Preparation of a Hybrid Membrane from Whey Protein Fibrils and Activated Carbon to Remove Mercury and Chromium from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Protein Fibrils
2.3. Preparation of Hybrid Membranes
2.4. Characterization of Protein Fibrils and Hybrid Membranes
2.5. Removal Efficiency of Heavy Metals from Water
2.6. Statistical Analysis
3. Results
3.1. Preparation of Whey Protein Fibrils
3.2. Characterization of Protein Fibrils and Hybrid Membranes
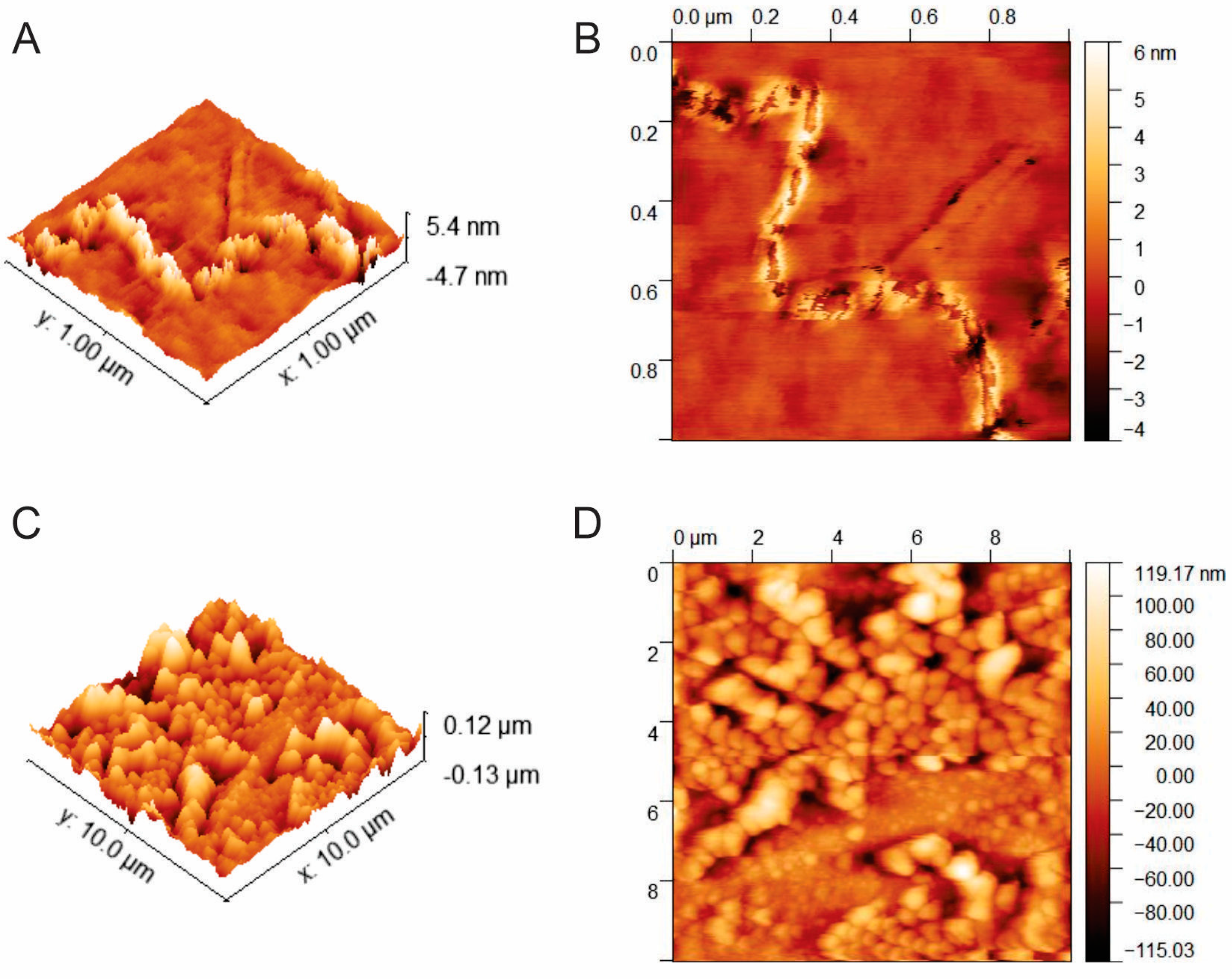
3.2.1. AFM Characterization of Whey Protein Aggregates
3.2.2. SEM Characterization of Hybrid Membrane of Whey Protein and Activated Carbon
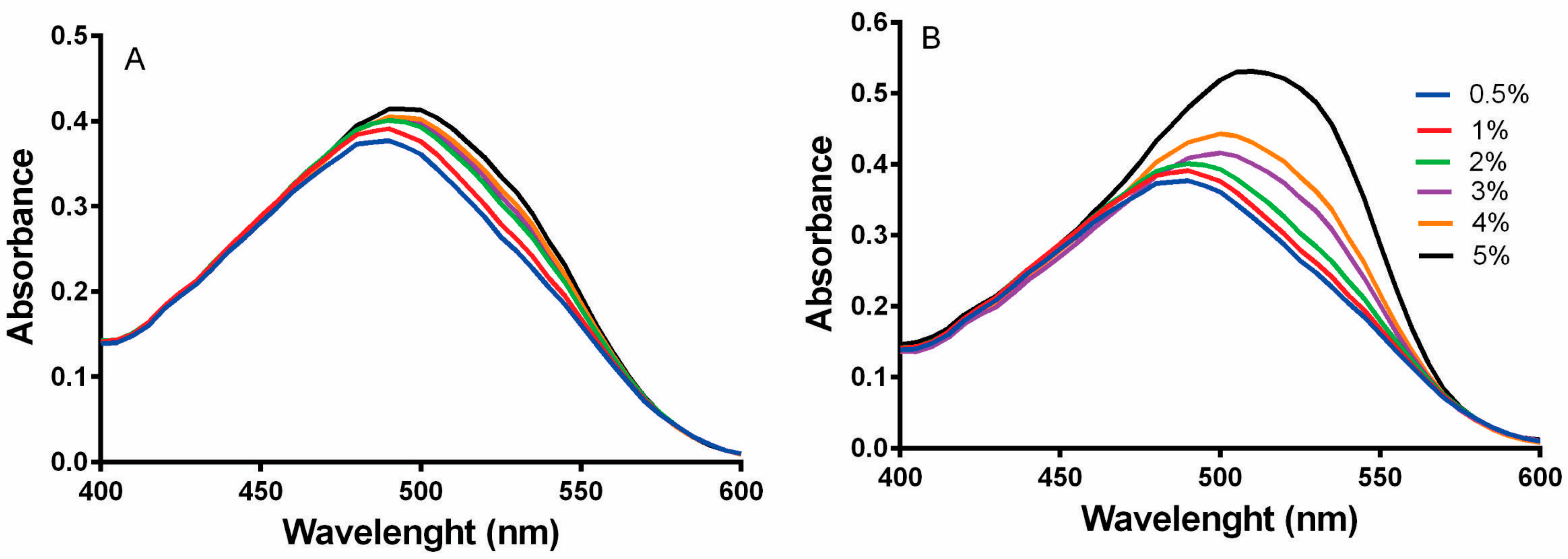
3.2.3. Red Congo
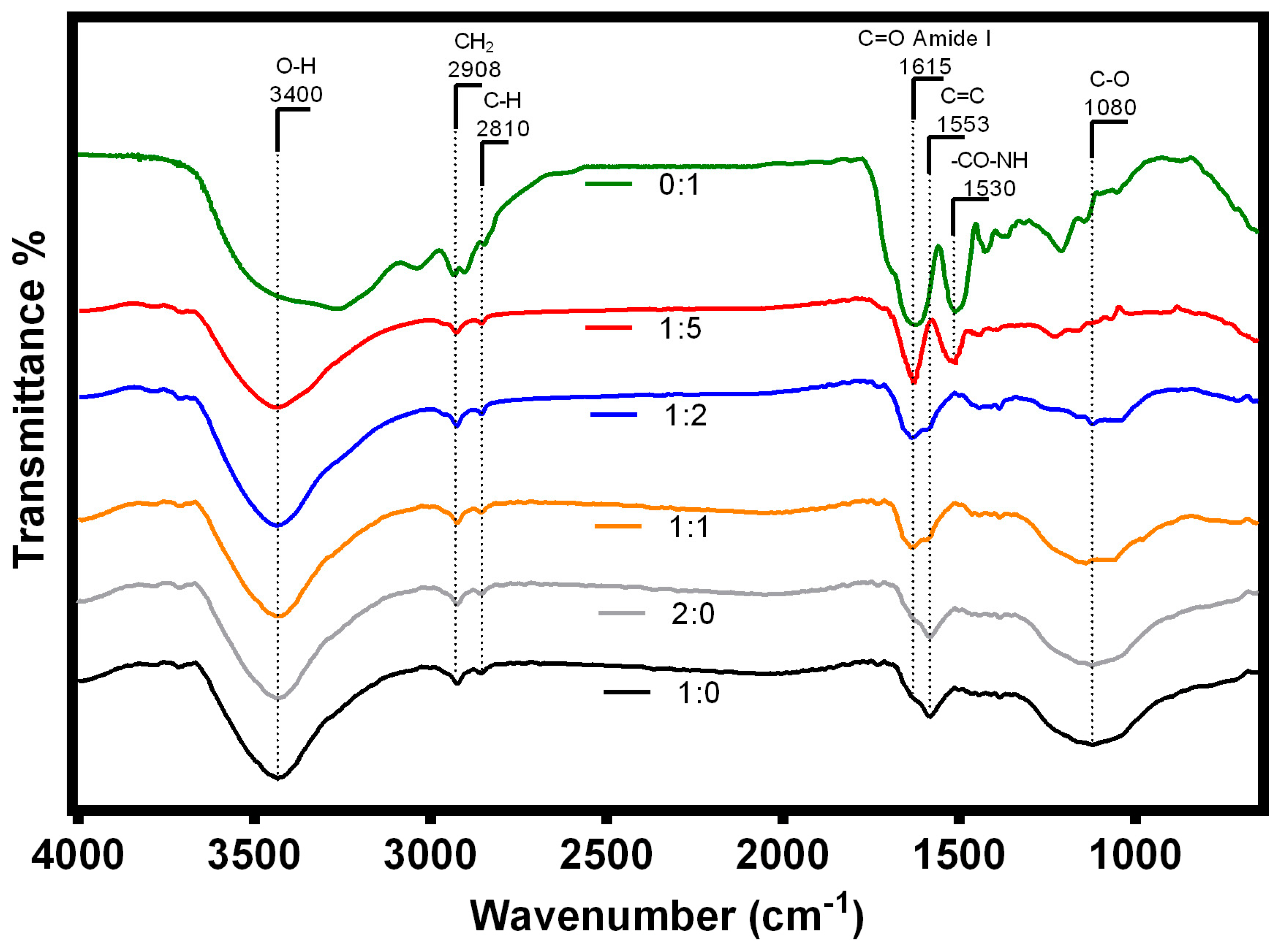
3.2.4. FTIR
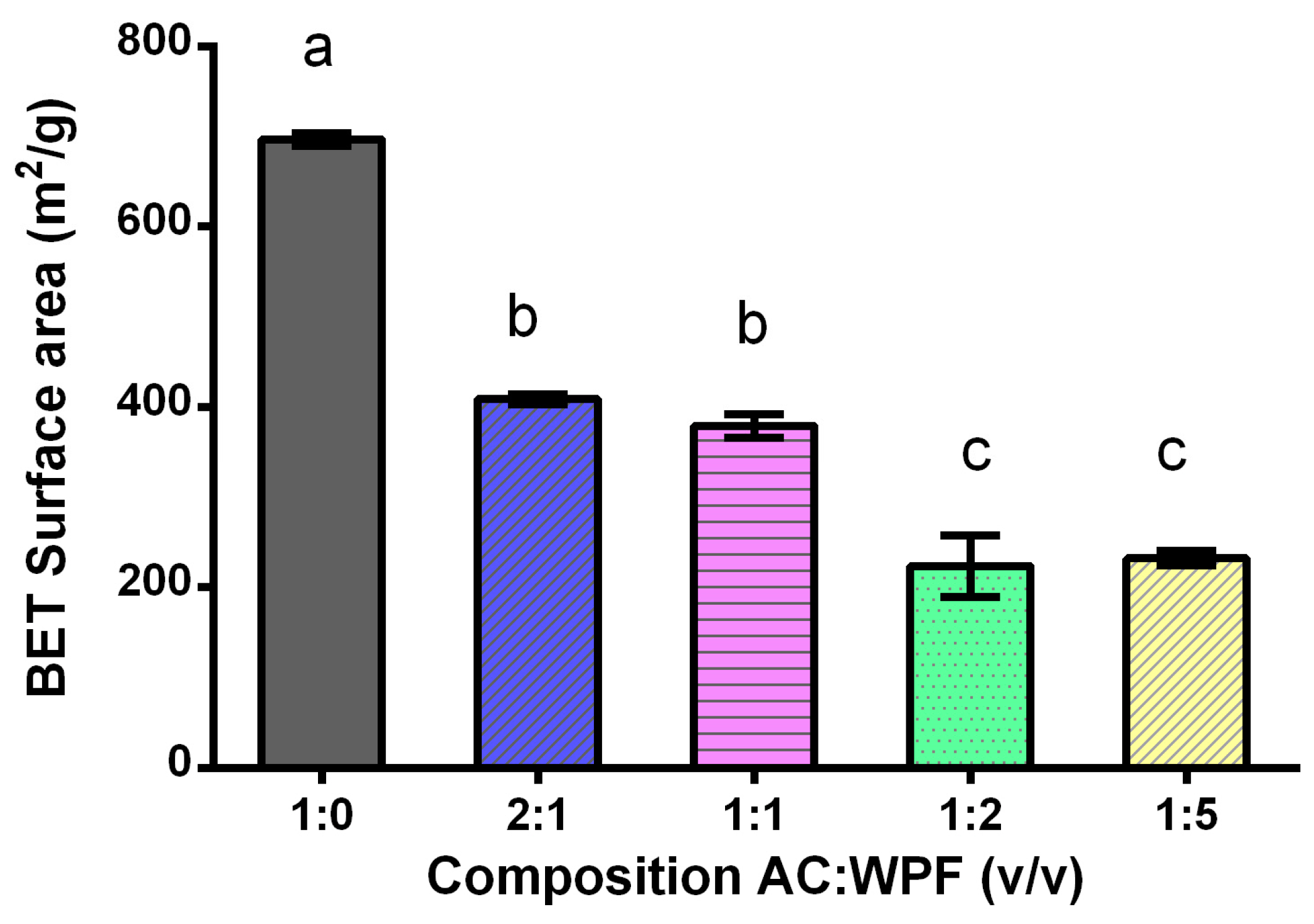
3.2.5. BET Surface Area
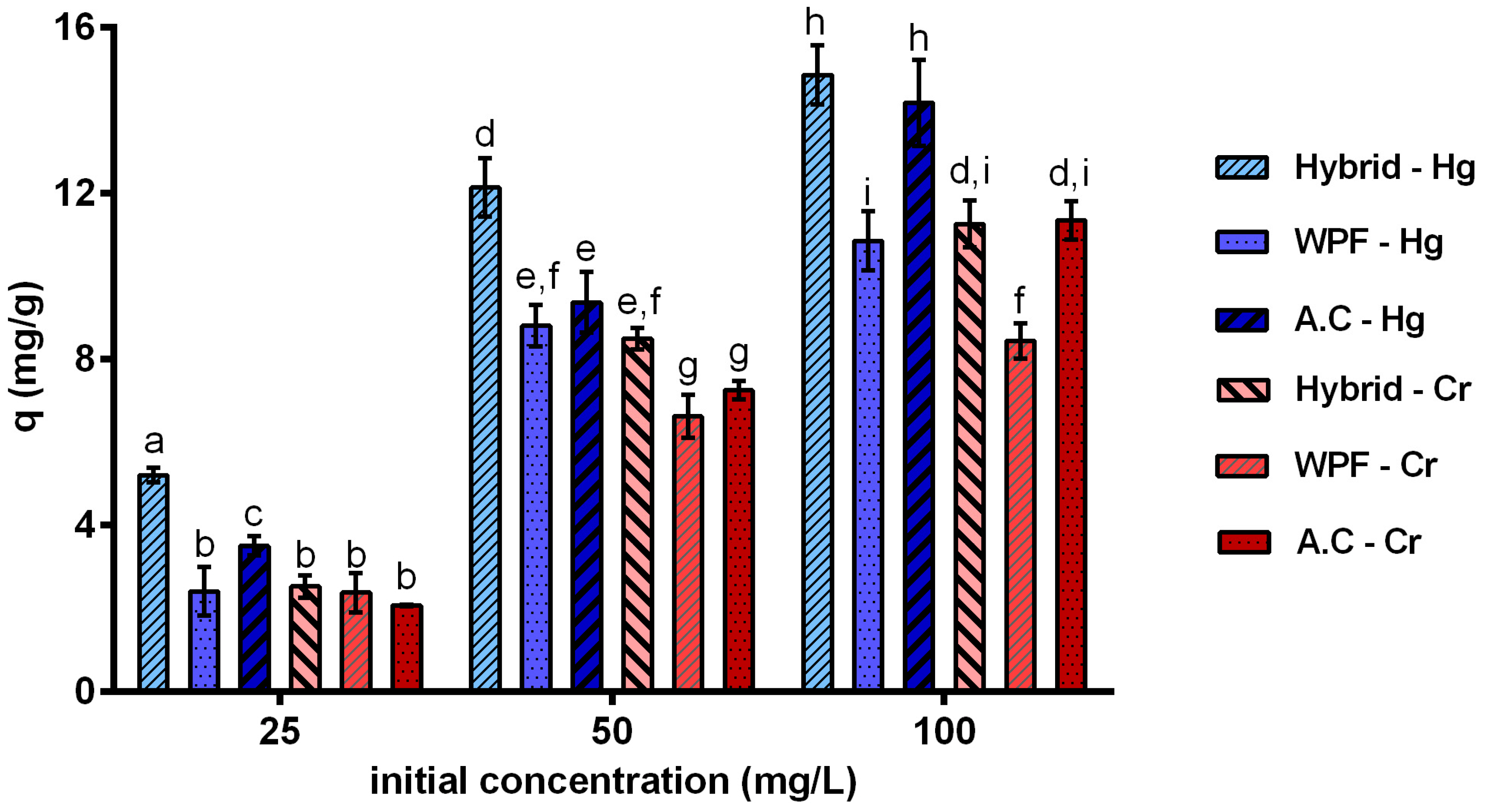
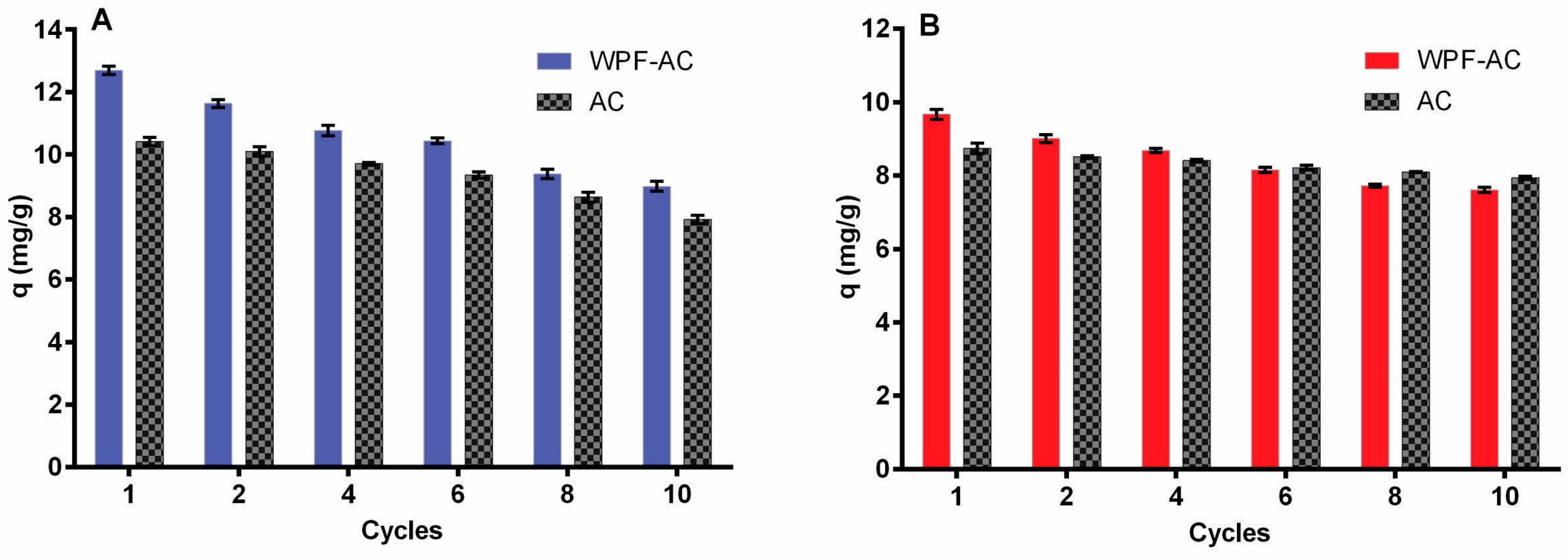
3.3. Removal Efficiency of Heavy Metals
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Genthe, B.; Kapwata, T.; Le Roux, W.; Chamier, J.; Wright, C.Y. The reach of human health risks associated with metals/metalloids in water and vegetables along a contaminated river catchment: South Africa and Mozambique. Chemosphere 2018, 199, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, S.; Zhou, L.; Miao, Z.; Zhang, X.; Su, Z.; Wei, G. Removing Metal Ions from Water with Graphene–Bovine Serum Albumin Hybrid Membrane. Nanomaterials 2019, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Khan, M.; Khan, A.; Zeb, S.; Khan, M.A.; Amin, N.; Sajid, M.; Khattak, A.M. Concentrations, dietary exposure, and human health risk assessment of heavy metals in market vegetables of Peshawar, Pakistan. J. Environ. Monit. Assess. 2018, 190, 505. [Google Scholar] [CrossRef] [PubMed]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Jin, X.; Wang, H.; Jin, X.; Wang, H.; Chen, L.; Wang, W.; Lin, T.; Zhu, Z. Preparation of keratin/PET nanofiber membrane and its high adsorption performance of Cr(VI). Sci. Total Environ. 2020, 710, 135546. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Liu, C.; Peng, J.; Zhang, L.; Wang, S.; Ju, S.; Liu, C. Mercury adsorption from aqueous solution by regenerated activated carbon produced from depleted mercury-containing catalyst by microwave-assisted decontamination. J. Clean. Prod. 2018, 196, 109–121. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Memb. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Rosa, M.A.; Egido, J.A.; Márquez, M.C. Enhanced electrochemical removal of arsenic and heavy metals from mine tailings. J. Taiwan Inst. Chem. Eng. 2017, 78, 409–415. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Z.; Yang, Q.; Huang, R. Efficient removal of lead, copper and cadmium ions from water by a porous calcium alginate/graphene oxide composite aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Elhafez, S.E.A.; Hamad, H.A.; Zaatout, A.A.; Malash, G.F. Management of agricultural waste for removal of heavy metals from aqueous solution: Adsorption behaviors, adsorption mechanisms, environmental protection, and techno-economic analysis. Environ. Sci. Pollut. Res. 2017, 24, 1397–1415. [Google Scholar] [CrossRef] [PubMed]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 1–30. [Google Scholar] [CrossRef]
- Tamagawa, H.; Morita, S. Membrane potential generated by ion adsorption. Membranes 2014, 4, 257–274. [Google Scholar] [CrossRef]
- Bolisetty, S.; Mezzenga, R. Amyloid-carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 2016, 11, 365–371. [Google Scholar] [CrossRef]
- Bolisetty, S.; Reinhold, N.; Zeder, C.; Orozco, M.N.; Mezzenga, R. Efficient purification of arsenic-contaminated water using amyloid-carbon hybrid membranes. Chem. Commun. 2017, 53, 5714–5717. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bolisetty, S.; Mohammadi, T.; Mezzenga, R. Assessing the Binding Performance of Amyloid–Carbon Membranes Towards Heavy Metal Ions. Langmuir 2019, 35, 4161–4170. [Google Scholar] [CrossRef]
- Mezzenga, R.; Zhang, Q.; Bolisetty, S.; Cao, Y.; Handschin, S.; Adamcik, J. Selective and efficient removal of fluoride from water by in-situ engineered amyloid fibrils-ZrO2 hybrid membranes. Angew. Chem. Int. Ed. 2019, 58, 6012–6016. [Google Scholar]
- Vega-Lugo, A.C.; Lim, L.T. Effects of poly(ethylene oxide) and pH on the electrospinning of whey protein isolate. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1188–1197. [Google Scholar] [CrossRef]
- Bacenetti, J.; Bava, L.; Schievano, A.; Zucali, M. Whey protein concentrate (WPC) production: Environmental impact assessment. J. Food Eng. 2018, 224, 139–147. [Google Scholar] [CrossRef]
- Guo, M.; Wang, G. Future Development of Whey Protein Production. In Whey Protein Production, Chemistry, Func- Tionality, and Applications; Guo, M., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 251–260. ISBN 9781119256038. [Google Scholar]
- Yver, A.L.; Bonnaillie, L.M.; Yee, W.; Mcaloon, A.; Tomasula, P.M. Fractionation of whey protein isolate with supercritical carbon dioxide-process modeling and cost estimation. Int. J. Mol. Sci. 2012, 13, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Menchik, P.; Moraru, C.I. Nonthermal concentration of liquid foods by a combination of reverse osmosis and forward osmosis. Acid whey: A case study. J. Food Eng. 2019, 253, 40–48. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Na, J.; Zhang, X.; Yuan, Y.; Liu, C.; Feng, Z. Environmentally-friendly strategy for separation of α-lactalbumin from whey by aqueous two phase flotation. Arab. J. Chem. 2020, 13, 3391–3402. [Google Scholar] [CrossRef]
- Wen-qiong, W.; Yun-chao, W.; Xiao-feng, Z.; Rui-xia, G.; Mao-lin, L. Whey protein membrane processing methods and membrane fouling mechanism analysis. Food Chem. 2019, 289, 468–481. [Google Scholar] [CrossRef]
- Alhashimi, H.A.; Aktas, C.B. Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis. Resour. Conserv. Recycl. 2017, 118, 13–26. [Google Scholar] [CrossRef]
- Gago, D.; Chagas, R.; Ferreira, M.; Velizarov, S.; Coelhoso, I. A Novel Cellulose-Based Polymer for Efficient Removal of Methylene Blue. Membranes 2020, 10, 13. [Google Scholar] [CrossRef]
- Loveday, S.M.; Anema, S.G.; Singh, H. β-Lactoglobulin nanofibrils: The long and the short of it. Int. Dairy J. 2017, 67, 35–45. [Google Scholar] [CrossRef]
- Bolder, S.G.; Hendrickx, H.; Sagis, L.M.C.; Van Der Linden, E. Fibril assemblies in aqueous whey protein mixtures. J. Agric. Food Chem. 2006, 54, 4229–4234. [Google Scholar] [CrossRef]
- Zare-Dorabei, R.; Ferdowsi, S.M.; Barzin, A.; Tadjarodi, A. Highly efficient simultaneous ultrasonic-assisted adsorption of Pb(II), Cd(II), Ni(II) and Cu (II) ions from aqueous solutions by graphene oxide modified with 2,2′-dipyridylamine: Central composite design optimization. Ultrason. Sonochem. 2016, 32, 265–276. [Google Scholar] [CrossRef]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bello-Vieda, N.J.; Pastrana, H.F.; Garavito, M.F.; Ávila, A.G.; Celis, A.M.; Muñoz-Castro, A.; Restrepo, S.; Hurtado, J.J. Antibacterial activities of azole complexes combined with silver nanoparticles. Molecules 2018, 23, 361. [Google Scholar] [CrossRef] [PubMed]
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, J.; Wang, R.; Jiao, T.; Huang, H.; Zhou, J.; Zhang, L.; Peng, Q. Facile preparation of self-assembled polydopamine-modified electrospun fibers for highly effective removal of organic dyes. Nanomaterials 2019, 9, 116. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Bourceanu, G.; Olariu, R.I.; Arsene, C. A novel polymer inclusion membrane applied in chromium (VI) separation from aqueous solutions. J. Hazard. Mater. 2011, 197, 244–253. [Google Scholar] [CrossRef]
- Anagnostopoulos, V.A.; Manariotis, I.D.; Karapanagioti, H.K.; Chrysikopoulos, C.V. Removal of mercury from aqueous solutions by malt spent rootlets. Chem. Eng. J. 2012, 213, 135–141. [Google Scholar] [CrossRef]
- Veerman, C.; Ruis, H.; Sagis, L.M.C.; van der Linden, E. Effect of electrostatic interactions on the percolation concentration of fibrillar β-lactoglobulin gels. Biomacromolecules 2002, 3, 869–873. [Google Scholar] [CrossRef]
- Bolder, S.G.; Vasbinder, A.J.; Sagis, L.M.C.; van der Linden, E. Heat-induced whey protein isolate fibrils: Conversion, hydrolysis, and disulphide bond formation. Int. Dairy J. 2007, 17, 846–853. [Google Scholar] [CrossRef]
- Arnaudov, L.N.; de Vries, R.; Ippel, H.; van Mierlo, C.P.M. Multiple steps during the formation of beta-lactoglobulin fibrils. Biomacromolecules 2003, 4, 1614–1622. [Google Scholar] [CrossRef]
- Aymard, P.; Nicolai, T.; Durand, D.; Clark, A. Static and Dynamic Scattering of β-Lactoglobulin Aggregates Formed after Heat-Induced Denaturation at pH 2. Macromolecules 1999, 32, 2542–2552. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Zappone, B.; De Santo, M.P.; Labate, C.; Guzzi, R. Catalytic activity of copper ions in the amyloid fibrillation of b-lactoglobulin Bruno. Soft Matter 2013, 9, 2412–2419. [Google Scholar] [CrossRef]
- Rodzik, A.; Pomastowski, P.; Sagandykova, G.N.; Buszewski, B. Interactions of whey proteins with metal ions. Int. J. Mol. Sci. 2020, 21, 2156. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Cheng, X.; Zhong, L.; Wu, R.; Zheng, Y. Preparation, characterization and performance of an electrospun carbon nanofiber mat applied in hexavalent chromium removal from aqueous solution. J. Environ. Sci. (China) 2018, 77, 75–84. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suter, M.K.; Bolisetty, S.; Boulos, S.; Handschin, S.; Nyström, L.; Mezzenga, R. Amyloid Fibrils Aerogel for Sustainable Removal of Organic Contaminants from Water. Adv. Mater. 2020, 32, 1907932. [Google Scholar] [CrossRef]
- Bhatt, R.; Padmaj, P. A chitosan-thiomer polymer for highly efficacious adsorption of mercury. Carbohydr. Polym. 2019, 207, 663–674. [Google Scholar] [CrossRef]
- Goers, J.; Permyakov, S.E.; Permyakov, E.A.; Uversky, V.N.; Fink, A.L. Conformational prerequisites for α-lactalbumin fibrillation. Biochemistry 2002, 41, 12546–12551. [Google Scholar] [CrossRef]
- Kontopidis, G.; Holt, C.; Sawyer, L. Invited Review: β-Lactoglobulin: Binding Properties, Structure, and Function. J. Dairy Sci. 2010, 87, 785–796. [Google Scholar] [CrossRef]
- Bhowmik, M.; Debnath, A.; Saha, B. Fabrication of mixed phase CaFe2O4 and MnFe2O4 magnetic nanocomposite for enhanced and rapid adsorption of methyl orange dye: Statistical modeling by neural network and response surface methodology. J. Dispers. Sci. Technol. 2019, 41, 1–12. [Google Scholar] [CrossRef]
- Bolder, S.G.; Sagis, L.M.C.; Venema, P.; Van Der Linden, E. Effect of stirring and seeding on whey protein fibril formation. J. Agric. Food Chem. 2007, 55, 5661–5669. [Google Scholar] [CrossRef]
- Adamcik, J.; Jung, J.-M.; Flakowski, J.; De Los Rios, P.; Dietler, G.; Mezzenga, R. Understanding amyloid aggregation by statistical analysis of atomic force microscopy images. Nat. Nanotechnol. 2010, 5, 423–428. [Google Scholar] [CrossRef]
- Wang, S.S.S.; Liu, K.N.; Wen, W.S.; Wang, P. Fibril Formation of Bovine α-Lactalbumin Is Inhibited by Glutathione. Food Biophys. 2011, 6, 138–151. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Zhou, B.R.; Chen, J.; Liang, Y. Oleic Acid Inhibits Amyloid Formation of the Intermediate of α-Lactalbumin at Moderately Acidic pH. J. Mol. Biol. 2006, 362, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M.; Wang, X.L.; Rao, M.A.; Anema, S.G.; Singh, H. Effect of pH, NaCl, CaCl2 and temperature on self-assembly of β-lactoglobulin into nanofibrils: A central composite design study. J. Agric. Food Chem. 2011, 59, 8467–8474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, S.; Zhao, Z.; Liu, M.; Yin, X.; Zhou, Y.; Wu, Y.; Peng, Q. Highly effective lead (II) removal by sustainable alkaline activated β-lactoglobulin nanofibrils from whey protein. J. Clean. Prod. 2020, 255, 120297. [Google Scholar] [CrossRef]
- Ji, W.; Xiao, L.; Ling, Y.; Ching, C.; Matsumoto, M.; Bisbey, R.P.; Helbling, D.E.; Dichtel, W.R. Removal of GenX and Perfluorinated Alkyl Substances from Water by Amine-Functionalized Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 12677–12681. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ma, Y.; Li, H.; Guan, X.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Qiu, S.; Valtchev, V. Postsynthetic Functionalization of Three-Dimensional Covalent Organic Frameworks for Selective Extraction of Lanthanide Ions. Angew. Chem. 2018, 130, 6150–6156. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.; Earl, L.D.; Abney, C.W.; Cheng, Y.; Wei, H.; Nguyen, N.; Wojtas, L.; Ma, S. Postsynthetically Modified Covalent Organic Frameworks for Efficient and Effective Mercury Removal. J. Am. Chem. Soc. 2017, 139, 2786–2793. [Google Scholar] [CrossRef]
- Ghanbarian, M.; Nabizadeh, R.; Nasseri, S.; Shemirani, F.; Mahvi, A.H.; Beyki, M.H.; Mesdaghinia, A. Potential of amino-riched nano-structured MnFe2O4@cellulose for biosorption of toxic Cr (VI): Modeling, kinetic, equilibrium and comparing studies. Int. J. Biol. Macromol. 2017, 104, 465–480. [Google Scholar] [CrossRef]
- Tshikovhi, A.; Mishra, S.B.; Mishra, A.K. Nanocellulose-based composites for the removal of contaminants from wastewater. Int. J. Biol. Macromol. 2020, 152, 616–632. [Google Scholar] [CrossRef]
- Huang, L.; Shen, R.; Liu, R.; Shuai, Q. Thiol-functionalized magnetic covalent organic frameworks by a cutting strategy for efficient removal of Hg2+ from water. J. Hazard. Mater. 2020, 392, 122320. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.R.H.; Devlin, G.L.; Donald, A.M. Amyloid fibril-like structure underlies the aggregate structure across the pH range for β-lactoglobulin. Biophys. J. 2009, 96, 5013–5019. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-M.; Savin, G.; Pouzot, M.; Schmitt, C.; Mezzenga, R. Structure of Heat-Induced B-Lactoglobulin Aggregates and their Complexes with Sodium-Dodecyl Sulfate. Biomacromolecules 2008, 9, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T.; Britten, M.; Schmitt, C. B-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Farrokhi, F.; Badii, F.; Ehsani, M.R.; Hashemi, M. Functional and thermal properties of nanofibrillated whey protein isolate as functions of denaturation temperature and solution pH. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 583, 124002. [Google Scholar] [CrossRef]
- Ikeda, S.; Morris, V.J. Fine-stranded and particulate aggregates of heat-denatured whey proteins visualized by atomic force microscopy. Biomacromolecules 2002, 3, 382–389. [Google Scholar] [CrossRef]
- Guan, C.; He, X.F.; Xu, H.H.; Shao, M.L.; Ma, J.Y.; Gao, Z.W. Comparative experiments of electrical conductivity from whey protein concentrates conventional film and nanofibril film. J. Dairy Res. 2020, 87, 103–109. [Google Scholar] [CrossRef]
- Maity, S.; Pal, S.; Sardar, S.; Sepay, N.; Parvej, H.; Begum, S.; Dalui, R.; Das, N.; Pradhan, A.; Halder, U.C. Inhibition of amyloid fibril formation of β-lactoglobulin by natural and synthetic curcuminoids. New J. Chem. 2018, 42, 19260–19271. [Google Scholar] [CrossRef]
- Pal, S.; Maity, S.; Sardar, S.; Chakraborty, J.; Halder, U.C. Insight into the co-solvent induced conformational changes and aggregation of bovine β-lactoglobulin. Int. J. Biol. Macromol. 2016, 84, 121–134. [Google Scholar] [CrossRef]
- O’Loughlin, I.B.; Kelly, P.M.; Murray, B.A.; Fitzgerald, R.J.; Brodkorb, A. Concentrated whey protein ingredients: A Fourier transformed infrared spectroscopy investigation of thermally induced denaturation. Int. J. Dairy Technol. 2015, 68, 349–356. [Google Scholar] [CrossRef]
- Gbassi, G.; Yolou, F.; Sarr, S.; Atheba, P.; Amin, C.; Ake, M. Whey proteins analysis in aqueous medium and in artificial gastric and intestinal fluids. Int. J. Biol. Chem. Sci. 2012, 6, 1828–1837. [Google Scholar] [CrossRef]
- Merodio-Morales, E.E.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Duran-Valle, C.J.; Bonilla-Petriciolet, A. Lanthanum- and cerium-based functionalization of chars and activated carbons for the adsorption of fluoride and arsenic ions. Int. J. Environ. Sci. Technol. 2020, 17, 115–128. [Google Scholar] [CrossRef]
- El-Hendawy, A.N.A. Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions. J. Anal. Appl. Pyrolysis 2006, 75, 159–166. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; Mattusch, J.; Peláez-Cid, A.A.; Wennrich, R. Characterization of adsorbent materials prepared from avocado kernel seeds: Natural, activated and carbonized forms. J. Anal. Appl. Pyrolysis 2007, 78, 185–193. [Google Scholar] [CrossRef]
- Torres, J.A.; Nogueira, F.G.E.; Silva, M.C.; Lopes, J.H.; Tavares, T.S.; Ramalho, T.C.; Corrêa, A.D. Novel eco-friendly biocatalyst: Soybean peroxidase immobilized onto activated carbon obtained from agricultural waste. RSC Adv. 2017, 7, 16460–16466. [Google Scholar] [CrossRef]
- Andrade, S.; Veloso, C.; Fontan, R.C.; Bonomo, R.C.; Santos, L.; Brito, M.J.; Diniz, G. Chemical-activated carbon from coconut (cocos nucifera) endocarp waste and its application in the adsorption of β-lactoglobulin protein. Rev. Mex. Ing. Química 2018, 17, 463–475. [Google Scholar] [CrossRef]
- Tabor, R.F.; Grieser, F.; Dagastine, R.; Chan, D.Y. The hydrophobic force: Measurements and methods. Phys. Chem. Chem. Phys. 2014, 16, 18065–18075. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Wang, H.; Dong, J.; Chen, W.; Wang, X.; Wang, S.; Hayat, T.; Alsaedi, A.; Wang, X. Preparation of Molybdenum Disulfide Coated Mg/Al Layered Double Hydroxide Composites for Efficient Removal of Chromium(VI). ACS Sustain. Chem. Eng. 2017, 5, 7165–7174. [Google Scholar] [CrossRef]
- Qian, L.; Wang, H.; Yang, J.; Chen, X.; Chang, X.; Nan, Y.; He, Z.; Hu, P.; Wu, W.; Liu, T. Amino Acid Cross-Linked Graphene Oxide Membranes for Metal Ions Permeation, Insertion and Antibacterial Properties. Membranes 2020, 10, 296. [Google Scholar] [CrossRef]
- Razmi, H.; Musevi, S.J.; Mohammad-Rezaei, R. Solid phase extraction of mercury(II) using soluble eggshell membrane protein doped with reduced graphene oxide, and its quantitation by anodic stripping voltammetry. Microchim. Acta 2016, 183, 555–562. [Google Scholar] [CrossRef]
- Kolbasov, A.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Heavy metal adsorption on solution-blown biopolymer nanofiber membranes. J. Memb. Sci. 2017, 530, 250–263. [Google Scholar] [CrossRef]
- Ling, S.; Qin, Z.; Huang, W.; Cao, S.; Kaplan, D.L.; Buehler, M.J. Design and function of biomimetic multilayer water purification membranes. Sci. Adv. 2017, 3, e1601939. [Google Scholar] [CrossRef] [PubMed]
- Manirethan, V.; Balakrishnan, R.M. Batch and continuous studies on the removal of heavy metals using biosynthesised melanin impregnated activated carbon. Environ. Technol. Innov. 2020, 20, 24723–24737. [Google Scholar] [CrossRef]
- Goscianska, J.; Fathy, N.A.; Aboelenin, R.M.M. Adsorption of solophenyl red 3BL polyazo dye onto amine-functionalized mesoporous carbons. J. Colloid Interface Sci. 2017, 505, 593–604. [Google Scholar] [CrossRef]
- Duan, X.L.; Yuan, C.G.; Jing, T.T.; Yuan, X.D. Removal of elemental mercury using large surface area micro-porous corn cob activated carbon by zinc chloride activation. Fuel 2019, 239, 830–840. [Google Scholar] [CrossRef]

| Variable. | Unit | Factors | Level | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |||
| Temperature | °C | A | 66 | 70 | 80 | 90 | 94 |
| Time | h | B | 5 | 6 | 8 | 10 | 11 |
| Whey concentration | wt% | C | 0.4 | 1 | 2.5 | 4 | 4.6 |
| Run | A | B | C | Adsorption Capacity and Efficiency for Hg | Adsorption Capacity and Efficiency for Cr | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Error | Experimental | Predicted | Error | ||||||||||
| q | % | q | % | q | % | q | % | q | % | q | % | ||||
| 1 | 66 | 8 | 2.5 | 23.4 | 79 | 23.5 | 79 | 0.5 | 0.5 | 17.8 | 56 | 17.9 | 56 | 0.6 | 0.6 |
| 2 | 70 | 6 | 1 | 13.9 | 47 | 13.8 | 46 | 0.9 | 0.9 | 11.4 | 36 | 11.3 | 35 | 1.1 | 1.1 |
| 3 | 70 | 10 | 4 | 12.6 | 42 | 12.4 | 42 | 1.1 | 1.0 | 9.5 | 30 | 9.3 | 29 | 1.4 | 1.4 |
| 4 | 80 | 5 | 2.5 | 23.0 | 78 | 23.1 | 78 | 0.5 | 0.5 | 9.8 | 31 | 9.9 | 31 | 1.1 | 1.1 |
| 5 | 80 | 8 | 0.4 | 2.6 | 8 | 2.7 | 9 | 4.7 | 4.7 | 1.5 | 4 | 1.6 | 5 | 7.5 | 7.6 |
| 6 | 80 | 8 | 2.5 | 21.5 | 73 | 20.1 | 68 | 6.9 | 6.9 | 7.5 | 23 | 7.7 | 24 | 2.5 | 2.5 |
| 7 | 80 | 8 | 2.5 | 20.0 | 68 | 20.1 | 68 | 0.4 | 0.4 | 8.0 | 25 | 7.7 | 24 | 3.7 | 3.7 |
| 8 | 80 | 8 | 2.5 | 20.2 | 68 | 20.1 | 68 | 0.7 | 0.7 | 7.4 | 23 | 7.7 | 24 | 4.0 | 4.0 |
| 9 | 80 | 8 | 2.5 | 18.9 | 64 | 20.1 | 68 | 6.1 | 6.1 | 8.0 | 25 | 7.7 | 24 | 3.0 | 3.0 |
| 10 | 80 | 8 | 2.5 | 20.1 | 68 | 20.1 | 68 | 0.2 | 0.3 | 7.9 | 25 | 7.7 | 24 | 2.2 | 2.2 |
| 11 | 80 | 8 | 4.6 | 15.5 | 52 | 15.6 | 53 | 0.6 | 0.7 | 17.2 | 54 | 17.3 | 54 | 0.6 | 0.6 |
| 12 | 80 | 11 | 2.5 | 13.0 | 44 | 13.1 | 44 | 0.8 | 0.9 | 6.6 | 21 | 6.8 | 21 | 1.6 | 1.6 |
| 13 | 90 | 6 | 4 | 6.5 | 21 | 6.3 | 21 | 2.1 | 2.1 | 10.7 | 33 | 10.6 | 33 | 1.2 | 1.2 |
| 14 | 90 | 10 | 1 | 24.2 | 82 | 24.1 | 81 | 0.6 | 0.5 | 6.6 | 21 | 6.5 | 20 | 2.0 | 2.0 |
| 15 | 94 | 8 | 2.5 | 17.4 | 59 | 17.5 | 59 | 0.6 | 0.7 | 2.1 | 6 | 2.2 | 7 | 4.9 | 5.0 |
| Best Preparation Conditions | ||
|---|---|---|
| Mercury | Chromium | |
| Temperature (°C) | 74.1 | 73.5 |
| Time (h) | 7.5 | 7.1 |
| Protein concentration (%) | 3.8 | 3.7 |
| q (mg/g) predicted | 29.4 | 19.3 |
| q (mg/g) validated | 24.9 ± 0.3 | 17.9 ± 0.8 |
| q (mg/g) mixed | 24.1 ± 0.8 | 11.5 ± 0.8 |
| q (mg/g) β-Lactoglobulin | 27.7± 0.2 | 19.35 ± 1.3 |
| Error (%) | 15.2 | 6.8 |
| Removal efficiency (%) predicted | 100 | 61 |
| Removal efficiency (%) validated | 81 ± 0.5 | 57 ± 1.8 |
| Removal efficiency (%) Mixed | 78 ± 1.4 | 37 ± 1.6 |
| Removal efficiency (%) β-Lactoglobulin | 90 ± 1.2 | 62 ± 2.1 |
| Error (%) | 19 | 5 |
| Hybrid Membrane | Heavy Metal | q (mg/g) | Evaluation | Co (mg/g) | Ref |
|---|---|---|---|---|---|
| Hybrid membrane AC-B-lactoblogulin | Au | 52.5 | 1st Cycle | 561 | [16] |
| Hg | 10.6 | 85 | |||
| Pb | 995.7 | 65 | |||
| Pd | 356.6 | 12 | |||
| Eggshell membrane protein doped with reduced graphene oxide | Hg | 77 | 1st Cycle | 0.01 | [81] |
| Hybrid membrane AC-B-lactoblogulin | Arsenate | 0.2 | 1st Cycle | 0.2 | [17] |
| Arsenite | 1.1 | ||||
| Hybrid membrane of lignin/nylon-6 membrane and oats/nylon-6 membrane | Pb | 37 and 11 | Batch for 2h | 10 | [82] |
| Hybrid membrane of silk nanofibril (SNF) and hydroxyapatite (HAP) | Au | 135.7 | Batch for 48 h | 100 | [83] |
| Ag | - | ||||
| Cu | 64.7 | ||||
| Ni | 63.0 | ||||
| Cr | 126.7 | ||||
| Pb | - | ||||
| Hybrid membrane AC-B-lactoblogulin | Cr | 148.6 | 1st Cycle | 0.17 | [18] |
| Ni | 13.8 | 0.21 | |||
| Ag | 86.7 | 0.20 | |||
| Pt | 234.7 | 0.13 | |||
| Graphene–Bovine Serum Albumin Hybrid Membrane | Co | 0.5 | 1st Cycle | 1000 | [2] |
| Cu | 0.3 | ||||
| Melanin-coated PVDF membranes | Hg | 9.2 | 1st Cycle | 3 | [84] |
| Cr | 5.0 | ||||
| Pb | 4.0 | ||||
| Cu | 6.9 | ||||
| This study | Hg | 14.8 | 1st Cycle | 100 | |
| Cr | 11.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Rodríguez, L.C.; Díaz Barrera, L.E.; Quintanilla-Carvajal, M.X.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Jiménez-Junca, C. Preparation of a Hybrid Membrane from Whey Protein Fibrils and Activated Carbon to Remove Mercury and Chromium from Water. Membranes 2020, 10, 386. https://doi.org/10.3390/membranes10120386
Ramírez-Rodríguez LC, Díaz Barrera LE, Quintanilla-Carvajal MX, Mendoza-Castillo DI, Bonilla-Petriciolet A, Jiménez-Junca C. Preparation of a Hybrid Membrane from Whey Protein Fibrils and Activated Carbon to Remove Mercury and Chromium from Water. Membranes. 2020; 10(12):386. https://doi.org/10.3390/membranes10120386
Chicago/Turabian StyleRamírez-Rodríguez, Laura Cristina, Luis Eduardo Díaz Barrera, María Ximena Quintanilla-Carvajal, Didilia Ileana Mendoza-Castillo, Adrián Bonilla-Petriciolet, and Carlos Jiménez-Junca. 2020. "Preparation of a Hybrid Membrane from Whey Protein Fibrils and Activated Carbon to Remove Mercury and Chromium from Water" Membranes 10, no. 12: 386. https://doi.org/10.3390/membranes10120386
APA StyleRamírez-Rodríguez, L. C., Díaz Barrera, L. E., Quintanilla-Carvajal, M. X., Mendoza-Castillo, D. I., Bonilla-Petriciolet, A., & Jiménez-Junca, C. (2020). Preparation of a Hybrid Membrane from Whey Protein Fibrils and Activated Carbon to Remove Mercury and Chromium from Water. Membranes, 10(12), 386. https://doi.org/10.3390/membranes10120386









