemann: Waltham, MA, USA, 2015; ISBN 978-0080983011. [Google Scholar]
- Xu, H.; Jin, W.; Wang, F.; Li, C.; Wang, J.; Zhu, H.; Guo, Y. Preparation and properties of PTFE hollow fiber membranes for the removal of ultrafine particles in PM2.5 with repetitive usage capability. RSC Adv. 2018, 8, 38245–38258. [Google Scholar] [CrossRef]
- Liang, W.; Xu, Y.; Li, X.; Wang, X.-X.; Zhang, H.-D.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Transparent polyurethane nanofiber air filter for high-efficiency PM2.5 Capture. Nanoscale Res. Lett. 2019, 14, 361. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cai, M.; Yang, X.; Yang, Y. Electret nanofibrous membrane with enhanced filtration performance and wearing comfortability for face mask. J. Colloid Interface Sci. 2018, 530, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Gong, H.; Zhang, X.; Wang, Y.; Jin, X. Polyethylene/polypropylene bicomponent spunbond air filtration materials containing magnesium stearate for efficient fine particle capture. ACS Appl. Mater. Interfaces 2019, 11, 40592–40601. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Tan, Z. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262. [Google Scholar] [CrossRef]
- Lian, Z.; Epstein, S.A.; Blenk, C.W.; Shine, A.D. Carbon dioxide-induced melting point depression of biodegradable semicrystalline polymers. J. Supercrit. Fluids 2006, 39, 107–117. [Google Scholar] [CrossRef]
- Kikic, I. Polymer–supercritical fluid interactions. J. Supercrit. Fluids 2009, 47, 458–465. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Su, J.-H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of supercritical carbon dioxide with polymers. I. Crystalline polymers. J. Appl. Polym. Sci. 1996, 59, 695–705. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Su, J.-H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of supercritical carbon dioxide with polymers. II. Amorphous polymers. J. Appl. Polym. Sci. 1996, 59, 707–717. [Google Scholar] [CrossRef]
- Li, M.; Huang, X.; Liu, H.; Liu, B.; Wu, Y.; Wang, L. Solubility prediction of supercritical carbon dioxide in 10 polymers using radial basis function artificial neural network based on chaotic self-adaptive particle swarm optimization and K-harmonic means. RSC Adv. 2015, 5, 45520–45527. [Google Scholar] [CrossRef]
- Aizawa, T. A new method for producing porous polymer materials using carbon dioxide and a piston. J. Supercrit. Fluids 2018, 133, 38–41. [Google Scholar] [CrossRef]
- Aizawa, T. Peel and penetration resistance of porous polyethylene terephthalate material produced by CO2-assisted polymer compression. Molecules 2019, 24, 1384. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T. Fabrication of porosity-controlled polyethylene terephthalate porous materials using a CO2-assisted polymer compression method. RSC Adv. 2018, 8, 3061–3068. [Google Scholar] [CrossRef]
- Wakui, Y.; Aizawa, T. Analysis of sustained release behavior of drug-containing tablet prepared by CO2-assisted polymer compression. Polymers 2018, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T. Process development of CO2-assisted polymer compression for high productivity: Improving equipment and the challenge of numbering-up. Technologies 2019, 7, 39. [Google Scholar] [CrossRef]
- Aizawa, T. Novel strategy for fabricating multi-layer porous membranes with varying porosity. ACS Omega 2020, 5, 24461–24466. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Martín, A.; Orihuela, M.P.; Becerra, J.A.; Martínez-Fernández, J.; Ramírez-Rico, J.T. Permeability and mechanical integrity of porous biomorphic SiC ceramics for application as hot-gas filters. Mater. Des. 2016, 107, 450–460. [Google Scholar] [CrossRef]
- Innocentini, M.D.M.; Pardo, A.R.F.; Menegazzo, B.A.; Bittencourt, L.R.M. Permeability of high-alumina refractory castables based on various hydraulic binders. J. Am. Ceram. Soc. 2002, 85, 1517–1521. [Google Scholar] [CrossRef]
- Kim, J.; Ha, J.-H.; Lee, J.; Song, I.-H. Effect of pore structure on gas permeability constants of porous alumina. Ceram. Int. 2019, 45, 5231–5239. [Google Scholar] [CrossRef]
- Li, X.; Yao, D.; Zuo, K.; Xia, Y.; Yin, J.; Liang, H.; Zeng, Y.-P. Fabrication, microstructural characterization and gas permeability behavior of porous silicon nitride ceramics with controllable pore structures. J. Eur. Ceram. Soc. 2019, 39, 2855–2861. [Google Scholar] [CrossRef]
- Simão, L.; Caldato, R.F.; Innocentini, M.D.M.; Montedo, O.R.K. Permeability of porous ceramic based on calcium carbonate as pore generating agent. Ceram. Int. 2015, 41, 4782–4788. [Google Scholar] [CrossRef]
- Simão, L.; Montedo, O.R.K.; Caldato, R.F.; Innocentini, M.D.M.; Paula, M.M.D.; Angioletto, E.; Dal-Bó, A.G.; da Silva, L. Porous ceramic structures obtained from calcium carbonate as pore generating agent. Mater. Sci. Forum 2014, 775–776, 755–760. [Google Scholar] [CrossRef]
- Dey, A.; Kayal, N.; Chakrabarti, O.; Caldato, R.F.; Andre, C.M.; Innocentini, M.D.M. Permeability and nanoparticle filtration assessment of cordierite-bonded porous SiC ceramics. Ind. Eng. Chem. Res. 2013, 52, 18362–18372. [Google Scholar] [CrossRef]


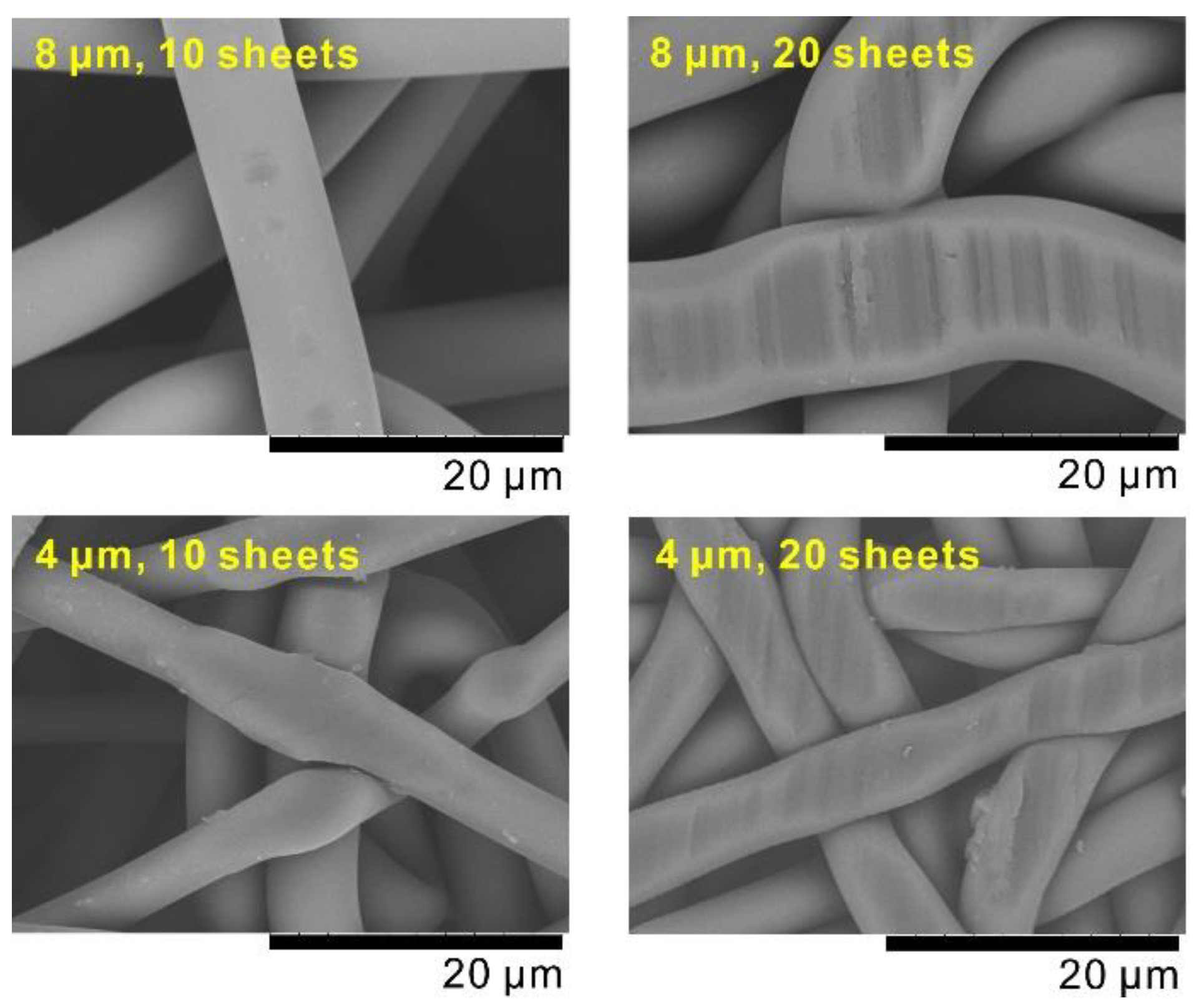
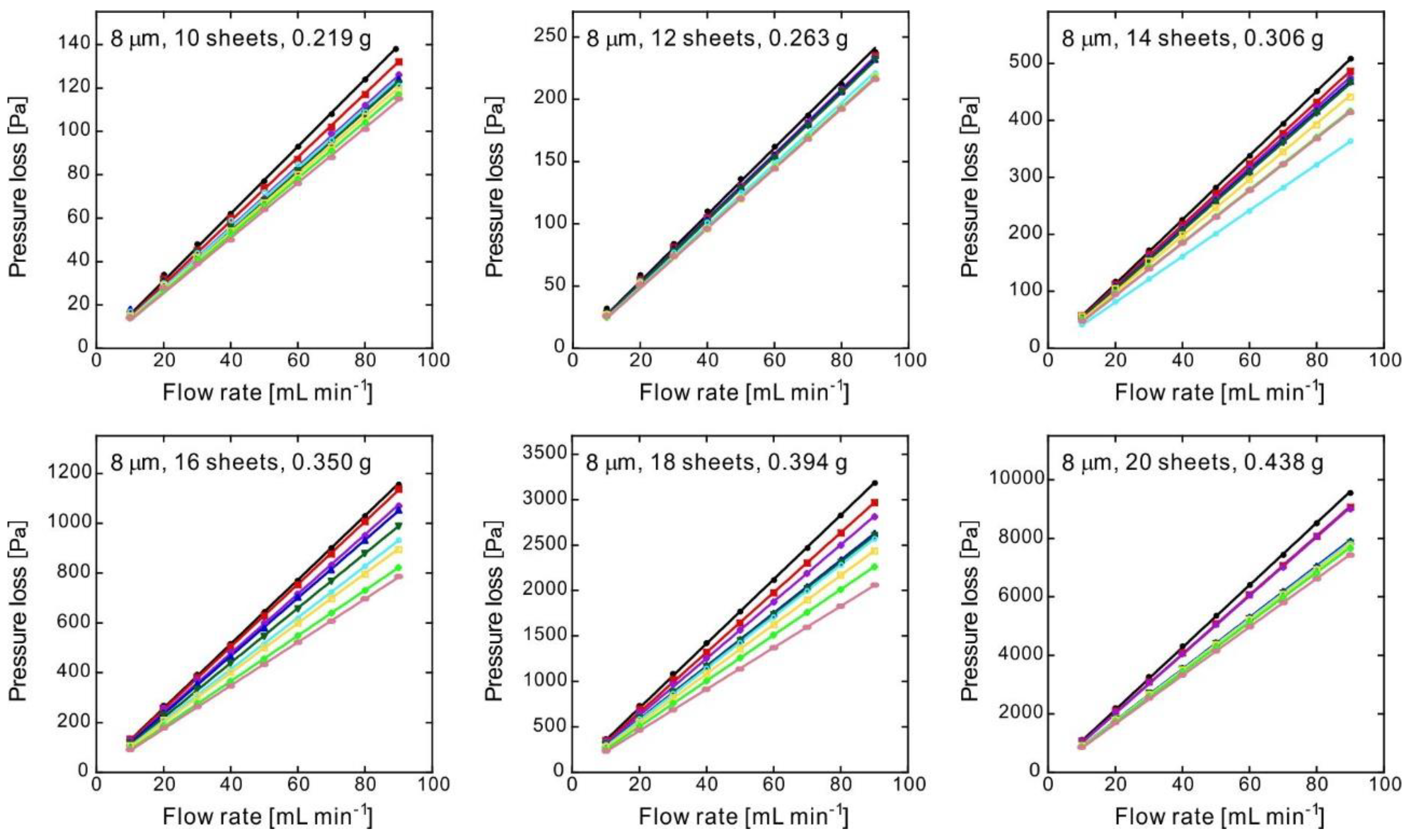
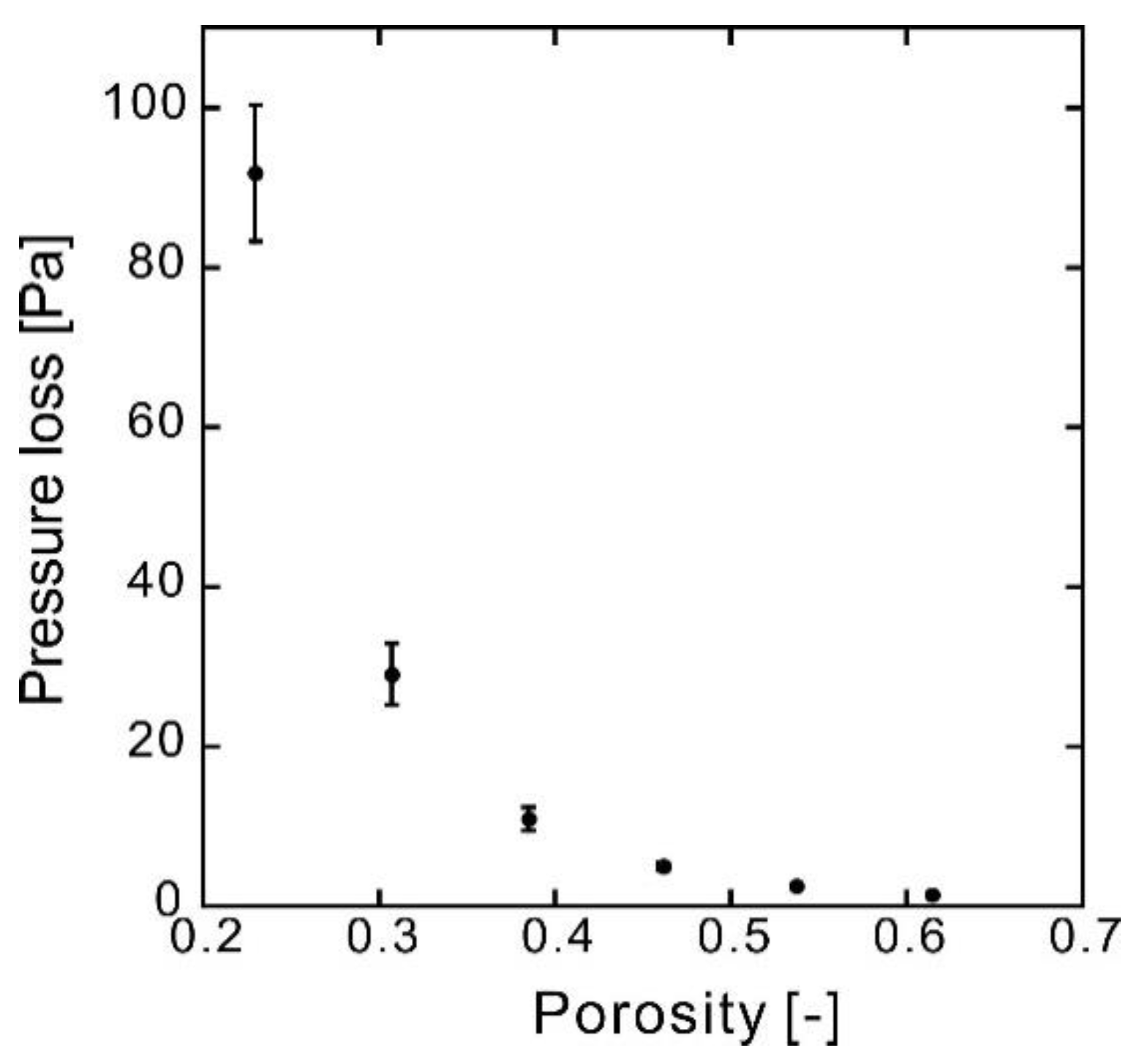
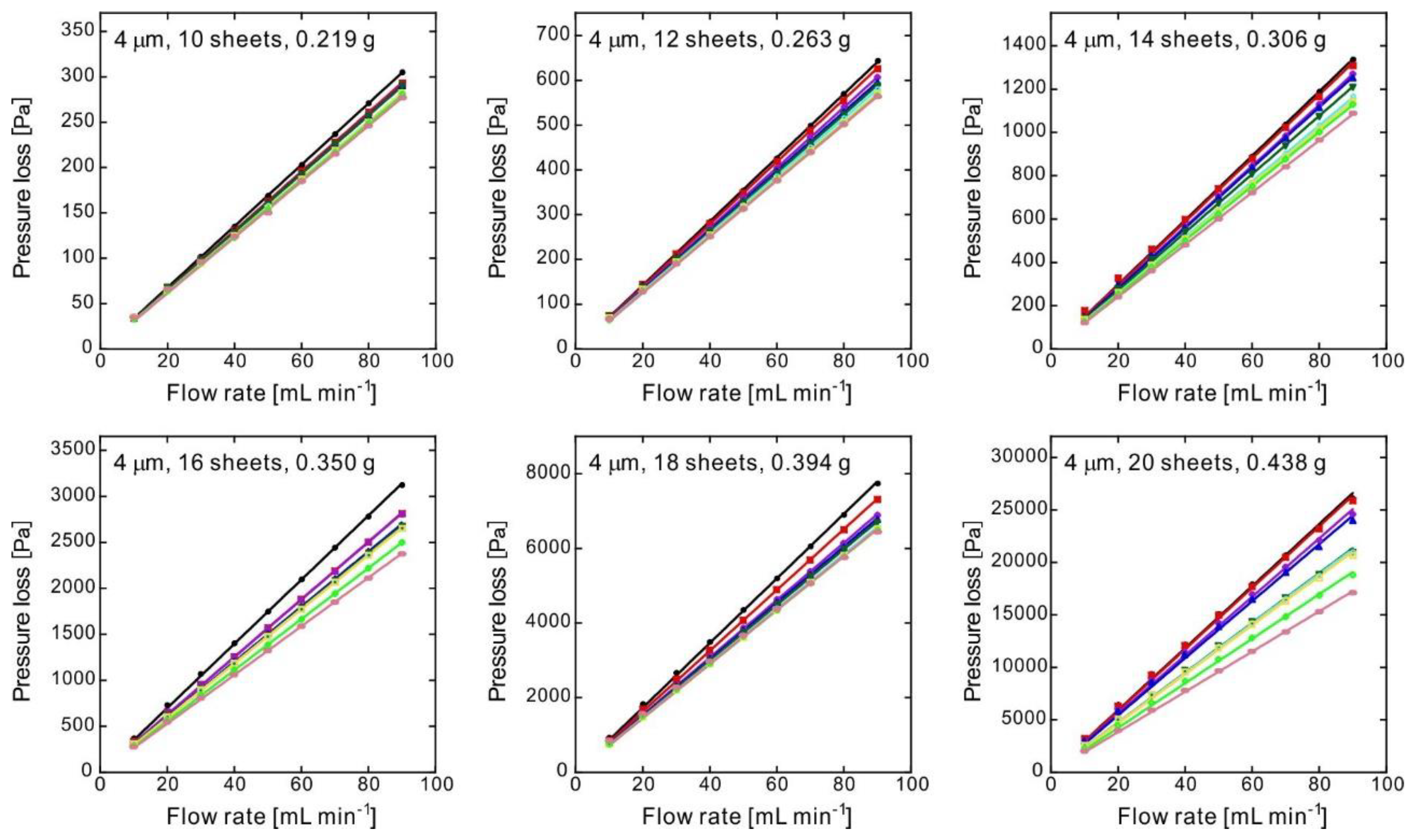
| Number of Sheets [-] | Weight [g] | Thickness [mm] | Porosity [-] | Pressure Loss at 1 mL min−1 (ΔP) [Pa] | Standard Deviation of ΔP [Pa] | Darcian Permeability Coefficient [m2] | Pore Diameter [μm] |
|---|---|---|---|---|---|---|---|
| 10 | 0.219 | 0.600 | 0.615 | 1.38 | 0.08 | 2.6 × 10−12 | 17 |
| 12 | 0.263 | 0.600 | 0.537 | 2.53 | 0.10 | 1.4 × 10−12 | 13 |
| 14 | 0.306 | 0.600 | 0.462 | 4.99 | 0.49 | 7.3 × 10−13 | 10 |
| 16 | 0.350 | 0.601 | 0.385 | 10.9 | 1.5 | 3.4 × 10−13 | 7.6 |
| 18 | 0.394 | 0.601 | 0.307 | 19.1 | 3.8 | 1.3 × 10−13 | 5.2 |
| 20 | 0.438 | 0.601 | 0.230 | 91.8 | 8.5 | 3.9 × 10−14 | 3.4 |
| Number of Sheets [-] | Weight [g] | Thickness [mm] | Porosity [-] | Pressure Loss at 1 mL min−1 (ΔP) [Pa] | Standard Deviation of ΔP [Pa] | Darcian Permeability Coefficient [m2] | Pore Diameter [μm] |
|---|---|---|---|---|---|---|---|
| 10 | 0.219 | 0.600 | 0.614 | 3.20 | 0.10 | 1.1 × 10−12 | 11 |
| 12 | 0.263 | 0.600 | 0.537 | 6.60 | 0.30 | 5.4 × 10−13 | 8.2 |
| 14 | 0.306 | 0.600 | 0.462 | 13.5 | 1.0 | 2.7 × 10−13 | 6.2 |
| 16 | 0.350 | 0.601 | 0.385 | 30.1 | 2.4 | 1.2 × 10−13 | 4.6 |
| 18 | 0.394 | 0.600 | 0.306 | 76.2 | 4.9 | 4.7 × 10−14 | 3.2 |
| 20 | 0.438 | 0.601 | 0.230 | 250 | 37 | 1.5 × 10−14 | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aizawa, T.; Wakui, Y. Correlation between the Porosity and Permeability of a Polymer Filter Fabricated via CO2-Assisted Polymer Compression. Membranes 2020, 10, 391. https://doi.org/10.3390/membranes10120391
Aizawa T, Wakui Y. Correlation between the Porosity and Permeability of a Polymer Filter Fabricated via CO2-Assisted Polymer Compression. Membranes. 2020; 10(12):391. https://doi.org/10.3390/membranes10120391
Chicago/Turabian StyleAizawa, Takafumi, and Yoshito Wakui. 2020. "Correlation between the Porosity and Permeability of a Polymer Filter Fabricated via CO2-Assisted Polymer Compression" Membranes 10, no. 12: 391. https://doi.org/10.3390/membranes10120391
APA StyleAizawa, T., & Wakui, Y. (2020). Correlation between the Porosity and Permeability of a Polymer Filter Fabricated via CO2-Assisted Polymer Compression. Membranes, 10(12), 391. https://doi.org/10.3390/membranes10120391





