Electrospun Hybrid Perfluorosulfonic Acid/Sulfonated Silica Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanofiber Electrospinning
2.2. Transforming Dual Fiber Mats into Dense Membranes
2.3. Fabrication of a Solution-Cast Blended Membrane (Membrane Type-D)
2.4. Characterization of Fiber Mats and Membranes
2.4.1. Scanning Electron Microscopy
2.4.2. Ion-Exchange Capacity (IEC)
2.4.3. Proton Conductivity
2.4.4. Gravimetric Water Uptake and In-Plane Swelling
2.4.5. Water Vapor Sorption
2.4.6. Membrane Mechanical Properties
3. Results
3.1. Preliminary Studies with Type-A Membranes
3.2. Type-A Membrane vs. Type-B Membrane—Location of Sulfonated Silica
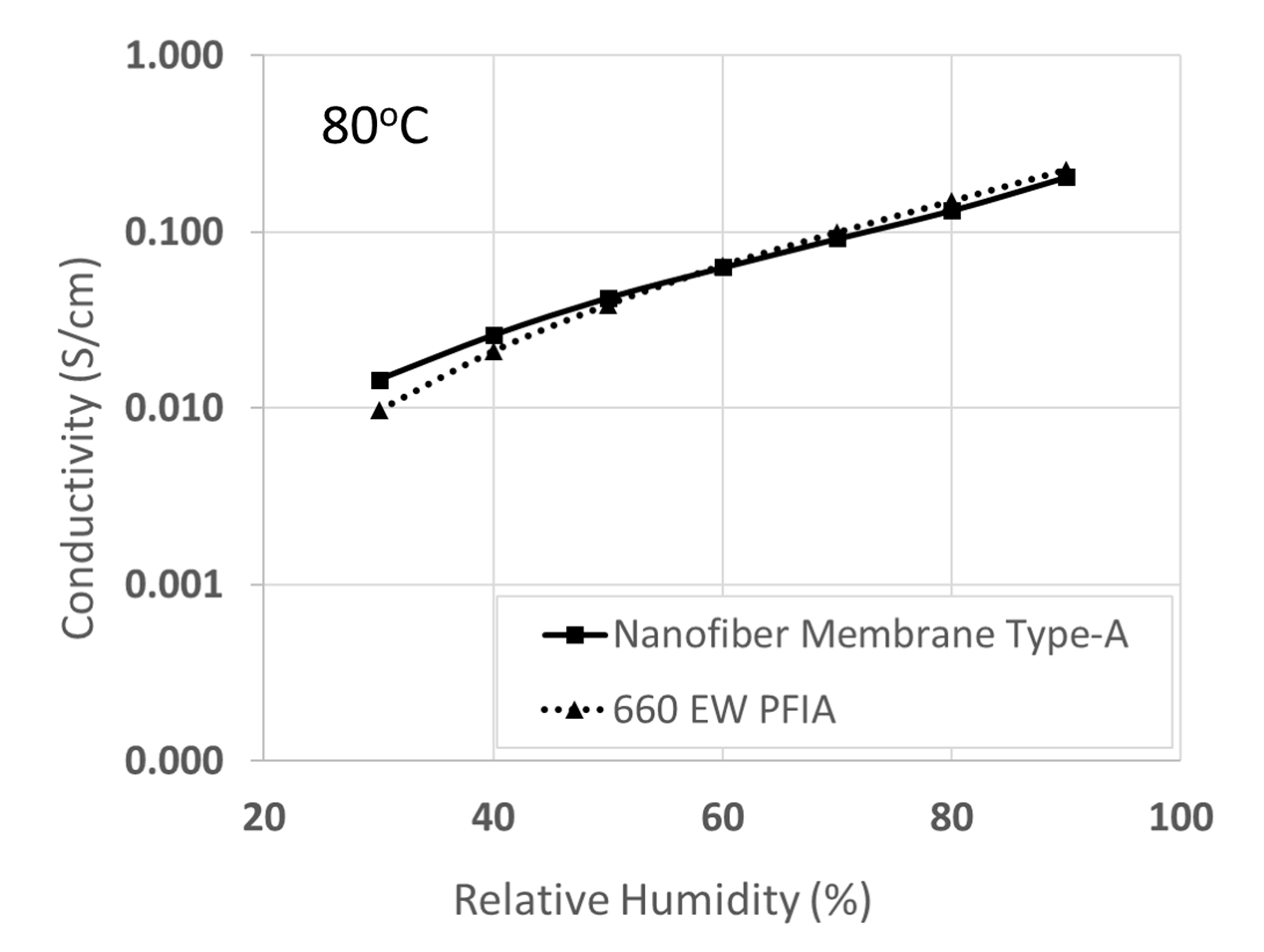
3.3. Comparison of Membrane Type-A with an Ultra-Low EW PFIA Film and with PFSA/Sulfonated Silica Composite Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wycisk, R.; Pintauro, P.N.; Park, J.W. New developments in proton conducting membranes for fuel cells. Curr. Opin. Chem. Eng. 2014, 4, 71–78. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, G.; Casciola, M. Composite Membranes for Medium-Temperature PEM Fuel Cells. Annu. Rev. Mater. Res. 2003, 33, 129–154. [Google Scholar] [CrossRef]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Fujimura, M.; Hashimoto, T.; Kawai, H. Small-angle x-ray scattering study of perfluorinated ionomer membranes. 1. Origin of two scattering maxima. Macromolecules 1981, 14, 1309–1315. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, G.E.; Wilson, F.C. The Morphology in Nafion Perfluorinated Membrane Products, as Determined by Wide- and Small- Angle X-ray Studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Maalouf, M.; Pyle, B.; Sun, C.-N.; Wu, D.; Paddison, S.J.; Schaberg, M.; Emery, M.; Lochhaas, K.H.; Hamrock, S.J.; Ghassemi, H.; et al. Proton exchange membranes for high temperature fuel cells: Equivalent weight and end group effects on conductivity. ECS Trans. 2009, 25, 1473–1481. [Google Scholar] [CrossRef]
- Yandrasits, M.A.; Hamrock, S.J. Membranes for PEM Fuel Cells. In Fuel Cell Chemistry and Operation; Chapter 2, ACS Symposium Series; American Chemical Society: Washginton, DC, USA, 2010; Volume 1040, pp. 15–29. [Google Scholar]
- Yandrasits, M.A.; Lindell, M.J.; Hamrock, S.J. New directions in perfluoroalkyl sulfonic acid–based proton-exchange membranes. Curr. Opin. Electrochem. 2019, 18, 90–98. [Google Scholar] [CrossRef]
- Dos Santos, L.; Rose, S.; Sel, O.; Maréchal, M.; Perrot, H.; Laberty-Robert, C. Electrospinning a versatile tool for designing hybrid proton conductive membrane. J. Membr. Sci. 2016, 513, 12–19. [Google Scholar] [CrossRef]
- Sahu, A.K.; Selvarani, G.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. A Sol-Gel Modified Alternative Nafion-Silica Composite Membrane for Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2007, 154, B123. [Google Scholar] [CrossRef]
- Adjemian, K.T.; Lee, S.J.; Srinivasan, S.; Benziger, J.; Bocarsly, B. Silicon Oxide Nafion Composite Membranes for Proton-Exchange Membrane Fuel Cell Operation at 80–140 °C. J. Electrochem. Soc. 2002, 149, A256. [Google Scholar] [CrossRef]
- Pereira, F.; Vallé, K.; Belleville, P.; Morin, A.; Lambert, S.; Sanchez, C. Advanced Mesostructured Hybrid Silica−Nafion Membranes for High-Performance PEM Fuel Cell. Chem. Mater. 2008, 20, 1710–1718. [Google Scholar] [CrossRef]
- Yen, C.Y.; Lee, C.H.; Lin, Y.F.; Lin, H.L.; Hsiao, Y.H.; Liao, S.H.; Chuang, C.Y.; Ma, C.C.M. Sol-gel derived sulfonated-silica/Nafion (R) composite membrane for direct methanol fuel cell. J. Power Sources 2007, 173, 36–44. [Google Scholar] [CrossRef]
- Jiang, R.; Kunz, H.R.; Fenton, J.M. Composite silica/Nafion® membranes prepared by tetraethylorthosilicate sol–gel reaction and solution casting for direct methanol fuel cells. J. Membr. Sci. 2006, 272, 116–124. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. In situ modification of Nafion® membranes with phospho-silicate for improved water retention and proton conduction. J. Membr. Sci. 2009, 333, 50–58. [Google Scholar] [CrossRef]
- Sahu, A.K.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. Co-assembly of a nafion-mesoporous zirconium phosphate composite membrane for PEM fuel cells. Fuel Cells 2009, 9, 139–147. [Google Scholar] [CrossRef]
- Hill, M.L.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Zirconium hydrogen phosphate/disulfonated poly(arylene ether sulfone) copolymer composite membranes for proton exchange membrane fuel cells. J. Membr. Sci. 2006, 283, 102–108. [Google Scholar] [CrossRef]
- Jiang, R.; Russell Kunz, H.; Fenton, J.M. Influence of temperature and relative humidity on performance and CO tolerance of PEM fuel cells with Nafion®–Teflon®–Zr(HPO4)2 higher temperature composite membranes. Electrochim. Acta 2006, 51, 5596–5605. [Google Scholar] [CrossRef]
- Silva, V.S.; Ruffmann, B.; Silva, H.; Silva, V.B.; Mendes, a.; Madeira, L.M.; Nunes, S. Zirconium oxide hybrid membranes for direct methanol fuel cells—Evaluation of transport properties. J. Membr. Sci. 2006, 284, 137–144. [Google Scholar] [CrossRef]
- Woo, M.H.; Kwon, O.; Choi, S.H.; Hong, M.Z.; Ha, H.-W.; Kim, K. Zirconium phosphate sulfonated poly (fluorinated arylene ether)s composite membranes for PEMFCs at 100–140 °C. Electrochim. Acta 2006, 51, 6051–6059. [Google Scholar] [CrossRef]
- Pica, M.; Donnadio, A.; Casciola, M.; Cojocaru, P.; Merlo, L. Short side chain perfluorosulfonic acid membranes and their composites with nanosized zirconium phosphate: Hydration, mechanical properties and proton conductivity. J. Mater. Chem. 2012, 22, 24902. [Google Scholar] [CrossRef]
- Kim, Y.S.; Wang, F.; Hickner, M.; Zawodzinski, T.A.; McGrath, J.E. Fabrication and characterization of heteropolyacid (H3PW12O40)/directly polymerized sulfonated poly(arylene ether sulfone) copolymer composite membranes for higher temperature fuel cell applications. J. Membr. Sci. 2003, 212, 263–282. [Google Scholar] [CrossRef]
- Ramani, V.; Kunz, H.R.; Fenton, J.M. Metal dioxide supported heteropolyacid/Nafion® composite membranes for elevated temperature/low relative humidity PEFC operation. J. Membr. Sci. 2006, 279, 506–512. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y. Proton conducting composite membranes from sulfonated polyethersulfone Cardo and phosphotungstic acid for fuel cell application. J. Power Sources 2006, 162, 541–546. [Google Scholar] [CrossRef]
- Choi, J.; Wycisk, R.; Zhang, W.; Pintauro, P.N.; Lee, K.M.; Mather, P.T. High conductivity perfluorosulfonic acid nanofiber composite fuel-cell membranes. ChemSusChem 2010, 3, 1245–1248. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.M.; Wycisk, R.; Pintauro, P.N.; Mather, P.T. Sulfonated Polysulfone/POSS Nanofiber Composite Membranes for PEM Fuel Cells. J. Electrochem. Soc. 2010, 157, B914. [Google Scholar] [CrossRef]
- Maneeratana, V.; Bass, J.D.; Azaïs, T.; Patissier, A.; Vallé, K.; Maréchal, M.; Gebel, G.; Laberty-Robert, C.; Sanchez, C. Fractal Inorganic−Organic Interfaces in Hybrid Membranes for Efficient Proton Transport. Adv. Funct. Mater. 2013, 23, 2872–2880. [Google Scholar] [CrossRef]
- Laberty-Robert, C.; Vallé, K.; Pereira, F.; Sanchez, C. Design and properties of functional hybrid organic-inorganic membranes for fuel cells. Chem. Soc. Rev. 2011, 40, 961–1005. [Google Scholar] [CrossRef]
- Dos Santos, L.; Maréchal, M.; Guillermo, A.; Lyonnard, S.; Moldovan, S.; Ersen, O.; Sel, O.; Perrot, H.; Laberty-Robert, C. Proton Transport in Electrospun Hybrid Organic-Inorganic Membranes: An Illuminating Paradox. Adv. Funct. Mater. 2016, 26, 594–604. [Google Scholar] [CrossRef]
- Ballengee, J.B.; Pintauro, P.N. Composite fuel cell membranes from dual-nanofiber electrospun mats. Macromolecules 2011, 44, 7307–7314. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.M.; Wycisk, R.; Pintauro, P.N.; Mather, P.T. Nanofiber composite membranes with low equivalent weight perfluorosulfonic acid polymers. J. Mater. Chem. 2010, 20, 6282. [Google Scholar] [CrossRef]
- Ballengee, J.B.; Haugen, G.M.; Hamrock, S.J.; Pintauro, P.N. Properties and Fuel Cell Performance of a Nanofiber Composite Membrane with 660 Equivalent Weight Perfluorosulfonic Acid. J. Electrochem. Soc. 2013, 160, F429–F435. [Google Scholar] [CrossRef]
- Laforgue, A.; Robitaille, L.; Mokrini, A.; Ajji, A. Fabrication and Characterization of Ionic Conducting Nanofibers. Macromol. Mater. Eng. 2007, 292, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Choi, J.; Wycisk, R.; Pintauro, P.N.; Mather, P. Nafion Nanofiber Membranes. ECS Trans. 2009, 25, 1451–1458. [Google Scholar] [CrossRef]
- Germer, W.; Harms, C.; Tullius, V.; Leppin, J.; Dyck, A. Comparison of conductivity measurement systems using the example ofnafion and anion exchange membrane. Solid State Ionics 2015, 275, 71–74. [Google Scholar] [CrossRef]
- Park, J.W.; Wycisk, R.; Pintauro, P.N. Nafion/PVDF Nanofiber Composite Membranes for Regenerative Hydrogen/Bromine Fuel Cells. J. Membr. Sci. 2015, 490, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Del Río, C.; Morales, E.; Escribano, P.G. Nafion/sPOSS hybrid membranes for PEMFC. Single cell performance and electrochemical characterization at different humidity conditiions. Int. J. Hydrogen Energy 2014, 39, 5326–5337. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Chanthad, C.; Gadinski, M.R.; Hickner, M.A.; Wang, Q. Acid-functionalized polysilsesquioxane-nafion composite membranes with high proton conductivity and enhanced selectivity. ACS Appl. Mater. Interfaces 2009, 1, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
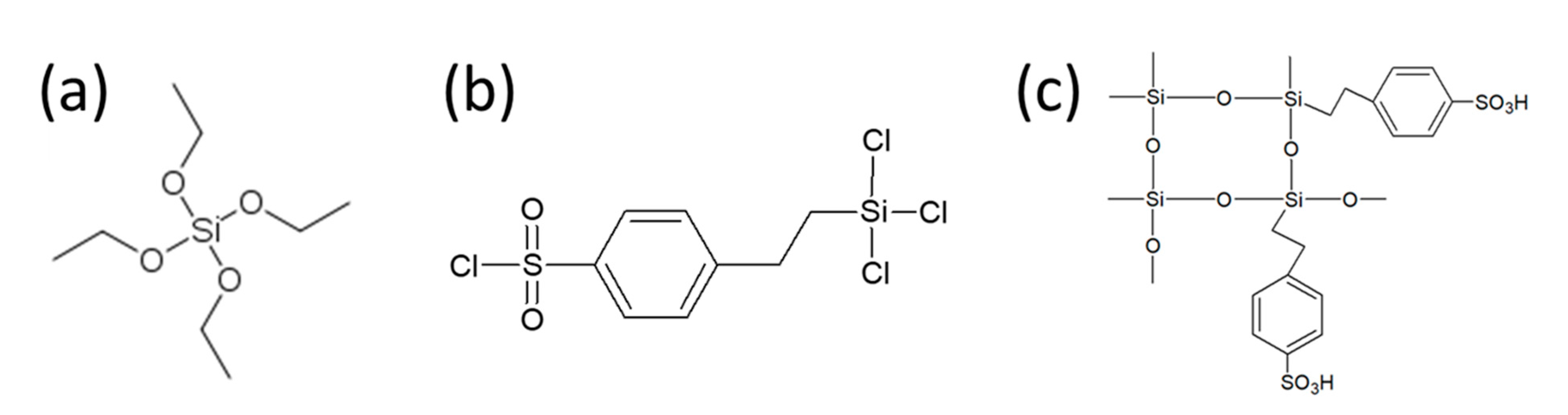


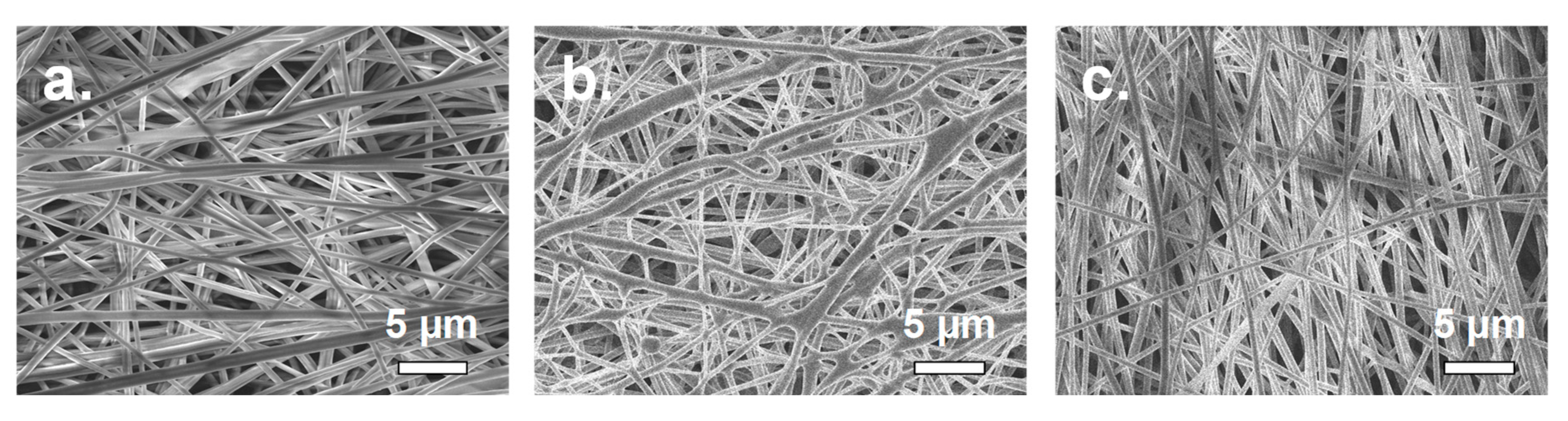



| Characteristics of Electrospinning Process | Membrane A | Membrane B | Membrane C | |||
|---|---|---|---|---|---|---|
| Fiber 1 | Fiber 2 | Fiber 1 | Fiber 2 | Fiber 1 | Fiber 2 | |
| PFSA/SiOxSO3H/PEO | PVDF | PFSA/PEO | PVDF/SiOxSO3H | PFSA/PEO | PVDF | |
| Solution Composition (wt./wt.) | 69/30/1 | - | 99/1 | 60/40 | 99/1 | - |
| Voltage (kV) | 9.0 | 9.0 | 8.0 | 13.0 | 8.0 | 9.0 |
| Solution Flow Rate (mL/h) | 0.5 | 0.16 | 0.25 | 0.25 | 0.5 | 0.16 |
| Spinneret to Collector Distance (cm) | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Relative Humidity (%) | 30 | 30 | 30 | |||
| Membrane Final Composition | 65-15/20 PFSA-SiOxSO3H/PVDF | 65/15-20 PFSA/SiOxSO3H-PVDF | 80/20 PFSA/PVDF | |||
| Ionomer Fiber Composition Before Sol-Gel Reaction | Ionomer/PVDF Fiber Wt. Ratio | Final Membrane Composition | |||
|---|---|---|---|---|---|
| 825 EW PFSA wt.% | (TEOS + CSPTC) wt.% | 825 EW PFSA wt.% | SiOxSO3H wt.% | PVDF wt.% | |
| 100 | 0 | 80/20 | 80 | 0 | 20 |
| 80 | 20 | 80/20 | 71 | 9 | 20 |
| 70 | 30 | 80/20 | 65 | 15 | 20 |
| 50 | 50 | 80/20 | 54 | 26 | 20 |
| Type-A Membranes (PFSA-SiOxSO3H/PVDF) | IEC (mmol/g) | |
|---|---|---|
| Measured | Theoretical | |
| 71-9/20 | 1.37 | 1.26 |
| 65-15/20 | 1.46 | 1.45 |
| 54-26/20 | 1.10 | 1.80 |
| 80/20 (Membrane Type-C) | 0.96 | 0.96 |
| Membrane Type | Membrane Composition (wt./wt.) | IEC (mmol/g) | Conductivity in Liquid Water at 20 °C (S/cm) | Swelling in Water at 20 °C | |
|---|---|---|---|---|---|
| Gravimetric (%) | In-Plane (%) | ||||
| Type-A | 65-15/20 PFSA-SiOxSO3H/PVDF | 1.46 | 0.089 | 49 | 12 |
| Type-B | 65/15-20 PFSA/SiOxSO3H-PVDF | 1.42 | 0.059 | 68 | 12 |
| Type-C | 80/20 PFSA/PVDF | 0.96 | 0.087 | 52 | 12 |
| Type-D | 65-15-20 PFSA-SiOxSO3H-PVDF | 1.44 | 0.059 | 23 | 16 |
| Type-E | Cast 825 EW PFSA | 1.21 | 0.102 | 47 | 38 |
| Membrane Type | Membrane Composition (wt./wt.) | Stress at Break (MPa) | Strain at Break (%) | Tensile Modulus (MPa) |
|---|---|---|---|---|
| Membrane Type-A | 65-15/20 PFSA-SiOxSO3H/PVDF | 14 | 134 | 152 |
| Membrane Type-B | 65/15-20 PFSA/SiOxSO3H-PVDF | 18 | 78 | 116 |
| Membrane Type-C | 80/20 PFSA/PVDF | 12 | 85 | 138 |
| Membrane Type-D | 65-15-20 PFSA-SiOxSO3H-PVDF | 10 | 49 | 187 |
| Membrane Type | IEC (mmol/g) | Conductivity in Water at 25 °C (S/cm) | Gravimetric Swelling in Water at 25 °C (%) | In-Plane Swelling in Water at 25 °C (%) | Stress at Break at 22 °C and 20% RH (MPa) |
|---|---|---|---|---|---|
| 660 EW PFIA | 1.51 | 0.13 | 120 | 35 | 7 |
| Nanofiber Membrane Type-A | 1.46 | 0.09 | 49 | 12 | 14 |
| Membrane Type | In-Plane Proton Conductivity (S/cm) | Comments |
|---|---|---|
| Membrane Type-A (this work) | 0.046 | 825 EW PFSA with a PFSA/SiOxSO3H ratio of 65/15 with PVDF reinforcing fibers |
| Sulfonated poly(arylene ether sulfone) (sPAES) fibers with sPOSS | 0.035 | 2.1 mmol/g IEC sPAES and a sPAES/sPOSS ratio of 60/40, with 30% Norland Optical Adhesive * as the reinforcing polymer (from reference [28]) |
| PFSA fibers with sPOSS | 0.083 | 825 EW PFSA and a PFSA/sPOSS with 26% Norland Optical Adhesive * as the reinforcing polymer (from reference [27]) |
| Solution-cast Nafion with sPOSS | 0.040 | Nafion with 2% sPOSS, From reference [39] |
| Solution-cast 1100 EW Nafion with sol-gel sulfonated silica | 0.020 | 1.28 mmol/g membrane IEC, from reference [40] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.D.; Powers, D.; Wycisk, R.; Pintauro, P.N. Electrospun Hybrid Perfluorosulfonic Acid/Sulfonated Silica Composite Membranes. Membranes 2020, 10, 250. https://doi.org/10.3390/membranes10100250
Santos LD, Powers D, Wycisk R, Pintauro PN. Electrospun Hybrid Perfluorosulfonic Acid/Sulfonated Silica Composite Membranes. Membranes. 2020; 10(10):250. https://doi.org/10.3390/membranes10100250
Chicago/Turabian StyleSantos, Leslie Dos, Devon Powers, Ryszard Wycisk, and Peter N. Pintauro. 2020. "Electrospun Hybrid Perfluorosulfonic Acid/Sulfonated Silica Composite Membranes" Membranes 10, no. 10: 250. https://doi.org/10.3390/membranes10100250
APA StyleSantos, L. D., Powers, D., Wycisk, R., & Pintauro, P. N. (2020). Electrospun Hybrid Perfluorosulfonic Acid/Sulfonated Silica Composite Membranes. Membranes, 10(10), 250. https://doi.org/10.3390/membranes10100250








